Abstract
Background
The Medicaid Incentives for Prevention of Chronic Diseases program was authorized by the Affordable Care Act to determine the effectiveness of providing financial incentives.
Objective
To examine the impact of incentives on adult Medicaid beneficiaries’ diabetes self-management using the Hawaii Patient Reward And Incentives to Support Empowerment project.
Methods
A randomized controlled trial study was conducted at Kaiser Permanente Hawaii with 320 participants (159 intervention group/161 control group). Participants could earn up to $320/y of financial incentives, distributed in the form of a debit card. Evaluation measures included 1) clinical outcomes of change in hemoglobin A1C, blood pressure, and cholesterol; 2) compliance with American Diabetes Association standards; 3) cost effectiveness; 4) quality of life; 5) self-management activities; and 6) satisfaction with incentives.
Results
No significant differences in clinical outcomes were found between groups. There were no differences in observance of American Diabetes Association standards of medical care between the intervention and control group. The project also did not show reduction in health cost. However, participants in the intervention group reported significantly higher adherence with the recommended general diet than those in the control group during the course of the study. They also reported statistically better physical health than their control counterparts at the midpoint of the study; however, the perception of increased physical health didn’t sustain to the end of the study. Participants’ satisfaction with incentives was high.
Conclusion
Overall, this study found no conclusive evidence that financial incentives alone had beneficial effects on improving standards of medical care in diabetes.
BACKGROUND
Beginning in 2014, the Patient Protection and Affordable Care Act (ACA)1 allowed states to expand Medicaid eligibility to cover individuals living up to 133% of the federal poverty level. A brief by the Kaiser Commission on Medicaid and the Uninsured predicted that the expansion of Medicaid under the health reform law would significantly increase the number of people covered by the program and reduce the uninsured in states across the country, with the federal government covering the vast majority of the cost.2 Health reform would offer Medicaid coverage to millions of low-income adults for the first time.
Millions of working adults in the US with chronic conditions do not have insurance and have less access to medical care than their insured counterparts.3 Adults with diabetes are disproportionately insured by Medicaid.4 Medicaid expansion under the ACA may further increase service utilization and improve access to care for low-income adults with diabetes.5,6 The estimated total economic cost of diagnosed diabetes in 2012 was $245 billion, including $176 billion in direct medical costs and $69 billion in reduced productivity.7 Diabetes is a public health problem with more than 20% of the health care spending in 2016 attributed to people with diagnosed diabetes.8 Section 4108 of the ACA created the Medicaid Incentives for Prevention of Chronic Diseases program to identify evidence-based practices that may impact the rising Medicaid costs of chronic diseases.9 Pre-ACA Medicaid beneficiary incentives programs have achieved mixed results on improving healthy behaviors, and some have faced skepticism from the health policy community.10 The goal of the Medicaid Incentives for Prevention of Chronic Diseases program was to determine the applicability of using incentives to improve health care for chronic diseases. The Medicaid Incentives for Prevention of Chronic Diseases provided a total of $85 million over 5 years to 10 states, including Hawaii, to test the effectiveness of providing incentives to Medicaid beneficiaries with chronic diseases who participated in evidence-based prevention programs to change their health risks through adoption of healthy behaviors and emphasized the importance of personal choice in determining health.
The overall objective of the Hawaii Patient Reward And Incentives to Support Empowerment (HI-PRAISE) project was to examine the impact of incentives on adult Medicaid beneficiaries’ diabetes self-management. Evaluation measures included the following: 1) clinical outcomes, 2) compliance with American Diabetes Association (ADA) standards of medical care in diabetes,11 3) cost-effectiveness, 4) well-being or quality of life, 5) self-management activities of diabetes, and 6) satisfaction with incentives.
METHODS
Setting and Participants
A randomized controlled trial (RCT) was conducted at Kaiser Permanente Hawaii (KPHI) from May 2014 to December 2015. Institutional review board approval was obtained from the University of Hawaii and KPHI. Eligible participants were established patients at KPHI aged 18 years or older, were Medicaid eligible, and had a known diagnosis of type 1 or type 2 diabetes. Those excluded from the HI-PRAISE project were the pediatric population and women with gestational diabetes. Subjects with conditions that would make participation infeasible (for example, inability to consent) and subjects with advanced terminal illness as well as those living in nursing homes, group homes, or other inpatient settings (eg, current treatment for substance abuse; metastatic cancer; enrolled in hospice) were excluded.
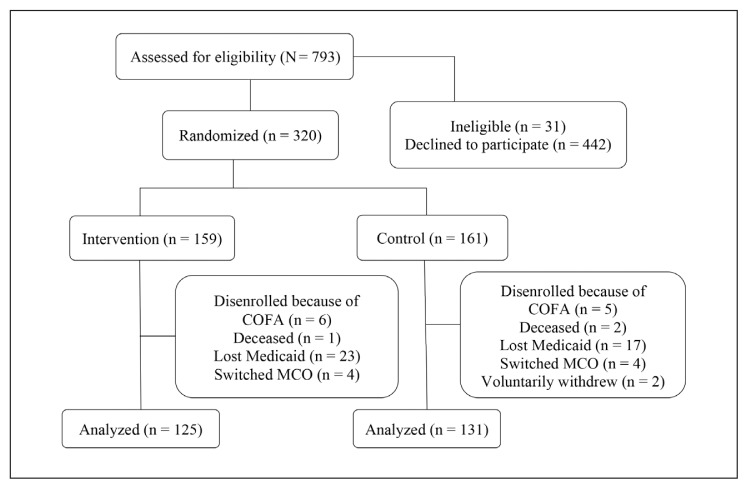
The RCT target population was adults enrolled in KPHI Quest Integration (Medicaid) receiving care coordination or standard care for diabetes. There were 793 potential participants assessed for eligibility. Participants were screened using designated criteria by the diabetes care coordinators and research team at KPHI. Eligible participants were contacted telephonically to determine their interest in the study. Participants who expressed a desire to enroll in the study provided oral consent and were assigned by the research team to either the intervention or control group through a random number table generated by the randomization function in statistics computing and graphics software R (R Foundation for Statistical Computing, Vienna, Austria). A packet was mailed to the participants, which included a written description about the HI-PRAISE project and surveys. The study recruited 320 (159 intervention group/161 control group) participants from May 2014 to January 2015. The recruitment period was extended beyond the initial 3 months because of slow enrollment. Neither the participants nor the study coordinator could be masked to group assignments owing to the nature of the intervention. Participants were followed-up for at least 12 months. Figure 1 shows the assessment for eligibility, randomization, and follow-up.
Figure 1.
Study flow diagram.1
1 Medicaid Incentives for Prevention of Chronic Diseases/Hawaii Patient Reward And Incentives to Support Empowerment Minimum Data Set.
COFA = Compact of Free Association; MCO = Managed Care Organization.
The research staff at KPHI received trainings on incentive distribution and data collection. Supplemental service payments were provided to KPHI for efforts in recruitment, enrollment, incentive distribution, and data collection. These payments amounted to $283 per participant/per year for the completion of these tasks for the intervention group and $175 per participant/per year for the control group. HI-PRAISE distributed a total of $138,625 in supplemental services to KPHI.
Intervention
Process and clinical outcome measures for diabetes were incentivized using a tiered approach to improve: Diabetes self-management, compliance with ADA standards of diabetes care, clinical outcomes, and promotion of a healthy lifestyle. The following items were incentivized: Monitoring of blood glucose ≤ $20, attendance of a diabetes education session ≤ $20, pneumococcal or influenza vaccination ≤ $10, retinal eye exam ≤ $20, urine for microalbumin test ≤ $10, cholesterol testing ≤ $20, glycated hemoglobin (HbA1C) testing ≤ $20, reduction of HbA1C by 1% ≤ $20, HbA1C at 7% goal ≤ $50, blood pressure control < 140/90 mmHg ≤ $20, and low-density lipoprotein cholesterol < 100 mg/dL ≤ $20. When applicable, participants were incentivized to adopt a healthy lifestyle change: Attend smoking cessation class ≤ $20, attend counseling for behavioral health ≤ $20, and achieve weight loss of 7% ≤ $50 for those with a body mass index (calculated as the weight in kilograms divided by the height in meters squared) ≥ 25.
Participants could earn up to a maximum of $320/y, from enrollment through December 2015. Only participants who were randomized into the intervention group were eligible for incentives for diabetes self-management or changes in clinical outcomes. Participants in the control group were not monetarily incentivized. Participants in both groups were compensated $25 each time for completion and return of the mail-in surveys at baseline, midpoint, and end of the study.
KPHI administered a debit card that allowed for electronic payment to participants upon achieving incentivized outcomes and/or for survey completion.12 The debit cards were mailed to the participants upon completion of enrollment. Before funds could be uploaded to any card, the card holder (research participant) was registered. Participant registration data was entered into the secure Web site of the debit card company. Data collected included name, address, telephone number, and date of birth. Cards had unique numbers that were assigned to individual participants and used for participant data tracking across the card’s technological system.13 The card was uploaded upon the participant’s completion of certain tests or achieving goals as aforementioned. Monetary uploads for earned incentives or survey compensation to the debit card was done by electronic bank fund transfer. The study coordinator at KPHI telephonically notified participants of goals achieved and distributed earned incentives.
Measures
Minimum Data Set included enrollment, demographic characteristics, services, incentives, and clinical outcomes. Demographic variables were collected on age, sex, race, ethnicity, insurance and Medicare status, and history of hypertension, heart disease, and smoking. Additionally, participation in diabetes education sessions, and type and amount of incentives distributed, were tracked. Clinical outcomes tracked by Minimum Data Set included weight, height, body mass index, blood pressure, fasting blood glucose, HbA1C, fasting lipid profile, renal function, smoking cessation, retinopathy, and influenza/pneumococcal vaccination status.
Survey data was collected from the participants in the intervention and control groups to assess and monitor changes over time in the well-being and self-management activities of diabetes. The surveys used include SF-36v2 Health Survey (Optum, Eden Prairie, MN),14 and Summary of Diabetes Self-Care Activities Measure (SDSCA).15 Surveys were mailed to participants at baseline, midpoint, and end of the study with a stamped return envelope included. A satisfaction survey was mailed to the participants in the intervention group at the end of the study.
The SF-36v2 is a multipurpose short-form survey with 36 questions that measures self-reported health status. Of the 36 total items, 35 items were transformed into 8 multi-item health domain scales containing 2 to 10 items each using Quality Metric Health Outcomes Scoring Software 4.5 (Quality Metrics, Inc; Lincoln, RI). These health domain scales are physical functioning, role limitation caused by physical health problems, bodily pain, general health, vitality (energy/fatigue), social functioning, role limitations caused by emotional problems, and mental health. The 8 health domain scales were further collapsed into 2 component (physical and mental) summary measures. Scores range from 0 to 100 with higher scores indicating better outcomes. Norm-based scoring was used so that scores for each health domain scale and component summary measure have a mean of 50 and standard deviation of 10 based on the 2009 US general population.
The SDSCA consists of 11 questions and addresses the following aspects of diabetes care: General diet, specific diet, exercise, blood-glucose testing, foot care, and smoking status and number of cigarettes smoked per day. The survey asks the respondent to recall and indicate the number of days the recommended diabetes self-care activities were performed during the past 7 days.
The HI-PRAISE satisfaction survey, developed by the project and administered at the end of the study, collected information from the participants regarding their level of satisfaction with respect to the incentives and program. The satisfaction with the incentives was scored on a Likert scale of 1 to 4, with 1 representing a low rating and 4 representing a high rating.
Statistical Analysis
Data were analyzed using SAS, version 9.4 (SAS Institute Inc, Cary, NC). Summary statistics and frequency distributions were used to describe the characteristics of the study participants. Generalized estimating equation (GEE) modeling techniques were used to examine the changes in health measures between groups at different time points after adjusting for age, sex, race, and hypertension at baseline. Likelihood ratio tests were used to choose the variance-covariance structure in the GEE models. Linear contrasts were used to examine the longitudinal changes in clinical outcomes for between-group differences. The average change in scores over time for the intervention group was compared with the average change in scores over time for the control group. The difference in these average changes is referred to as the difference-in-differences (difference in Δ) values, which was assessed for significance at p = 0.05. The estimated coefficients and corresponding 95% confidence intervals were used to quantify the differences in changes between the intervention and the control groups.
For SF-36v2 survey results, a GEE model with group, time, and interaction between group and time was used to examine the longitudinal changes between groups after adjusting for the potential confounding effects of age and sex. Similarly, for the SDSCA survey, a GEE model was fitted to the data with group, time, and the interaction between group and time. Linear contrasts and corresponding 95% confidence intervals were used to evaluate the differences between the intervention and control groups in their longitudinal changes from baseline to midpoint, from midpoint to endpoint, and from baseline to endpoint. In both GEE models, the variance-covariance structure was selected on the basis of the results from the likelihood ratio tests.
For cost analysis, expenses related to outpatient, inpatient, emergency room, skilled nursing, hospice, prescription drugs, and dental care were included. The summarized costs per patient/d were first log transformed to meet the assumption of normality in general linear models. General linear models with group, time, and interaction between group and time were used to estimate the cost difference between groups after adjusting for age, sex, race, Medicare status, and Compact of Free Association status. The estimated coefficients and standard errors for the interaction term (difference-in-differences) in the general linear models were used to estimate the causal effect of the intervention on medical costs per patient/d and the cost-effectiveness ratio was calculated.16
RESULTS
The mean age of the participants was 48.5 years and 47.8 years in the intervention and control groups, respectively. The sex distribution was similar between groups (Table 1). Native Hawaiian and Other Pacific Islander (34.0%) followed by multiple races (25.2%) were the majority in the intervention group. Multiple races (32.9%) followed by Native Hawaiian and Other Pacific Islander (29.8%) were most reported in the control group. Participants of Hispanic or Latino origin were 6.3% of the intervention group and 8.7% of the control group. Overall, 8.8% of the intervention group and 9.9% of the control group were Medicare and Medicaid dual-eligible. Comorbidities did not significantly differ (both p > 0.05) between groups on hypertension (72.3% in the intervention group and 65.8% in the control group) or heart disease (8.8% in the intervention group and 12.4% in the control group).
Table 1.
Descriptive statistics of sample in Hawaii Patient Reward and Incentives to Support Empowerment project (N = 320)1
| Characteristic | Intervention group, no. (%) (n = 159) | Control group, no. (%) (n = 161) |
|---|---|---|
| Sex | ||
| Women | 88 (55.3) | 86 (53.4) |
| Men | 71 (44.7) | 75 (46.6) |
| Race | ||
| White | 27 (17.0) | 22 (13.7) |
| Black | 1 (0.6) | 1 (0.6) |
| American Indian or Alaska Native | 1 (0.6) | 0 (0) |
| Asian | 32 (20.1) | 36 (22.4) |
| Native Hawaiian or Other Pacific Islander | 54 (34.0) | 48 (29.8) |
| Multiple races | 40 (25.2) | 53 (32.9) |
| Missing/unknown | 4 (2.5) | 1 (0.6) |
| Ethnicity | ||
| Hispanic or Latino | 10 (6.3) | 14 (8.7) |
| Missing/unknown | 4 (2.5) | 3 (1.9) |
| Eligibility | ||
| Compact of Free Association migrants | 6 (3.8) | 5 (3.1) |
| Dual-eligible (Medicaid/Medicare) | 14 (8.8) | 16 (9.9) |
| Age, years | ||
| 18–19 | 0 (0) | 0 (0) |
| 20–29 | 12 (7.5) | 10 (6.2) |
| 30–39 | 26 (16.4) | 25 (15.5) |
| 40–49 | 47 (29.6) | 49 (30.4) |
| 50–59 | 54 (34.0) | 61 (37.9) |
| 60–69 | 20 (12.6) | 16 (9.9) |
| ≥ 70 | 0 (0) | 0 (0) |
| Mean age, years (SD)a | 48.5 (10.7) | 47.8 (10.0) |
| Comorbidities | ||
| History of hypertension | 115 (72.3) | 106 (65.8) |
| History of heart disease | 14 (8.8) | 20 (12.4) |
| History of smoking or tobacco dependence | 47 (29.6) | 48 (29.8) |
All data are no. (%) except Mean age, years (SD).
CMS.gov. Medicaid incentives for the prevention of chronic disease model [Internet]. Baltimore, MD: Centers for Medicare and Medicaid Services; updated 2017 Aug 9 [cited 2016 Oct 29]. Available from: https://innovation.cms.gov/initiatives/mipcd.
SD = standard deviation.
During the course of the entire study, the total amount of incentives distributed to participants in the intervention group was $32,270. The average amount of incentives earned was $203; the highest amount was $360 and the lowest amount was $30. The average number of incentives received was 11. Most incentives were earned for completion of the HbA1C test, completion of the cholesterol test, and achievement of blood pressure target goal.
No significant improvements in the clinical measures were observed (Table 2). Overall there were no statistically significant difference-in-differences between groups for weight, blood pressure, or cholesterol levels. However, the control group showed improvement in HbA1C (8.88% to 8.53%), whereas the intervention group remained the same (8.20% to 8.27%), with the difference-in-differences value approaching statistical significance (p = 0.0553). There was no difference in observance of ADA standards of medical care between the intervention group and control group over time.
Table 2.
Changes in clinical measures according to group assignment in Hawaii Patient Reward and Incentives to Support Empowerment projecta1
| Variable | Intervention group | Control group | Δ within intervention | Δ within control | Difference in Δ | 95% CI | p value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||||
| Weight, lbs | 200.05 | 198.28 | 213.54 | 213.34 | −1.77 | −0.20 | −1.57 | −4.53 | 1.38 | 0.2972 |
| BMI, kg/m2 | 32.12 | 31.85 | 33.91 | 33.99 | −0.28 | 0.08 | −0.36 | −0.92 | 0.20 | 0.2091 |
| SBP, mmHg | 126.61 | 125.25 | 125.13 | 126.06 | −1.36 | 0.93 | −2.29 | −6.13 | 1.54 | 0.2416 |
| DBP, mmHg | 76.11 | 73.93 | 75.46 | 74.36 | −2.19 | −1.10 | −1.09 | −3.56 | 1.38 | 0.3881 |
| HbA1C, % | 8.20 | 8.27 | 8.88 | 8.53 | 0.06 | −0.34 | 0.41 | −0.01 | 0.83 | 0.0553 |
| Total cholesterol, mg/dL | 176.04 | 172.12 | 183.96 | 181.39 | −3.92 | −2.57 | −1.34 | −16.45 | 13.77 | 0.8617 |
| Triglycerides, mg/dL | 182.72 | 183.21 | 137.09 | 155.36 | 0.49 | 18.27 | −17.78 | −73.75 | 38.17 | 0.5333 |
| LDL, mg/dL | 93.58 | 93.13 | 103.50 | 102.14 | −0.45 | −1.36 | 0.91 | −9.35 | 11.17 | 0.8620 |
| HDL, mg/dL | 43.99 | 44.66 | 45.12 | 45.07 | 0.67 | −0.05 | 0.72 | −1.33 | 2.76 | 0.4915 |
Analysis was adjusted for age, gender, race, and history of hypertension.
CMS.gov. Medicaid incentives for the prevention of chronic disease model [Internet]. Baltimore, MD: Centers for Medicare and Medicaid Services; updated 2017 Aug 9 [cited 2016 Oct 29]. Available from: https://innovation.cms.gov/initiatives/mipcd.
BMI = body mass index (calculated as weight in kilograms divided by height in meters squared); CI = confidence interval; DBP = diastolic blood pressure; HBA1C = glycated hemoglobin; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SBP = systolic blood pressure.
A total of 277 participants completed at least 1 SF-36v2 survey; 145 (52%) were in the intervention arm and 132 (48%) were in the control arm. From baseline to midpoint, there was a significant difference in the average change of scores on the physical component summary measure (difference in Δ = 2.12, p = 0.0399) between groups, with scores increasing for respondents in the intervention group but decreasing for respondents in the control group. A similar pattern was observed for the bodily pain scale (difference in Δ = 2.97, p = 0.0184). The difference in the average change of scores on the general health scale was marginally significant (difference in Δ = 2.29, p = 0.0517), with those in the intervention group improving more than those in the control group. This indicates greater improvement for the intervention group compared with the control group for the physical component summary measure and 2 subscales: Bodily pain and general health. From the midpoint to the endpoint, there was a decrease in scores on the role-physical and role-emotional scales for the intervention group, whereas scores on these scales increased for the control group. This resulted in significant differences in the average change of scores for both the role-physical scale (difference in Δ = −5.49, p = 0.0009) and the role-emotional scale (difference in Δ = −4.24, p = 0.0316). From baseline to the endpoint, there were significant differences in the average change of scores for both the role-physical scale (difference in Δ = −3.61, p = 0.0306) and the role-emotional scale (difference in Δ = −4.48, p = 0.0271), indicating greater improvement in the control group compared to the intervention group between these time points.
Of the 278 participants who completed at least 1 SDSCA survey, 147 (53%) were in the intervention arm and 131 (47%) were in the control arm. No significant differences were identified for SDSCA domains between the intervention group changes and the control group changes from the baseline to the midpoint of the study. From baseline to endpoint, there was a significant difference in the change of average number of days the recommended general diet was followed (difference in Δ = −0.64, p = 0.0420) when comparing the intervention with the control group, with the average number of days increasing more dramatically for respondents in the intervention group (3.6 to 4.4 days) but remaining the same for respondents in the control group (4.1 to 4.2 days).
Only 77 (48%) of 159 participants in the intervention group completed the satisfaction survey. The overall satisfaction with HI-PRAISE project was rated high, averaging 9.1 on a 10-point scale with 10 indicating the best program ever and 1 indicating the worst program ever. All survey respondents indicated they received incentives. Almost half of the respondents (46%) used the incentives for themselves, 39% of respondents used the incentive for their family, and 12% of respondents indicated that they used the incentive for both themselves and their family. Also, 2 respondents (3%) indicated they wanted to save the incentive for later use. More than 90% of participants agreed that incentives were given to them on time, helped with setting goals, and helped make positive changes in their lives. Participants appreciated being incentivized for taking good care of their diabetes, and were happy with the amount and frequency of the incentive. Participants also found it easy to use the incentive and agreed on the fairness of the incentive. However, 19 people (25%) stated that the incentives did not help them keep up with their diabetes care, and 23 people (30%) agreed that they were unable to earn some of the incentives. The overall mean on incentive satisfaction was 3.4 (standard deviation = 0.4), as assessed using a 4-point Likert scale with higher scores corresponding to higher satisfaction.
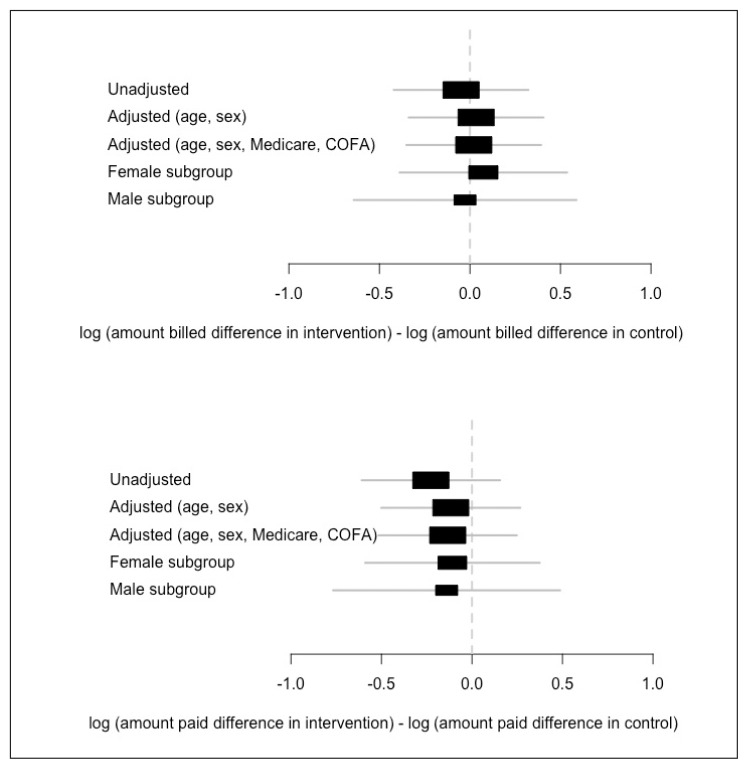
For the cost analysis, the outcomes included both the amount billed and the amount paid to KPHI. The difference-in-differences estimate is the interaction between group and time (Figure 2). Adjusting for sex, Medicare eligibility, and race, the difference-in-differences estimates in the RCT were 1.8% and −8.4% for the amounts billed and paid. Even though neither of these estimates were statistically significant, the amount paid showed a decreasing trend and was moving in a positive direction.
Figure 2.
Effect of the intervention on amount billed and amount paid – estimates.1
1 State of Hawaii, Department of Human Services Claims Data.
COFA = Compact of Free Association.
DISCUSSION
HI-PRAISE conducted diabetes behavioral economics research among the medically underserved Medicaid population in the state of Hawaii including racial and ethnic minorities. Although there are numerous studies evaluating the impact of financial incentives on smoking cessation and weight loss, to our knowledge HI-PRAISE is the first RCT to assess the impact of incentives on persons with diabetes in socioeconomically disadvantaged populations.16–20
The RCT found no statistical improvements in clinical outcomes of diabetes. The control group demonstrated improved A1C levels whereas the intervention group did not. However, the control group entered the study with an average A1C value of 8.88% compared with 8.20% for the intervention group. It is possible that both groups may have simply regressed toward a common mean. The intervention group failed to achieve target ADA guidelines and goals for diabetes. The reasons for this are discussed below.
The project also showed neither significant reduction nor increase in health care cost at the end of the study. In 2008, Oregon initiated an expansion of its Medicaid program for low-income adults through a lottery drawing of 30,000 persons. The Oregon Health Study showed increased utilization of health care services as well as rates of diabetes detection and management in Medicaid populations but no significant improvements in physical health outcomes.21,22
Previous research on the impact of financial incentives has raised concerns about incentives infringing upon an individual’s decision-making autonomy. Financial incentives might represent unjust inducements on the most vulnerable low-income populations.23 Another concern is that introducing external incentives may reduce people’s intrinsic motivation and desire to improve their own health.24 The HI-PRAISE satisfaction survey administered to the intervention group at the end of the study found most participants agreed that rewards helped them set goals and make positive changes in their lives. The participants appreciated being rewarded for taking good care of their diabetes and were happy with the amount and frequency of the incentives. Bazerman et al25 refer to the common struggle between choosing what we desire in the heat of the moment and what would be best for us in the long run as “want/should conflict.” To provide health plans with training on how to facilitate should decision making, additional research such as longitudinal behavior studies on want/should conflict is needed. Policy makers may be able to use these types of interventions to “nudge” individuals toward should behaviors.26 The dollar amount of the financial incentives in HI-PRAISE were small and may not have been enough to meet the threshold to “nudge” participants’ compliance with ADA guidelines. The amount may not have been sufficient to induce new behaviors or to change behavior. However, financial incentives also increase the costs for pharmacy and ambulatory visits with an unclear long-term impact on clinical outcomes as well as hospitalizations and emergency room visits. Improving the health of persons with diabetes requires reducing old unhealthy habits and building new healthy habits, which seems to be beyond the capacity of financial incentives alone.
HI-PRAISE constitutes an initial attempt to investigate quality of life in patients with diabetes within a managed care organization and is one of the first to examine the impact of financial incentives. The mean scores of the physical component summary measure and two subscales of bodily pain and general health showed a trend toward improvement at the midpoint of the study; however, this was not sustained at the end. Previous studies have shown that higher levels of distress correlate to lower quality-of-life scores in persons with diabetes for those treated with insulin, longer duration of diabetes, and a history of cardiovascular comorbidity.27,28 The incentives temporarily improved the quality-of-life scores.
Some RCT participants in both groups were followed-up by nurse care coordinators who assisted telephonically with diabetes self-management and goal setting. They were encouraged to consider goals appropriate and pertinent to their condition. Nurse case management is a collaborative process that provides and coordinates health care services to meet an individual’s health needs.29 This is a promising approach to the management of patients with diabetes and considered a key element in the adoption of a patient-centered medical home model.30 The aim of the HI-PRAISE incentives was to encourage the adoption of diabetes self-management behaviors. A wallet-sized goal and incentive tracking card was created by HI-PRAISE for the intervention group participants. Participants in the intervention group showed more improvement in following a recommended general diet for diabetes from baseline to the end of the study when compared with those in the control group, as assessed by the SDSCA.
Standardization of care through Patient-Centered Medical Home and the use of standing orders at KPHI may have led to no difference in patient compliance with the ADA standards of medical care in diabetes or clinical outcomes between the intervention and control groups. This suggests that a modified incentive schedule with higher dollar amounts focusing on key clinical measures (HbA1C, blood pressure, cholesterol) may lead to improvement in health outcomes among Medicaid beneficiaries at KPHI.
Some of the limitations the HI-PRAISE project faced were delays establishing fully executed contracts with KPHI. Initial rolling enrollment was stretched from three months to nine months because of slow recruitment of participants into the study. The original target population was patients with diabetes receiving care coordination services. Enrollment was delayed initially because of the small pool of persons receiving diabetes care coordination who met the general eligibility; the pool was later expanded to also include persons receiving standard care for diabetes. Participants were contacted telephonically, and many did not respond to the messages left on voice mail. KPHI staff had other competing priorities and projects, which led to HI-PRAISE assigning a graduate student to assist KPHI with recruitment. Additionally, KPHI had a change of Principal Investigator, which temporarily halted the RCT study.
The HI-PRAISE project was conducted in the setting of usual care, which meant that the clinicians were the ones to order the necessary laboratory tests on the basis of ADA standards of medical care in diabetes. This may have led to a high number of missing orders and test results because there were no specific HI-PRAISE study visit requirements for the participants. Additionally, the project did not engage the primary care clinicians, which inhibited the connection of the incentive to the participants’ overall health care experience. Financial incentives alone likely fail to recognize the degree to which patient motivation depends on goals shared within therapeutic alliance between patients and their primary care clinicians. This was a confounder in the study, which was adjusted by the nurse care coordinators involved in diabetes care coordination who provided motivation and goal setting to some participants. All persons with diabetes at KPHI have yearly standing orders for necessary blood tests such as HbA1C; however, compliance is self-determined.
Fluctuating eligibility status of Medicaid beneficiaries led to reduction in sample size in both groups. Compact of Free Association migrants (n = 11) lost Medicaid on March 1, 2015, and were no longer eligible to participate in the study. In the late 1980s, the former Trust Territory of the Pacific Island formed the 3 Freely Associated States through separate Compacts: Palau, Marshall Islands, and the Federated States of Micronesia. The compacts granted these islands sovereignty in domestic and foreign affairs in return for defense rights in the islands to the US. The citizens of these islands could enter the US without visa requirements, and many chose to migrate to Hawaii in search of better economic and health care opportunities. Because of the ruling of the Ninth Circuit Court of Appeals and availability of ACA marketplace health plans, the State of Hawaii was no longer obligated to provide Medicaid-like services to Compact of Free Association noncitizen adults. Others (n = 40) lost Medicaid for various reasons, such as change of address, recent travel, or change in income status. Limited English proficiency and low health literacy were some of the barriers to reapplying for Medicaid.
Unequally spaced intervention periods across participants increased the variability of the outcomes and decreased the power of the study. Reduction in sample size reduced the power to detect significant differences-in-differences. The response rate for the SF-36v2 and SDSCA surveys decreased by approximately 40% from baseline to end of the study, whereas the one-time satisfaction survey was 48%. Factors such as reduced sample size, limited survey response time for the participants, lack of involvement of primary care clinicians, and lack of time for the research team to follow-up with the nonresponders may have contributed to the low response at end of the study.
Future studies on financial incentives could increase the dollar amounts of the financial incentives rewarding the outcome measures rather than process measures. Financial incentives could be expanded to clinicians too, encouraging clinician-patient dyads in commonly shared goals. Fidelity of the intervention could be improved by having nurse care coordinators physically present at each clinician site to oversee goals and achieved behavior outcomes pairing with the incentives. Positive feedback, motivational interviewing, and goal setting by nurse care coordinators would enhance the internal motivation of participants.
CONCLUSION
The patient-centered medical home model for diabetes will continue at KPHI. This study did not provide conclusive evidence to support the use of financial incentives to improve diabetes standards of care or clinical outcomes in a managed care setting. Further long-term studies are needed to better assess the impact of financial incentives along with other behavioral economic tools to improve clinical outcomes and quality of life of patients with diabetes. Micro- and macrovascular changes from diabetes take several years to develop. Contemplating change, getting motivated, and committing to desired changes in lifestyle may take longer. Primary care clinicians build long-term relationships through patience and persistence, winning trust, and ultimately timing interventions to patients’ readiness to change. Future studies could expand to non-Medicaid adult patients with diabetes. The project was not effective at reducing costs during the study period. However, conducting future studies that allow for a longer follow-up period may help determine the long-term impact of financial incentives on health costs.
Acknowledgements
The project described was supported by Grant Number 1B1CMS330884 from the Department of Health and Human Services, Centers for Medicare & Medicaid Services (CMS). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies. The research presented here was conducted by the awardee. Findings might or might not be consistent with or confirmed by the findings of the independent evaluation contractor. We are grateful to the patients of Kaiser Permanente Hawaii for their participation. We appreciate the guidance from our community partners who served on the advisory council and evaluation council. We wish to acknowledge the support received from the State of Hawaii Department of Human Services, Honolulu, HI; IMPAQ International, Columbia, MD; RTI International, Research Triangle Park, NC; Econometrica, Inc, Bethesda, MD; and Centers for Medicaid and Medicare Services, Baltimore, MD. We thank the HI-PRAISE project coordinators Misha Morioka, MEd, MBA; and Robin Arndt, MSW; health economist Timothy Halliday, PhD, and data specialist Zi Wang, MS, for their contributions to this study.
Mary Corrado, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.The Patient Protection and Affordable Care Act of 2010. Public Law 111-148, 111th Congress, 124 Stat 119, HR 3590, enacted 2010 Mar 23.
- 2.Paradise J. Medicaid moving forward [Internet] Menlo Park, CA: Kaiser Commission on Medicaid and the Uninsured, Kaiser Family Foundation; c2017. [cited 2017 Mar 23]. Available from: http://kff.org/health-reform/issue-brief/medicaid-moving-forward. [Google Scholar]
- 3.Harris MI. Racial and ethnic differences in health insurance coverage for adults with diabetes. Diabetes Care. 1999 Oct;22(10):1679–82. doi: 10.2337/diacare.22.10.1679. DOI: https://doi.org/10.2337/diacare.22.10.1679. [DOI] [PubMed] [Google Scholar]
- 4.Wilper AP, Woolhandler S, Lasser KE, McCormick D, Bor DH, Himmelstein DU. A national study of chronic disease prevalence and access to care in uninsured US adults. Ann Intern Med. 2008 Aug 5;149(3):170–6. doi: 10.7326/0003-4819-149-3-200808050-00006. DOI: https://doi.org/10.7326/0003-4819-149-3-200808050-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gilmer T. Costs of chronic disease management for newly insured adults. Med Care. 2011 Sep;49(9):e22–7. doi: 10.1097/MLR.0b013e318215d280. DOI: https://doi.org/10.1097/MLR.0b013e318215d280. [DOI] [PubMed] [Google Scholar]
- 6.Garfield RL, Damico A. Medicaid expansion under health reform may increase service use and improve access for low-income adults with diabetes. Health Aff (Millwood) 2012 Jan;31(1):159–67. doi: 10.1377/hlthaff.2011.0903. DOI: https://doi.org/10.1377/hlthaff.2011.0903. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013 Apr;36(4):1033–46. doi: 10.2337/dc12-2625. DOI: https://doi.org/10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes [Internet] Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion Centers for Disease Control and Prevention; updated 2016 Jul 25 [cited 2017 May 1]. Available from: www.cdc.gov/chronicdisease/resources/publications/aag/diabetes.htm. [Google Scholar]
- 9.CMSgov. Medicaid incentives for the prevention of chronic disease model [Internet] Baltimore, MD: Centers for Medicare and Medicaid Services; updated 2017 Aug 9 [cited 2016 Oct 29]. Available from: https://innovation.cms.gov/initiatives/mipcd. [Google Scholar]
- 10.Blumenthal KJ, Saulsgiver SA, Norton L, et al. Medicaid incentive programs to encourage healthy behavior show mixed results to date and should be studied and improved. Health Aff (Millwood) 2013 Mar;32(3):497–507. doi: 10.1377/hlthaff.2012.0431. DOI: https://doi.org/10.1377/hlthaff.2012.0431. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012 Jan;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. DOI: https://doi.org/10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenphire [Internet] King of Prussia, PA: Greenphire, Inc; c2017. [cited 2016 Oct 31]. Available from: https://greenphire.com/solutions. [Google Scholar]
- 13.Yawn BP, Madison S, Bertram S, et al. Automated patient and medication payment method for clinical trials. Open Access Journal of Clinical Trials. 2013 Jan 29;2013(5):23–31. DOI: https://doi.org/10.2147/OAJCT.S38489. [Google Scholar]
- 14.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–83. DOI: https://doi.org/10.1097/00005650-199206000-00002. [PubMed] [Google Scholar]
- 15.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care. 2000 Jul;23(7):943–50. doi: 10.2337/diacare.23.7.943. DOI: https://doi.org/10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 16.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4th ed. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 17.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009 Feb 12;360(7):699–709. doi: 10.1056/NEJMsa0806819. DOI: https://doi.org/10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 18.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: A randomized controlled trial. JAMA. 2008 Dec 10;300(22):2631–7. doi: 10.1001/jama.2008.804. DOI: https://doi.org/10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kullgren JT, Troxel AB, Loewenstein G, et al. Individual-versus group-based financial incentives for weight loss: A randomized, controlled trial. Ann Intern Med. 2013 Apr 2;158(7):505–14. doi: 10.7326/0003-4819-158-7-201304020-00002. DOI: https://doi.org/10.7326/0003-4819-158-7-201304020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendzor DE, Businelle MS, Poonawalla IB, et al. Financial incentives for abstinence among socioeconomically disadvantaged individuals in smoking cessation treatment. Am J Public Health. 2015 Jun;105(6):1198–205. doi: 10.2105/AJPH.2014.302102. DOI: https://doi.org/10.2105/AJPH.2014.302102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baicker K, Taubman SL, Allen HL, et al. Oregon Health Study Group. The Oregon experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013 May 2;368(18):1713–22. doi: 10.1056/NEJMsa1212321. DOI: https://doi.org/10.1056/NEJMsa1212321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkelstein AN, Taubman SL, Allen HL, Wright BJ, Baicker K. Effects of Medicaid coverage on ED use—further evidence from Oregon’s experiment. N Engl J Med. 2016 Oct 20;375(16):1505–7. doi: 10.1056/NEJMp1609533. DOI: https://doi.org/10.1056/NEJMp1609533. [DOI] [PubMed] [Google Scholar]
- 23.Halpern SD, Madison KM, Volpp KG. Patients as mercenaries?: The ethics of using financial incentives in the war on unhealthy behaviors. Circ Cardiovasc Qual Outcomes. 2009 Sep;2(5):514–6. doi: 10.1161/CIRCOUTCOMES.109.871855. DOI: https://doi.org/10.1161/CIRCOUTCOMES.109.871855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyman J, Ariely D. Effort for payment. A tale of two markets. Psychol Sci. 2004 Nov;15(11):787–93. doi: 10.1111/j.0956-7976.2004.00757.x. DOI: https://doi.org/10.1111/j.0956-7976.2004.00757.x. [DOI] [PubMed] [Google Scholar]
- 25.Bazerman MH, Tenbrunsel AE, Wade-Benzoni K. Negotiating with yourself and losing: Making decisions with competing internal preferences. Acad Manage Rev. 1998 Apr;23(2):225–41. DOI: https://doi.org/10.2307/259372. [Google Scholar]
- 26.Thaler RH, Sunstein CR. Nudge: Improving decisions about health, wealth, and happiness. New Haven, CT: Yale University Press; 2008. [Google Scholar]
- 27.Delahanty LM, Grant RW, Wittenberg E, et al. Association of diabetes-related emotional distress with diabetes treatment in primary care patients with Type 2 diabetes. Diabet Med. 2007 Jan;24(1):48–54. doi: 10.1111/j.1464-5491.2007.02028.x. DOI: https://doi.org/10.1111/j.1464-5491.2007.02028.x. [DOI] [PubMed] [Google Scholar]
- 28.Papathanasiou A, Shea S, Koutsovasilis A, Melidonis A, Papavasiliou E, Lionis C. Reporting distress and quality of life of patients with diabetes mellitus in primary and secondary care in Greece. Ment Health Fam Med. 2008 Jun;5(2):85–93. [PMC free article] [PubMed] [Google Scholar]
- 29.Gabbay RA, Añel-Tiangco RM, Dellasega C, Mauger DT, Adelman A, Van Horn DH. Diabetes nurse case management and motivational interviewing for change (DYNAMIC): Results of a 2-year randomized controlled pragmatic trial. J Diabetes. 2013 Sep;5(3):349–57. doi: 10.1111/1753-0407.12030. DOI: https://doi.org/10.1111/1753-0407.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bojadzievski T, Gabbay RA. Patient-centered medical home and diabetes. Diabetes Care. 2011 Apr;34(4):1047–53. doi: 10.2337/dc10-1671. DOI: https://doi.org/10.2337/dc10-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]