Abstract
Background
Metformin is associated with low levels of vitamin B12 (VitB12) in patients with diabetes. The CCTG/MA.32 trial investigates effects of metformin vs placebo on breast cancer (BC) outcomes in non-diabetic high risk BC patients. We analyzed VitB12 at baseline and after 6 months of metformin (versus placebo) in the first 492 patients with paired blood samples.
Methods
VitB12 was analyzed centrally in baseline and 6 month fasting plasma. Levels<181pmol/L were considered deficient, 181 to 221pmol/L borderline and ≥ 222pmol/L sufficient. Methylmalonic acid(MMA) and homocysteine (HC) were assayed in those with VitB12 levels<222pmol/L. Statistical analyses used Spearman’s ranked correlation coefficients and the Wilcoxon signed rank test for continuous variables and Chi-square test for categorical variables.
Results
237 patients received metformin and 255, placebo; Median (Inter Quartile Range) baseline VitB12 levels were 390(290, 552) and 370(290, 552) pmol/L in the metformin and placebo arms, respectively, (p=0.97). At six months, the median levels were 320(244, 419) in the metformin versus 380(286, 546) pmol/L in the placebo arm (p=0.0001). At baseline, 15 patients (11 metformin and 4 placebo) had VitB12<181pmol/L; at 6 months, 18 patients (15 metformin and 3 placebo) (p=0.004).
Median hemoglobin was similar at baseline, metformin, 130g/L (124-137), and placebo arms, 131g/L (124-137), (p=0.38) and at 6 months, metformin, 131g/L (91-162), and 131g/L (106-169) in placebo group, (p=0.11). Of the 74 subjects with vitamin B12<222pmol/L at either time point (45 metformin, 29 placebo), at baseline MMA was normal in all patients and 2 had elevated HC (>15umol/L). At 6 months, 1 patient (metformin) had MMA >0.4umol/L and 3 (2 metformin, 1 placebo) had HC>15umol/L.
Conclusions
There was an increased rate of biochemical VitB12 deficiency after 6 months of metformin; this was not associated with anemia. Further research will investigate VitB12 levels in all subjects at baseline, 6 and 60 months.
Keywords: breast cancer, metformin, vitamin B12, methylmalonic acid and homocysteine, megaloblastic anemia, anemia
Introduction
Metformin, a biguanide used in the treatment of type II diabetes, has emerged as a potential anti-cancer agent [1]. Multiple pre-clinical, epidemiological and clinical studies have reported that metformin is associated with decreased cancer incidence and cancer related mortality in diabetic patients [2–4]. Although definitive data are lacking, phase II and III trials have been launched to evaluate the effects of metformin on cancer outcomes. In breast cancer, Canadian Clinical Trial Group (CCTG) MA.32 is designed to assess the effectiveness of metformin vs placebo on invasive disease-free survival and other outcomes in 3,649 non-diabetic women with early stage breast cancer [5,6]. Accrual to this trial has completed and follow-up is ongoing.
Recently, a meta-analysis by Chapman et al reported an association of low vitamin B12 plasma levels in patients with diabetes [7]. A mean difference in vitamin B12 levels in metformin versus controls of 57pmol/L after 6 weeks to 3 months of use in patients with type 2 diabetic was seen (fixed weighted mean difference, −0.57; 95%CI, −35 to 79pmol/L)[7]. A meta-analysis by Niafar et al identified an increased incidence of vitamin B12 deficiency in diabetic patients, defined as levels <150pmol/L, (OR=2.45, 95%Confidence interval, CI 1.74 to 3.44, p<0.0001) as well as lower mean vitamin B12 levels in those who received metformin (mean difference= −65.8, 95%CI −78.1 to −53.6 pmol/L, p<0.00001) versus control [8]. Since metformin has been associated with decreased plasma levels of vitamin B12 in diabetic patients [7, 8], a safety concern has been raised regarding its use in cancer patients.
Maintaining blinding of investigators to treatment allocation, we investigated the effects of metformin versus placebo on vitamin B12 status in the CCTG MA.32 trial. Vitamin B12 was assayed at baseline and after 6 months of metformin or placebo, in the first 492 patients with paired blood samples. Methylmalonic acid (MMA) and homocysteine (HC), often elevated in clinical vitamin B12 deficiency, were also analysed in those patients with vitamin B12 levels below 222 pmol/L at either time point.
Material and Methods
Study Design
The CCTG MA.32 Clinical Trial (ClinicalTrials.gov identifier: NCT01101438 and EudraCT number: 2011-005230-18) [5]; is a phase III trial that randomly assigned patients from Canada, the USA, the United Kingdom and Switzerland to receive metformin 850 mg po bid or placebo po bid for five years. This study has enrolled 3649 non-diabetic women diagnosed with T1-3, N0-3, M0 breast cancer who had received standard surgical, chemotherapeutic, hormonal, biologic, and radiation treatment. Patients with T1c N0 breast cancer were eligible if they had at least one of the following risk factors: histologic grade III, lymphovascular invasion, negative estrogen (ER) and progesterone (PgR) receptors, HER2 positivity, Oncotype Recurrence Score greater than or equal to 25 or Ki-67 over 14%. The exclusion criteria included fasting glucose ≥7.0 mmol/L, clinical diagnosis of diabetes, history of lactic acidosis, current use of diabetes medication, recurrence of breast cancer or prior breast cancer, excessive alcohol intake, or marked hepatic, kidney, or cardiac dysfunction.
This study was approved by the Adult Central Research Board ethics (US, NIH) and by boards of CCTG and participating centers, and is overseen by the CCTG Data Safety Monitoring Committee. In the core study, consenting women provided fasting blood (plasma, serum, whole blood), collected before initiation of study drug treatment and at six months (includes four-week ramp up of one caplet per day plus 20 weeks of full dose). The requirement for fasting blood reflects other goals, including investigation of metabolic factors. As part of correlative studies, the first 507 patients for whom the blood was available participated in the vitamin B12 substudy (CONSORT diagram, figure 1). Fifteen patients were excluded (12 from metformin arm and 3 from placebo arm, 8 were ineligible and 7 patients provided plasma samples at incorrect date) which led to 492 evaluable subjects. There were two patients with missing baseline vitamin B12 and three patients at 6 month in the final study population.
Figure 1.
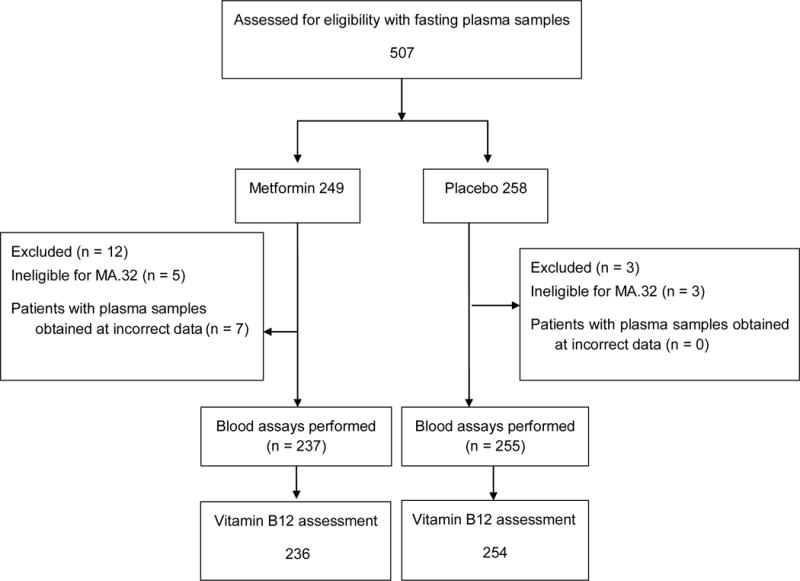
CONSORT diagram for metabolic sub-study: CCTGMA.32. Vitamin B12 substudy
Measurements
Vitamin B12 was analyzed centrally in baseline and 6 month fasting plasma; levels <181pmol/L were considered potentially deficient, 181 to 221pmol/L borderline and ≥ 222 pmol/L sufficient according to blood assays. MMA and HC (elevated in clinical vitamin B12 deficiency) were assayed in those with vitamin B12 levels <222 pmol/L at either time point.
Fasting blood was collected, and plasma was frozen at −80C, shipped on dry ice to ice to the central repository at CCTG in Kingston, Canada and subsequently transported on dry ice to Mount Sinai Hospital (Toronto) at −80C. Vitamin B12 (pmol/L) was quantified in one batch by a Roche E MODULAR ANALYTICS E170 (Elecsys module) immunoassay analyzers170 ECLIA, ElectroChemiLuminescence ImmunoAssay (sensitivity <37 pmol/L and assay coefficient of variation <5%) in the Dr. Azar Azad laboratory at Mount Sinai Hospital (Toronto). Paired bloods (baseline and six months) were assayed in one batch.
HC and MMA were measured in patients with vitamin B12 < 222 pmol/L at baseline or 6 months in reference laboratories with paired samples in the same batch using radioimmunoassay kits (Diazyme Enzymatic kit from Block Scientific for HC and GC-MSD for MMA) (assay coefficient of variation < 8% and <10.43% for HC and MMA, respectively). HC testing was performed at the Department of Laboratory Medicine at St Michael’s Hospital, Toronto and MMA testing at the Laboratory Medicine Program, University Health Network, Toronto. Hemoglobin was measured at local centres.
Statistical Analysis
Continuous variables were summarized with median and interquartile range (IQR), reflecting non-normal distributions. Correlations among continuous variables were calculated using Spearman ranked correlation coefficient. The Wilcoxon signed rank test was used to compare difference of continuous variables between Metformin and Placebo arms. Categorical variables were summarized with percentage of each group. Chi-square test was used to compare differences of categorical variables between Metformin and Placebo arms. All analyses were conducted using SAS software (version 9.3).
Results
Population
Baseline characteristics of the study population, which are comparable to the whole patient population (data not shown), are summarized in table 1. Mean age was 52.4±9.7 years, 52.1 (±9.5) years in the metformin and 52.6 (±9.8) years in the placebo arm. The majority of patients were Caucasian, 95.8% (227) in the metformin arm and 93.7% (239) in the placebo arm. The majority had T2 or T3 breast cancer (132 T2 and 28 T3 of 237 in the metformin arm, 137 T2 and 30 T3 of 255 in the placebo arm), with positive axillary nodes (in 56.5% and 51.4% in the metformin and placebo arms, respectively). Estrogen and/or progesterone receptors were positive in 73.0% and 72.8%, and HER2 was positive in 17.3% and 14.1% of cases in the metformin and placebo arms, respectively. Approximately half of the patients, 52.7% (125) in the metformin arm and 51% (130) in the placebo arm had grade III breast cancer and just under half the subjects in each arm had undergone mastectomy (49.4% and 41.6% metformin and placebo arms, respectively). Chemotherapy was administered to 88.6% (210), 13.1% neoadjuvant and 75.5% adjuvant, in the metformin arm, and, 87.9% (224), 10.6% neoadjuvant and 77.3% adjuvant in the placebo arm. Most patients received hormone therapy: 65% (154) and 67.8% (173) in the metformin and placebo arms, respectively. Adjuvant trastuzumab was delivered in 16.9% (40) in the metformin arm and 12.9 % (33) and the placebo arm.
Table 1.
Baseline characteristics of the metabolic sub-study population
| Metformin (n = 237) | Placebo (n = 255) | |||
|---|---|---|---|---|
| Age (mean, SD) | 52.1 (±12) | 52.6 (±) | ||
|
| ||||
| Race | ||||
| Asian | 4 | (1.7%) | 6 | (2.4%) |
| Black | 2 | (0.8%) | 3 | (1.2%) |
| Native | 3 | (1.2%) | 3 | (1.2%) |
| White | 227 | (95.8%) | 239 | (93.7%) |
| Unknown | 1 | (0.4%) | 4 | (1.6%) |
|
| ||||
| BMI (median, range) | 27.37 | (17.68, 46.48) | 27.27 | (18.73, 46.07) |
|
| ||||
| T stage (neoadjuvant) | ||||
| cT1 | 3 | (9.6%) | 1 | (3.7%) |
| cT2 | 18 | (58.1%) | 13 | (48.1%) |
| cT3 | 10 | (32.3%) | 13 | (48.1%) |
|
| ||||
| N stage (neoadjuvant) | ||||
| cN0 | 6 | (19.4%) | 8 | (29.6%) |
| cN1-3 | 25 | (80.6%) | 19 | (70.4%) |
|
| ||||
| T stage (adjuvant) | ||||
| T1 | 74 | (36.0%) | 87 | (38.2%) |
| T2 | 114 | (55.3%) | 124 | (54.4%) |
| T3 | 18 | (8.7%) | 17 | (7.4%) |
|
| ||||
| N stage (adjuvant) | ||||
| N0 | 97 | (47.1%) | 116 | (50.9%) |
| N1-3 | 109 | (52.9%) | 112 | (49.1%) |
|
| ||||
| Hormone receptor | ||||
| ER and/or PgR positive | 173 | (73.0%) | 190 | (72.8%) |
| ER and PgR negative | 64 | (27.0%) | 71 | (27.2%) |
|
| ||||
| HER2 | ||||
| Positive | 41 | (17.3%) | 36 | (14.1%) |
| Negative | 196 | (82.7%) | 219 | (85.9%) |
|
| ||||
| Histologic Grade | ||||
| Grade I | 24 | (10.1%) | 24 | (9.4%) |
| Grade II | 87 | (36.7%) | 100 | (39.2%) |
| Grade III | 125 | (52.7%) | 130 | (51.0%) |
| Unknown | 1 | (0.4%) | 1 | (0.4%) |
|
| ||||
| Surgery | ||||
| Mastectomy | 117 | (49.4%) | 106 | (41.6%) |
| Lumpectomy | 120 | (50.6%) | 149 | (58.4%) |
|
| ||||
| Chemotherapy | ||||
| Yes - neoadjuvant | 31 | (13.1%) | 27 | (10.6%) |
| - adjuvant | 179 | (75.5%) | 197 | (77.3%) |
| No | 27 | (11.4%) | 31 | (12.1%) |
|
| ||||
| Hormone Therapy | ||||
| Yes - neoadjuvant | 3 | (1.3%) | 0 | (0%) |
| - adjuvant | 151 | (63.7%) | 173 | (67.8%) |
| No | 83 | (35.0%) | 82 | (32.2%) |
|
| ||||
| Adjuvant Trastuzumab | ||||
| Yes | 40 | (16.9%) | 33 | (12.9%) |
| No | 197 | (83.1%) | 222 | (87.1%) |
Range = Interquartile Range; SD= standard deviation; ER = estrogen receptor; PgR = progesterone receptor.
Baseline and Change at Six Months Measurements
Median baseline levels of vitamin B12 were 390 pmol/L, interquartile range (IQR) 290 to 552 pmol/L in the metformin arm, and 370 pmol/L (IQR 290, 552 pmol/L) in the placebo arm, (p=0.97), seen table 2. At 6 months, the median levels were lower −320 pmol/L (IQR 244, 419) in the metformin arm versus 380 pmol/L (IQR 286, 546) in the placebo arm (p=0.0001). The median change from baseline to 6 months was −51pmol/L (IQR 6, −182) in the metformin arm versus 0 (IQR −61, +65) in the placebo arm, p<0.001 (see table 3) and the mean percentage change of vitamin B12 from baseline to month 6 was −6.3% (standard deviation, SD 71, 65) and 7.52% (SD 51, 20) in the metformin and placebo arms, respectively. Fifteen patients (11 metformin and 4 placebo) had vitamin B12 <181pmol/L at diagnosis (p=0.3), and 18 patients (15 metformin and 3 placebo) had vitamin B12 <181pmol/L at 6 months (p=0.002). Figure 2 shows scatter plots of vitamin B12 levels at baseline versus 6 months and at baseline versus change in the metformin and placebo arms.
Table 2.
Vitamin B12 and related variables at baseline and at 6 months
| At Baseline | At 6 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Total Median (IQR) | Metformin Median (IQR) | Placebo Median (IQR) | P | Total Median (IQR) | Metformin Median (IQR) | Placebo Median (IQR) | P |
| Vitamin B12 (pmol/L) | 378 (288,553) | 390 (290,552) | 370 (290,552) | 0.97 | 353 (262,495) | 320 (244,419) | 380 (286,546) | 0.0001 |
| Proportion of Low Vitamin B12 (<181 pmol/L) | n=15 (3.0%) | n=11 (4.6%) | n=4 (1.6%) | 0.048 | n=18 (3.7%) | n=15 (6.3%) | n=3 (1.2%) | 0.002 |
| Hemoglobin (g/L) | 131 (124,137) | 130 (124,137) | 131 (124,137) | 0.38 | 132 (91,169) | 131 (91,162) | 131 (106,169) | 0.115 |
| Methylmalonic acid* (umol/L) | 0.16 (0.13,0.24) | 0.16 (0.13,0.24) | 0.16 (0.13,0.23) | 0.71 | 0.15 (0.11,0.2) | 0.15 (0.12, 0.2) | 0.15(0.11,0.2) | 0.70 |
| Homocysteine* (umol/L) | 10 (9,12) | 10 (9,12) | 10 (9,12) | 0.74 | 9 (8,12) | 9.5 (8.5,12.5) | 9 (8,12) | 0.57 |
IQR = interquartile range, N= number of patients,
measured in patients with vitamin B12 < 222pmol/L at baseline or 6 months
Table 3.
Change from baseline at 6 months
| Metformin
|
Placebo
|
Total
|
||
|---|---|---|---|---|
| Change Median (IQR) | Change Median (IQR) | Change Median (IQR) | P | |
| Vitamin B12 | n= 234 | n = 253 | n= 487 | <.001 |
| −51 (6, −182) | +0 (−61, +65) | −26 (−107, 45) | ||
| Hemoglobin | n=232 | n=252 | n=484 | 0.35 |
| + 1 (−4, 5.5) | +2 (−3, 8) | +1.5 (−3.5, 6.5) | ||
IQR = interquartile range, N= number of patients
Figure 2. Scatter plots of vitamin B12.
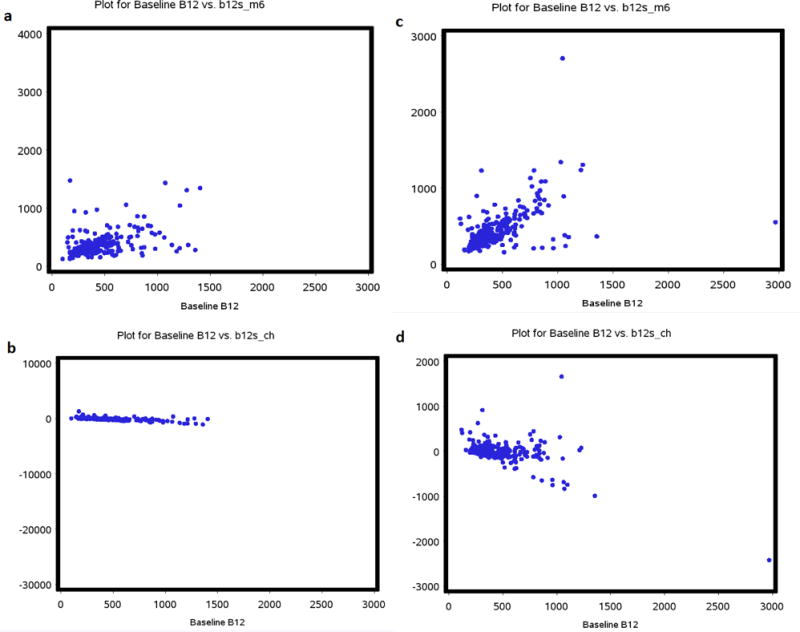
a- Vitamin B12 levels at baseline versus at 6 months in the metformin arm, b- Vitamin B12 levels at baseline versus change at 6 months in the metformin arm, c- vitamin B12 at baseline versus 6 months in the placebo arm and d- Vitamin B12 levels at baseline versus change in the placebo arm
The 74 patients, who had vitamin B12 <222 pmol/L (45 in the metformin arm and 29 in the placebo arm) at baseline and/or 6 months, had MMA and HC measured. At baseline, MMA was normal in all these patients (median 0.16 umol/L IQR 0.13, 0.24, table 2), p=0.8. Median baseline HC was 10 (IQR 9, 12); 2 patients (both in the metformin arm) had elevated HC (>15 umol/L). At 6 months, the median MMA was 0.15 (IQR 0.12, 0.2) and median HC was 9 (IQR 8, 12) overall, one patient receiving metformin had elevated MMA (>0.4 umol/L), p=0.6 (this patient also had elevated HC > 15 umol/L), and 3 (2 receiving metformin and 1 receiving placebo) had HC >15 umol/L, p=0.5.
Median hemoglobin at baseline was 130 g/L (IQR 124-137) in the metformin group and 131 (124-137) g/L, placebo group, (p=0.38). At 6 months, medians were 131 (91-162) and 133 (106-139) g/L for metformin and placebo groups respectively (p=0.11) (table 2). The median change from baseline to 6 months was + 1 (range −4, 5.5) g/L and +2 (range −3, 8) g/L in the metformin and placebo arms respectively (p=0.35) (Table 3). Changes in Hemoglobin and Vitamin B12 were not significantly correlated (Spearman Correlation coefficient 0,05, p = 0.25).
Discussion
We have shown that, in the first 492 participants in the CCTG MA.32 trial, baseline vitamin B12 was in a normal range in the vast majority of subjects (97.3%). After 6 months of treatment, those patients who received metformin had significantly lower plasma levels of vitamin B12 compared to placebo. However, few patients in either arm had elevated MMA or HC (measured in those with vitamin B12 levels<222pmol/L at either time point), suggesting that lower vitamin B12 levels were not indicative of clinical vitamin B12 deficiency. Haemoglobin was not significantly different at either time point and by treatment arm. Our results are consistent with the medical literature in patients without cancer. In the National Health and Nutrition Examination Survey (NHANES), 5.8% of diabetic patients using metformin compared with 2.4% of those with diabetes not using metformin had vitamin B12 levels ≤148pmol/L (p= 0.0026) [9] and metformin use was associated with increased likelihood of vitamin B12 levels ≤148pmol/L (adjusted odds ratio 2.92; 95% CI 1.26–6.78) [9]. Similarly, Jager et al reported a mean decrease in vitamin B12 concentration of −19% (95%CI −24% to −14%, p<0.001) in long term (4.3 years) treatment with metformin compared to placebo in the Hyperinsulinemia: the Outcome of its Metabolic Effects (HOME) trial [10]. They also reported a 5% increase in HC levels (95% CI, −10% to −0.4%; p<0.001) during the follow-up period [10]. In addition, in non-diabetic women with polycystic ovary syndrome taking metformin for 6 months, Greibe at al reported that metformin lowered vitamin B12 levels versus placebo (median 298, range 183–478, vs 346, 155–601, p=0.003, respectively) [11].
Although treatment of diabetic patients with metformin is associated with a decline in plasma vitamin B12, it is unknown whether this results in clinical vitamin B12 deficiency, with neurological manifestations related to demyelination of spinal cord, cranial, peripheral nerves or with megaloblastic anemia [12]. Although it has been suggested that metformin reduces vitamin B12 absorption, a recent study in rats showed that animals treated with metformin accumulate vitamin B12 in the liver which could also explain its lower plasma level [13].
Additionally, because low plasma levels of vitamin B12 are not sufficient to diagnose the deficiency syndrome (which requires the neurologic and/or hematologic signs and symptoms outlined above), reductions in blood levels of vitamin B12, particularly in the absence of elevations in MMA or HC have low diagnostic value [14]. Given that MMA and HC are elevated in the majority (98%) of patients with clinical vitamin B12 deficiency, it is recommended that these be measured in those patients with clinical symptoms [14]. MMA is more specific for vitamin B12 deficiency than HC which can be elevated in other conditions such as folate deficiency, classic homocystinuria and renal failure [14]. In our study, only one patient (on metformin, 6 month measurement) had elevated MMA, which suggests clinically relevant vitamin B12 deficiency may not be present in the majority of patients with low levels of vitamin B12, at least after six months of metformin.
Our study has limitations. We conducted measurements only at diagnosis and 6 months. It is not known whether vitamin B12 varied between these times or after 6 months. Study measurements at 5 years will become available in future. Although standard adverse event reporting system was in place, data on clinical symptoms of vitamin B12 deficiency was not specifically collected. Additionally, no information about vitamin B12 supplementation is available. In future research we will explore vitamin B12 levels, MMA and HC after 5 years of metformin or placebo.
Conclusion
In this substudy of the CCTG MA.32 clinical trial, we identified an increased frequency of biochemical, vitamin B12 deficiency after 6 months of metformin in breast cancer patients, but this did not appear to be associated with clinical vitamin B12 deficiency. These preliminary results suggest that metformin use in patients with early breast cancer could be considered safe, although measurement of vitamin B12 on a regular basis, similar to recommendations in diabetic patients may be prudent [15].
Acknowledgments
Dr. Lohmann’s work is supported Hold’Em for Life Translating Discoveries into Breast Cancer Cures (Canada). Dr. Stambolic’s work is supported by the Canadian Institutes of Health Research (CIHR), Canadian Cancer Society Research Institute (CCSRI) and Hold’Em for Life Translating Discoveries into Breast Cancer cures (Canada). Dr. Goodwin’s work is supported by The Breast Cancer Research Foundation (United States) and Hold’Em for Life Translating Discoveries into Breast Cancer Cures (Canada).
Funding: The MA.32 Study is supported by the following grants: Canadian Cancer Society Research Institute – CCSRI grant (#021039), National Institute of Health -NIH Grant (#CA180863), Canadian Breast Cancer Foundation-CBCF and Breast Cancer Research Foundation (US). Apotex (in kind donation of placebo and metformin).
The study sponsors have no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
The results of this manuscript were presented as part of poster Abstract Session at the American Society of Clinical Oncology Annual Meeting 2014 and the contents of this manuscript are not under consideration for publication elsewhere.
Compliance with Ethical Standards
Conflict of Interest: Drs. Lohmann, Liebman, Brien, Parulekar, Gelmon, Shepherd, Ligibel, Hershman, Rastogi, Hobday, Lemieux, Thompson, Whelan, Mukherjee, Chalchal, Stambolic, Chen, Goodwin declare no conflict of interest.
Dr. Pritchard reported receiving consulting fees from AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline and Eisai. Dr. Mayer received consulting fees from Novartis and Pfizer. Dr. Bernstein received consulting fees from Bristol-Myers Squibb.
Ethical approval: All procedures performed in the study were in accordance with the ethical standards of the institutional review board of the participating institutions and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: All patients provided written informed consent to participate, including conduct of the correlative analyses reported here.
References
- 1.Dowling RJ, Niraula S, Chang MC, et al. Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res. 2015;17:0. doi: 10.1186/s13058-015-0540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 4.Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous A Phase III Randomized Trial of Metformin vs Placebo in Early Stage Breast Cancer.
- 6.Goodwin PJ, Parulekar WR, Gelmon KA, et al. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv006. Print 2015 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman LE, Darling AL, Brown JE. Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2016 doi: 10.1016/j.diabet.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Niafar M, Hai F, Porhomayon J, et al. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med. 2015;10:93–102. doi: 10.1007/s11739-014-1157-5. [DOI] [PubMed] [Google Scholar]
- 9.Reinstatler L, Qi YP, Williamson RS, et al. Association of biochemical B(1)(2) deficiency with metformin therapy and vitamin B(1)(2) supplements: the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2012;35:327–333. doi: 10.2337/dc11-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010;340:c2181. doi: 10.1136/bmj.c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greibe E, Trolle B, Bor MV, et al. Metformin lowers serum cobalamin without changing other markers of cobalamin status: a study on women with polycystic ovary syndrome. Nutrients. 2013;5:2475–2482. doi: 10.3390/nu5072475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obeid R. Metformin causing vitamin B12 deficiency: a guilty verdict without sufficient evidence. Diabetes Care. 2014;37:22. doi: 10.2337/dc13-2278. [DOI] [PubMed] [Google Scholar]
- 13.Greibe E, Miller JW, Foutouhi SH, et al. Metformin increases liver accumulation of vitamin B12 - an experimental study in rats. Biochimie. 2013;95:1062–1065. doi: 10.1016/j.biochi.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368:2041–2042. doi: 10.1056/NEJMc1304350. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment. Diabetes Care. 2017;40:S74. doi: 10.2337/dc17-S011. [DOI] [PubMed] [Google Scholar]


