Abstract
Background
Cognitive impairment is a major health concern among older Mexican Americans, associated with significant morbidity and mortality, and may be influenced by environmental exposures.
Objectives
To investigate whether agricultural based ambient organophosphorus (OP) exposure influences 1) the rate of cognitive decline and mortality and 2) whether these associations are mediated through metabolic or inflammatory biomarkers.
Methods
In a subset of older Mexican Americans from the Sacramento Area Latino Study on Aging (n=430), who completed modified mini-mental state exams (3MSE) up to 7 times (1998–2007), we examined the relationship between estimated ambient OP exposures and cognitive decline (linear repeated measures model) and time to dementia or being cognitively impaired but not demented (CIND) and time to mortality (cox proportional hazards model). We then explored metabolic and inflammatory biomarkers as potential mediators of these relationships (additive hazards mediation). OP exposures at residential addresses were estimated with a geographic information system (GIS) based exposure assessment tool.
Results
Participants with high OP exposure in the five years prior to baseline experienced faster cognitive decline (β=0.038, p=0.02) and higher mortality over follow-up (HR=1.91, 95% CI=1.12, 3.26). The direct effect of OP exposure was estimated at 241 (95% CI = 27 to 455) additional deaths per 100,000 person-years, and the proportion mediated through the metabolic hormone adiponectin was estimated to be 4% (1.5 to 19.2). No other biomarkers were associated with OP exposure.
Conclusions
Our study provides support for the involvement of OP pesticides in cognitive decline and mortality among older Mexican Americans, possibly through biologic pathways involving adiponectin.
Keywords: Organophosphates, cognitive decline, mortality, mediation, adiponectin, Mexican Americans
Introduction
Cognitive impairment is a major health concern for older adults, which threatens to become even more prominent with increasing life expectancy and the aging of populations1,2. Due to the current limitations of treatments for impairment, primary prevention is imperative for reducing this burden. Few risk factors for cognitive impairment or dementia have been established. These include age, apolipoprotein E allele ε4 (APOE4), cerebrovascular diseases, and type 2 diabetes3–6.
Unlike other chemicals, pesticides are designed to impact living systems (http://www.cdc.gov/niosh/docs/81-123/). Many target the nervous system, with potential health consequences among exposed populations7. More than a billion pounds of pesticides are used annually in the United States8, the majority in agricultural applications. Organophosphorus pesticides (OPs) are among the most acutely toxic and commonly used insecticides8,9. Acute OP exposure is widely associated with significant increases in morbidity, including neurocognitive impairment and mortality9. The potential toxic effects of long-term low-level OP exposure are less clear. Though, there is increasing evidence linking low-level exposure to OPs with impaired cognitive and neurobehavioral function, among other health outcomes10–12. OPs may influence cognitive function both via the targeted neurotoxic cholinergic stimulation as well as their ability to induce inflammation, oxidative stress, and mitochondrial dysfunction in the nervous system or other less well understood neuropathologic mechanisms9,13. Furthermore, multiple studies have associated OP exposure with type 2 diabetes, a well-recognized risk factor for cognitive decline14,15.
Certain populations are known to disproportionately experience OP exposure. According to the Centers for Disease Control and Prevention, Hispanics of Mexican descent living in the United States have 1.3 to 5 times the amount of OP pesticide metabolites in urine than non-Hispanic whites16,17, suggesting higher levels of exposure. In California, as many as 91 percent of agricultural workers are of Mexican descent17,18. Communities and family members of agricultural workers may also be exposed from drift of ambient pesticides following aerial crop spraying or from pesticides in dust and from volatilization after applications to fields19. Furthermore, individuals of Hispanic ancestry are nearly 1.5 times more likely to develop dementia or Alzheimer’s disease than non-Hispanic whites20,21. Much of this has been attributed to high rates of diabetes among other risk factors.
Here we aim to examine the impact of residential proximity to agricultural OP application on cognitive functional decline and mortality during 10 years of follow-up among older Mexican Americans living in the Sacramento region of California. Further, to help understand potential biologic pathways, in secondary, exploratory analysis we will examine metabolic, inflammatory, and neurodegenerative biomarkers as possible mediators of OP exposure and morbidity/mortality associations.
Methods
All procedures described here were approved by the Institutional Review Boards of the University of California San Francisco, Los Angeles, and Davis and the University of Michigan.
Study Population
For these analyses we relied on a subset of the Sacramento Area Latino Study on Aging (SALSA). SALSA is a population-based cohort of older Mexican Americans living in the Sacramento Valley area of California designed to investigate metabolic and cardiovascular risk factors for dementia. A total of 1,789 participants, aged 60 years and over and self-identified as Latino, were enrolled between 1998 and 1999. Participants were interviewed in their homes every 12–15 months for up to seven study visits, ending in 2007. A detailed description of study sampling and procedures has been published22.
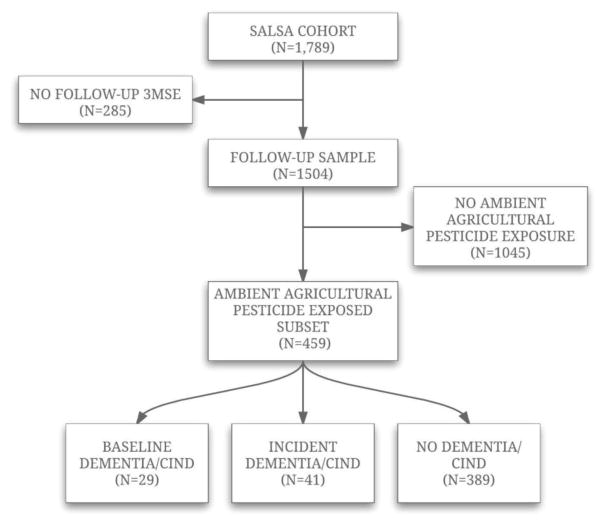
In order to assess ambient pesticide exposure, we used California state mandated pesticide use reports (see below). However, California does not require pesticide applicators to submit the coordinates of non-agricultural pesticide applications (right-of way, structural, etc.) mainly found in urban environments. Thus, it is difficult to estimate ambient pesticide exposure from these major sources for urban participants at a better than county-wide spatial scale. Therefore, we restricted our study population to a subset of the SALSA population comprised of 459 participants who were exposed to at least one agricultural use chemical – thus designating their residences as being in proximity to agricultural fields - and who had at least one follow-up cognitive evaluation. We further excluded 29 participants who had baseline dementia/CIND, leaving 430 participants for analysis. A flow chart of study participants is detailed in figure 1.
Figure 1.
Flow chart to SALSA participants used in analysis.
Pesticide Exposure Assessment
For our record-based, environmentally constructed exposure assessment, we estimated ambient exposure to OP pesticides from commercial agricultural pesticide applications in proximity to each participant’s residential address. This exposure assessment method uses a geographic information systems (GIS) based computer model (Cockburn et al. 2011), which links California state mandated (since 1974) pesticide use reports (CA-PUR) from commercial agricultural application23, land use surveys providing locations of specific crop24, and geocoded residential addresses for each participant. For each pesticide reported to the CA-PUR, we estimated the pounds applied each year within a 500-m buffer of each residential address of our participants.
For each participant, we have residential addresses at baseline interview and self-reported information on how long the participant lived at the location. We limited exposure assessment to the 5 years prior to baseline, as 93.5% of the study population reported living at their baseline residential location for 5 years or more, while only 83% reported living at the address for 10 or more and 62% for 20 or more years. There are 24 different pesticides that were applied within residential proximity to our population that are considered OPs according to the pesticide action network (PAN) pesticide database, see supplemental table S1 for a list of chemicals25. For each of these 24 OP pesticides, we calculated the yearly average pounds of pesticide applied within 500-m of each participants residence over the 5 years prior to baseline (i.e. total pounds applied over the 5 years/5). For participants who reported having moved within the 5 year exposure window, we calculated the yearly average based only on the years they reported living at the address, and also conducted sensitivity analysis excluding these participants.
Given the uncertainty in this assessment method (e.g. assuming the participant was at the recorded location during the relevant time period, wind patterns, etc.), we did not use the yearly average pounds of pesticide applied as a continuous variable. We dichotomized this average to specify those with high application near their residence and thus more likely to have ambient exposure. As the toxicity per poundage of each chemical is not necessarily similar across all OPs, we dichotomized the yearly average for each of the 24 OP pesticides according to the chemical-specific median of all exposed participants living in non-urban locations (all non-metropolitan areas based on Census tract 2000) (http://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx). We then counted the number of individual OP pesticides (of the 24) with high application in proximity to each participant’s individual residence; the range of this count was 0 to 9 OP pesticides per residence. We also dichotomized this OP count at or above the median, to create a high vs none/low OP indicator.
To address issues of application of non-OP pesticides within the buffers, for each participant, we also counted how many different non-OP pesticides were applied within 500-m of the individual residences (estimated exposure assessed in the same manner as described above); the range for this count is 0–65 non-OP pesticides.
Cognitive Function and Health Status
Cognitive function was assessed with the Modified Mini Mental State Exam (3MSE), a commonly used test of global cognitive function designed to minimize ceiling effects and enhance reliability of the Mini-Mental State Exam26. Higher scores, which range from 0 to 100, represent better cognitive function. A full neuropsychological test battery (Spanish English Neuropsychological Assessment Scales (SENAS)) with five scales, verbal and nonverbal measures of semantic memory, verbal attention span, verbal abstraction, and visual-perceptual ability27, was conducted on a subset of the participants determined by 3MSE and Spanish and English Verbal Learning Test (SEVLT) exams. All participants whose adjusted score was < 84 on the 3MSE or had a SEVLT delayed recall trial adjusted score < 7, 20th percentile on respective exams, were then referred for examination by a team of neurologists and neuropsychologists for a clinical evaluation. After baseline, all participants who declined from the baseline score by >3 points (SE of measurement) on the Verbal Episodic Memory test (delayed word list recall 0–15) or by >8 points on the 3MSE or whose current Verbal Episodic Memory or 3MSE test score was below the 20th percentile were referred for clinical evaluation.
All cases were classified as cognitively normal, cognitively impaired but not demented (CIND), or demented by the team of neurologists and neuropsychologists. Standard diagnostic criteria were applied for a diagnosis of dementia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition)28, AD (National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association)29, and vascular dementia (California Alzheimer’s Disease Diagnostic and Treatment Centers)30.
Diabetes was based on participant self-report of a diagnosis, fasting blood glucose ≥126, use of diabetes medication at each study visit, or diabetes listed as a cause on their death certificate. Mortality was monitored in the study population, with online surveillance of death notices, review of the Social Security Death Index, the National Death Index, vital statistics data from the state of California, and information from family members. Cause of death was extracted from death certificates using a multiple cause of death procedure. Of the 430 participants, 135 participants had passed away (83% of these deaths were confirmed with a death certificate).
Biomarker Measurement
Adiponectin, Homocysteine, Leptin, IL6, IL6 receptor 1, TNFα, TNFα receptor 1, hs-CRP, and Cortisol were measured and used in this analysis. At baseline, fasting blood was collected by venipuncture into evacuated tubes with and without EDTA. Tubes were transported on ice to the Medical Center Clinical Laboratory at the University of California, Davis within 4 hours of collection, where it was isolated and store at −80 °C. See supplementary materials for biomarker measurement methods. SALSA was originally designed to explore metabolic and cardiovascular risk factors for cognitive decline, thus biomarkers for environmental toxicants such as acetyl cholinesterase function for OP exposure were not assessed.
Statistical Analysis
We examined differences in demographic, exposure, and baseline health characteristics between high and low OP exposed groups using chi-square or t-tests. We assessed associations between OP exposure and the rate of change in cognitive function with repeated-measures regression analyses (Proc MIXED; SAS 9.4, SAS Institute, Cary, NC). We utilized an unstructured correlation structure for within-subject associations. As seen commonly for the Modified Mini Mental State Exams, the distribution of scores was right-skewed, thus we log-transformed errors on the 3MSE (log (101-3MSE score)) to move it to a normal distribution. Positive effect estimates from our statistical model thus correspond to more predicted log-transformed errors (lower 3MSE score).
To help account for practice effects, referring to improvements in cognitive test performance attributable to increased familiarity with the cognitive testing procedures, we included variables (yes/no) to indicate if the MSE was the first testing, where one would not expect practice effects, or second testing31. The interaction term between OP exposure and follow-up time (years) allows us to estimate the yearly difference in annual change in 3MSE score according to exposure groups. We show results for two OP exposure assessments, 1) the count score of the number of different OP pesticides applied heavily within 500-m of each residence, treated as a continuous variable (range 0–9), with associations shown per 1 unit increase, and 2) high/low OP exposure categories based on dichotomizing the OP count at the median in exposed participants. The models also controlled for baseline age, gender, baseline prevalent diabetes (yes/no), occupation during most of life (agricultural/non-agricultural), years of schooling, urban/rural residential location indicator, baseline body mass index (BMI), and grouped census tract. During model selection, we assessed interactions between each of these covariates and time (supplementary table S2). The only term which influenced the outcome (log (101-3MSE score)) was time*baseline age (p=0.089). However, the association estimates of interest (OP, OP x time) were very similar both with and without this interaction, and the AICC was lower in the model excluding time*baseline age. Thus, for our final model we did not include any interactions between covariates with time, other than the exposure of interest (OP exposure). In sensitivity analysis, we also controlled for potential ambient exposure to non-OP pesticides by including a count of non-OP pesticides applied in the 500-m buffer (continuous).
To further examine the impact of OP exposure in the population, we also conducted time to event analyses with Cox regression (Proc PHREG; SAS 9.4, SAS Institute, Cary, NC), for time to dementia/CIND and time to death. The time scale for dementia/CIND was years from baseline to date of dementia/CIND, last interview or death, and time from baseline to death or censoring for survival analysis. We estimated hazard ratios and 95% confidence intervals.
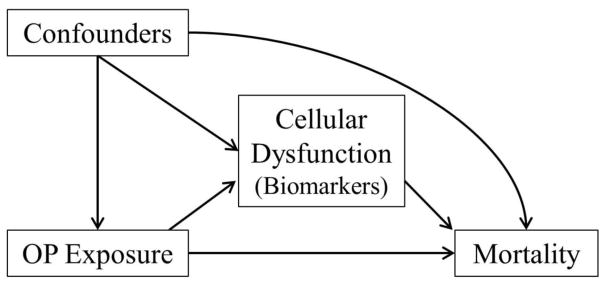
In secondary, hypothesis-generating analyses, we explored potential biologic pathways of OP exposure and time to dementia/CIND and survival with mediation analysis. We considered baseline inflammatory and metabolic biomarkers as potential mediators of OP-survival (see figure 2). With the mediation analysis, we aimed to quantify the degree to which the association between OP exposure dementia/CIND and mortality was mediated by levels of measured biomarkers. We used the marginal structural approach first proposed by Lange et al32, based on the counterfactual framework (see Pearl 2012)33. This method involves two steps, first, estimation of effects of OP exposure on the potential mediators (by linear regression; table 3), and then estimation of both OP exposure and the mediator on survival by fitting an Aalen additive hazard model adjusted for the same baseline confounders. Only biomarkers associated with OP exposure (step 1; table 3) were fit with the Aalen mediator model (step 2). We used this method as it allows us to investigate continuous mediators, and produces a readily interpretable risk difference between exposure groups, information that is complimentary to the hazard ratios.
Figure 2.
Model for mediation analysis exploring potential biologic pathways of the association between OP pesticide exposure and time to event outcomes (dementia/CIND and mortality).
Table 3.
Regression coefficients to describe relationship between high and no/low OP exposure and potential mediators, baseline biomarkers (dependent variables in regression models).
|
|
|||||
|---|---|---|---|---|---|
| Mean Biomarker Levels by OP Exposure | Linear Regression Outputa | ||||
|
|
|||||
| Biomarker (mean ± SD) | High OP Exposure (n=50) | Low/No OP Exposure (n=380) | β | SE | P-value |
| Adiponectin ng/mL | 10471.7 ± 4940.7 | 12297.1 ± 5632.1 | −1698.43 | 787.08 | 0.032 |
| Leptin ng/mL | 14.9 ± 10.2 | 20.7 ± 16.9 | −2.17 | 1.81 | 0.232 |
| Homocysteine μmol/L | 12.1 ± 8.0 | 10.7 ± 4.5 | 1.02 | 0.77 | 0.185 |
| IL6 pg/mL | 5.2 ± 5.9 | 4.9 ± 4.8 | 0.34 | 0.76 | 0.658 |
| TNFα pg/mL | 4.6 ± 2.6 | 4.2 ± 3.4 | 0.40 | 0.53 | 0.460 |
| hs-CRP mg/L | 5.1 ± 8.0 | 5.2 ± 8.5 | −0.11 | 1.32 | 0.931 |
| Cortisol ug/dL | 13.5 ± 5.0 | 13.3 ± 5.0 | 0.23 | 0.78 | 0.766 |
Abbreviations: hs-CRP=High-sensitivity C-reactive protein
β represents the difference in biomarker level in high OP exposure relative to none/low
Models also adjust for baseline age, gender, baseline diabetes, baseline BMI, rural/urban residential location, occupation in agriculture, years of schooling, census tract indicator
This model yields estimates of absolute change in the dementia/CIND or death rate in high exposure vs none/low OP exposure (reference) groups. The estimates are interpreted as the number of additional deaths per 100,000 person-years at risk, when compared with the reference group. The mediated proportion was computed as IE/TE. For the direct effect, 95% confidence intervals (CIs) were readily available from the additive hazards model, while 95% CIs for the indirect and mediated proportion were estimated by repeating the analysis on 10,000 bootstrapped samples. We tested for exposure-by-mediator interactions by including cross-product terms, but found no indication of interaction. See Lange et al, 2012 and Nordahl et al 2014 for a more detailed discussion and coding tutorials using R32,34.
Repeated measures and Cox regression analysis was done with SAS 9.4 (SAS Institute Inc., Cary, NC) and mediation in R version 2.10.1 (http://cran.rproject.org).
Results
We did not find any differences in demographic and baseline health indicators between the whole SALSA population and the subset of participants exposed to at least one agricultural use pesticide (supplementary table S3). A higher proportion of participants lived in micro/rural locations in the exposed subset relative to the whole SALSA cohort. The median follow-up in our subset was 6.5 years (SD=2.3) and the mean baseline age 70.4 years (SD=6.8).
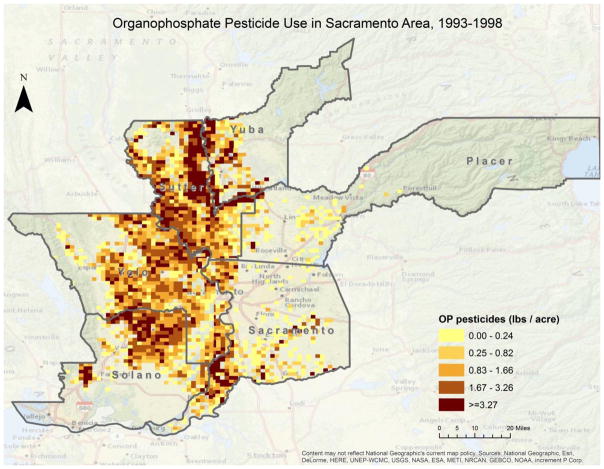
Among the cohort of participants with any residential pesticide exposure (n=430), 20% had worked in agriculture (supplemental table S3). A larger proportion of participants with high OP exposure lived in rural environments and worked in agricultural occupations compared with those in low/no OP exposures. The high exposure group was also comprised of more men and participants born in the United States, though these differences were not statistically significant (table 1). Of the subset, 223 participants lived within 500-meters of any application of at least one of the OP chemicals in the 5 years prior to baseline. In total, just over 33,670.60 lbs of OP pesticides were applied within 500-meters of participant’s residential addresses in this timeframe. In terms of pounds applied in proximity to the individual participant’s residences, among the 223 participants who lived in proximity to OP application, the 5-year range was 0.001 lbs to 1508.9 lbs, mean 151.0 lb, and median 11.9 lbs. The distribution of agricultural OP pesticide use in the Sacramento Study area can be seen in figure 3.
Table 1.
Health, exposure, and demographic characteristics of the pesticide exposed SALSA participant cohort, with at least two 3MSE evaluations.
| Variable Mean ± SD/n (%) |
High OP Exposure | Low/No OP Exposure | |
|---|---|---|---|
|
| |||
| N=50 | N=380 | P-value* | |
| Baseline Age (y) | 70.50 ± 6.7 | 70.44 ± 6.7 | 0.95 |
| Urban Residential Location | 12 (24) | 255 (67) | <.0001 |
| Baseline Diabetes | 15 (30) | 118 (31) | 0.71 |
| Born in Mexico | 18 (37) | 190 (50) | 0.08 |
| Male | 27 (54) | 158 (42) | 0.10 |
| Education (y) | 5.89 ± 4.4 | 6.79 ± 5.3 | 0.26 |
| Occupation | |||
| Non-Agricultural | 34 (38) | 309 (81) | |
| Agricultural | 16 (32) | 71 (19) | |
| OP Count | 6.18 ± 1.2 | 0.34 ± 0.90 | <.0001 |
| Range | (5–9) | (0–4) | |
| Mean among OP exposed | 6.18 ± 1.2 | 2.11 ± 1.2 | <.0001 |
| Any OP Exposure | 50 (100) | 61 (16) | <.0001 |
Abbreviations: OP=organophosphorus, CIND=cognitive impairment no dementia
P-values based on t-test or chi-square between high and low/no OP exposure groups
Figure 3.
Distribution of agricultural OP application in the Sacramento Area study area; 33,670.60 lbs were applied within 500m of participant’s residence. Descriptive measures of OP application: range per residence=0.001 lbs/5y to 1508.9 lbs/5y, mean 151.0 lbs/5y, and median 11.9 lbs/5y.
Using chemical specific medians, we identified 111 participants with likely ambient exposure to at least 1 OP chemical (residential proximity to agricultural use (lbs/acre applied) above the median). The mean OP count score among these participants was 3.9 (SD=2.4; median=4). Based on dichotomizing the OP count, 50 of these participants were considered highly OP exposed, with a mean count of 6.2 OP chemicals (SD=1.2), compared with 0.3 (SD=0.9) in the low/no exposure group (table 1).
Using repeated measures linear regression, we estimated that those with residential proximity to high agricultural OP application experienced faster cognitive decline over time on the 3MSE. This was found across both our continuous OP count score (exposure 1) and high OP dichotomized measure (exposure 2). According to predicted values, those with higher exposures started out with similar errors on the 3MSE, but declined faster over follow-up, e.g. had more log transformed errors (OP x time (y): exposure 1: β=0.006 per 1 OP per year, p=0.030; exposure 2: β=0.038 per year, p=0.017; table 2).
Table 2.
Regression coefficients (longitudinal models: log (errors on Modified Mini Mental State Exam)) and hazard ratios (Cox time to event models) to describe relationship between OP exposure and time to dementia/CIND and mortality.
|
|
||||||
|---|---|---|---|---|---|---|
| Parameters | Exposure Assessment 1: OP Count, continuous (per 1 unit, range 0–9) | Exposure Assessment 2: OP Count dichotomized at median | ||||
|
| ||||||
| Longitudinal Model | β | 95% CI | P-value | β | 95% CI | P-value |
| OP Exposure | −0.020 | (−0.047, 0.007) | 0.163 | −0.139 | (−0.315, 0.037) | 0.123 |
| Time (y) | 0.064 | (0.046, 0.082) | <.0001 | 0.064 | (0.046, 0.082) | <.0001 |
| OP Exposure x time | 0.006 | (0.0001, 0.012) | 0.030 | 0.038 | (0.005, 0.071) | 0.021 |
| Cox Models – Time to Event | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Time to Dementia/CIND | 1.09 | (0.94, 1.25) | 0.260 | 1.94 | (0.75, 5.03) | 0.171 |
| Time to Death | 1.12 | (1.03, 1.21) | 0.006 | 1.91 | (1.12, 3.26) | 0.017 |
Models included baseline age, gender, baseline diabetes, baseline BMI, rural/urban residential location, occupation in agriculture, years of schooling, census tract indicator, and wave 1 and wave 2 indicator (longitudinal model)
We estimated the same direction of association with our Cox models and time to dementia/CIND. High OP exposure was associated with faster time to dementia/CIND (exposure 1: HR=1.09 per 1 OP, 95% CI=0.94, 1.25; exposure 2: HR=1.94, 95% CI=0.75, 5.03). The confidence intervals contain the null likely due to our sample size was limited – of the 41 incident dementia/CIND cases only 6 were highly exposed to OPs. Both exposure assessments were also associated with an increased risk of death over follow-up (exposure 1: HR=1.12 per 1 OP, 95% CI=1.03, 1.21; exposure 2: HR=1.91, 95% CI=1.12, 3.26; table 2). These associations did not change notably when we limited analyses to participants who lived at their baseline address for the full 5 years prior to baseline interview or controlled for non-OP pesticide application (see supplemental table S4).
Table 3 shows both the mean levels of baseline inflammatory and metabolic biomarker levels, explored as potential mediators, by OP exposure group (high/low OP, exposure 2), and the results of linear regression analyses, treating the biomarker as the dependent variable and OP exposure as a predictor. While we did not find any associations with inflammatory markers (including IL6 and TNFα), we found OP pesticide exposure to be associated with adiponectin levels measured at baseline (table 3). As adiponectin levels had a wide range (579–30920 ng/mL), for subsequent mediation analysis we divided by the IQR (7364 ng/mL) such that our range was 0.21–4.18. Similar to the Cox models, comparing high OP exposure to none/low, we estimated a direct effect from high OP exposure of 92 (95% CI=−73 to 257) additional dementia/CIND cases per 100,000 person-years, and no indirect effect through adiponectin (table 4). For mortality, we estimated a direct effect of 241 (95% CI=27 to 455) additional deaths per 100,000 person-years, and a modest indirect effect of OP exposure mediated through adiponectin of 10 (95% CI=5 to 14) additional deaths per 100,000 person-years and each 1 unit increase in adiponectin IQR (or 3.8%; 95% CI=1.5% to 19.2%; table 4).
Table 4.
Rate Difference in Additional Deaths per 100,000 Person-Years by OP exposure, separated into direct and indirect effects through Adiponectin (mediator).
| Event | High OP Exposurea RD (95% CI) |
Proportion Mediated through Adiponectinb % (95% CI) |
|---|---|---|
| Dementia/CIND | ||
| Direct effect | 92 (−73 to 257) | |
| Indirect effectb | 0.3 (−0.6 to 1.2) | 0.3% (−3.6 to 4.5) |
| Survival | ||
| Direct effect | 241 (27 to 455) | |
| Indirect effectb | 10 (5 to 14) | 3.8% (1.5 to 19.2) |
RD=Risk Difference (Aalen additive hazards model)
Dichotomized OP exposure assessment; Reference category=None/Low
Indirect effect mediated per 1 IQR unit. Adiponectin levels (range 1579–30920 ng/mL) were divided by IQR (7364 ng/mL; IQR range 0.21–4.18). Proportion mediated is the ratio of indirect effect to total effect.
Models adjust for baseline age, gender, baseline diabetes, baseline BMI, rural/urban residential location, occupation in agriculture, years of schooling, census tract indicator
Discussion
Examining this SALSA sub-cohort of older Hispanic Mexican-Americans living near agricultural fields with pesticide applications, we found that residential proximity to high levels of agricultural OP pesticide applications was associated with faster rates of cognitive decline and an increased risk of death during follow-up. Our results also suggest high ambient exposure is associated with an increased risk of clinically defined dementia/CIND, though our sample size was limited and the 95% CIs contain the null. Furthermore, high OP exposure was predictive of lower levels of the metabolic hormone adiponectin, and we estimated a modest proportion of the OP-mortality association to be mediated through the lowering of these hormone levels.
Although OP use has declined in the last 20 years, an estimated 33 million pounds are still used in the United States annually, accounting for 35% of all insecticides applied8. OPs are designed to specifically target and inhibit acetylcholinesterase enzyme activity of insects, resulting in an excess of cholinergic stimulation, acutely affecting the motor and central nervous system35. This intended neurotoxic function also likely contributes to harm in the nervous system of humans. Additionally, after decades of research, it is also well accepted that OPs can induce oxidative stress, mitochondrial dysfunction, and neuroinflammation9,36. Furthermore, research targeting epigenetic effects of environmental toxicants and abnormal gene expression may present another direction for future research of chronic effects from OP exposure37.
Acute OP exposure has been implicated in a number of health problems, including cognitive outcomes such as deficits in information processing, sustained attention, memory, sequencing and problem solving, abstraction, flexibility of thinking, and also depressed mood9,38. Though research investigating low level exposure to OPs is less conclusive, chronic OP exposure at levels not considered acutely toxic has been associated with a variety of health issues, including cognitive deficits9,10,39–41. Two meta-analyses have reported associations between low level OP exposure and reduction in cognitive function10,39. The Agricultural Health Study, a large study of licensed pesticide applicators in the US, found that cumulative OP exposure, at long-term moderate levels, was cross-sectionally associated with an increased risk of experiencing neurologic symptoms, including cognitive dysfunction42. Two community-based cohorts have reported associations between pesticide exposure and dementia/Alzheimer’s disease (AD). One in Cache County, UT, found occupational pesticide exposure to be associated with an increased risk of dementia (n=3,084; HR=1.38, 95% CI=1.09–1.76)43. Another in France reported cognitive performance was worse among those occupationally exposed to pesticides (insecticides, herbicides, or fungicides), and found in analysis restricted to men that occupational exposure was associated with AD (n= 1,507; RR=2.39, 95% CI=1.02, 5.63)44. Though studies of chronic OP exposure are not univocal, and some report no associations (review: Colosio et al 2003)45.
Our OP exposures were not only associated with cognition, but also mortality. OP exposure has been associated with adverse health outcomes beyond neurotoxicity and neurologic dysfunctions, including metabolic dysfunction (hyperglycemia, type 2 diabetes)46,15. Our mortality findings may be a reflection of this, with OP exposure adversely impacting different biologic pathways in different people ultimately leading to an increased risk in all-cause mortality in the population. To explore such potential biologic pathways of OP exposure, we performed mediation analysis, first examining the influence of OP exposure on baseline inflammatory and metabolic biomarker levels. In our population, we found OP exposure was associated with lower measured levels of the metabolic hormone adiponectin, but not with other markers of inflammation.
Adiponectin has important roles in metabolic regulation through insulin sensitivity and glucose homeostasis mechanisms across many cell types47. Abnormal circulating adiponectin concentrations are associated with a variety of diseases, including type 2 diabetes, cognitive decline, and cardiac and pulmonary disease48,49. It is also implicated in two mechanisms of cellular dysfunction, oxidative stress and the induction of inflammatory cytokines50–53. However, we did not find a relationship between OP exposure and biomarkers for inflammation (including homocysteine, TNFα, or IL6) in our population. In mediation analysis, we estimated a modest indirect effect of OP exposure on survival through adiponectin, about 4% (95% CI=1.5 to 19.2%). This suggests that OP exposure may act through metabolic dysfunction. However it should be noted, that we cannot draw causal conclusions from mediation analysis as these models assume no unmeasured confounding of the OP-mortality, OP-adiponectin, and adiponectin-mortality relationships. Further research is needed to replicate and extend the current findings.
As cognitive decline is predictive of mortality, loss to follow-up due to earlier death may bias the reported associations between OP exposure and cognitive decline. We estimated positive associations between OP exposure and both cognitive decline and mortality and would expect any bias to result in an underestimation of the true effect of OPs on cognitive decline.
Since the California pesticide use reports do not include locations for non-agricultural applications, we opted to only investigate a subgroup of participants exposed to any type of agricultural pesticide as a proxy for residences near agricultural fields, which limited our sample size and statistical power. We were unable to assess ambient exposures at occupational addresses, though we controlled for occupation in analyses. We were also limited in our exposure window, as we only had access to residential locations at baseline. While 93.5% of participants reported living at the baseline address for at least the five years prior, only 62% had lived at their baseline address for 20 years. Thus we could not reliably estimate longer exposure windows. Additionally, our record-based exposure method is contingent on residential proximity to OP pesticide application. Residential proximity to application likely indicates some degree of ambient exposure, but we do not have actual biomarkers levels, so we expect some level of non-differential exposure misclassification. Socioeconomic status (SES) and other factors which co-vary with geographic locations may also confound these results. We controlled for SES related factors including education, occupation, and census tract; however unmeasured confounding due to geographic factors is possible.
SALSA, a longitudinal population-based study, with regular, long-term follow-up (up to 7 interviews, 10 years of follow-up), is one of few population-based prospective studies now investigating environmental exposures and cognitive function. We are the first to our knowledge to investigate these relationships among Mexican Americans, a population disproportionately exposed to pesticides. As discussed, the exposure assessment relied on a GIS tool and pesticide use and land use records. As a result we do not rely on participant recall, minimizing exposure misclassification from recall bias. The record-based method also allows us to investigate specific pesticides or chemical classes of interest, like OPs.
Conclusions
While further research is required to replicate and elucidate the role of OP pesticides in cognitive dysfunction and decline and mortality in elderly populations, we present evidence among older Mexican Americans that ambient OP exposure derived from residential proximity to agricultural pesticide application is associated with both faster cognitive decline and mortality. Furthermore, mediation analysis suggested that some of the association may be mediated through the metabolic hormone adiponectin. Thus, chronic OP exposure may influence cognition through multiple pathophysiologic pathways, including but not only metabolic function. Given the complexity of studying lifetime exposures that may influence cognitive decline in the elderly, future epidemiologic research dedicated to understanding how chronic exposures to OPs may act on intermediate biomarkers linked to cognitive decline may be key to disease prevention and future policy actions.
Supplementary Material
Highlights.
In a subset of older Mexican Americans from the Sacramento Area Latino Study on Aging, we examined the relationship between estimated ambient organophosphorus (OP) exposures and cognitive decline and mortality
Participants with high OP exposure in the five years prior to baseline experienced faster cognitive decline and higher mortality over follow-up
High OP exposure was predictive of lower levels of the metabolic hormone adiponectin, and we estimated a modest proportion of the OP-mortality association to be mediated through the lowering of adiponectin hormone levels
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences R01 ES023451, National Institute on Aging (grants AG012975 and AG033751), National Institute of Diabetes, Digestive, and Kidney Diseases DK060753, and KP was funded through a Burroughs Wellcome Fund Population and Laboratory Based Sciences Award and F32 ES028087. The authors would like to acknowledge and thank study participants, without whom the work would not be possible.
Footnotes
Financial Disclosure/Conflict of Interest
The authors declare no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weuve J, Hebert LE, Scherr PA, Evans DA. Prevalence of Alzheimer disease in US states. Epidemiology. 2015;26(1):e4–e6. doi: 10.1097/EDE.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 3.Ishii M, Iadecola C. Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochim Biophys Acta - Mol Basis Dis. 2016;1862(5):966–974. doi: 10.1016/j.bbadis.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalaria RN. Vascular basis for brain degeneration: Faltering controls and risk factors for dementia. Nutr Rev. 2010;68(SUPPL 2) doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res Int. 2014:2014. doi: 10.1155/2014/908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilvis RS, Kähönen-Väre MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59(3):268–274. doi: 10.1093/gerona/59.3.M268. [DOI] [PubMed] [Google Scholar]
- 7.Blair A, Ritz B, Wesseling C, Freeman LB. Pesticides and human health. 2015. [DOI] [PubMed] [Google Scholar]
- 8.Grube A, Donaldson D, Timothy Kiely A, Wu L. Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates. US Environ Prot Agency. 2011:41. http://nepis.epa.gov/Adobe/PDF/3000659P.pdf.
- 9.Terry aV. Functional consequences of repeated organophosphate exposure: Potential non-cholinergic mechanisms. Pharmacol Ther. 2012;134(3):355–365. doi: 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross SM, McManus IC, Harrison V, Mason O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol. 2013;43(1):21–44. doi: 10.3109/10408444.2012.738645. [DOI] [PubMed] [Google Scholar]
- 11.Zaganas I, Kapetanaki S, Mastorodemos V, et al. Linking pesticide exposure and dementia: What is the evidence? Toxicology. 2013;307:3–11. doi: 10.1016/j.tox.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10(6):RA141–A147. http://www.medscimonit.com/pub/vol_10/no_6/4163.pdf. [PubMed] [Google Scholar]
- 13.Banks CN, Lein PJ. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology. 2012;33(3):575–584. doi: 10.1016/j.neuro.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery MP, Kamel F, Saldana TM, Alavanja MCR, Sandler DP. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993–2003. Am J Epidemiol. 2008;167(10):1235–1246. doi: 10.1093/aje/kwn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starling AP, Umbach DM, Kamel F, Long S, Sandler DP, Hoppin JA. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup Environ Med. 2014;71(9):629–635. doi: 10.1136/oemed-2013-101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC, Centers for Disease Control and P. Third National Report on Human Exposure to Environmental Chemicals. Third Natl Rep Hum Expo to Environ Chem. 2005:475. http://www.cdc.gov/exposurereport/report.htm.
- 17.Quintero-Somaini A. Hidden Danger: Environmental Health Threats in the Latino Community. Natural Resources Defense Council; 2004. [Google Scholar]
- 18.Villarejo D, Lighthall D, Williams D, III, et al. Suffering in silence: A report on the health of California’s agricultural workers. 2000 [Google Scholar]
- 19.Baker LW, Fitzell DL, Seiber JN, et al. Ambient air concentrations of pesticides in California. Environ Sci Technol. 1996;30(4):1365–1368. doi: 10.1021/es950608l. [DOI] [Google Scholar]
- 20.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial group. Int J Geriatr Psychiatry. 1999;14(6):481–493. doi: 10.1002/(SICI)1099-1166(199906)14:6<481::AID-GPS959>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Post B, Merkus MP, De Haan RJ, Speelman JD. Prognostic factors for the progression of Parkinson’s disease: A systematic review. Mov Disord. 2007;22(13):1839–1851. doi: 10.1002/mds.21537. [DOI] [PubMed] [Google Scholar]
- 22.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: The influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51(2):169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 23.CDPR. [Accessed July 28, 2015];California Department of Pesticide Regulation Product/Label Database. 2013 http://www.cdpr.ca.gov/docs/label/labelque.htm.
- 24.CDWR. [Accessed July 28, 2015];California Department of Water Resources Land Use Surveys. 2013 http://www.water.ca.gov/landwateruse/lusrvymain.cfm.
- 25.Kegley SE, Hill BR, Orme S, Choi AH. PAN Pesticide Database. Pesticide Action Network; North America (Oakland, CA): 2014. [Google Scholar]
- 26.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. http://www.ncbi.nlm.nih.gov/pubmed/3611032. [PubMed] [Google Scholar]
- 27.Mungas D, Reed BR, Marshall SC, González HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14(2):209–223. doi: 10.1037/0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Am Psychiatr Assoc. 2000 doi: 10.1176/appi.books.9780890423349. [DOI]
- 29.McKhann G, Drachman D, Folstein M, Katzman R, Price DSE. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 30.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42(3):473–480. doi: 10.1212/WNL.42.3.473. [DOI] [PubMed] [Google Scholar]
- 31.Mayeda ER, Haan MN, Yaffe K, Kanaya AM, Neuhaus J. Does Type 2 Diabetes Increase Rate of Cognitive Decline in Older Mexican Americans? Alzheimer Dis Assoc Disord. 2015;29(3):206–212. doi: 10.1097/WAD.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176(3):190–195. doi: 10.1093/aje/kwr525. [DOI] [PubMed] [Google Scholar]
- 33.Pearl J. The mediation formula: A guide to the assessment of causal pathways in nonlinear models. Causality: Wiley Series in Probability and Statistics. 2012:151–179. doi: 10.1002/9781119945710.ch12. [DOI] [Google Scholar]
- 34.Nordahl H, Lange T, Osler M, et al. Education and cause-specific mortality: the mediating role of differential exposure and vulnerability to behavioral risk factors. Epidemiology. 2014;25(3):389–396. doi: 10.1097/EDE.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 35.Pope CN. ORGANOPHOSPHORUS PESTICIDES: DO THEY ALL HAVE THE SAME MECHANISM OF TOXICITY? J Toxicol Environ Heal Part B. 1999;2(2):161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- 36.Soltaninejad K, Abdollahi M. Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Med Sci Monit. 2009;15(3):RA75–A90. [PubMed] [Google Scholar]
- 37.Hodjat M, Rahmani S, Khan F, et al. Environmental toxicants, incidence of degenerative diseases, and therapies from the epigenetic point of view. Arch Toxicol. 2017;91(7):2577–2597. doi: 10.1007/s00204-017-1979-9. [DOI] [PubMed] [Google Scholar]
- 38.Malekirad AA, Faghih M, Mirabdollahi M, Kiani M, Fathi A, Abdollahi M. Neurocognitive, Mental Health, and Glucose Disorders in Farmers Exposed to Organophosphorus Pesticides. Arch Ind Hyg Toxicol. 2013;64(1):1–8. doi: 10.2478/10004-1254-64-2013-2296. [DOI] [PubMed] [Google Scholar]
- 39.Meyer-Baron M, Knapp G, Schäper M, van Thriel C. Meta-analysis on occupational exposure to pesticides - Neurobehavioral impact and dose-response relationships. Environ Res. 2015;136:234–245. doi: 10.1016/j.envres.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Burns CJ, McIntosh LJ, Mink PJ, Jurek AM, Li AA. Pesticide Exposure and Neurodevelopmental Outcomes: Review of the Epidemiologic and Animal Studies. J Toxicol Environ Heal Part B. 2013;16(3–4):127–283. doi: 10.1080/10937404.2013.783383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayrami M, Hashemi T, Malekirad AA, Ashayeri H, Faraji F, Abdollahi M. Electroencephalogram, cognitive state, psychological disorders, clinical symptom, and oxidative stress in horticulture farmers exposed to organophosphate pesticides. Toxicol Ind Health. 2012;28(1):90–96. doi: 10.1177/0748233711407243. [DOI] [PubMed] [Google Scholar]
- 42.Kamel F, Engel LS, Gladen BC, Hoppin Ja, Alavanja MCR, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum Exp Toxicol. 2007;26(3):243–250. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- 43.Hayden KM, Norton MC, Darcey D, et al. Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology. 2010;74(19):1524–1530. doi: 10.1212/WNL.0b013e3181dd4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003;157(5):409–414. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- 45.Colosio C, Tiramani M, Maroni M. Neurobehavioral effects of pesticides: State of the art. Neuro Toxicology. 2003;24:577–591. doi: 10.1016/S0161-813X(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 46.Karami-Mohajeri S, Abdollahi M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates. Hum Exp Toxicol. 2011;30(9):1119–1140. doi: 10.1177/0960327110388959. [DOI] [PubMed] [Google Scholar]
- 47.Turer AT, Scherer PE. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 48.Kishida K, Funahashi T, Shimomura I. Adiponectin as a routine clinical biomarker. Best Practice and Research: Clinical Endocrinology and Metabolism. 2014;28:119–130. doi: 10.1016/j.beem.2013.08.00611. [DOI] [PubMed] [Google Scholar]
- 49.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 50.Chan KH, Lam KSL, Cheng OY, et al. Adiponectin is Protective against Oxidative Stress Induced Cytotoxicity in Amyloid-Beta Neurotoxicity. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamogawa K, Kohara K, Tabara Y, et al. Abdominal fat, adipose-derived hormones and mild cognitive impairment: The J-SHIPP study. Dement Geriatr Cogn Disord. 2010;30(5):432–439. doi: 10.1159/000321985. [DOI] [PubMed] [Google Scholar]
- 52.Song J, Lee JE. Adiponectin as a new paradigm for approaching Alzheimer’s disease. Anat Cell Biol. 2013;46(4):229–234. doi: 10.5115/acb.2013.46.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teixeira AL, Diniz BS, Campos AC, et al. Decreased levels of circulating adiponectin in mild cognitive impairment and alzheimer’s disease. Neuro Molecular Med. 2013;15(1):115–121. doi: 10.1007/s12017-012-8201-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.