Abstract
Objective
To describe the systematic development of the Stroke Coach, a theory- and evidence-based intervention to improve control of lifestyle behaviour risk factors in stroke patients.
Design
Intervention development.
Setting
Community.
Participants
Individuals who have had a stroke.
Intervention
We used Intervention Mapping to guide the development of the Stroke Coach. Intervention Mapping is a systematic process used for intervention development and comprised of steps that progress from the integration of theory and evidence to the organization of realistic strategies to facilitate the development of a practical intervention supported by empirical evidence. Social Cognitive Theory was the underlying premise for behaviour change, while Control Theory methods were directed towards sustaining the changes to ensure long-term health benefits. Practical evidence-based strategies were linked to behavioural determinants to improve stroke risk factor control.
Main outcome measures
Not applicable.
Results
The Stroke Coach is a patient-centred, community-based, telehealth intervention to promote healthy lifestyles after stroke. Over six months, participants receive seven 30 to 60 minute telephone sessions with a lifestyle coach who provides education, facilitates motivation for lifestyle modification, and empowers participants to self-management their stroke risk factors. Participants also receive a self-management manual and a self-monitoring kit.
Conclusion
Through the use of Intervention Mapping we developed a theoretically sound and evidence-grounded intervention to improve risk factor control in stroke patients. If empirical evaluation of the Stroke Coach produces positive results, the next step will be to develop an implementation intervention to ensure successful uptake and delivery of the program in community and outpatient settings.
Keywords: behaviour change, intervention mapping, stroke, secondary prevention, health promotion, chronic disease management
After 55 years of age, one in five women and one in six men will have a stroke.1 Due to population aging the total number of people with first ever stroke is increasing.2 After a stroke, there is a 13% risk of a subsequent stroke within one year, and a 25 to 33% risk after five years.3–5 These high rates of recurrence, largely due to poor risk factor control,6,7 place increasing emphasis on the importance of developing, evaluating, and implementing preventive strategies.
Stroke prevention guidelines8–10 and epidemiological studies11 consistently report both behavioural and physiological processes as stroke risk factors. The efficacy of behaviour modification at improving physiological factors is well established,12–14 and therefore, the existing paradigm for secondary stroke prevention is to emphasize improvements to lifestyle behaviours, which in turn will lead to improved control of physiological risk factors, and eventually improved secondary prevention.
There is increasing recognition that interventions to change behaviour should have strong theoretical foundations because such programs will more likely target causal determinants of behaviour change, contribute to the testing and further development of theory, and lead to an increase in the understanding of strategies that facilitate change.15 The majority of secondary stroke prevention programs, however, do not appear to be based on behaviour change theory.16 Thus, it is not surprising that meta-analyses of results from existing behaviour modification programs after stroke show minimal effects on behavioural stroke risk factors,17 and no effect on mortality, cardiovascular event rates, or cardio-metabolic risk factor profiles.18
The complex nature of behaviour change complicates the process of intervention development, evaluation, and implementation.19 Several theoretical frameworks have been proposed to help researchers navigate through the complexities by providing guidance during the planning phases (e.g., Intervention Mapping,20 Behaviour Change Wheel21). These frameworks are comprehensive in that they provide guidance on the development of theoretically sound and evidence-guided interventions, and also emphasize the importance of considering implementation and evaluation strategies. Given the high rates of secondary stroke and inadequacies of existing prevention programs, the use of theoretical frameworks to aid in the development of new preventative interventions seems prudent.
In this paper, we report on the development of a theory- and evidence-based intervention, the Stroke Coach, using an Intervention Mapping approach.22 The purpose of the Stroke Coach is to empower individuals to improve lifestyle behaviours and maintain the improvements after the conclusion of the intervention.
Methods
Intervention Mapping is comprised of six steps that serves as a blueprint for designing, implementing and evaluating practical interventions supported by theory, empirical evidence, and clinical experiences.22
Step 1 - Needs assessment
We used several methods to assess needs, including: 1) Establishing and working with a planning group to provide input throughout intervention development; and 2) Conducting a detailed literature search to assess the issues associated with secondary prevention efforts, and conceptualize a framework for an intervention to address the needs derived from theory, empirical evidence, and practical knowledge.
Step 2 - Proximal intervention objectives
To determine proximal objectives, we followed two steps: 1) Identify performance objectives that specify lifestyle behaviours to change to improve stroke risk factor control; and 2) Identify theoretical determinants of those behaviours as the proximal objectives.22
Step 3 - Theory-based intervention methods and practical strategies
We identified evidence-based intervention methods to target the proximal objectives, and then translated the methods into practical strategies for intervention delivery. Whereas methods are general evidence-based techniques (e.g. self-monitoring) used to influence behavioural determinants, strategies are more specific and practical techniques used to operationalize and deliver the method (e.g. using an activity monitor to track daily steps and instruction on how to use the monitor to provide motivation for continued physical activity).22
Step 4 - Organizing the strategies into an intervention
We integrated the practical strategies into an organized intervention that addresses the proximal objectives. In doing so, we conceptualized the: 1) dose; 2) delivery; and 3) organization of the intervention. We also obtained 4) feedback from stakeholders, including end-users as well as from decision-makers from organizations that could potentially implement the intervention, and refined the program structure and materials based on the feedback.22
Step 5 - Implementation Plan
We initiated plans for initial implementation, including training materials for the coaches, as well as methods to assess program fidelity.
Step 6 - Evaluation Plan
We develop an evaluation plan to test whether the intervention is successful at addressing the proximal objectives.22
Results
Below we present the considerations and decisions made during each of the six intervention mapping steps22 in developing the Stroke Coach.
Step 1: Needs Assessment
1) Establish and work with a planning group
Our planning group (n=8) was comprised of health care professionals, including a neurologist, psychologist, physiatrist, dietician, and physical therapist, and researchers with expertise in neurosciences, stroke and cardiac prevention, human nutrition, behaviour change theories, self-management, and research and evaluation methodologies. Three member of our planning group have health authority leadership roles in which they could potentially implement the program within their setting.
2) Conduct a literature search
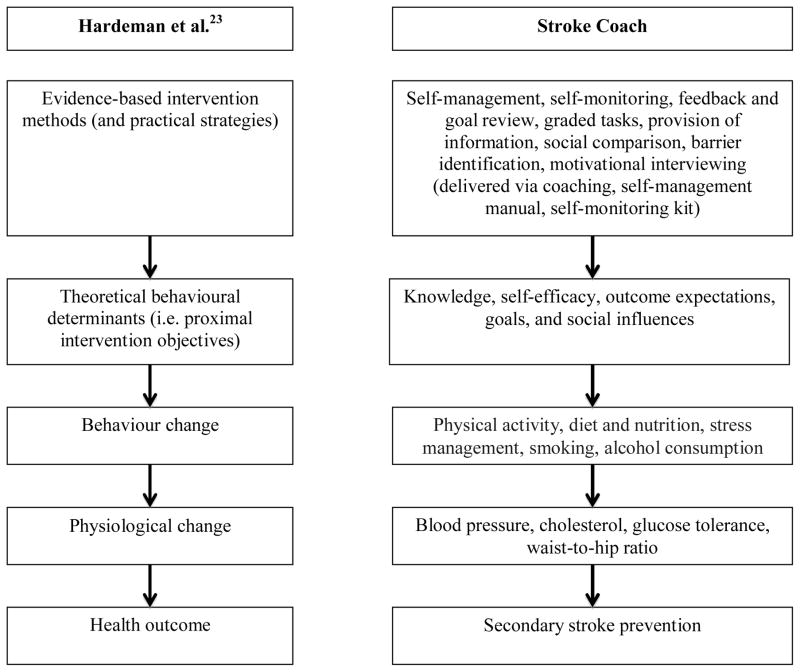
We performed a literature search to develop an understanding of the population and health issue of secondary stroke prevention, which formed the basis of the introduction to this manuscript. Furthermore, we conceptualized the Stroke Coach to be theoretically sound and use evidence-based strategies. To guide our work in the development of the Stroke Coach, we followed the Causal Modelling Behaviour Change conceptual framework proposed by Hardeman et al..23 This framework proposes a causal path in which behaviour change leads to physiological changes, which in turn lead to changes in health outcomes. As well, the Stroke Coach focuses on behaviour change and maintenance by targeting theoretical determinants of behaviour using evidence-based change and maintenance strategies. Figure 1 presents the general causal framework in parallel to the specific causal path for secondary stroke prevention hypothesized by Stroke Coach.
Figure 1.
Framework for causal behavioural modeling. This figure presents the general Causal Modelling Behaviour Change conceptual framework on the left, and the specific causal path towards secondary prevention hypothesized by the Stroke Coach intervention on the right.
We also conceptualized the Stroke Coach to align with key principles of health care quality24,25 to facilitate the program’s eventual implementation, including:
i. Patient centred
Everyone has different beliefs about healthy living, as well as different personal and environmental issues that may influence behaviour change. For these reasons, we decided to use a one-on-one delivery method, and follow the concept of patient centredness.24
ii. Highly accessible
After stroke many individuals are reported to have transportation and geographic barriers to attending health service programs.26–29 To address these possible barriers and to increase the accessibility of our intervention, we planned to deliver the Stroke Coach using highly accessible consumer technologies, such as the telephone.
iii. Timely and Community-based
The highest incidence of secondary strokes is within one-year of the initial event.3 It is during this time that patients have returned to community living and are receiving few or no follow-up health services.2 For this reason, we decided to design an intervention that would be timely for secondary prevention (i.e., within one year) and could be easily implemented in a community-based setting.
Step 2: Proximal Intervention Objectives
We planned to develop a comprehensive and patient-centred intervention that would allow participants to focus on any behavioural stroke risk factor (i.e., physical activity, diet and nutrition, stress management, smoking and alcohol consumption).
The proximal intervention objectives were thus considered to be theoretical social cognitive determinants of behaviour change (e.g., knowledge, self-efficacy), as shown in Figure 1. We focus on social cognitive determinants because Social Cognitive Theory30,31 is the most comprehensive theory of behaviour change32 and has much evidence demonstrating the predictive value of its determinants.
Step 3: Evidence-based Intervention Methods and Practical Strategies
1) Evidence-based intervention methods
To modify the behavioural determinants and ensure sustained behaviour change, we identified evidence-based behaviour change methods originating from Bandura’s Social Cognitive Theory31 as well as Carver and Scheier’s Control Theory.33 Whereas social cognitive methods were deemed important to initiate modification of the determinants derived from the same theory, Control Theory methods were more directed towards sustaining the changes (i.e., self-management) to ensure long-term health benefits.
To help identify evidence-based intervention methods, we used the foundational works of Michie et al.15 and Abraham and Michie,34 who have created a taxonomy of behaviour change and maintenance methods and linked those methods to theoretical determinants of behaviour. Table 1 presents the evidence-based methods used in Stroke Coach, and identifies the behavioural determinants that each method is targeting.
Table 1.
Linking evidence-based intervention methods to practical strategies and behavioural determinants
| Behavioural determinants | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Theory | Evidence-based intervention methods | Practical strategies for delivery | Knowledge | Self-efficacy | Outcome expectations | Goals | Sociostructural factors – social influences |
| Control Theory to sustain/maintain behaviour | Self-management (goal setting; decision making/problem solving; action planning) | Self-management manual; Coaching | x | x | |||
| Self-monitoring | Self-monitoring kit; Coaching | x | x | ||||
| Feedback and goal review | Coaching | x | x | ||||
|
| |||||||
| Social Cognitive Theory to initiate new behaviour | Graded tasks | Coaching | x | x | |||
| Information about behaviour, outcome, consequences | Self-management manual; Coaching | x | x | x | |||
| Social comparison and encouragement | Coaching | x | x | x | |||
| Barrier identification | Coaching | x | x | x | x | ||
|
| |||||||
| Various | Motivational interviewing | Coaching | x | x | |||
NOTE: Coaching: A patient-centred approach used to motivate patients to change behaviour, and achieve goals that improve health using the 5A (Assess, Advise, Agree, Assist, Arrange) counselling model;42 Self-management manual: Used to increase knowledge about behavioural stroke risk factors and the concept of self-management, and detail important self-management skills; Self-monitoring kit: Used to monitor various behavioural stroke risk factors and keep track of progress.
2) Practical strategies
We use three practical strategies to deliver Stroke Coach’s evidence-based intervention methods, and to serve as the foundation of the intervention. All participants will receive:
i. Lifestyle Coaching
Lifestyle coaching is a patient-centred approach to motivate patients to change behaviour and improve their health.35,36 The coach’s role involves active listening and non-judgmental inquiry to build patients’ desire to become healthier.36 Our coaches will be health workers who have experience working with individuals with stroke, knowledge of chronic disease self-management (e.g., kinesiologists), and who have completed our training specific to the project.
ii. Self-management manual
The purpose of the self-management manual is to provide detailed information on how to self-manage lifestyle behaviours for improved stroke risk factor control. This manual, prepared by the Stroke Coach development/research team will be given to participants as a resource and used as a discussion document during the coaching sessions.
iii. Self-monitoring kit
Self-monitoring is the single most important behaviour change and self-management method.37 The purpose of the self-monitoring kit is for participants to monitor various behaviours (as well as physiological indicators) and keep track of progress. This kit includes a: health report card; pedometer (Fitbit Zip; Fitbit, Inc., San Francisco, CA, USA); blood pressure monitor (Omron 3 series model: BP710CANN; Omron Healthcare Inc., Hoffman Estates, IL, USA); tape measure for waist and hip measurement; food and physical activity diaries; body mass index chart; and instructions.
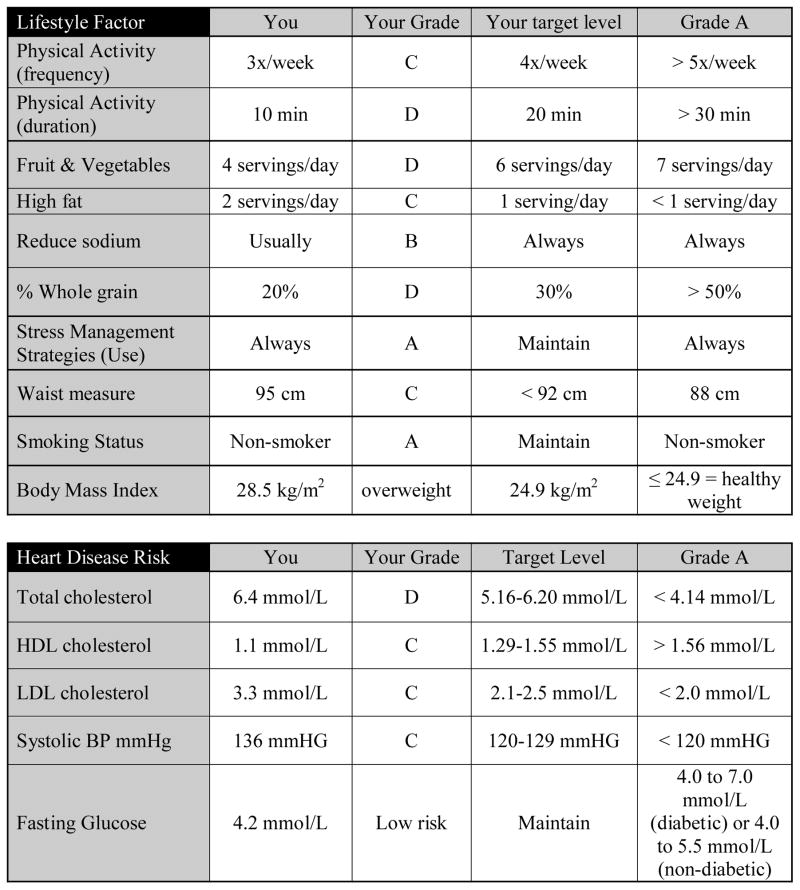
A key component of the self-monitoring kit is the health report card, shown in Figure 2. Health reports cards have successfully been used in previous cardiovascular prevention research,38 and we have adapted the use of report cards for stroke prevention. Each participant receives grades varying from A to F on their ‘lifestyle behaviour’ and ‘cardiovascular’ stroke risk factors. They also receive information on the definition of each grade, which have been adapted from clinical guidelines (e.g., Canadian Physical Activity and Food Guidelines). Prior to enrolling in the program participants will complete a brief survey from which we will determine their grades.
Figure 2.
Example of a health report card. This figure presents a completed health report card that participants would receive and discuss with their coach.
Table 1 identifies which practical strategy is expected to deliver each of the evidence-based intervention methods.
Step 4: Organizing the Strategies into an Intervention
1) Dose
Participants will receive seven 30 to 45 minute coaching sessions on a one-to-one basis with a lifestyle coach over a six-month period. The duration, frequency, and intensity of our intervention are approximately the averages used in other stroke self-management programs reported in a recent systematic review.17
2) Delivery
To increase accessibility, Stroke Coach will be delivered via telehealth.39 In our 2015 survey on willingness to receive stroke rehabilitation using technology, we found that telephones are one of the most widely owned and used communication technologies, and that people had a high interest to receive education through phone calls.40 Evidence also exists that telephone coaching improves health behaviours and health status in people with chronic conditions.41
3) Organization
To structure the coaching sessions, we adopted the evidence-based Five A’s (Assess, Advise, Agree, Assist, Arrange) organizational construct for clinical counselling.42 In sessions one to six, the coaches will review the participants’ health report card with them and assess their knowledge about stroke risks, and current behaviours (Assess). The coaches will also provide information about stroke risks and benefits of improving lifestyle behaviours (Advise). Any number of long-term health report card goals prioritized by the Health Report Card will be established through a collaborative process (Agree). The coaches and participants will discuss options for implementing short-term strategies to reach the long-term goals, and refer to the self-management manual for resources. Furthermore, participants will be informed about the benefits of self-monitoring, and instructed on how to monitor their own blood pressure, body composition, diet and physical activity (Assist). Follow-up will include the scheduling of the next monthly session (Arrange). At the last session (session 7) the coaches will work with the participants to determine long-term strategies to maintain or further improve lifestyle behaviours on their own.
4) Stakeholder review and revision
After conceptualizing the intervention, we sought feedback from various stakeholders including: stroke patient groups; advocacy groups; health professionals and other researchers. Feedback from stroke patients emphasized the importance that the service be free (which it will be). It was also felt that one month in-between sessions was too long and that participants needed more contact with their coach to review progress and revise action plans if needed. As a result, it was decided to add in brief (5 to 10 minute) follow-up phone calls to check on progress in between the longer monthly sessions. Thus, in the final Stroke Coach intervention, participants have either a telephone coaching session or a follow-up call every two weeks for six months.
Step 5: Implementation Plan
We devised written materials that describe how the Stroke Coach is to be delivered, as well as a training presentation and coaching manual for the coaches to use during each session (available upon request). The training presentation included information on stroke epidemiology, stroke risk factors and the importance of lifestyle behaviour modification to improve secondary prevention, all components of the Stroke Coach, the delivery of each of the telephone sessions using the 5A counseling model, as well as review of the coaching manual and practice. To assess our training materials, and to ensure fidelity of the delivery of the program in our evaluation, we decided that the coaches would record various sessions for the intervention developers to listen to and critique, or developers would listen to ‘live’ sessions. The lead researcher would then review the coaching sessions to ensure the dose, delivery, and organization (as noted above) of the sessions are implemented as detailed in the coaching manual. Any discrepancies would be brought to the coaches’ attention immediately after review. It was also decided that the coaches and developers would meet on an on-going basis to discuss progress, challenges, and issues.
Step 6: Evaluation Plan
We developed a research protocol detailing the evaluation of Stroke Coach using a multi-site single-blind randomized controlled trial study design.43 Our primary hypothesis is that among stroke patients within one-year post stroke, the Stroke Coach intervention will improve a global measure of lifestyle behaviour (i.e., Health Promoting Lifestyle Profile II44) compared to an attention-control program. Our secondary hypotheses will test the effects of the Stroke Coach on specific lifestyle behaviours, depressive symptoms, cardiovascular risk, cognition, and health-related quality of life. To obtain information on the experiences and satisfaction with Stroke Coach, all participants will also complete an exit survey to determine their perspectives. Furthermore, we will undertake a qualitative study to explore the coaches’ perceptions of their skills to coaching and ability to provide coaching that is consistent over time and across participants. Such knowledge will inform the recruitment of coaches, the development of supplementary materials to train the coaches and help ensure reliable delivery of the intervention. While ethics approval was not necessary during intervention development, we will seek ethics approval from all participating sites prior to beginning the trial.
Discussion
In this paper we describe the development of a theoretically driven and evidence-based Stroke Coach intervention to improve lifestyle behaviours and thus risk factor control in stroke patients using an Intervention Mapping approach. Intervention Mapping has been previously used to develop effective self-management, prevention and health promotion programs for people with various conditions,22 however up until now it has not been used to guide the development of secondary stroke preventions programs.
In our experience, the principles and steps delineated by Intervention Mapping are useful to address current issues regarding the development, implementation, and evaluation of secondary stroke prevention programs. First, Intervention Mapping provides a systematic approach for the development of health programs and establishes a clear set of tasks that help to focus developers. It enabled us to systematically report on the use theory, empirical evidence, and practical perspectives in the development of the Stroke Coach, which at present is lacking in the existing secondary stroke prevention literature. It also helped facilitate a clear description of the different components of the intervention which will enable meaningful replication. Second, the formation of our multidisciplinary planning group proved to be valuable. With expert knowledge, input, and shared decision making throughout the process, though to engaging our stakeholder group that included patients, we were able to develop a highly relevant and patient-oriented program. Third, Intervention Mapping facilitated early consideration of program implementation. As such, the Stroke Coach adheres to key principles of health service quality, and we have developed materials to train the lifestyle coaches to help ensure a standardized delivery, as well as considered methods to test for and ensure program fidelity. Finally, given the amount of consideration the Intervention Mapping process required for the development of the Stroke Coach, we have developed a detailed research protocol to evaluate the program.
Limitations
There are several limitations to our intervention development process that are worth noting. For example, the participation of patients in the development of the Stroke Coach was to review and comment on a program that had already been conceptualized. While there was widespread interest and support of the program, had patients been more involved at the conceptualization stage, the program might have been developed differently. However, our planning group had three health authority stakeholders who were in leadership roles where they could potentially implement the program within their setting. As well, the program itself utilizes only a few methods deemed to modify theoretical determinants of behaviour. There are other existing evidence-based methods, such as peer support and mentoring, that the program does not include. In future, it is likely worthwhile to test alternative delivery models where peers are the coaches, and possibly group formats that make use of group video or teleconferencing technologies. Next, because of the complexity of the program, its delivery by different coaches with diverse skill sets and personalities may lead to variable outcomes. To understand these issues, we will formally evaluate the implementation of the program (including the fidelity of the intervention and perceptions of the coaches) and develop supplemental training materials for the coaches as needed to ensure consistent delivery of Stroke Coach. Finally, because we wanted to develop a comprehensive yet patient-oriented program, we identified target lifestyle behaviours for change at a global level, and not on specific behaviours. We decided on this approach to avoid prescription and to encourage patient to self-identify specific behaviours as goals to focus on and work with their coaches to develop meaningful action plans to achieve those goals.
Conclusion
Stroke Coach is a novel secondary prevention program designed to improve stroke risk factor control via lifestyle behaviour modification. By working with participants to actively improve the management of their behavioural stroke risk factors, it is postulated that the study participants will be empowered to improve their behaviours during the 6-month program, and importantly to maintain the improvements after the program has ended. If the evaluation of Stroke Coach produces positive results, the next step will be to develop an implementation intervention to ensure successful uptake and delivery of the program in community and outpatient settings.
Acknowledgments
This work was supported by: Canadian Institutes of Health Research Postdoctoral Fellowship (BMS) and Operating Grant; Michael Smith Foundation for Health Research Postdoctoral Fellowship (BMS) and Senior Scholar Award (JJE); Canada Research Chair in Neurological Rehabilitation (JJE); Pfizer/Heart and Stroke Foundation Chair in Cardiovascular Prevention Research at St. Paul’s Hospital (SAL); and the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery Operating Grant.
References
- 1.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. on behalf of the Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Diseases Study 2010. Lancet. 2014;383(9913):245–255. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The oxfordshire community stroke project. Stroke. 1994;25(2):333–37. doi: 10.1161/01.str.25.2.333. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–33. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 5.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. on behalf of the American Heart Association Stroke Council. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke. 2011;42:227–76. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 6.Heuschmann PU, Kircher J, Nowe T, Dittrich R, Reiner Z, Cifkova R, et al. Control of risk factors after ischaemic stroke across Europe: data from the stroke-specific module of the EUROASPIRE III survey. European Journal of Preventive Cardiology. 2015;22(10):1354–62. doi: 10.1177/2047487314546825. [DOI] [PubMed] [Google Scholar]
- 7.Brewer L, Mellon L, Hall P, Dolan E, Horgan F, Shelley E. Secondary prevention after ischaemic stroke: the ASPIRE-S study. BMC Neurology. 2015;15:216. doi: 10.1186/s12883-015-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsay P, Furie KL, Davis SM, Donnan GA, Norrving B. World Stroke Organization global stroke services guidelines and action plan. Int J Stroke. 2014;9:4–13. doi: 10.1111/ijs.12371. [DOI] [PubMed] [Google Scholar]
- 9.Coutts SB, Wein TH, Lindsay MP, Buck B, Cote R, Ellis P, et al. Canadian Stroke Best Practice Recommendations: secondary prevention of stroke guidelines, update 2014. Int J Stroke. 2015;10:282–291. doi: 10.1111/ijs.12439. [DOI] [PubMed] [Google Scholar]
- 10.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet Neurol. 2010;376:112–23. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan NM. Lifestyle modification for prevention and treatment of hypertension. J Clin Hypertens. 2004;6:716–9. doi: 10.1111/j.1524-6175.2004.03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong Q, Kang J, Ying Y, Li H, Zhang X, Wu Y, et al. Lifestyle interventions for adults with impaired glucose tolerance: A systematic review and meta-analysis of the effects on glycemic control. Internal Med. 2015;54:303–10. doi: 10.2169/internalmedicine.54.2745. [DOI] [PubMed] [Google Scholar]
- 14.Mannu GS, Zaman MJS, Gupta A, Rehman HU, Myint PK. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr Cardiol Rev. 2013;9:2–14. doi: 10.2174/157340313805076313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michie S, Johnson M, Francis J, Hardeman W, Eccles M. From theory to intervention: Mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Pyschol. 2008;57(4):660–680. [Google Scholar]
- 16.Redfern J, McKevitt C, Wolfe CDA. Development of complex interventions in stroke care. A systematic review. Stroke. 2006;37(9):2410–9. doi: 10.1161/01.STR.0000237097.00342.a9. [DOI] [PubMed] [Google Scholar]
- 17.Sakakibara BM, Kim AJ, Eng JJ. A systematic review and meta-analysis on self-management for improving risk factor control in stroke patients. Int J Behav Med. doi: 10.1007/s12529-016-9582-7. (in-press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lennon O, Galvin R, Smith K, Doody C, Blake C. Lifestyle interventions for secondary disease prevention in stroke and transient ischaemic attack: a systematic review. Eur J Prev Cardiol. 2014;21(8):1026–39. doi: 10.1177/2047487313481756. [DOI] [PubMed] [Google Scholar]
- 19.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Perricrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartholomew LK, Parcel GS, Gerjo K. Intervention mapping: A process for developing theory- and evidence-based health education program. Health Edu Behav. 1998;25(5):545–563. doi: 10.1177/109019819802500502. [DOI] [PubMed] [Google Scholar]
- 21.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartholomew-Eldredge LK, Markham CM, Ruiter RAC, Fernandex ME, Kok G, Parcel GS. Planning health promotion programs: An intervention mapping approach. 4. New York: Jossey-Bass; 2016. [Google Scholar]
- 23.Hardeman W, Sutton S, Griffin S, Johnson M, White N, Waream NJ, et al. A causal modelling approach to the development of theory-based behaviour change programmes for trial evaluation. Health Educ Res. 2005;20(6):676–687. doi: 10.1093/her/cyh022. [DOI] [PubMed] [Google Scholar]
- 24.Committee on Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington: Institute of Medicine; 2001. [Google Scholar]
- 25.Health Council of Canada. Which way to quality? Key perspectives on quality improvement in Canadian health care systems. Ottawa: Health Council of Canada; 2013. [Google Scholar]
- 26.Fisk GD, Owsley C, Vonne Pulley L. Driving after stroke: Driving exposure, advice, and evaluations. Arch Phys Med Rehabil. 1997;78:1338–45. doi: 10.1016/s0003-9993(97)90307-5. [DOI] [PubMed] [Google Scholar]
- 27.Ing MM, Vento MA, Nakagawa K, Linton KF. A Qualitative Study of Transportation Challenges Among Intracerebral Hemorrhage Survivors and Their Caregivers. Hawaii J Med Public Health. 2014;73(11):353–357. [PMC free article] [PubMed] [Google Scholar]
- 28.Shultis W, Graff R, Chamie C, Hart C, Louangketh P, McNamara M, et al. Striking rural-urban disparities observed in acute stroke care capacity and services in the pacific northwest: implications and recommendations. Stroke. 2010;41:2278–2282. doi: 10.1161/STROKEAHA.110.594374. [DOI] [PubMed] [Google Scholar]
- 29.Statistics Canada [Internet] Ottawa: Statistics Canada; 2011. [cited 2016 April 26] available from: https://www12.statcan.gc.ca/census-recensement/2011/as-sa/98-310-x/2011003/fig/fig3_2-2-eng.cfm. [Google Scholar]
- 30.Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- 31.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 32.Redding CA, Rossi JS, Rossi SR, Velicer WF, Prochaska JO. Health behaviour models. Int Electr J Health Educ. 2000;3:180–193. [Google Scholar]
- 33.Carver CS, Scheier MF. Control Theory: A useful conceptual framework for personality-social, clinical and health psychology. Psychol Bull. 1982;92(1):111–35. [PubMed] [Google Scholar]
- 34.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27(3):379–387. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- 35.Miller WR, Rollnick S. Motivational Interviewing. 3. Guilford Press; 2012. [Google Scholar]
- 36.Kivela K, Elo S, Kyngas H, Kaariainen M. The effects of health coaching on adult patients with chronic diseases: A systematic review. Patient Educ Couns. 2014;97:147–157. doi: 10.1016/j.pec.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009;28:690–701. doi: 10.1037/a0016136. [DOI] [PubMed] [Google Scholar]
- 38.Wister A, Loewen N, Kennedy-Symonds H, McGowan B, McCoy B, Singer J. One-year follow up of a therapeutic lifestyle intervention targeting cardiovascular disease risk. Can Med Assoc J. 2007;177:859–65. doi: 10.1503/cmaj.061059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White LUE, Krousel-Wood MA, Mather F. Technology meets healthcare: Distance learning and telehealth. Ochsner J. 2001;2(1):22–29. [PMC free article] [PubMed] [Google Scholar]
- 40.Edgar MC, Monsees S, Rhebergen J, Waring J, Van der Star T, Eng JJ, et al. Feasibility of telerehabilitation in stroke recovery: A survey on access and willingness to use low-cost consumer technologies. doi: 10.1089/tmj.2016.0129. (in-press) [DOI] [PubMed] [Google Scholar]
- 41.Dennis SM, Harris M, Lloyd J, Powell G, Faruqi N, Zwar N. Do people with existing chronic conditions benefit from telephone coaching? A rapid review. Aust Health Rev. 2013;37:381–388. doi: 10.1071/AH13005. [DOI] [PubMed] [Google Scholar]
- 42.Whitlock EP, Orleans CT, Pender N, Allan J. Evaluating primary care behavioral counseling interventions: an evidence-based approach. Am J Prev Med. 2002;22:267–84. doi: 10.1016/s0749-3797(02)00415-4. [DOI] [PubMed] [Google Scholar]
- 43.Sakakibara BM, Eng JJ, Benavente O, Barr SI, Silberberg ND, Goldsmith CH, et al. A telehealth intervention to promote healthy lifestyles after stroke: The Stroke Coach protocol. Stroke. 2014;45:E285–E285. doi: 10.1177/1747493017729266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker SN, Sechrist KR, Pender NJ. The Health-Promoting Lifestyle Profile: Development and psychometric characteristics. Nurs Res. 1987;36:76–81. [PubMed] [Google Scholar]