Abstract
Purpose
To evaluate corneal morphology using ultrasonic pachmetry (USP), Fourier-domain optical coherence tomography (FD-OCT) and in vivo confocal microscopy (IVCM) in two related canine breeds – German shorthaired pointers (GSHPs) and German wirehaired pointers (GWHPs) - with and without corneal endothelial dystrophy (CED). This condition is characterized by premature endothelial cell degeneration leading to concomitant corneal edema and is similar to Fuchs endothelial corneal dystrophy (FECD).
Methods
Corneas of 10 CED-affected (4 GSHP, 6 GWHP) and 19 unaffected, age-matched (15 GSHP, 4 GWHP) dogs were examined using USP, FD-OCT and IVCM. A two-sample t-test or Mann-Whitney rank sum test statistically compared parameters between the two groups. Data are presented as mean ± SD or median (range).
Results
Central corneal thickness using USP was significantly greater in CED-affected versus unaffected dogs at 1179 (953–1959) and 646 (497–737) μm, respectively (P < 0.001). Central epithelial thickness was significantly decreased in CED-affected versus unaffected dogs at 47 ± 7.1 and 55 ± 7.1 μm, respectively (P = 0.011) using FD-OCT. With IVCM, corneal endothelial density was significantly less (P < 0.001) in 5 dogs with CED versus 19 unaffected controls at 499 ± 315 versus 1805 ± 298 cells/mm2, respectively. CED-affected dogs exhibited endothelial pleomorphism and polymegathism while CED unaffected dogs had a regular hexagonal arrangement of cells.
Conclusion
German shorthaired pointers and GWHPs with CED exhibit marked differences in corneal morphology in comparison to age-matched control dogs. These two CED-affected breeds can be utilized as spontaneous, large animal models for human FECD.
Keywords: Fuchs endothelial corneal dystrophy, Canine, Corneal endothelial dystrophy, Dog, German shorthaired pointer, German wirehaired pointer
Introduction
Fuchs endothelial corneal dystrophy (FECD) is a common, late onset condition in humans characterized by progressive loss of endothelial cells and the formation of guttae or collageneous deposits on Descemet’s membrane (DM). With progressive loss of endothelial cells, a point is reached where the remaining cells are unable to maintain stromal deturgescence1 leading to secondary thickening of the cornea from edema and loss of corneal transparency, decreased visual acuity, and bullous keratopathy.2
Similar to human patients with FECD, adult dogs suffer from a bilateral, sometimes asymmetric disease in which endothelial cells degenerate prematurely, termed corneal endothelial dystrophy (CED). With canine CED, corneal endothelial decompensation can occur with concomitant corneal edema and secondary vision compromise, bullous keratopathy, and corneal ulceration.3,4 Multiple breeds appear to be at increased risk for CED, in particular small breed dogs such as Boston terriers, chihuahuas, and dachshunds, an observation that suggests an underlying genetic component for this disease in dogs.4,5 In reviewing the records at the University of California-Davis Veterinary Medical Teaching Hospital, we identified two large breed dogs, German shorthaired pointers (GSHP) and German wirehaired pointers (GWHP), were overrepresented in compared to the hospital reference population (unpublished data). To the authors’ knowledge, CED has been only rarely described in large canine breeds6 and never been described or characterized in these two closely related breeds. Thus, the purpose of this study was to describe and characterize CED in GSHPs and GWHPs using noninvasive advanced ophthalmic imaging techniques and histopathology.
Materials and Methods
Animals
The study was performed in accordance with the Association for Research in Vision and Ophthalmology resolution on the use of animals in research and approved by the Institutional Animal Care and Use Committee at University of California-Davis (#17847). All dogs included were GSHPs or GWHPs; none had received medications for at least 48 h prior to study inclusion. For unaffected dogs, criteria for inclusion were clear corneas in both eyes and a regular arrangement of corneal endothelial cells with in vivo confocal microscopy (IVCM). For CED-affected dogs, criteria for inclusion was the presence of corneal edema in both eyes. For both groups, criteria for exclusion were diabetes mellitus, prior intraocular surgery or history and/or clinical signs consistent with glaucoma, anterior uveitis and/or lens instability.
An ophthalmic examination was performed including digital and handheld slit lamp biomicroscopy (Imaging Module 900®, Haag-Streit USA Inc, Mason, OH; SL-15, Kowa Company Ltd, Nagoya, Aichi, Japan). A Schirmer Tear Test-1 (STT-1; Intervet Inc, Summit, NJ) was performed followed by measurement of corneal sensitivity with a Cochet-Bonnet asesthesiometer (12/100mm, Luneau Ophthalmologie, Prunay-le-Gillon, France) using previously described methods.7 Proparacaine 0.5% ophthalmic solution (Akorn Inc, Lake Forest, IL) was applied prior to performing applanation tonometry (Tono-Pen XL, Mentor Ophthalmics, Norwell, MA) to measure intraocular pressure (IOP). Ultrasonic pachymetry (Pachette 3, DGH Technology Inc, Exton, PA) was performed as previously described.5 Dogs were sedated with acepromazine (0.01 mg/kg) and buprenorphine (0.01 mg/kg) administered intravenously.
Ophthalmic Imaging
Animals were placed in sternal recumbency. Fourier-domain optical coherence tomography (FD-OCT) imaging (RTVUE® 100, software version 6.1; Optovue Inc., Fremont, CA; 26000 A scan/sec, 5 um axial resolution, 840 nm superluminescent diode) of the central cornea was performed as previously described.8
Next, ICVM (ConfoScan 4; Nidek Technologies, Gamagori, Japan) was performed in the central cornea with a 40x/0.75 objective lens as previously described.9 To determine density of keratocyte and endothelial cells, a region of interest of 0.075 and 0.05 mm2 was used, respectively. Last, corneas were stained with fluoroscein sodium (Ful-Glo® strips USP 1 mg; Akorn Inc, Lake Forest, IL).
Owner survey
A survey was collected from each client who had a dog enrolled in the study (Supplementary Figure 1). This survey was reviewed by the UC Davis Institutional Review Board and deemed exempt.
Histopathology
One 14-year-old intact female CED-affected GSHP and one unaffected 15-year-old intact male GSHP were euthanized for reasons unrelated to their eyes; owner consent was obtained in accordance with hospital policy for use of tissues following euthanasia. The right globe from each dog was enucleated, placed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin or Periodic acid-Schiff. The sections were examined by a board-certified veterinary pathologist (CMR).
Statistical Analysis
For all measurements, values from each eye were averaged. A Student’s t-test was used to compare age, weight, epithelial thickness, endothelial-DM complex thickness, keratocyte density, endothelial density, STT-1 values, and IOP between CED-unaffected and affected dogs and age and weight between GSHPs and GWHPs; a Fisher’s exact test was used to compare gender distribution between groups. A Kruskal-Wallis one-way analysis of variance on ranks was used to compare differences in total corneal thickness between CED-unaffected and affected dogs by location. A Mann-Whitney rank sum test was used to compare central corneal thickness (CCT), stromal thickness, corneal touch threshold, and time spent outdoors between CED-unaffected and affected dogs. Least squares linear regression assessed the relationship between central epithelial thickness and CCT. Data are presented as mean ± SD or median (range).
Results
Study Population
The corneas of 10 affected (4 GSHP, 6 GWHP) and 19 unaffected (15 GSHP, 4 GWHP) dogs were included; 3 affected GWHPs were from the same litter. Gender distribution did not significantly differ between the affected (4 female, 6 male) and unaffected (9 female, 10 male) groups (P = 1.00). Mean age and weight did not significantly differ between CED-affected and unaffected dogs at 11.0 ± 1.9 and 28 ± 4.0 kg and 10.7 ± 1.8 years and 28 ± 5.8 kg, respectively (P = 0.729 and 0.737, respectively). Mean age and weight did not significantly differ between GSHPs and GWHPs at 10.8 ± 1.9 years and 27 ± 4.8 kg and 10.9 ± 1.7 years and 30 ± 5.5 kg, respectively (P = 0.880 and 0.112, respectively). Accordingly, data from both breeds were combined for all analyses.
USP
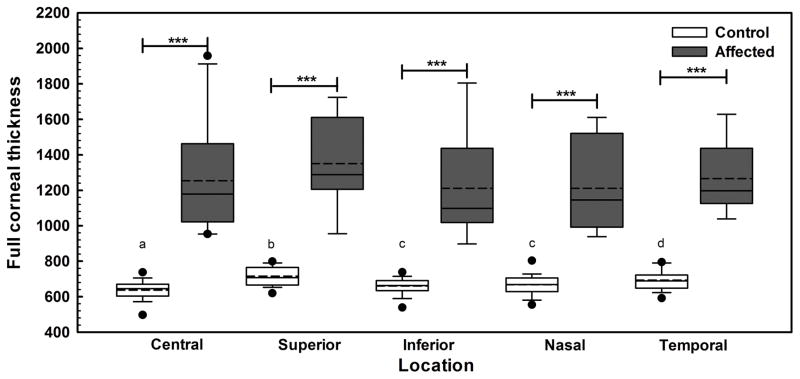
The CED-unaffected dogs had central, superior, inferior, nasal, and temporal perilimbal corneal thicknesses of 646 (497–737), 708 (620–799), 663 (539–738), 669 (555–803), and 690 (592–795) μm, respectively, which significantly differed at all locations (P < 0.005, Figure 1) but the inferior and nasal cornea (P = 0.224). CED-affected dogs had significantly thicker corneas at all locations versus unaffected dogs with central, superior, inferior, nasal, and temporal perilimbal measurements of 1179 (953–1959), 1289 (955–1725), 1098 (898–1805), 1146 (938–1612) and 1198 (1039–1629) μm, respectively (P < 0.001); there were no significant differences in corneal thickness between locations (P > 0.05, Figure 1).
Fig. 1. Corneal thickness in 10 CED-affected dogs as measured by USP markedly differed in comparison to 19 unaffected controls.
At all 5 locations measured, CED-affected dogs had significantly greater corneal thickness in comparison to CED-unaffected dogs. Corneal thickness significantly differed between all locations in the CED-unaffected dogs but the inferior and nasal locations (P = 0.224). Box plots depict median (solid line), mean (dashed line), 25th and 75th percentiles, while whiskers show 10th and 90th percentiles; black circles indicate outliers. The P values were determined by a Kruskal Wallis ANOVA on ranks, ***P < 0.001 between CED-unaffected and affected dogs. a,b,c,dP < 0.005 between the different locations of CED-unaffected dogs.
FD-OCT
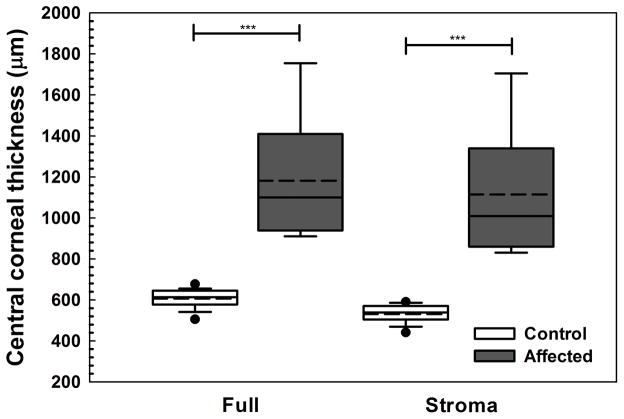
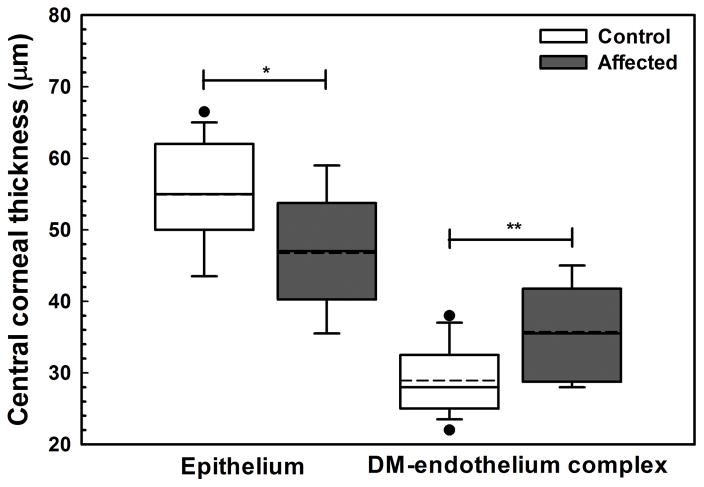
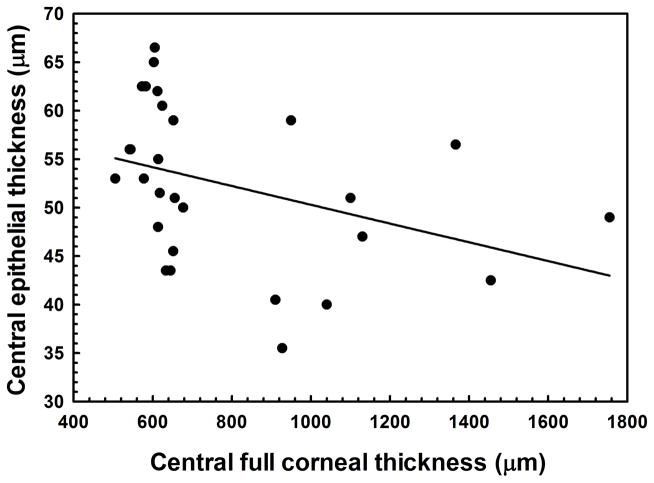
In CED-affected dogs, corneal thickening was caused by edema with loss of the orderly arrangement of collagen in the corneal stroma and increase in reflectivity as observed by multiple imaging modalities (Figure 2). Consistent with USP, CCT as measured by FD-OCT significantly differed between CED-affected and unaffected dogs (P < 0.001, Figure 3A). The increase in CCT was primarily due to a significant increase in stromal thickness in CED-affected versus unaffected dogs (P < 0.001, Figure 3A). Thickness of DM-endothelium complex was significantly greater in CED-affected versus unaffected dogs (P = 0.003, Figure 3B). By contrast, central epithelial thickness was significantly less in CED-affected and unaffected dogs (P = 0.011, Figure 3B). A decrease in central epithelial thickness was significantly correlated with CCT (P = 0.049, R2 = 0.141, Figure 3C).
Fig. 2. Corneal structure and endothelial morphology as determined by color photography, digital slit lamp biomicroscopy, FD-OCT and IVCM dramatically differed between control and CED-affected dogs with variable disease severity.
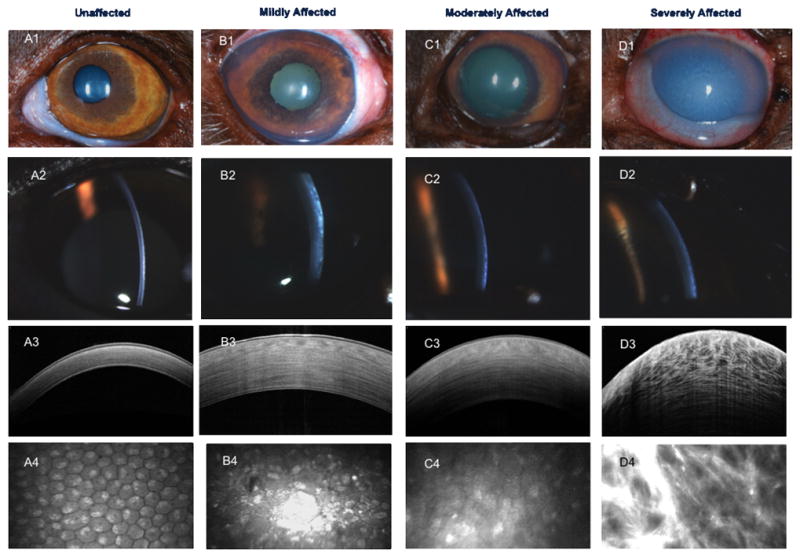
A 14 year old spayed female GSHP with a clear cornea (A1-2), normal corneal thickness and lamellar arrangement of the collagen fibrils (A3) and regular, hexagonal arrangement of corneal endothelium (A4); this dog was categorized as a control. An 11 year old intact male with mild diffuse corneal edema (B1-2), mildly thickened cornea (B3) and moderate pleomorphism and polymegathism of the corneal endothelium (B4); an 8 year old spayed female GSHP with moderate diffuse edema of the cornea (C1-2), increased corneal thickness (C3) and moderate pleomorphism and polymegathism of the corneal endothelium (C4); a 13 year old spayed female GSHP with marked, diffuse corneal edema (E1-2), marked increase in corneal thickness (E3), and loss of the orderly arrangement of collagen fibrils within the anterior stroma (E3-4) such that the corneal endothelium and keratocytes could not be visualized (E4).
Fig. 3. Central corneal full, stromal, epithelial and DM-endothelium complex thickness significantly differed between dogs without and with CED as measured by FD-OCT.
Central corneal thickness (CCT) was significantly greater in 9 CED-affected versus 19 unaffected dogs with a median (range) of 1100 (910–1755) and 614 (506–677) μm, respectively, primarily due to a significant increase in stromal thickness in CED-affected versus unaffected dogs at 1009 (831–1705) and 539 (442–591) μm, respectively (A). Epithelial thickness in the central cornea was significantly less in dogs with versus without CED at 47 ± 7.1 and 55 ± 7.1 μm, respectively (B). In the central cornea, DM-endothelium complex thickness was significantly greater in dogs with versus without CED at 36 ± 6.3 and 29 ± 4.5 μm, respectively. Box plots depict median (solid line), mean (dashed line), 25th and 75th percentiles, while whiskers show 10th and 90th percentiles; black circles indicate outliers. The P values were determined by a Mann-Whitney rank sum test, *P < 0.05, **P < 0.01, and ***P < 0.001. (C) A significant inverse relationship was identified between epithelial thickness and CCT as measured by FD-OCT using least squares linear regression (P = 0.049, R2 = 0.141).
ICVM
With IVCM, marked differences in endothelial cell morphology were identified with a regular, hexagonal pattern in unaffected dogs, and pleomorphism and polymegathism in 5 CED-affected dogs that varied depending on their disease severity; severe corneal edema prevented visualization of the endothelium in the 5 remaining dogs. Endothelial cell density (ECD) was significantly less in CED-affected (n = 5) versus unaffected (n = 19) dogs (P < 0.001, Figure 4). In the anterior and posterior stroma, keratocyte density did not significantly differ (P = 0.162 and 0.684, respectively) in dogs with CED (n = 3) at 394 ± 73 and 424 ± 77 cells/mm2 versus unaffected controls (n = 19) at 464 ± 77 and 442 ± 71 cells/mm2. Severe corneal edema prevented visualization of keratocytes in 6 CED-affected dogs.
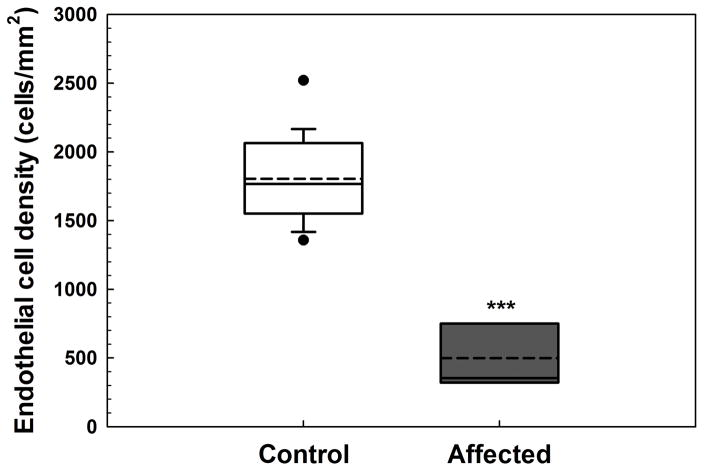
Fig. 4. Endothelial cell density as measured by IVCM was markedly less in dogs with CED.
Corneal endothelial density was significantly less in 5 dogs with CED (499 ± 315 cells/mm2) versus 19 unaffected controls (1805 ± 298 cells/mm2). Endothelial cell density could not be assessed in 5 dogs with CED due to severe, bilateral corneal edema. Box plots depict median (solid line), mean (dashed line), 25th and 75th percentiles, while whiskers show 10th and 90th percentiles; black circles indicate outliers. The P value was determined by a Students’s t-test, ***P < 0.001.
Ancillary Diagnostic Tests
Aqueous tear production, central corneal touch threshold and IOP did not significantly differ between affected and unaffected dogs at 22 ± 2.7 mm/min, 3.95 (2.3–13.1) g/mm2 and 8.3 (4.5–17) mm Hg and 21 ± 2.7 mm/min, 3.4 (1.4–13.1) g/mm2 and 7.0 (4–11.5) mm Hg, respectively (P = 0.617, 0.466 and 0.581, respectively). No dogs had corneal fluorescein retention at the examination.
Owner survey
Owners of 28 of the 29 dogs (97%) included in this study completed the survey, with the one unanswered survey from the owner of an affected GWHP. Ophthalmic conditions reported by owners included CED (n = 8), meibomian gland adenoma (n = 1) and retrobulbar mass (n = 1). In the CED-affected dogs, median (range) age of onset of clinical signs was 10 (6–12) years. Time outdoors did not significantly differ between CED-affected and unaffected dogs with a median (range) of 3–6 (1–2 to >12) and 1–2 (1–2 to >12) h, respectively (P = 0.177). Of the 10 affected dogs, 6 had previously received treatment for CED. At initiation of treatment, severity of clinical signs were median (range) of 7.5 (3–10) for 6 dogs. Five affected dogs were topically treated with 5% sodium chloride (NaCl) ophthalmic ointment and a medication containing a corticosteroid. One dog was treated solely with an antibiotic-steroid solution. Four owners reported that topical 5% NaCl with a corticosteroid was the most effective treatment, one owner did not indicate a most effective treatment, and one owner reported that the patient did not improve on any medical therapies. Four owners indicated severity of clinical signs following medical therapy with a median (range) of 6 (3.5–7). Three of the patients with submitted surveys had received surgical intervention, a superficial keratectomy and conjunctival advancement hood flap (SKCAHF), for their disease.10 In these 3 patients, owner reported disease severity was 8 and 7 prior to surgery and 2 and 5.5 following surgery, respectively; one owner did not rate disease severity with regard to surgery. Ultrasonic pachymetry, FD-OCT and IVCM data was available and included for 2 dogs prior to SKCAHF surgery while 1 dog only had central USP data available and included prior to SKCAHF surgery. One GSHP included in the present study that received SKCAHF surgery was described in a separate publication.10
Histopathology
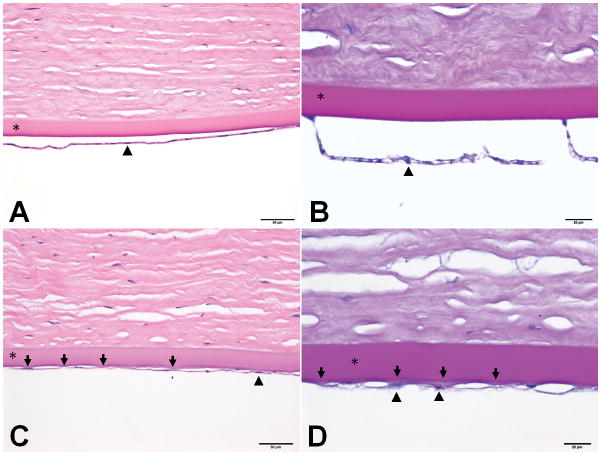
In the dog with CED, DM was slightly thicker with guttae-like protrusions of disorganized, fibrillar extracellular matrix (ECM), compared to a smooth and uniform DM in the CED-unaffected dog (Figure 5). Corneal endothelial cells were flattened, attenuated and much less frequent along the inner aspect of DM in the CED-affected versus unaffected dog (Figure 5).
Figure 5. Histology morphology of the corneal endothelium and DM markedly differed between a dog with and without CED.
Photomicrographs of sections of the inner cornea from the right eye of a 15 year old intact male GSHP (A & B) unaffected with CED and a 14 year old intact female CED-affected GSHP (C & D) stained with hematoxylin and eosin (200x magnification) or Periodic acid-Schiff (400x magnification) where DM is bright magenta, respectively. In A & B, note the smooth contour of the inner aspect of DM (*), and the frequent nuclei in the cuboidal endothelial cells (example at arrowhead) in a dog unaffected with CED. In the CED-affected dog (C), note the irregularities (arrows) on the inner surface of DM (*) and the slight increase in thickness of DM in comparison to the CED-unaffected dog (A). The endothelium is more sparsely cellular, and the cells are attenuated (example at arrowhead) in the CED-affected (C) versus unaffected dog (A). In D, note the pale-staining guttae-like excrescences of loose, fibrillar ECM (arrows) on the inner surface of DM (magenta) in the CED-affected dog; two of these excrescences are associated with flattened endothelial cells (examples at arrowheads). In D, DM (*) is also slightly thicker than in B (CED-unaffected).
Discussion
The results of this study demonstrate numerous clinical and histologic similarities between canine CED and human FECD. Other human endothelial disorders such as congenital hereditary endothelial dystrophy, X-linked endothelial corneal dystrophy and posterior polymorphous corneal dystrophy are inconsistent with canine CED given the adult onset, affected females and specific morphology in DM and the endothelium observed.4,5,11 Using ICVM, marked morphologic changes were observed in CED-affected dogs, including polymegathism and pleomorphism which tended to increase in correlation with disease severity. Microscopically, endothelial cell morphology was markedly attenuated and spread with a decrease in density in a CED-affected versus unaffected dog consistent with a previous study documenting fibrous metaplasia in canine CED.4 Similarly, endothelial cells undergo epithelial-mesenchymal transformation (EMT) in FECD and exhibit a fibroblast-like phenotype,12 suggesting that EMT may play an important role in the pathophysiology of both diseases.13 Furthermore, ECD was markedly decreased in CED-affected GSHPs and GWHPs versus age-matched controlsand less than values from previous reports of CED-affected Boston terriers5 as well as normal adult dogs14 and Boston terriers5, respectively. It is possible that large breed dogs such as GSHPs and GWHPs have a lower ECD and/or accelerated endothelial cell loss compared to small breed dogs such as Boston terriers. It is well known that large breed dogs age faster and die earlier than small breed dogs15 and a decline in ECD is expected with aging. Thus, endothelial decompensation from CED may occur at an earlier age in GSHPs and GWHPs and possibly other large canine breeds. Further studies of ECD and body weight over time in normal and CED-affected dogs are warranted.
Numerous studies have documented that DM is markedly thicker in human patients with versus without FECD using a variety of techniques.16,17 We similarly demonstrated with FD-OCT that the DM-endothelium complex was significantly thicker in CED-affected dogs in the present and a previous study.5 Furthermore, histology demonstrated a thickened DM with a posterior fibrillar layer and guttae-like deposits in a GSHP with CED consistent with a previous study.4 A prominent feature of FECD is the formation of guttae or excrescences of ECM on DM that is often observed with IVCM,18 suggesting that this dysregulated DM may play an important role in disease progression.19 Therefore, studying the pathogenesis of canine CED may offer new insight into FECD that animal models of endothelial injury would not provide.
Unaffected GSHPs and GWHPs have a mean CCT of 614 μm which is greater than the mean CCT of 527 μm reported in human patients aged 60 years or older.20 Central corneal thickness increases with body weight; thus, small breed dogs such as beagles8 and Boston terriers5 will have a CCT that is closer to a human versus large canine breeds. In CED-affected dogs,5 endothelial decompensation results in a marked increase of CCT due to moderate to severe corneal edema. By contrast, humans with FECD tend to have a mild increase in CCT from corneal endothelial decompensation presumably due to human FECD patients seeking ophthalmic care at an earlier disease stage from decreased visual acuity2 versus owners of CED-affected dogs.3,21 Intrinsic differences between the human and canine cornea exist including less extensive collagen fiber intertwining in the canine (unpublished data, M. Winkler, J.V. Jester, UC Irvine, S.M. Thomasy, V.K. Raghunathan, C.J. Murphy, UC Davis) versus human cornea22 and a lack of Bowmans layer23 in the dog that would make the canine cornea more susceptible to swelling. Interestingly, central corneal epithelium was significantly thinner in CED-affected versus unaffected GSHPs and GWHPs consistent with a previous study.5 This finding in combination with formation of corneal bullae likely contributes to the tendency for these dogs to ulcerate and thus experience ocular discomfort.24 While it is possible that a thinner epithelium is intrinsic to canine CED, it is more likely related to corneal edema given the inverse linear relationship between CCT and epithelial thickness observed. To the authors’ knowledge, a difference in epithelial thickness between human patients with or without FECD has not been studied but investigations may be warranted given the present findings.
Corneal edema in CED-affected GSHPs or GWHPs was predominantly diffuse and contrasts with CED-affected Boston terriers where edema typically begins temporally and progresses to involve the entire cornea, affecting the nasal aspect last.3–5 In humans, even mild, diffuse corneal edema can result in a marked decrease in visual acuity,2 thus GSHPs and GWHPs may experience a decline in vision earlier than Boston terriers with temporal edema that does not affect the visual axis. Penetrating keratoplasty is the gold standard treatment for canine CED3 but is rarely performed due to high risk of complications, lack of appropriate donor tissue, and high cost of the surgery to owners. Other interventions to treat CED include superficial keratectomy and conjunctival advancement hood flap (SKCAHF),10 thermokeratoplasty,21 penetrating or non-penetrating keratoprosthesis25,26 and corneal crosslinking with riboflavin and ultraviolet light.27 In the present study, three dogs received a SKCAHF with moderate to marked improvement noted by 2 owners; the third owner did not complete this portion of the survey. These results are consistent with a recent clinical trial whereby owners reported increased corneal clarity and vision in their dogs following SKCAHF surgery.10 The most common palliative treatment was 5% NaCl which is consistent with previous reports of CED-affected dogs.28 Four owners reported worsening (n = 1), no (n = 1), or mild to moderate (n = 2) improvement with 5% NaCl consistent with a previous study demonstrating only a 2% decrease in corneal thickness following at least 4 times daily treatment with this medication in normal dogs.28
There are numerous advantages to a spontaneous, large animal model for FECD. Corneal endothelial dystrophy in dogs occurs spontaneously and thus is more likely to mimic the genetic and environmental variability within the human population, especially in comparison to the highly inbred and uniform environment of laboratory animals.29 Dogs are commonly used in ocular drug development and to investigate and develop treatments for corneal disease due to their large eyes, ease of handling, and relatively similar corneal anatomy to humans.30–32 There are well characterized murine FECD models, Col8a2L450W/L450W and Col8a2Q455K/Q455K, which exhibit guttae, decreased ECD and altered endothelial cell morphology33 but these models have limited value for investigating surgical and cell-based interventions due to the small murine eye. Additionally, a large discrepancy exists between murine and human CCT likely contributing to great variability in the kinetics of topical ocular drug delivery between the two species.34,35 Veterinary clinical trials of CED-affected patients may thus provide a more predictive model for evaluation of pharmacologic, surgical and cell-based therapies for human FECD from preclinical development to human clinical trials.29
In conclusion, this study utilized advanced ophthalmic imaging to describe the in vivo characteristics of CED in two closely related breeds – the GSHP and GWHP. Similar to FECD, CED is a bilateral, adult-onset condition that exhibits endothelial cell loss concurrently with an abnormal DM. Thus CED could serve as a useful disease model in order to study the pathogenesis, progression, and novel therapies for FECD.
Supplementary Material
Survey provided to owners of 10 CED-affected (4 GSHP, 6 GWHP) and 19 unaffected, age-matched (15 GSHP, 4 GWHP) dogs.
Acknowledgments
Supported by NIH grants K08 EY021142, R01 EY019970, R01 EY016134, and P30 EY12576, UC Davis Academic Federation Innovative Development Award, and Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis.
The authors thank Kathleen Stewart, Alyssa Hoehn, Allison Calderon, Geneva Tripp, Amelia Stanley, Connor Chang, Ariana Marangakis, and Monica Motta for assisting with the patient examinations.
Footnotes
Conflict of interest: None
References
- 1.Chi HH, Teng CC, Katzin HM. Histopathology of primary endothelial-epithelial dystrophy of the cornea. Am J Ophthalmol. 1958;45:518–535. doi: 10.1016/0002-9394(58)90521-x. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RA, Raza S, Walford E, et al. Fuchs endothelial corneal dystrophy: clinical characteristics of surgical and nonsurgical patients. Clin Ophthalmol. 2014;8:1761–1766. doi: 10.2147/OPTH.S68217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gwin RM, Polack FM, Warren JK, et al. Primary canine corneal endothelial cell dystrophy: Specular microscopic evaluation, diagnosis and therapy. Journal of the American Animal Hospital Association. 1982;18:471–479. [Google Scholar]
- 4.Martin CL, Dice PF. Corneal endothelial dystrophy in the dog. J Am Anim Hosp Assoc. 1982;18:327–336. [Google Scholar]
- 5.Thomasy SM, Cortes DE, Hoehn AL, et al. In Vivo Imaging of Corneal Endothelial Dystrophy in Boston Terriers: A Spontaneous, Canine Model for Fuchs’ Endothelial Corneal Dystrophy. Invest Ophthalmol Vis Sci. 2016;57:OCT495–503. doi: 10.1167/iovs.15-18885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks DE, Samuelson DA, Smith RJ. Corneal endothelial cell degeneration in a German shepherd dog. Journal of Small Animal Practice. 1990;31:31–34. [Google Scholar]
- 7.Good KL, Maggs DJ, Hollingsworth SR, et al. Corneal sensitivity in dogs with diabetes mellitus. Am J Vet Res. 2003;64:7–11. doi: 10.2460/ajvr.2003.64.7. [DOI] [PubMed] [Google Scholar]
- 8.Strom AR, Cortes DE, Rasmussen CA, et al. In vivo evaluation of the cornea and conjunctiva of the normal laboratory beagle using time- and Fourier-domain optical coherence tomography and ultrasound pachymetry. Vet Ophthalmol. 2016;19:50–56. doi: 10.1111/vop.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strom AR, Cortes DE, Thomasy SM, et al. In vivo ocular imaging of the cornea of the normal female laboratory beagle using confocal microscopy. Vet Ophthalmol. 2016;19:63–67. doi: 10.1111/vop.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horikawa T, Thomasy SM, Stanley AA, et al. Superficial Keratectomy and Conjunctival Advancement Hood Flap (SKCAHF) for the Management of Bullous Keratopathy: Validation in Dogs With Spontaneous Disease. Cornea. 2016 doi: 10.1097/ICO.0000000000000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss JS, Moller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies--edition 2. Cornea. 2015;34:117–159. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 12.Hidayat AA, Cockerham GC. Epithelial metaplasia of the corneal endothelium in Fuchs endothelial dystrophy. Cornea. 2006;25:956–959. doi: 10.1097/01.ico.0000228786.84581.ee. [DOI] [PubMed] [Google Scholar]
- 13.Okumura N, Minamiyama R, Ho LT, et al. Involvement of ZEB1 and Snail1 in excessive production of extracellular matrix in Fuchs endothelial corneal dystrophy. Lab Invest. 2015 doi: 10.1038/labinvest.2015.111. [DOI] [PubMed] [Google Scholar]
- 14.Gwin RM, Lerner I, Warren JK, et al. Decrease in canine corneal endothelial cell density and increase in corneal thickness as functions of age. Invest Ophthalmol Vis Sci. 1982;22:267–271. [PubMed] [Google Scholar]
- 15.Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52:B171–178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- 16.Shousha MA, Perez VL, Wang J, et al. Use of ultra-high-resolution optical coherence tomography to detect in vivo characteristics of Descemet’s membrane in Fuchs’ dystrophy. Ophthalmology. 2010;117:1220–1227. doi: 10.1016/j.ophtha.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heindl LM, Hofmann-Rummelt C, Schlotzer-Schrehardt U, et al. Histologic analysis of descemet stripping in posterior lamellar keratoplasty. Arch Ophthalmol. 2008;126:461–464. doi: 10.1001/archophthalmol.2007.75. [DOI] [PubMed] [Google Scholar]
- 18.Chiou AG, Kaufman SC, Beuerman RW, et al. Confocal microscopy in cornea guttata and Fuchs’ endothelial dystrophy. Br J Ophthalmol. 1999;83:185–189. doi: 10.1136/bjo.83.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali M, Raghunathan V, Li JY, et al. Biomechanical relationships between the corneal endothelium and Descemet’s membrane. Exp Eye Res. 2016;152:57–70. doi: 10.1016/j.exer.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eysteinsson T, Jonasson F, Sasaki H, et al. Central corneal thickness, radius of the corneal curvature and intraocular pressure in normal subjects using non-contact techniques: Reykjavik Eye Study. Acta Ophthalmol Scand. 2002;80:11–15. doi: 10.1034/j.1600-0420.2002.800103.x. [DOI] [PubMed] [Google Scholar]
- 21.Michau TM, Gilger BC, Maggio F, et al. Use of thermokeratoplasty for treatment of ulcerative keratitis and bullous keratopathy secondary to corneal endothelial disease in dogs: 13 cases (1994–2001) J Am Vet Med Assoc. 2003;222:607–612. doi: 10.2460/javma.2003.222.607. [DOI] [PubMed] [Google Scholar]
- 22.Winkler M, Chai D, Kriling S, et al. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Invest Ophthalmol Vis Sci. 2011;52:8818–8827. doi: 10.1167/iovs.11-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nautscher N, Bauer A, Steffl M, et al. Comparative morphological evaluation of domestic animal cornea. Vet Ophthalmol. 2015 doi: 10.1111/vop.12298. [DOI] [PubMed] [Google Scholar]
- 24.Ledbetter EC, Munger RJ, Ring RD, et al. Efficacy of two chondroitin sulfate ophthalmic solutions in the therapy of spontaneous chronic corneal epithelial defects and ulcerative keratitis associated with bullous keratopathy in dogs. Vet Ophthalmol. 2006;9:77–87. doi: 10.1111/j.1463-5224.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 25.Isard PF, Dulaurent T, Regnier A. Keratoprosthesis with retrocorneal fixation: preliminary results in dogs with corneal blindness. Vet Ophthalmol. 2010;13:279–288. doi: 10.1111/j.1463-5224.2010.00800.x. [DOI] [PubMed] [Google Scholar]
- 26.Allgoewer I, McLellan GJ, Agarwal S. A keratoprosthesis prototype for the dog. Vet Ophthalmol. 2010;13:47–52. doi: 10.1111/j.1463-5224.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 27.Pot SA, Gallhofer NS, Walser-Reinhardt L, et al. Treatment of bullous keratopathy with corneal collagen cross-linking in two dogs. Vet Ophthalmol. 2015;18:168–173. doi: 10.1111/vop.12137. [DOI] [PubMed] [Google Scholar]
- 28.Samuel ML, Thomasy SM, Calderon AS, et al. Effects of hypertonic saine on central corneal thickness in dogs. Proceedings of the 45th Annual Meeting of the American College of Veterinary Ophthalmologists; San Antonio. 2014. [Google Scholar]
- 29.Kol A, Arzi B, Athanasiou KA, et al. Companion animals: Translational scientist’s new best friends. Sci Transl Med. 2015;7:308ps321. doi: 10.1126/scitranslmed.aaa9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaswan RL, Salisbury MA, Ward DA. Spontaneous canine keratoconjunctivitis sicca. A useful model for human keratoconjunctivitis sicca: treatment with cyclosporine eye drops. Arch Ophthalmol. 1989;107:1210–1216. doi: 10.1001/archopht.1989.01070020276038. [DOI] [PubMed] [Google Scholar]
- 31.Murphy CJ, Bentley E, Miller PE, et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest Ophthalmol Vis Sci. 2011;52:3174–3180. doi: 10.1167/iovs.09-5078. [DOI] [PubMed] [Google Scholar]
- 32.Bentley E, Murphy CJ, Li F, et al. Biosynthetic corneal substitute implantation in dogs. Cornea. 2010;29:910–916. doi: 10.1097/ICO.0b013e3181c846aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng H, Matthaei M, Ramanan N, et al. L450W and Q455K Col8a2 knock-in mouse models of Fuchs endothelial corneal dystrophy show distinct phenotypes and evidence for altered autophagy. Invest Ophthalmol Vis Sci. 2013;54:1887–1897. doi: 10.1167/iovs.12-11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Wang L, Xie Y, et al. The measurement of corneal thickness from center to limbus in vivo in C57BL/6 and BALB/c mice using two-photon imaging. Exp Eye Res. 2013;115:255–262. doi: 10.1016/j.exer.2013.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey provided to owners of 10 CED-affected (4 GSHP, 6 GWHP) and 19 unaffected, age-matched (15 GSHP, 4 GWHP) dogs.