Abstract
Recent studies have shown that fat accumulation is associated with insulin resistance; however, the risks associated with long-term changes and fluctuations in central fatness are less clear. This study examined the longitudinal relationship between waist circumference (WC) and insulin resistance using three dimensions of WC: baseline WC, slope of linear changes in WC, and fluctuation of WC around the slope during 20 years of follow-up. Anthropometry, insulin resistance (homeostasis model assessment (HOMAIR)), and lifestyle factors were obtained in a population-based, prospective observational study (Coronary Artery Risk Development in Young Adults (CARDIA)) during 1985–2006, excluding participants who had been diagnosed with diabetes at any examination. After adjusting for socio-demographic and lifestyle factors, the evolution of HOMAIR from CARDIA year 15 to 20 was 6.9% higher per standard deviation of year 0 WC (P trend <0.0001) and 6.3% higher per standard deviation increase in the change in WC over the long term (P trend <0.0001). However, WC fluctuations around the linear change were not associated with insulin resistance or its evolution. The level of HOMAIR increased substantially with steeper linear WC slope among initially thinner participants at baseline, whereas this association tended to be weaker in those with higher initial WC (P interaction <0.0001). We conclude that year 0 WC and long-term increment in WC are associated with worsening insulin resistance. However, the association of HOMAIR with slope of WC change may vary across the range of initial WC.
INTRODUCTION
The growing incidence and prevalence of obesity in the United States (1) and other developed countries worldwide (2) is associated with insulin resistance (3–12), a prediabetic state that can predict incident type 2 diabetes relatively far into the future (13–17). Studies have shown that fat accumulation leads to the dysregulation of adipocytokines, which participate in the pathogenesis of obesity and insulin resistance (7–12). Additionally, accumulation of adipose tissue in a particular anatomical compartment or region, most distinctively visceral adiposity contained within the abdominal cavity (deep in the body) (18,19), appears to confer increased insulin resistance risk, compared to that conferred by the gluteofemoral compartment (e.g., subcutaneous fat).
However, the risks associated with long-term changes and fluctuations in central fatness are less clear, controlling for initial abdominal adiposity. Several studies found that weight gain is related to the risk of diabetes (20–27), but most of these studies have used self-reported information for anthropometrics and/or a diagnosis of diabetes (20–26), which could be less valid than direct measurements. Furthermore, it is not clear whether any association between change in abdominal fat and insulin resistance is similar across different levels of initial adiposity.
To better understand the relationships between changes in body fat distribution and insulin resistance, we examined the association between waist circumference (WC) and insulin resistance estimated by the Homeostasis Model Assessment (HOMAIR, (28)) in multivariable generalized linear models using three dimensions of WC: baseline WC, linear changes in WC, and fluctuation of WC, in young adults through 20 years of follow-up. We hypothesized that increasing levels of WC are associated with increasing levels of HOMAIR, and that the impact of WC change on HOMAIR is different, depending on baseline WC.
METHODS AND PROCEDURES
Subjects and measurements
We used data from the Coronary Artery Risk Development in Young Adults (CARDIA) study to examine the long-term association between changes and fluctuations in WC and HOMAIR. In brief, 5,115 free-living African-American and white participants aged 18–30 years were recruited at baseline from 1985 to 1986 from the populations of four US cities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). Since initiation of the study, follow-up examinations were completed at years 2, 5, 7, 10, 15, and 20 with 90, 86, 81, 79, 74, and 72%, respectively, of the surviving cohort returning.
Self-reported demographic information and medical history, such as a history of hypertension, type 2 diabetes, and the use of medications, as well as anthropometrics, blood constituents, and blood pressure were obtained across CARDIA examination visits. Tobacco and alcohol consumption were self-reported at each examination. Alcoholic beverages were quantified as average milliliters of alcohol consumed per day, and smoking status was classified as never, former, or current smoker. Habitual physical activity was measured by using the CARDIA Study Physical Activity History over the course of a year, weighting frequency and intensity in order to obtain a total activity score.
Height and weight were measured. BMI was calculated as weight (kg)/height (m2). WC was measured as the abdominal girth midway between the iliac crest and the bottom of the rib cage, using a Gulick II vinyl tape that was kept horizontal.
The participants were asked to fast for at least 12 h, and to avoid heavy physical activity and smoking for at least 2 h before their examination. After blood samples were drawn by venipuncture, they were centrifuged, and aliquots were stored at −70 °C until they were shipped on dry ice to a central laboratory.
Glucose was measured in stored blood samples using the hexokinase ultraviolet method on a Cobas Mira Plus chemistry analyzer. The insulin measurements were performed by using a radioimmunoassay with an overnight, equilibrium incubation format. Based on reassays of glucose in 2006 and 2007 in about 200 samples per examination drawn at years 7, 10, 15, and 20, and of insulin in 100 samples stored since year 15, glucose and insulin were recalibrated to harmonize them with the previous measurements. Recalibrated glucose values were 6.98 + 0.94 × year 7 glucose concentration, 7.15 + 0.96 × year 10 glucose concentration, 6.99 + 1.01 × year 15 glucose concentration, and 4.06 + 0.97 × year 20 glucose concentration. Recalibrated insulin was −0.36 + 0.93 × year 20 insulin concentration.
We diagnosed diabetes at each examination as a fasting glucose of ≥126 mg/dl (7 mmol/l) or receiving antidiabetic medication. Once this diagnosis was made, diabetes was assumed to be present at all future examinations.
Statistical analysis
The homeostasis model assessment of insulin resistance (HOMAIR: fasting plasma insulin (μU/l) × fasting plasma glucose (mmol/l)/22.5 (ref. 28)) was used to estimate insulin resistance. HOMAIRs was logarithmically transformed, given that its distribution was skewed to the right.
The WC was set to be missing at each examination in which a woman was pregnant. We investigated patterns of repeated measurements for individuals with large within-person standard deviations from year 0 to year 20 by visual inspection of the raw data to detect whether there were any substantial departures (e.g., outliers) from patterns. These outliers of WC (four at year 10 and six at year 15) and HOMAIR (one at year 7) were replaced with missing values.
In analyses predicting HOMAIR, the primary focus of this article, we excluded diabetic patients at any examination from year 0 to year 20 (n = 346). We only included participants with at least three WC measurements (WC at baseline, year 15 and at least one value among WC measurements at years 2, 5, 7, and 10, n = 1,438 excluded) in order to examine the long-term trends of WC over 15 years. This resulted in including 3,331 men and women for these analyses.
We examined longitudinal associations between WC and HOMAIR with three dimensions of WC, using generalized linear models. We used three WC decomposition terms, consisting of WC at baseline (at year 0), linear slopes in WC from year 0 to year 15, and WC fluctuation around the linear slope during 15 years of follow-up. Estimates of the slope and fluctuation in WC during 15 years of follow-up were obtained from a simple linear regression, estimated for each individual’s changes. The linear trend of an individual’s WC changes was estimated using the slope coefficient of this model, and the magnitude of WC fluctuation was represented by the standard deviation of the residuals around the fitted line. Multivariable generalized linear models were used to test the associations of three components of WC with HOMAIR, using as a dependent variable HOMAIR at year 20. Each regression adjusts the given WC component for the other two components, in addition to socio-demographic and lifestyle factors, including age, sex, race, study center, smoking status, physical activity, alcohol consumption, and education, all of which were measured at year 15. Since year 15 HOMAIR was included in the model; the dependent variable is evolution of HOMAIR between years 15 and 20. There were no significant interactions in the prediction of HOMAIR between any of year 0 WC, WC slope, or WC fluctuation, and other covariates, including sex, race, education, smoking, alcohol consumption, and physical activity, except with year 0 WC and WC slope. The subjects were classified into quartiles, according to the level of baseline WC, slopes, and fluctuations in WC.
Recognizing that there might be a feedback loop between WC and HOMAIR, secondary analyses examined the reverse: predictability of WC at year 20 from HOMAIR from year 0 to year 15 with three dimensions (HOMAIR at year 0, linear slopes in HOMAIR from year 0 to year 15, and HOMAIR fluctuation around the linear slope during 15 years of follow-up).
As a final step, we conducted analysis concerning the association of baseline and 10-year changes in waist and HOMAIR with incident diabetes between years 10 and 20. We performed proportional hazards regression with four main predictors: baseline WC, change in WC from year 0 to year 10, baseline HOMAIR, and change in HOMAIR from year 0 to year 10, among whom there were 143 incident diabetes cases out of 3,099 participants.
SAS version 9.1 (SAS Institute, Cary, NC) was used to examine the longitudinal association between WC and HOMAIR with three dimensions of WC.
RESULTS
Prediction of HOMAIR from WC
The mean age of the participants at year 15 was ~40 years; 54% were white and 44% were male. Overall, mean and standard deviation of year 20 ln(HOMAIR) was 1.44 ± 0.50 μU/l × mmol/l, whereas year 0 mean WC was 77.7 ± 11.4 cm, linear slope was 0.75 ± 0.62 cm/year over 15 years, and fluctuation was 3.25 ± 2.03 cm over 15 years. As previously reported (29), baseline WC was positively correlated with BMI, alcohol intake, and fasting insulin (data not shown). Tables 1 and 2 show the distributions of socio-demographic, body fat–related, and insulin resistance–related variables, according to the categories of linear slopes in WC during the first 15 years, and fluctuation in WC during 15 years of follow-up, respectively. Black and less educated people experienced greater increases in both WC change and WC fluctuation during 15 years of follow-up. Women tended to have greater WC fluctuation, whereas this gender difference was not evident in WC change. Current smokers experienced greater WC fluctuation, and alcohol consumption decreased with increment in WC slope. Physical activity showed an inverse association with both increment in WC slope and WC fluctuation. Body fat measurements showed strong positive associations with both higher increment in WC slope and higher WC fluctuation, but they were more strongly associated with increment in WC slope than with WC fluctuation.
Table 1.
Means (or percentages) of demographic and lifestyle factors measured at year 15 by quartile of change (cm/year) in waist circumference during 15 years of follow-up
| Quartiles of change (cm/year) in WC during 15 years of follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| −2.770 to +0.321 | +0.322 to +0.674 | +0.675 to +1.112 | +1.113 to +3.895 | ||||||
|
|
|
|
|
||||||
| Mean or % | s.d. | Mean or % | s.d. | Mean or % | s.d. | Mean or % | s.d. | P trend | |
| N | 832 | 833 | 833 | 833 | |||||
| Median | 0.11 | 0.49 | 0.86 | 1.45 | |||||
| Demographics | |||||||||
| Age (years) | 40.4 | 3.5 | 40.4 | 3.5 | 40.2 | 3.6 | 39.3 | 3.7 | <0.0001 |
| White (%) | 65.0 | 60.3 | 49.2 | 43.0 | <0.0001 | ||||
| Male (%) | 37.5 | 49.0 | 51.3 | 39.4 | 0.3 | ||||
| Education (grade of school completed) | 15.2 | 2.7 | 15.3 | 2.6 | 14.9 | 2.5 | 14.6 | 2.3 | <0.0001 |
| Lifestyle | |||||||||
| Alcohol consumption (g/day) | 13.0 | 24.4 | 12.2 | 24.1 | 11.3 | 27.9 | 8.6 | 24.9 | 0.003 |
| Physical activity (exercise units) | 415.9 | 294.1 | 396.6 | 306.5 | 330.8 | 260.7 | 274.2 | 256.1 | <0.0001 |
| Current smoker (%) | 24.2 | 21.9 | 19.9 | 20.6 | 0.06 | ||||
| Body fat variables | |||||||||
| Waist circumference (cm) | 76.8 | 10.0 | 83.8 | 10.1 | 90.9 | 10.7 | 101.4 | 12.5 | <0.0001 |
| BMI (kg/m2) | 23.6 | 4.1 | 26.1 | 4.4 | 29.0 | 4.9 | 34.3 | 6.9 | <0.0001 |
| HOMAIR (μU/l × mmol/l) | |||||||||
| ln(HOMAIR) at year 0 | 1.09 | 0.28 | 1.11 | 0.26 | 1.14 | 0.27 | 1.17 | 0.27 | <0.0001 |
| ln(HOMAIR) at year 7 | 1.16 | 0.30 | 1.23 | 0.33 | 1.32 | 0.33 | 1.45 | 0.39 | <0.0001 |
| ln(HOMAIR) at year 10 | 1.10 | 0.28 | 1.20 | 0.30 | 1.31 | 0.33 | 1.49 | 0.39 | <0.0001 |
| ln(HOMAIR) at year 15 | 1.06 | 0.29 | 1.18 | 0.33 | 1.37 | 0.36 | 1.64 | 0.44 | <0.0001 |
| ln(HOMAIR) at year 20 | 1.14 | 0.34 | 1.28 | 0.37 | 1.44 | 0.41 | 1.62 | 0.43 | <0.0001 |
All variables measured at year 15 unless otherwise specified. The number of participants with HOMAIR values at years 0, 7, 10, 15, and 20 were 2,863, 2,878, 3,015, 3,254, and 2,849, respectively, in this sample (subsets of 3,331).
HOMAIR, homeostasis model assessment of insulin resistance; ln, natural logarithm; WC, waist circumference.
Table 2.
Means (or percentages) of demographic and lifestyle factors measured at year 15 by quartile of WC fluctuation (standard deviation around the linear change, cm) during 15 years of follow-up
| Quartiles of WC fluctuation from year 0 to year 15 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 0.050–1.859 | 1.860–2.788 | 2.789–4.077 | 4.078–17.455 | ||||||
|
|
|
|
|
||||||
| Mean or % | s.d. | Mean or % | s.d. | Mean or % | s.d. | Mean or % | s.d. | P trend | |
| N | 832 | 833 | 833 | 833 | |||||
| Median | 1.41 | 2.32 | 3.35 | 5.30 | |||||
| Demographics | |||||||||
| Age (years) | 40.1 | 3.5 | 40.2 | 3.6 | 40.1 | 3.6 | 39.8 | 3.7 | 0.2 |
| White (%) | 59.9 | 58.6 | 53.4 | 45.6 | <0.0001 | ||||
| Male (%) | 49.2 | 50.2 | 44.9 | 33.0 | <0.0001 | ||||
| Education (grade of school completed) | 15.4 | 2.6 | 15.2 | 2.6 | 14.9 | 2.5 | 14.4 | 2.4 | <0.0001 |
| Lifestyle | |||||||||
| Alcohol consumption (g/day) | 11.7 | 21.6 | 11.6 | 22.9 | 9.5 | 19.2 | 12.3 | 34.8 | 0.9 |
| Physical activity (exercise units) | 391.3 | 295.5 | 383.6 | 292.7 | 345.8 | 290.0 | 297.0 | 252.8 | <0.0001 |
| Current smoker (%) | 17.9 | 19.1 | 21.4 | 28.0 | <0.0001 | ||||
| Body fat variables | |||||||||
| Waist circumference (cm) | 82.1 | 12.1 | 84.9 | 12.3 | 89.8 | 13.1 | 96.0 | 15.1 | <0.0001 |
| BMI (kg/m2) | 25.3 | 4.7 | 26.5 | 4.9 | 28.8 | 6.0 | 32.5 | 7.6 | <0.0001 |
| HOMAIR (μU/l × mmol/l) | |||||||||
| ln(HOMAIR) at year 0 | 1.08 | 0.25 | 1.09 | 0.24 | 1.13 | 0.26 | 1.21 | 0.30 | <0.0001 |
| ln(HOMAIR) at year 7 | 1.20 | 0.30 | 1.24 | 0.32 | 1.30 | 0.36 | 1.41 | 0.39 | <0.0001 |
| ln(HOMAIR) at year 10 | 1.19 | 0.32 | 1.22 | 0.32 | 1.30 | 0.36 | 1.39 | 0.39 | <0.0001 |
| ln(HOMAIR) at year 15 | 1.19 | 0.38 | 1.26 | 0.40 | 1.34 | 0.41 | 1.46 | 0.45 | <0.0001 |
| ln(HOMAIR) at year 20 | 1.27 | 0.41 | 1.31 | 0.41 | 1.42 | 0.42 | 1.47 | 0.44 | <0.0001 |
All variables measured at year 15 unless otherwise specified. The number of participants with HOMAIR values at year 0, 7, 10, 15, and 20 were 2,863, 2,878, 3,015, 3,254, and 2,849, respectively, in this sample (subsets of 3,331).
HOMAIR, homeostasis model assessment of insulin resistance; ln, natural logarithm; WC, waist circumference.
Table 3 includes adjusted estimates for HOMAIR at year 20, according to three components of WC at year 15 (WC at year 0, slope of linear change in WC from year 0 to year 15, and WC fluctuation about the linear change line from year 0 to year 15). Both the baseline WC and changes in WC during 15 years of follow-up showed strong positive relationships with HOMAIR at year 20, adjusted for HOMAIR at year 15 (so representing evolution of HOMAIR), independent of the other two WC components, even after adjusting for lifestyle factors, including smoking, drinking, and physical activity (P for trend <0.0001). For both baseline WC and WC slope, HOMAIR was 12–16% higher for those in the highest category of the predictor variable than for those in the lowest. In a model with continuous WC measures, year 20 HOMAIR adjusted for year 15 HOMAIR in addition to lifestyle factors was 6.9% higher per standard deviation of baseline WC and 6.3% higher per standard deviation of the slope of WC. However, fluctuations in WC during 15 years of follow-up were not associated with HOMAIR when examined by quartiles (Table 3) or with continuous measures (data not shown).
Table 3.
Adjusted regression estimates for year 20 HO MAIR (insulin resistance) with three decomposition components of WC at year 15 (observed WC at year 0, linear change in WC from year 0 to year 15, and WC fluctuation about the linear change during 15 years), n = 2,777
| Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Categories | Estimates | s.e. | P trend | Estimates | s.e. | P trend | |
| WC at year 0 | Male: 85+ Female: 78+ |
0.1213 | 0.0200 | <0.0001 | 0.1211 | 0.0199 | <0.0001 |
| Male: 80 to <85 Female: 70 to <78 |
0.0442 | 0.0182 | 0.0427 | 0.0182 | |||
| Male: 75 to <80 Female: 66 to <70 |
0.0113 | 0.0180 | 0.0130 | 0.0180 | |||
| Male: <75 Female: <66 |
Ref. | Ref. | |||||
| Changes in WC from year 0 to year 15 | +1.113 to +3.895 | 0.1669 | 0.0204 | <0.0001 | 0.1628 | 0.0206 | <0.0001 |
| +0.675 to +1.112 | 0.1290 | 0.0181 | 0.1274 | 0.0182 | |||
| +0.322 to +0.674 | 0.0749 | 0.0172 | 0.0739 | 0.0171 | |||
| −2.770 to +0.321 | Ref. | Ref. | |||||
| Fluctuations in WC from year 0 to year 15 | 4.078 to 17.46 | −0.0115 | 0.0186 | 0.08 | −0.0065 | 0.0188 | 0.1 |
| 2.789 to 4.077 | 0.0147 | 0.0176 | 0.0141 | 0.0176 | |||
| 1.860 to 2.788 | −0.0095 | 0.0170 | −0.0087 | 0.0170 | |||
| 0.050 to 1.859 | Ref. | Ref. | |||||
Model 1: each regression adjusts the given WC component for the other two components, in addition to age, sex, race, study center, education, and year 15 HOMAIR.
Model 2: Model 1 plus smoking, drinking, and physical activity. Each non-WC covariate was measured at year 15. Each estimate is approximately the proportionate increase in HOMAIR in the indicated category compared to its reference category.
HOMAIR, homeostasis model assessment of insulin resistance; Ref., reference; WC, waist circumference.
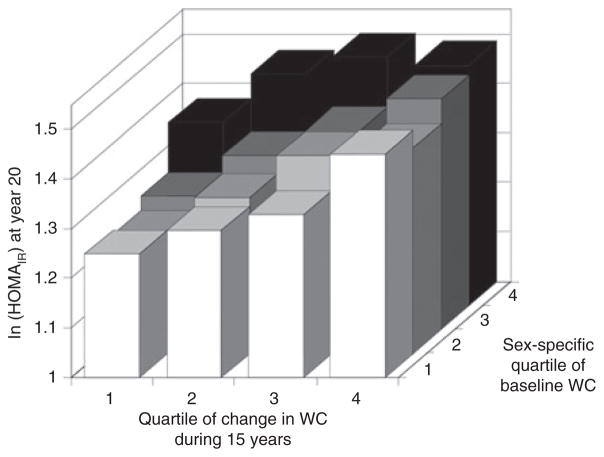
The association of change in WC during 15 years of follow-up with HOMAIR at year 20 adjusted for HOMAIR at year 15 varied depending on the level of WC at year 0 (Figure 1; P for interaction <0.0001). Estimated HOMAIR at year 20 increased substantially for steeper linear slope in WC among participants with smaller WC at year 0, whereas this association tended to be weaker at higher levels of WC at year 0. In particular, the level of HOMAIR did not increase further after the third quartile of the linear slope in WC among the participants in the largest quartile (fourth quartile) of baseline WC (WC ≥85 cm in men or ≥78 cm in women).
Figure 1.
Adjusted estimates for year 20 HOMAIR (ln(μU/l × mmol/l)) by quartiles of change in waist circumference (WC) stratified by WC at year 0 (N = 2,777). P for interaction <0.0001. Model adjusted for year 15 HOMAIR, age, sex, race, study center, education, cigarette smoking, physical activity, and fluctuations of changes in WC from year 0 to year 15.
Prediction of WC from HOMAIR
In analysis of possible reverse causality, long-term increment in HOMAIR during 15 years of follow-up did not predict future WC levels at year 20, controlling for year 0 HOMAIR, fluctuations in HOMAIR during 15 years of follow-up, year 15 WC, socio-demographic and lifestyle factors in our data (β-coefficient = −3.81 cm/(mU/l × mmol/l/year), P = 0.6).
Prediction of incident diabetes
Baseline and 10-year change in WC and HOMAIR all predicted future diabetes in a proportional hazards regression analysis that included all four variables plus covariates year 0 values of age, race, sex, clinical center, education, smoking, physical activity, and alcohol intake. The relative hazard of incident diabetes was 1.48 (95% confidence interval: 1.25, 1.75) per 11.0 cm (1 s.d.) of baseline waist; 1.25 (1.06, 1.46) per 8.3 cm of 10-year change in waist; 1.89 (1.58, 2.26) per 0.29 ln(μU/l × mmol/l) of HOMAIR at baseline; and 1.45 (1.26, 1.67) per 0.35 ln(μU/l × mmol/l) of 10-year change in HOMAIR.
DISCUSSION
Our findings show that abdominal adiposity, measured by WC, and a steady increase in abdominal adiposity (measured by slope of WC) over the long term were associated with increasing insulin resistance, even after adjusting for socio-demographic and lifestyle factors. However, WC fluctuations around the linear change during 15 years of follow-up were not associated with insulin resistance or its evolution in this nondiabetic adult population. Additional analyses showed that year 0 HOMAIR and long-term increment in HOMAIR during 15 years of follow-up did not predict future WC.
The present study showed results consistent with a recent report in these data that increased BMI during 15 years of follow-up was associated with a greater incidence of metabolic syndrome, and fluctuating BMI was not associated with this risk (30). These findings are in line with theory about the relation of body fatness with the evolution of metabolic dysregulation and diabetogenesis, insofar as insulin resistance is well estimated by HOMAIR and is involved in the pathogenesis of metabolic dysregulation and diabetes. Our findings provide additional information regarding the impact of abdominal obesity and its long-term effect on the risk of insulin resistance, a prediabetic state that can predict incident type 2 diabetes. This positive association may be explained by the dysregulation of adipocytokines, active molecules produced by adipocytes (3–6), brought about by excess fat accumulation. Adipokines participate in the pathogenesis of obesity and insulin resistance (7–12).
One of our observations raises a question about whether this clear positive relationship of body fatness and gain in abdominal adiposity with insulin resistance is uniformly applicable. Specifically, we observed the interesting interaction that the association of long-term WC increments with insulin resistance was stronger among thinner participants than those with larger WC at baseline. Several observational studies have shown that weight gain is associated with the risk for type 2 diabetes in normal weight participants (27), but not in overweight (22,27) individuals. Resnick et al. (22) reported that weight gain did not further increase the risk of type 2 diabetes, especially for those who were in the highest BMI group (BMI ≥37), using data from the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Similar findings were observed in Pima Indians: weight gain was positively associated with the incidence of type 2 diabetes in a population of Pima Indians, but not in women who were initially overweight (27). This interaction could be explained via several possible mechanisms. Obese persons are already at a higher risk of insulin resistance, so that increasing adiposity may not contribute an added risk beyond a certain threshold. A second mechanism follows Frayn et al. (31), where an interesting role of adipose tissue was noted, namely that it functions as a buffer for daily lipid flux of fatty acids in the circulation. This prevents accumulation of triacylglycerol in liver, skeletal muscle, and pancreatic β-cells; if this buffering action was impaired, then the process to insulin resistance might be accelerated. In addition, a new viewpoint has been introduced that fat-soluble toxins in adipose tissue, such as persistent organic pollutants accumulated in the food chain, persisting in the environment, are stored in human adipose tissue for a lifetime (32), and, eventually, lead to worsening insulin resistance, metabolic syndrome, and diabetes (33–37). Therefore, we can offer a possible scenario in which there were diminished relationships of increasing WC with insulin resistance in certain subgroups. Fat-soluble toxins can be released to be circulated and absorbed into critical organs (38) under some circumstances, and they can be diluted or sequestered by increasing adipose tissue mass. Thus, there could be a tension between an adverse pathway (adipokine or other dysregulation due to excess adiposity) and a beneficial pathway (dilution of persistent organic pollutants or other fat-soluble toxins in adipose tissue). However, we saw only a hint of such a tension in the reduced rate of change in HOMAIR across WC slope categories in the initially fatter compared to thinner participants. These speculations regarding fat-soluble toxins or other potential mechanisms could not be addressed in the present study, and additional research is warranted.
Our study has limitations. The study was conducted only among black and white individuals, with no representation of Hispanic, Asian, or individuals of other racial or ethnic backgrounds. As a result, the conclusions from this study may not be applicable to all populations. In particular, there are some findings that Asians are more responsive to visceral fat than are whites, and one might expect the associations of WC with insulin resistance to be different in this subgroup. In addition, there could be residual confounding due to this study’s observational nature. A strength is that we were able to examine changes in WC and HOMAIR over a reasonably long time period, including analyses of whether there is a mutually reciprocal relation between HOMAIR and WC.
In summary, our results show that abdominal obesity and a long-term increase of central fat are associated with increased insulin resistance; however, larger increases in WC were not related to increased insulin resistance among those with greatest baseline WC. Further research is needed to elucidate the underlying etiological relationships between adiposity, an increase of adipose tissue, and the genesis and progression of insulin resistance in diverse populations.
Acknowledgments
This research was supported by contracts N01-HC-95095, N01-HC-48047, N01-HC-48048, N01-HC-48049, and N01-HC-48050, and a grant R01-HL-53560, all from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Gravas S. Epidemiologic trends indicate the prevalence of obesity will rise globally. Eur Urol. 2007;52:204–205. [PubMed] [Google Scholar]
- 3.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 4.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie SA, Ewart MA, Perry CG, Connell JM, Salt IP. The role of insulin and the adipocytokines in regulation of vascular endothelial function. Clin Sci. 2004;107:519–532. doi: 10.1042/CS20040190. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 8.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 9.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 10.Farooqi IS, Keogh JM, Kamath S, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–35. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci. 2006;110:267–278. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 12.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 13.Skarfors ET, Selinus KI, Lithell HO. Risk factors for developing non-insulin dependent diabetes: a 10 year follow up of men in Uppsala. BMJ. 1991;303:755–760. doi: 10.1136/bmj.303.6805.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charles MA, Fontbonne A, Thibult N, et al. Risk factors for NIDDM in white population. Paris prospective study. Diabetes. 1991;40:796–799. doi: 10.2337/diab.40.7.796. [DOI] [PubMed] [Google Scholar]
- 15.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 16.Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin secretion and increased insulin resistance are independently related to the 7-year risk of NIDDM in Mexican-Americans. Diabetes. 1995;44:1386–1391. doi: 10.2337/diab.44.12.1386. [DOI] [PubMed] [Google Scholar]
- 17.Martin BC, Warram JH, Krolewski AS, et al. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 18.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 19.Lebovitz HE. The relationship of obesity to the metabolic syndrome. Int J Clin Pract Suppl. 2003;(134):18–27. Review. [PubMed] [Google Scholar]
- 20.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997;146:214–222. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- 22.Resnick HE, Valsania P, Halter JB, Lin X. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health. 2000;54:596–602. doi: 10.1136/jech.54.8.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. J Epidemiol Community Health. 2005;59:134–139. doi: 10.1136/jech.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh-Banerjee P, Wang Y, Hu FB, et al. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159:1150–1159. doi: 10.1093/aje/kwh167. [DOI] [PubMed] [Google Scholar]
- 25.Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2006;84:427–433. doi: 10.1093/ajcn/84.1.427. [DOI] [PubMed] [Google Scholar]
- 26.Oguma Y, Sesso HD, Paffenbarger RS, Jr, Lee IM. Weight change and risk of developing type 2 diabetes. Obes Res. 2005;13:945–951. doi: 10.1038/oby.2005.109. [DOI] [PubMed] [Google Scholar]
- 27.Hanson RL, Narayan KM, McCance DR, et al. Rate of weight gain, weight fluctuation, and incidence of NIDDM. Diabetes. 1995;44:261–266. doi: 10.2337/diab.44.3.261. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Lee CD, Jacobs DR, Jr, Schreiner PJ, Iribarren C, Hankinson A. Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2007;86:48–54. doi: 10.1093/ajcn/86.1.48. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd-Jones DM, Liu K, Colangelo LA, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation. 2007;115:1004–1011. doi: 10.1161/CIRCULATIONAHA.106.648642. [DOI] [PubMed] [Google Scholar]
- 31.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 32.Fisher BE. Most unwanted. Environ Health Perspect. 1999;107:A18–A23. doi: 10.1289/ehp.99107a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DH, Lee IK, Song K, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 34.Lee DH, Jacobs DR, Jr, Porta M. Could low-level background exposure to persistent organic pollutants contribute to the social burden of type 2 diabetes? J Epidemiol Community Health. 2006;60:1006–1008. doi: 10.1136/jech.2006.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007;30:622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- 36.Lee DH, Lee IK, Steffes M, Jacobs DR., Jr Extended analyses of the association between serum concentrations of persistent organic pollutants and diabetes. Diabetes Care. 2007;30:1596–1598. doi: 10.2337/dc07-0072. [DOI] [PubMed] [Google Scholar]
- 37.Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR., Jr Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetologia. 2007;50:1841–1851. doi: 10.1007/s00125-007-0755-4. [DOI] [PubMed] [Google Scholar]
- 38.Chevrier J, Dewailly E, Ayotte P, et al. Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. Int J Obes Relat Metab Disord. 2000;24:1272–1278. doi: 10.1038/sj.ijo.0801380. [DOI] [PubMed] [Google Scholar]