Abstract
Background
Cognitive training may contribute to the ability to maintain cognitive function in healthy elderly adults. Whether genotype modifies training effects remains unknown.
Objective
Assess influence of APOE on cognitive function over time in community-dwelling elderly adults participating in multi-domain cognitive training.
Methods
Healthy individuals ≥70 years of age were screened from one urban community in Shanghai. 145 healthy Chinese older adults met inclusion criteria and were assigned to intervention (n = 88) or control (n = 57) groups. Multi-domain cognitive training involved 24 sessions of different content taking place over 12 weeks. Neuropsychological testing was administered at baseline, immediately after training, six months and twelve months post-intervention; composite measures of cognitive function were identified via factor analysis.
Results
Three factors explained the majority of variance in function (verbal memory, processing speed, executive function). The intervention attenuated 12-month declines in processing speed, regardless of APOE genotype (p = 0.047). Executive function declined in APOE ε4 carriers over 12 months, regardless of intervention (p = 0.056). There was a significant interaction after 12 months where intervention ε4 carriers had better processing speed than ε4 controls (p = 0.003). Intervention ε2 carriers had better executive function immediately after training (p = 0.02) and had better verbal memory 6-months post-intervention (p = 0.04). These effects remained significant after false-discovery rate correction.
Conclusion
Multi-domain cognitive training reduces declines in processing speed over time. APOE ε4 is associated with reductions in executive function over time, and training may attenuate ε4-associated declines in processing speed. APOE ε2 carriers may also benefit from training, particularly on measures of executive function and verbal memory.
Keywords: Apolipoprotein E, cognitive training, elderly, neuropsychology
INTRODUCTION
Cognitive training, an efficacious intervention that has been widely studied during recent years, can target specific cognitive domains in order to maintain or improve cognitive functioning [1]. Several studies have demonstrated that cognitive training can improve various domains of cognitive function in elderly populations, such as reasoning [2, 3], memory [4, 5], processing speed [6, 7], and executive function [8].
The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial, one of the largest randomized cognitive training trials to date, showed that the effects of single-domain cognitive training (memory, reasoning, and processing speed training) are specific to the trained cognitive ability, persist for 10 years, and may delay difficulties in daily function [9–11]. While single-domain cognitive training often focuses on one cognitive function, multi-domain cognitive interventions are more attractive and may generalize to provide benefits on multiple functions, which is particularly important for elderly adults who often have deficits in several domains of cognition [12–14]. Multi-domain cognitive interventions are also often designed to be enjoyable, which aids in motivating older adults to adhere to the training regimen.
Apolipoprotein E (ApoE) is a plasma protein involved in regulating lipoprotein metabolism and cholesterol balance. The lipid transport protein is encoded by the polymorphic APOE gene, which has three allelic variants, ε2, ε3, and ε4, due to cysteine-arginine interchanges at codons 112 and 158 [15]. The most frequently occurring allele is ε3, followed by ε4 and ε2 [16]. The ε4 isoform has been related to several conditions involving normal aging, cognitive impairment, and, most notably, has been consistently associated with increased risk for Alzheimer’s disease (AD) [17–21]. In a recent, small, randomized controlled trial testing the short-term (two week post-intervention) effects of cognitive stimulation on cognition in mild cognitive impairment and healthy older adults with a family history of dementia, healthy older adults without APOE ε4 showed improvements on a measure of visuospatial memory post-intervention when compared to the sham group, whereas ε4 carriers did not show any intervention effects [22]. However, whether the ε4 allele alters long-term cognitive training effects in healthy elderly people remains unknown. In contrast, the least common APOE allele, ε2, has been associated with protection against AD, as well as longevity, and has been suggested to reduce age-associated cognitive decline in domains such as episodic memory and information processing [23].
Previously, we reported the successful use of multi-domain cognitive training in improving cognition of healthy elderly adults in China. Compared to cognitive functioning in a control (non-intervention) group, individuals in the intervention group had better reasoning, memory, and executive function at the end of the training, and these differences persisted for one year after the training concluded [24]. The present study was undertaken to extend our previous research by investigating the longitudinal associations between APOE genotype and cognitive change in these participants. Our underlying hypothesis, based on previous literature, was that the effect of the intervention would differ based on APOE genotype—with ε4 carriers showing worse cognitive outcomes and ε2 carriers showing better cognitive outcomes—and postulated that these effects would be detectable across the duration of the 12-month follow-up period.
MATERIALS AND METHODS
Sample
Study participants were recruited and screened from two neighborhoods of the Putuo District in Shanghai in 2006. Inclusion criteria were: 1) at least 70 years of age; 2) ability of self-care with no physical disability or severe physical disease; 3) no psychiatric disorders or dementia; 4) ability to read, write, see, and hear. Self-care was assessed via activities of daily living (ADL) score, and individuals with scores of 19 or below were included in the study. Scores of 19 or more on the Chinese version of the Mini-Mental State Examination (MMSE) were required for enrollment to exclude dementia. The normal cut-off point of the MMSE is lower in China than in the U.S. due to a lower educational level; the Chinese education level is 1 year and above. Medical eligibility was assessed via interview with a health status checklist designed specifically for this study. Three psychiatrists performed clinical interviews and assessed past medical history to exclude individuals with psychiatric disorders, dementia, visual disorders, and hearing disorders. Individuals were sequentially assigned in groups of 50 to either the intervention or control group to avoid possible bias due to communication between intervention and control participants.
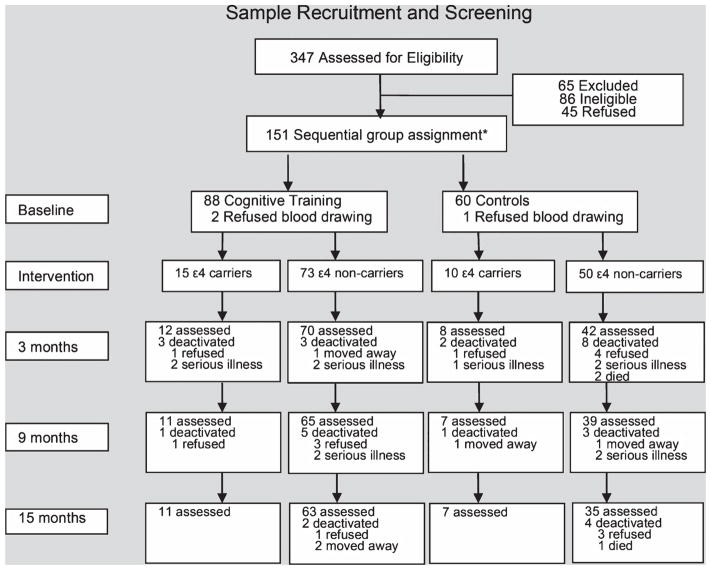
A total of 151 elderly individuals met these inclusion criteria and were divided into two groups: 90 in the intervention group and 61 in the control group; their age ranged from 70 to 89 years with a mean age of 74.8 (standard deviation [SD] = 3.7) years. Details of the recruitment and the protocols have been published elsewhere [24, 25]. All participants were assessed by physical and neuropsychological tests at baseline, and 147 individuals (88 in the intervention and 59 controls) agreed to have blood drawn for DNA extraction and genotyping at baseline. All participants were followed-up with physical examinations and neuropsychological testing. The follow-up time points were 3 months, 9 months, and 15 months after enrollment (Fig. 1). This study was approved by the Ethics Committee of Tongji Hospital of Tongji University and all participants provided informed consent.
Fig. 1.
Sample recruitment and screening. Flowchart shows participant recruitment and screening at baseline, intervention, and 3, 9, and 15 months from baseline. *Individuals were sequentially assigned in groups of 50 to either the intervention or control group to avoid possible bias due to communication between intervention and control participants. For more details, see Materials and Methods section and [24].
Multi-domain cognitive training
Elderly people in the intervention group received multi-domain cognitive training as described in detail below, which consisted of a total of 24 face-to-face training sessions at a frequency of twice per week for 12 weeks (from Months 0–3) [24, 25]. The length of each session was 60 minutes and consisted of an average class size of 15 individuals led by one instructor. A study observer also monitored individual performance, preventing participants from developing poor practice habits that may inadvertently reinforce detrimental training, assessed the quality of their skill-related practice throughout the intervention, and provided feedback to the instructor. No face-to-face training was provided to control group members.
For the intervention, the first 15 minutes of each session consisted of a lecture focusing on education about common diseases in older adults. The following 30 minutes involved structured training via PowerPoint presentation in one specific technique per session. The instructor taught the participants the particular strategy or technique, including its methods, function, and how it could be used in daily life (e.g., face/name memory training). Instructors followed a manual of structured curricular format for each training session but were open to discussion based on the needs and interests of their group. The majority of these classes (17 of 24 [71%]) focused on cognitive training in domains such as memory, reasoning, problem solving, and processing speed. The 24 sessions of intervention were: story recall training (3 times), face and name training (2 times), words recall training (2 times), vocabulary learning (2 times), reasoning training (4 times), problem solving (2 times), processing speed training (map searching training, 2 times), handcrafts (2 times), physical exercise (2 times), handwriting (1 time), and painting (2 times). The last 15 minutes of each class were used to consolidate the newly acquired skills by solving real-life problems. At the end of each session, participants provided feedback on the type of intervention used for that session including performance achievement, their opinion of the training, and suggestions for how the intervention could be improved. Participants were assigned homework once a week, which included cognitive tasks related to the latest week’s course content, health education readings, Chinese calligraphy, and simple graph drawing. Homework was reviewed each week. They were also asked to record, daily, any self-training or practice they performed outside of formal class sessions in a diary which they handed in to the study personnel after completing all 24 training sessions. Finally, participants were also encouraged to exercise in any way they liked as much as possible when at home and instructed to record those activities in their daily diary entry.
Cognitive assessment
The Chinese version of the World Health Organization Neuropsychological Battery of Cognitive Assessment Instruments for the elderly (WHO-BCAI) included nine tests of cognitive functioning: Auditory Verbal Learning Test (AVLT; memory); Sorting Test (executive function); Cancellation Test (attention; processing speed); Articulation and Naming Tests (language); Mini-token Test (language); Motor Test (memory and executive function); Visual Matching and Reasoning Test (reasoning); Spatial Construction Test (constructional ability); Trail Making Test (executive function) [26, 27]. The Stroop Color-Word test was also used to assess executive function by testing the accuracy and speed of reading color words presented in conflicting colors [28]. Neuropsychological scores were standardized (z-scores, representing number of positive or negative SDs from the mean) across all individuals for each test. Positive scores represent better performance.
APOE genotyping
Genomic DNA was isolated from peripheral blood according to standard procedures and stored at −20°C. Genotyping of APOE was performed using a PCR-RFLP method. In brief, a portion of the gene was first amplified with the primer pair: forward, 5′-GAT GGC GCT GAG GCC GCG CT-3′ and reverse, 5′-GGC ACG GCT GTC CAA GGA GCT-3′. The following PCR reaction was then used: 95°C for 5 min, 5 cycles of (94°C for 30 s + 67°C for 30 s + 70°C for 120 s), 30 cycles of (94°C for 30 s + 60°C for 30 s + 70°C for 120 s), 72°C for 7 min, then held at 40°C. A total 11 μl PCR product and 10 ul CfoI restriction endonuclease were then incubated overnight at 30°C. Resulting fragments were then separated on a 20% polyacrylamide gel via electrophoresis (15 min at 150 V) and specific fragment size patterns were observed under ultraviolet lamp after 1 h of ethidium bromide staining.
Statistical analysis
Epidata 3.0 software was used for data entry and SPSS 17.0 software was used for preliminary data analyses. Descriptive statistics were performed using chi-squared test (categorical data), one sample t-test, paired t-test, and analysis of covariance (continuous variables) depending on the type of data. Intention-to-treat analysis was used to estimate the intervention effect. That is, all elderly individuals who participated in at least one training session and had follow-up data were included in the analysis, regardless of whether or not they completed the entire 12-week intervention. Factor analysis using the principal-component method was used to analyze the correlation matrix of all standardized neuropsychological test scores at baseline. In the principal-component method, communalities are assumed to be 1. We assessed the first 10 factors via scree plot to identify the minimal number that explained the most variance in the data (i.e., all factors with eigenvalues above the mean). We then used the resulting scoring coefficients for each test from the baseline factor analysis to create scores for each top Factor (1–3) at each follow-up time point (Months 3, 9, and 15).
For cross-sectional analyses, linear regression was used to assess the effect of the intervention or APOE genotype (ε4 or ε2 carrier status, scored as 0/1 such that all ε4 carriers were compared to all other genotype groups [ε3/ε3, ε3/ε2, and ε2/ε2] and all ε2 carriers were compared to all other genotype groups [ε3/ε3 and ε3/ε4] in separate analyses) on cognitive outcome measures, adjusting for gender, age at baseline, and years of education. In analysis of post-intervention time points, models were also adjusted for baseline test performance. We also tested interaction effects of intervention × genotype, adjusting for demographic covariates, baseline test performance (for post-intervention time points), and main effects. For longitudinal data, a time series was created starting with the post-intervention time point (Month 3) and extending for one year (Month 9 and Month 15). Mixed effect linear regression models were used to test the interaction effect of intervention or genotype with time. Fixed effects included intervention, genotype, and the two-way interaction between these variables and time (i.e., intervention × time; ε4 carrier × time; ε2 carrier × time). Models were also adjusted for fixed effects of age at baseline, gender, years of education, baseline test performance, and main effects in interaction analyses. Random effects included individuals and time (i.e., random slopes and intercepts). Models were fit via maximum likelihood. Log-likelihood nested tests were used to evaluate the significance of adding significant intervention, genotype and interaction variables to the mixed model. Adjusted values were calculated from mixed models as fitted predictions, accounting for both fixed and random effects. All analyses were two-tailed. Significance was established using the Benjamini-Hochberg procedure, controlling for a false discovery rate (FDR) of Q = 0.2 [29–32]. Factor and regression analyses were performed in Stata 10.1/MP (StataCorp LP, College Station, TX). Post hoc power calculations for the same models were conducted in R. Data and linear predictions for time series data (Months 3–15) were visualized in Prism 6 (Graphpad Software, Inc., La Jolla, CA).
RESULTS
Demographic and genotype distributions in intervention and control groups
A total of 147 individuals underwent genotyping for APOE. All genotypes were in Hardy-Weinberg equilibrium (p = 0.60). Of genotyped individuals, 88 participated in the intervention and 59 were controls (Fig. 1). Across the entire cohort, one person’s APOE genotype was ε2/ε2 (0.7%), 26 were ε2/ε3 (17.7%), 95 were ε3/ε3 genotype (64.6%), 23 were ε3/ε4 (15.6%), and two were ε2/ε4 (1.4%). No individuals carried two APOE ε4 alleles. Participants were classified according to the presence or absence of at least one copy of the APOE ε4 allele in the control and intervention groups. Participants were also scored according to the presence or absence of at least one copy of the APOE ε2 allele in both groups (Table 1). Two individuals (both control [non-intervention] participants) had ε2/ε4 genotypes and were removed from the analysis to maximize our ability to evaluate the role of specific APOE alleles to cognition in aging, for a total of 88 intervention and 57 control individuals. There were no statistically significant differences in demographic characteristics by intervention or APOE carrier status for either allele group (p > 0.05, Table 1).
Table 1.
Demographic characteristics
| Intervention | Control | APOE | Intervention | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Non-carrier | Carrier | Non-carrier | Carrier | p-value | p-value | |
| By APOE ε4 carrier status | ||||||
| n | 73 | 15 | 49 | 8 | ||
| age (mean ± SE) | 74.9 ± 0.5 | 73.4 ± 0.5 | 75.1 ± 0.6 | 74.3 ± 0.9 | 0.14 | 0.68 |
| n female (%) | 28 (38.4%) | 7 (46.7%) | 25 (51.0%) | 5 (62.5%) | 0.41 | 0.12 |
| education (mean ± SE years) | 9.7 ± 0.5 | 8.7 ± 0.6 | 9.4 ± 0.6 | 8.6 ± 1.6 | 0.29 | 0.71 |
| By APOE ε2 carrier status | ||||||
| n | 73 | 15 | 45 | 12 | ||
| age (mean ± SE) | 74.7 ± 0.5 | 74.4 ± 0.8 | 74.7 ± 0.5 | 76.0 ± 1.4 | 0.61 | 0.66 |
| n female (%) | 28 (38.4%) | 7 (46.7%) | 22 (48.9%) | 8 (66.7%) | 0.24 | 0.15 |
| education (mean ± SE years) | 9.7 ± 0.4 | 8.7 ± 1.2 | 9.3 ± 0.6 | 9.5 ± 1.3 | 0.56 | 0.77 |
Cognitive assessment
We performed factor analysis to mathematically classify baseline neuropsychological test scores into composite variables that reflect broad domains of cognition. The first three factors captured the majority of the variance explained by test scores (Supplementary Figure 1). The first factor measured verbal memory, with primary loading from the AVLT (Supplementary Table 1). The second factor measured processing speed, with primary loadings from Cancellation Test Completion Time (Supplementary Table 1). The third factor represented executive function, primarily defined by tasks related to set shifting (e.g., Trail Making Test, Contact Function Test, Visual Matching and Reasoning, Spatial Construction, Cancellation Test - Correct Responses; Supplementary Table 1). At baseline, control APOE ε4 carriers had significantly worse Factor 1 (verbal memory) scores compared to all other groups (Supplementary Table 2).
Genotype effects over 12-months post-intervention
When the entire follow-up period was analyzed as a continuous trajectory of cognitive function over time, the main effect of the intervention and the interaction effect of Intervention × Time made significant contributions to modeling processing speed scores over 12 months (p = 0.03, 2 degrees of freedom, log-likelihood test). Over time, the intervention attenuated declines in processing speed scores of participants as compared to controls (raw p = 0.047, beta ± SE = 0.16 ± 0.08 via 2-tailed mixed model; Fig. 2, Table 2). This effect remained significant after FDR adjustment.
Fig. 2.
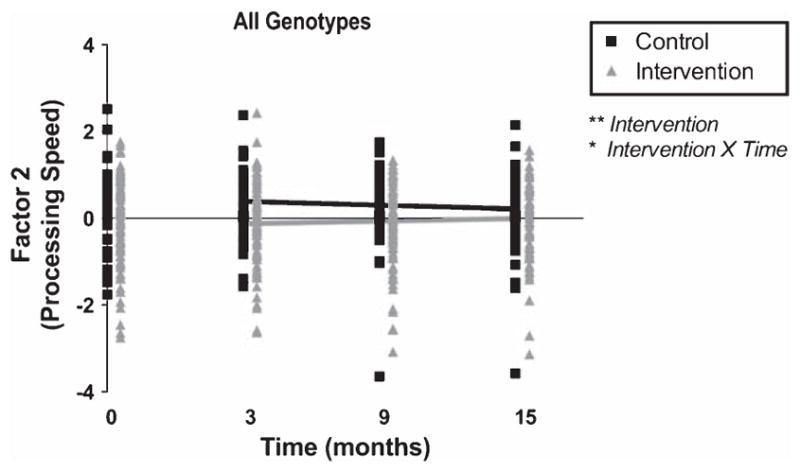
Multi-domain intervention modified processing speed over 12 months. Unadjusted Factor 2 scores, which represent performance on multiple neuropsychological tests assessing processing speed, are plotted at each time point (black squares for controls and gray triangles for intervention participants). Lines represent fitted values from the mixed model, which include both linear predictions of the fixed portion plus contributions based on predicted random effects. This reflects the trajectory of cognitive testing performance during the 12 months following the intervention, which took place from months 0–3. Scores are shown for individuals of all genotypes. There was an interaction between Intervention × Time as well as a significant main effect of intervention via two-tailed mixed effect linear regression accounting for age at baseline, gender, years of education, main effects and baseline score. The main effect of the intervention and the interaction effect of Intervention × Time made significant contributions to modeling processing speed scores over 12 months (p = 0.03; 2 degrees of freedom, log-likelihood test). The interaction remained significant after adjustment for false discovery rate (FDR). Raw *p < 0.05, **p < 0.01.
Table 2.
Effect of intervention on Factors 1–3 over time
| Factor 1 (Verbal Memory) Coef./SE |
Factor 2 (Processing Speed) Coef./SE |
Factor 3 (Executive Function) Coef./SE |
|
|---|---|---|---|
| Intervention × Time | −0.04 (0.06) | 0.16* (0.08) | 0.01 (0.08) |
| Intervention | 0.26 (0.14) | −0.49** (0.18) | 0.04 (0.18) |
| Time | −0.01 (0.04) | −0.10 (0.06) | −0.03 (0.06) |
| Gender | 0.05 (0.11) | 0.14 (0.12) | −0.38** (0.13) |
| Age (years) | −0.04** (0.01) | −0.03* (0.02) | 0.03* (0.02) |
| Education (years) | 0.04* (0.02) | 0.004 (0.02) | 0.03 (0.02) |
| Baseline score | 0.74*** (0.06) | 0.47*** (0.05) | 0.49*** (0.06) |
| n | 343 | 343 | 343 |
The effect of interest is the interaction of Intervention × Time, which remains significant after false discovery rate (FDR) correction for Factor 2 (in bold). Raw *p < 0.05, **p < 0.01, ***p < 0.001.
Across intervention and control groups, carrying APOE ε4 had a significant effect on trajectory of executive function over time as measured by Factor 3 after FDR correction (raw p = 0.056, beta ± SE = −0.20 ± 0.10 via 2-tailed mixed model; Fig. 3, Table 3). Across the entire cohort, carrying ε4 was associated with a decline in executive function over time when compared to those without ε4. There was no additional effect of intervention on executive function after accounting for the APOE ε4 × time interaction (p = 0.57, beta ± SE 0.06 ± 0.11 via 2-tailed mixed model).
Fig. 3.
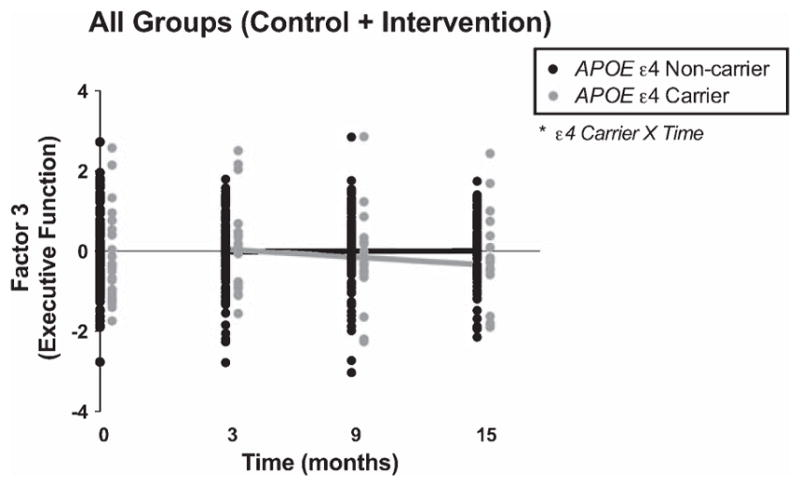
Carrying APOE ε4 modified executive function over 12 months. Unadjusted Factor 3 scores, which represent performance on multiple neuropsychological tests assessing executive function, are plotted at each time point (black circles for APOE ε4 non-carriers and gray circles for APOE ε4 carriers). Lines represent fitted values from the mixed model, which include both linear predictions of the fixed portion plus contributions based on predicted random effects. This reflects the trajectory of cognitive testing performance during the 12 months following the intervention, which took place from months 0–3. Scores are shown for individuals from both the intervention and control groups. There was an interaction between APOE ε4 × Time via two-tailed mixed effect linear regression accounting for age at baseline, gender, years of education, main effects, and baseline score. The interaction remained significant after adjustment for false discovery rate (FDR). Raw *p < 0.05.
Table 3.
Effect of APOE ε4 on Factors 1–3 over time
| Factor 1 (Verbal Memory) Coef./SE |
Factor 2 (Processing Speed) Coef./SE |
Factor 3 (Executive Function) Coef./SE |
|
|---|---|---|---|
| APOE ε4 × Time | 0.03 (0.07) | −0.01 (0.11) | −0.20 (0.10)‡ |
| Time | −0.04 (0.03) | 0.005 (0.04) | 0.008 (0.04) |
| APOE ε4 Carrier | 0.09 (0.20) | −0.02 (0.25) | 0.42 (0.24) |
| Gender | 0.02 (0.11) | 0.17 (0.12) | −0.39** (0.13) |
| Age (years) | −0.04** (0.01) | −0.03* (0.02) | 0.03* (0.02) |
| Education (years) | 0.03* (0.02) | 0.007 (0.02) | 0.03 (0.02) |
| Baseline score | 0.75*** (0.06) | 0.49*** (0.05) | 0.49*** (0.06) |
| n | 343 | 343 | 343 |
The effect of interest is the interaction of APOE ε4 × Time. In this analysis, individuals carrying at least one APOE ε4 allele were compared to all individuals without APOE ε4.
Raw p = 0.056, significant after false discovery rate (FDR) correction. Raw *p < 0.05, **p < 0.01, ***p < 0.001.
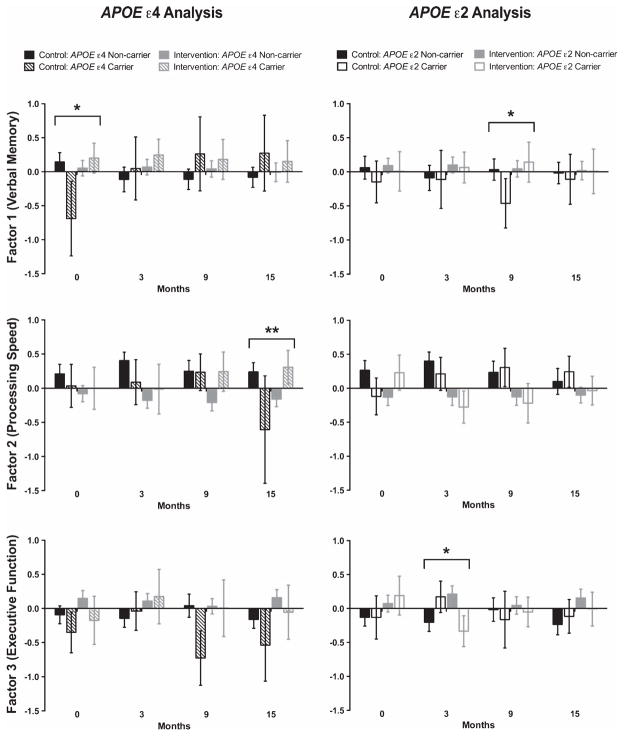
In order to assess effects of APOE genotype on effects of the intervention, we next performed cross-sectional analyses at each follow-up time point. There was an interaction effect of carrying APOE ε4 and participating in the intervention at the final follow-up time point 12-months after the intervention ended (Month 15, raw p = 0.003, beta ± SE = 1.31 ± 0.43; Fig. 4, Table 4, Supplementary Table 3). In this interaction, APOE ε4 carriers who participated in the intervention did slightly better than average on measures of processing speed (Factor 2), while ε4-carrying controls [no intervention] did slightly worse than average. This effect remained significant after adjustment for FDR. We had 75% power to detect the observed effect (beta ± SE) of 1.31 ± 0.43 for the APOE ε4 × Intervention interaction in the 107 individuals available for analysis at the 15 Month time point.
Fig. 4.
Intervention and APOE genotype have differential effects on neuropsychological test performance 12 months later. Factor scores (mean ± SE) are provided at baseline (month 0) and for each of three testing time points in the year following the intervention (months 3, 9, and 15) by genotype (on left: APOE ε4 carriers—hatched, ε4 non-carriers—solid; on right: APOE ε2 carriers—open, ε2 non-carriers—solid). The intervention took place between Months 0–3. Factor 1 represents verbal memory, Factor 2 measures processing speed, and Factor 3 assesses executive function. At baseline, there was no significant difference in performance between controls (in black) versus intervention participants (in gray) for any factor. There was an interaction effect between APOE ε4 × Intervention on Factor 1 at baseline and on Factor 2 at Month 15. There was an interaction effect between APOE ε2 × Intervention on Factor 1 at Month 9 and on Factor 3 at Month 3. All tests were two-tailed linear models accounting for age at baseline, gender, years of education, main effects and baseline score (for analyses of data at months 3, 9, and 15). All effects remained significant after adjustment for false discovery rate (FDR). Raw *p < 0.05, **p < 0.01, ***p < 0.001.
Table 4.
Main cross-sectional results for APOE carrier status × Intervention interactions at each follow-up time point
| 3 Months Coef./SE |
9 Months Coef./SE |
15 Months Coef./SE |
|
|---|---|---|---|
| Factor 1 (Verbal Memory) | |||
|
| |||
| APOE ε4 × Intervention | −0.36 (0.34) | −0.40 (0.38) | −0.33 (0.36) |
| Intervention | 0.25 (0.13) | 0.23 (0.14) | 0.13 (0.13) |
| APOE ε4 | 0.36 (0.28) | 0.41 (0.32) | 0.35 (0.30) |
|
| |||
| APOE ε2 × Intervention | 0.19 (0.30) | 0.64* (0.31) | 0.15 (0.30) |
| Intervention | 0.17 (0.13) | 0.05 (0.14) | 0.05 (0.14) |
| APOE ε2 | −0.21 (0.23) | −0.57* (0.24) | −0.14 (0.24) |
|
| |||
| Factor 2 (Processing Speed) | |||
|
| |||
| APOE ε4 × Intervention | 0.56 (0.39) | 0.59 (0.43) | 1.31** (0.43) |
| Intervention | −0.34* (0.15) | −0.31 (0.16) | −0.19 (0.16) |
| APOE ε4 | −0.48 (0.32) | −0.25 (0.36) | −0.98** (0.35) |
|
| |||
| APOE ε2 × Intervention | −0.33 (0.35) | −0.46 (0.37) | −0.51 (0.38) |
| Intervention | −0.21 (0.16) | −0.13 (0.17) | 0.10 (0.18) |
| APOE ε2 | −0.04 (0.27) | 0.13 (0.28) | 0.32 (0.29) |
|
| |||
| Factor 3 (Executive Function) | |||
|
| |||
| APOE ε4 × Intervention | 0.09 (0.37) | 0.70 (0.48) | 0.22 (0.44) |
| Intervention | 0.08 (0.14) | −0.10 (0.17) | 0.12 (0.17) |
| APOE ε4 | 0.23 (0.30) | −0.59 (0.40) | −0.29 (0.37) |
|
| |||
| APOE ε2 × Intervention | −0.79* (0.33) | 0.14 (0.41) | −0.10 (0.38) |
| Intervention | 0.25 (0.14) | −0.05 (0.18) | 0.17 (0.18) |
| APOE ε2 | 0.38 (0.26) | −0.09 (0.32) | 0.13 (0.30) |
Effects (beta coefficients, Coef.) and standard errors (SE) are provided for the interaction effect of interest (interactions of APOE ε4 or ε2 carrier status × Intervention) and main effects of that interaction for each neuropsychological test Factor at each time point post-intervention. Interactions that remain significant after false discovery rate (FDR) correction are in bold. Models were also adjusted for gender, age, education and baseline score (full model results are provided in Supplementary Table 3). Raw *p < 0.05, **p < 0.01.
There were also significant effects of an interaction between APOE ε2 × Intervention after FDR correction (Fig. 4, Table 4, Supplementary Table 3). Carrying APOE ε2 was associated with better verbal memory (Factor 1) in intervention participants compared to ε2-carrying controls after six months (Month 9, raw p = 0.04, beta ± SE = 0.64 ± 0.31). Carrying APOE ε2 was also associated with slightly lower executive function (Factor 3) in intervention participants versus ε2-carrying controls immediately after the intervention (Month 3, raw p = 0.02, beta ± SE = −0.79 ± 0.33), though performance for both groups was not significantly different at either of the following time points (Fig. 4, Table 4, Supplementary Table 3). We had power of 66% at Month 3 and 53% at Month 9 to detect effects of these magnitudes in our cohort for the APOE ε2 × Intervention interactions.
DISCUSSION
To our knowledge, this is the first study to explore the interaction between the longer-term effects of cognitive training and APOE genotype in community-dwelling elderly individuals. The persistence of training-associated improvements in cognitive testing over one year post-intervention have previously been observed following training in healthy older adults on reasoning, memory, and processing speed [11, 33]. However, the interaction of this phenomenon with APOE genotype has not yet been thoroughly investigated. This study bolsters a small but growing body of work to characterize the effects of APOE and training interventions on cognitive aging in Chinese individuals.
In our study, the multi-domain cognitive training intervention reduced declines in a data-driven composite measure of processing speed over 12 months of time. Carrying the AD risk factor, APOE ε4, was associated with declines in executive function over time, regardless of participating in the intervention. Cross-sectional analysis of each follow-up time point identified a significant interaction effect of carrying APOE ε4 with the intervention; ε4 carriers who did not participate in the intervention showed worse scores in processing speed performance than ε4 carriers who participated in multi-domain cognitive training after 12 months. We also identified significant interactions between the putative protective allele, APOE ε2, and the multi-domain cognitive training such that intervention participants carrying ε2 had better verbal memory scores than ε2-carrying controls six months after the intervention. There was also a significant interaction such that immediately after training, ε2 intervention participants had worse executive function than ε2-carrying controls, although this effect did not persist in the following 12 months of follow-up.
We evaluated different domains of cognition: memory, processing speed, and executive function so that we could identify which of these cognitive domains are the most sensitive markers of training effects. Our findings suggest that processing speed may be the most sensitive marker for multi-domain cognitive training. It is possible that memory and executive function are less amenable to training interventions because they depend more on cortical gray matter regions—which show linear rates of atrophy over time—whereas processing speed could be more amenable to training because it is more dependent on white matter integrity—which shows non-linear declines that plateau in middle-age then show additional decline later in life [34]. Our data suggests that this cognitive intervention could have a positive impact on reducing declines in processing speed that occur with aging, and that ε4 carriers may particularly benefit from this type of training, although our limited sample size only allowed us to detect genotype × Intervention interactions at single follow-up time points. One reason for this finding specifically in APOE ε4 carriers could be due to increased risk this allele confers on age-related cognitive decline, which we observed for our measure of executive function. For this measure, participating in the intervention did not affect ε4-associated declines in our cohort, further supporting the idea that cognitive domains associated with non-cortical regions may be more amenable to training.
We did not detect any effect of carrying APOE ε2 over time on the three measures of cognition identified by factor analysis. Carrying APOE ε2 and participating in the intervention was associated with lower executive function scores, but only at one time point immediately following the intervention, suggesting this effect may have been due to other factors. The most intriguing finding for ε2 was an interaction with the intervention on measures of verbal memory, which was significant for the 6-month follow-up time point. This result is somewhat in line with previous data suggesting that APOE ε2 may protect against declines in episodic memory in aging [23] and suggests we may have been underpowered to detect a persistent effect of ε2 in our study. Due to our small sample size, ε2- or ε4-carriers were compared to all non-carriers, inclusive of individuals carrying the other allele (i.e., ε2-carriers versus ε3/ε3 and ε3/ε4 individuals). It is possible that subtle effects of the two alleles—which are proposed to have effects in opposite directions—could attenuate associations with cognitive measures, and may explain why genotype × Intervention interactions could only be identified at single follow-up time points for specific cognitive measures. Although memory is the most sensitive cognitive domain affected in the early stages of AD [35–37], the possible interaction effect of cognitive training with APOE genotype on neuropsychological performance over time has not been extensively investigated. This line of study warrants future study in larger cohorts with sufficient power to test two- and three-way interaction effects of specific APOE alleles and cognitive training over time.
In this study, the impact of APOE alleles on cognitive training response over time was only observed at single follow-up time points, but these effects did not necessarily persist for the duration of follow-up testing. There are three possible reasons. First, the number of APOE ε4 and ε2 carriers was small, and there were no ε4 homozygotes and only one ε2 homozygote in the cohort, so the study had limited power. As the frequency of ε4 in non-demented elderly in China is lower than European and U.S. [38], a larger sample will be required to replicate these findings and allow for direct comparisons of ε2-carrying or ε4-carrying individuals to a reference population composed of only ε3/ε3 individuals in future studies. Second, cognitive effects of this intervention may decline over time without additional training. In the ACTIVE study, 60% of initially trained subjects were offered a booster training at 11 months after the initial training. Booster training enhanced training gains in speed and reasoning, which were maintained at two-year follow-up [11]. The decline of our training results after 12 months suggests it might be beneficial to increase the number of sessions or add booster sessions in the future. Third, participants in our study were all 70 years old or above, which represents an older age bracket than some previous intervention studies [39, 40]. Sensitivity to training effects and brain plasticity may decline in advanced age, which could reduce or eliminate potential positive effects of a short cognitive training program in this age group. Future studies of larger groups of diverse older adults that span a broader age range and incorporate multi-domain cognitive booster training may help to further elucidate the effects of training-specific changes in cognitive function in APOE ε4 and ε2 carriers, and to assess whether these effects are specific to Chinese or generalize to all populations.
The findings of this study support other research that finds value in cognitive training for older adults to improve processing speed [7, 12, 33]. There were two sessions of speed of processing training in our total 24 sessions that focused on increasing individuals’ ability to quickly identify locations on a map. In future studies, computerized training programs—which may be more effective at improving function of this cognitive domain in the elderly [41]—could be implemented, such as in ACTIVE [1]. Whether the enhancements in processing speed we observed are the direct effect of training on this specific cognitive domain or are an integrated effect of multi-disciplinary training cannot be tested in the present study given its design and requires further research. Testing the effects of single processing speed training versus multi-domain training may be one way to address this question. In future studies, different training frequency and duration could also be administered based on APOE genotype. For example, based on the results of this study, longer training sessions and additional booster trainings may be particularly warranted for APOE ε4 carriers since ε4-carrying controls showed worse scores in processing speed than non-ε4 carrier controls at 12-month follow-up.
As the first report of APOE genotype effects on post-intervention cognitive assessments in a Chinese cohort, we hope this study provides useful information to other groups designing cognitive intervention studies or with access to larger cohorts so they can further investigate these findings and assess them in an independent elderly population. In addition to providing evidence for a specific neuropsychological domain that may be most sensitive to multi-domain cognitive intervention—which in our case appears to be processing speed—we also hope that the reported interaction effects in APOE ε4 and ε2 carriers provide insight to others who may wish to assess the utility of stratifying training and/or booster sessions in a genotype-specific manner.
Supplementary Material
Acknowledgments
This study was supported by the National Science Foundation of China (81200831, 81371505 and 30770769) and Shanghai Health Bureau (20124y038). JSY was supported by the Larry L. Hillblom Foundation 2012-A-015-FEL, an NIA Diversity Supplement to P50 AG023501 (PI:BL Miller) and the AFTD Susan Marcus Memorial Fund Clinical Research Grant. Zhiqiang Xue and Fang Fang contributed to the cognitive training validity test. Other members of the research group, Mudan Wu, Liang Liu, Jingyu Shi, Jia Xu, Qingwei Li, and Xu Zhang, contributed to data collection.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0039r3).
Footnotes
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150039.
References
- 1.Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E ACTIVE Study Group. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman SB, Mudar RA. Enhancement of cognitive and neural functions through complex reasoning training: Evidence from normal and clinical populations. Front Syst Neurosci. 2014;8:69. doi: 10.3389/fnsys.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams K, Herman R, Bontempo D. Reasoning exercises in assisted living: A cluster randomized trial to improve reasoning and everyday problem solving. Clin Interv Aging. 2014;2014:981–996. doi: 10.2147/CIA.S62095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youn JH, Lee JY, Kim S, Ryu SH. Multistrategic memory training with the metamemory concept in healthy older adults. Psychiatry Investig. 2011;8:354–361. doi: 10.4306/pi.2011.8.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebok GW, Langbaum JBS, Jones RN, Gross AL, Parisi JM, Spira AP, Kueider AM, Petras H, Brandt J. Memory training in the ACTIVE study: How much is needed and who benefits? J Aging Health. 2012;25(8 Suppl):21S–42S. doi: 10.1177/0898264312461937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burge WK, Ross LA, Amthor FR, Mitchell WG, Zotov A, Visscher KM. Processing speed training increases the efficiency of attentional resource allocation in young adults. Front Hum Neurosci. 2013;7:684. doi: 10.3389/fnhum.2013.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ball KK, Ross LA, Roth DL, Edwards JD. Speed of processing training in the ACTIVE study: How much is needed and who benefits? J Aging Health. 2014;25(8 Suppl):65S–84S. doi: 10.1177/0898264312470167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res Rev. 2014;15:28–43. doi: 10.1016/j.arr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, Marsiske M, Morris JN, Tennstedt SL, Unverzagt FW, Willis SL Study ACTIVE Group. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstedt SL. The ACTIVE cognitive training trial and health-related quality of life: Protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61:1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]
- 11.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL Advanced Cognitive Training for Independent and Vital Elderly Study Group. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Wu W, Feng W, Wang J, Chen Y, Shen Y, Li Q, Zhang X, Li C. The effects of multi-domain versus single-domain cognitive training in non-demented older people: A randomized controlled trial. BMC Med. 2012;10:30. doi: 10.1186/1741-7015-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci. 2010;65 A:1338–1343. doi: 10.1093/gerona/glq127. [DOI] [PubMed] [Google Scholar]
- 14.Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front Aging Neurosci. 2013;5:1–12. doi: 10.3389/fnagi.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanlon CS, Rubinsztein DC. Arginine residues at codons 112 and 158 in the apolipoprotein E gene correspond to the ancestral state in humans. Atherosclerosis. 1995;112:85–90. doi: 10.1016/0021-9150(94)05402-5. [DOI] [PubMed] [Google Scholar]
- 16.Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science (80-) 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 17.Smith JD. Apolipoproteins and aging: Emerging mechanisms. Ageing Res Rev. 2002;1:345–365. doi: 10.1016/s1568-1637(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 18.Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiol Aging. 2011;32:63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Rubinsztein DC, Easton DF. Apolipoprotein E genetic variation and Alzheimer’s disease - A meta-analysis. Dement Geriatr Cogn Disord. 1999;10:199–209. doi: 10.1159/000017120. [DOI] [PubMed] [Google Scholar]
- 20.Michaelson DM. APOE ε4: The most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimers Dement. 2014;10:861–868. doi: 10.1016/j.jalz.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Bruni AC, Conidi ME, Bernardi L. Genetics in degenerative dementia: Current status and applicability. Alzheimer Dis Assoc Disord. 2014;28:199–205. doi: 10.1097/WAD.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 22.Polito L, Abbondanza S, Vaccaro R, Valle E, Davin A, Degrate A, Villani S, Guaita A. Cognitive stimulation in cognitively impaired individuals and cognitively healthy individuals with a family history of dementia: Short-term results from the “Allena-Mente” randomized controlled trial. Int J Geriatr Psychiatry. 2014;30:631–638. doi: 10.1002/gps.4194. [DOI] [PubMed] [Google Scholar]
- 23.Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE e2. Neurosci Biobehav Rev. 2013;37:2878–2886. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Feng W, Li C, Chen Y, Cheng Y, Wu W. Integrative cognitive training for healthy elderly Chinese in community: A controlled study. Biomed Res India. 2013;24:223–229. [Google Scholar]
- 25.Feng W, Li C, Chen Y, Cheng Y, Wu W. Five-year follow-up study of multi-domain cognitive training for healthy elderly community members. Shanghai Arch Psychiatry. 2014;26:30–42. doi: 10.3969/j.issn.1002-0829.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health, Organization. Battery of Cognitive Assessment Instrument for Elderly. World Health Organization; Geneva: 1996. [Google Scholar]
- 27.Xue HB, Xiao SF, Li CB, He YL, Wu WYZM. The neuropsychological test battery for elderly people. Zhonghua Yi Xue Za Zhi. 2005;85:2961–2965. [PubMed] [Google Scholar]
- 28.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 29.Benjamini Y, Yekutieli D. Quantitative trait loci analysis using the false discovery rate. Genetics. 2005;171:783–790. doi: 10.1534/genetics.104.036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald JH. Handbook of Biological Statistics. 3. Sparky House Publishing; Baltimore: 2014. [Google Scholar]
- 31.Efron B. Size, power and false discovery rates. Ann Stat. 2007;35:1351–1377. [Google Scholar]
- 32.Craiu RV, Sun L. Choosing the lesser evil: Trade-off between false discovery rate and non-discovery rate. Stat Sin. 2008;18:861–879. [Google Scholar]
- 33.Willis SL, Caskie GIL. Reasoning training in the ACTIVE study: How much is needed and who benefits? J Aging Health. 2014;25(8 Suppl):43S–64S. doi: 10.1177/0898264313503987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenwood PM, Sunderland T, Lambert C, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiepers OJ, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, Deary IJ. APOE E4 status predicts age-related cognitive decline in the ninth decade: Longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry. 2012;17:315–324. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- 37.Della Sala S, Parra MA, Fabi K, Luzzi S, Abrahams S. Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia. 2012;50:833–840. doi: 10.1016/j.neuropsychologia.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Allaire JC, Marsiske M. Well- and ill-defined measures of everyday cognition: Relationship to older adults’ intellectual ability and functional status. Psychol Aging. 2002;17:101–115. doi: 10.1037/0882-7974.17.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diehl M, Willis SL, Schaie KW. Everyday problem solving in older adults: Observational assessment and cognitive correlates. Psychol Aging. 1995;10:478–491. doi: 10.1037//0882-7974.10.3.478. [DOI] [PubMed] [Google Scholar]
- 40.Katzman R, Zhang MY, Chen PJ, Gu N, Jiang S, Saitoh T, Chen X, Klauber M, Thomas RG, Liu WT, Yu ES. Effects of apolipoprotein E on dementia and aging in the Shanghai Survey of Dementia. Neurology. 1997;49:779–785. doi: 10.1212/wnl.49.3.779. [DOI] [PubMed] [Google Scholar]
- 41.Nouchi R, Taki Y, Takeuchi H, Hashizume H, Nozawa T, Kambara T, Sekiguchi A, Miyauchi CM, Kotozaki Y, Nouchi H, Kawashima R. Brain training game improves executive functions and processing speed in the elderly: A randomized controlled trial. PLoS One. 2013;8:e55518. doi: 10.1371/journal.pone.0029676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.