Abstract
Energy densities (ED, mJ/mm3) quantify mechanical work imposed on articular cartilages during function. This cross-sectional study examined differences in temporomandibular joint (TMJ) ED during asymmetric versus symmetric jaw closing in healthy females versus males. ED component variables were tested for differences between and within sexes for two types of jaw closing. Seventeen female and 17 male subjects gave informed consent to participate. Diagnostic Criteria for Temporomandibular Disorders and images (magnetic resonance (MR), computed tomography) were used to confirm healthy TMJ status. Numerical modeling predicted TMJ loads (Fnormal) consequent to unilateral canine biting. Dynamic stereometry combined MR imaging and jaw tracking data to measure ED component variables during 10 trials of each type of jaw closing in each subject’s TMJs. These data were then used to calculate TMJ ED during jaw closing asymmetrically and symmetrically. Paired and Student’s t-tests assessed ED between jaw closing movements and sexes, respectively. Multivariate data analyses assessed ED component variable differences between jaw closing movements and sexes (α=0.05). Contralateral TMJ ED were 3.6-fold and significantly larger (P<0.0001) during asymmetric versus symmetric jaw closing, due to significantly larger (P≤0.001) distances of TMJ stress-field translation in asymmetric versus symmetric movement. During asymmetric jaw closing, contralateral TMJ ED were two-fold and significantly larger (P=0.036) in females versus males, due to 1.5-fold and significantly smaller (P≤0.010) TMJ disc cartilage volumes under stress-fields in females versus males. These results suggest that in healthy individuals asymmetric compared to symmetric jaw closure in females compared to males have higher TMJ mechanical fatigue liabilities.
Keywords: biomechanical phenomena, cartilage, females, human, males, temporomandibular joint
Background
Evidence for the role of mechanics in the precocious development of temporomandibular joint (TMJ) cartilage failure has been reported.1, 2 In brief, the articular tissue integrity of synovial joints is likely to be dependent, at least in part, on energy densities (ED, mJ/mm3), which are the amounts of mechanical work per volume of cartilage that are imposed during function. In particular, TMJ disc fibrocartilage is avascular and depends on mechanical loading for nutrient and waste exchange. However, mechanical over-loading can elicit oxidative stresses and inflammation3 leading to damage, and if the limited repair capacity of the cartilage is out-stripped, ultimately ending in tissue failure due to mechanical fatigue. In parallel with the mechanical fatigue of the articular tissues, mechanically induced expression of inflammatory mediators disrupt localized spatial downregulation of Wnt/β Catenin signaling which is critical for maintenance of fibrocartilage stem cells and inhibition of metalloproteinases that could otherwise challenge fibrocartilage matrix homeostasis.4–7 Why degeneration of the articular tissues occurs earlier in the TMJ compared to post-cranial joints and why females are more afflicted than males,8, 9 may be due to the TMJ’s unique vulnerability to combined mechanical and biological variables. That is, there is an innate susceptibility of the TMJ disc to anisotropic mechanical fatigue,10, 11 as demonstrated by normal function which routinely produces tractional (plowing plus frictional) forces along the low yield-strength mediolateral axis of the TMJ disc.12 This, combined with the regional distribution of fibroblast cartilage stem cells in the articulating surface layers that are most affected by tractional forces13, 14 may be the foundational mechanisms which explain the precocious development of degenerative joint disease of the TMJ compared to hips and knees.
Data reported to date1, 2 demonstrated that during symmetric jaw closing with a 20 N unilateral mandibular canine load, ED were significantly different between diagnostic groups and between healthy females and males. For example, subjects with chronic pain and bilateral TMJ disc displacement showed average ED (±standard deviation) in the contralateral TMJ that were more than double those of healthy subjects (12.7±1.5 compared to 5.8±0.9 mJ/mm3; p=0.0006).1 In addition, amongst healthy subjects, females showed average ED in the contralateral TMJ that were significantly larger by 33% than males (8.4±5.5 compared to 5.6±4.2 mJ/mm3; p=0.001).2 It is known that normal function typically involves loading of the TMJ during lateral as well as symmetric jaw movements15 but what TMJ ED occur during such lateral jaw movements are unknown. Previous reports on healthy subjects indicated that the average amounts of applied mechanical work in the TMJ increased during symmetric jaw opening-closing movements at 0.5 Hz (191±166 mJ) compared to 1.0 Hz (251±211 mJ)16 and were larger still in the contralateral TMJ during laterotrusion (374±740 mJ).12 However, it is unknown if, during the closing phase from jaw laterotrusion, ED are larger than during symmetric jaw closing and larger in females than males. Addressing these unknowns could elucidate the observed precocious degeneration of TMJ articulating tissues, especially in females compared to males.
The objectives of the current project were to test: (i) the hypotheses that ED in contralateral TMJs are significantly larger (a) during jaw closing from laterotrusion than during symmetric jaw closing, and (b) during jaw closing from laterotrusion in healthy females than healthy males, and ii) if there are differences in the component variables that contribute to TMJ ED (a) between females and males for the two types of jaw closing and (b) within each sex for jaw closing from laterotrusion and symmetric jaw closing.
Methods
This cross-sectional study complied with “Strengthening the reporting of observational studies in epidemiology” (STROBE) recommendations. Subjects were recruited at the University at Buffalo School of Dental Medicine (UBSDM) from the patient and general populations from the surrounding area. Subjects were recruited in a pilot phase from September 2006–June 2008 that resulted in five female and three male participants and then the remainder were recruited between November 2011–February 2014. All subjects provided written informed consent before participating and study protocols were approved by the UB (#388770-1) and University of Missouri-Kansas City (UMKC, #13-656) Institutional Review Boards. Inclusion, exclusion, and classification of subjects were based on comprehensive histories, physical examinations, and imaging via magnetic resonance (MR; Echelon 1.5T, Hitachi America, Tarrytown NY) and computed tomography (CT; Galileos Comfort, Dentsply Sirona, York PA), using Diagnostic Criteria for Temporomandibular Disorders (TMD).17, 18 Specific inclusion criteria were adults without TMD while exclusion criteria were: pregnancy, systemic rheumatological or musculoskeletal disease, TMJ disc displacement or degenerative disease based on MR or CT images respectively, decayed or multiple missing teeth, large dental restorations, fixed orthodontic appliances, claustrophobia, and history of frank TMJ trauma.
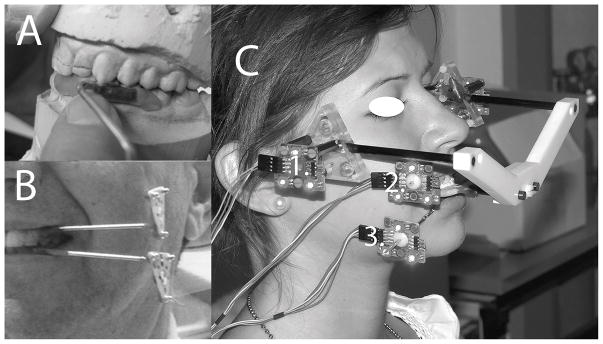
All subjects made one clinical visit at the UBSDM and one imaging visit at a private imaging clinic in Buffalo, NY (PIC) to determine diagnoses between November 2007–November 2011 for pilot subjects and January 2012–February 2014 for the remainder (Table 1). Subjects with healthy TMJs bilaterally qualified and made additional clinical and imaging visits (Table 1) for the dynamic stereometry protocols, which have been previously described.1, 19 At Imaging Visit #2, conducted at the PIC between December 2007–January 2012 for pilot subjects and between February 2012–June 2014 for the remainder, MR images of each subject’s TMJs were made using a 1.5 T machine and surface coils of 12 cm radius while the subject bit into a custom occlusal registration appliance that carried a head reference system with 3 MR-contrast spheres. At Clinical Visit #2, conducted at the UBSMD between November 2007–November 2011 for pilot subjects and between December 2011–May 2014 for the remainder, jaw-tracking was accomplished by recording the positions of each subject’s jaws while biting into the occlusal registration appliance with head reference system and without the appliance while performing jaw opening-closing movements. During the jaw-tracking protocol, each subject had a maxillary and mandibular custom acrylic splint temporarily luted to vestibular surfaces of the anterior and premolar teeth in the maxillary and mandibular dental arches, respectively, on one side at a time (Fig. 1A,B). Each splint had a metal arm attached that extended outside of the mouth and housed a set of three light-emitting diodes (LED). Similarly, the head reference system had a set of three LED in fixed relation to the MR-contrast spheres. Positions of the LED were recorded on one side of the subject at a time via three cameras. The cameras were arranged linearly and attached to a base, which was supported by a table. The table height was adjusted to ensure visualization of all LED on one side of the subject by all 3 cameras. While recording on one side, each subject held their jaws in a static position by biting into the occlusal registration appliance (Fig. 1C), then the appliance was removed and the subject performed 10 trials of two types of jaw opening-closing movements: symmetric and from laterotrusion. The protocol was repeated for recording on the other side.
Table 1.
Overview of Methods (modified from Iwasaki et al. 201719)
| Activity [Location] | Purpose | Application |
|---|---|---|
| Clinical Visit #1 [UB] Sept. 2006–June 2008 (pilot phase) and Nov. 2011–Feb. 2014 approximately 161 subjects were screened; 34 met study criteria and completed the protocols | Subject recruitment and enrollment Acquire:
|
|
| Imaging visit #1 [PIC] Nov. 2007–Nov. 2011 (pilot phase) and Jan. 2012–Feb. 2014 | Acquire bilateral MRI of TMJs (Echelon 1.5T, Hitachi America Ltd., Tarrytown NY) |
|
| Imaging visit #2 [PIC] Dec. 2007–Jan. 2012 (pilot phase) and Feb. 2012–June 2014 | Acquire bilateral MRI of TMJs and reference system (with surface coils) |
|
| Clinical Visit #2 [UB] Nov. 2007–Nov. 2011 (pilot phase) and Dec. 2011–May 2014 | Record:
|
|
| Data Analysis [UB, UMKC, UZ] 34 subjects completed study protocols; data analysis was on-going as collection was completed for each subject | Analyses of variance, Tukey honest significant difference post hoc tests Independent Variables
|
|
3D, three-dimensional; ΔD, distance of stress-field translation (mm); DC/TMD, diagnostic criteria for temporomandibular disorders; MRI, magnetic resonance images; PIC, private imaging center (WNY MRI, Buffalo, NY); Q, cartilage volume (mm3); TMJ, temporomandibular joint; UB, University at Buffalo School of Dental Medicine; UMKC, University of Missouri-Kansas City School of Dentistry; UZ, University of Zurich School of Dental Medicine; V, velocity of stress-field translation (mm/s); x, aspect ratio=radius of the stress-field/instantaneous TMJ disc thickness.
Fig. 1.
A. Maxillary left splint in position on plaster models from dental impressions made during Clinical Visit #1; then B. shown with mandibular left splint luted in place on a subject’s teeth. C. Subject is shown wearing the occlusal registration appliance and head reference system with light-emitting diodes (1). Light-emitting diodes are also connected to the maxillary (2) and mandibular (3) teeth via the splints affixed to the teeth. Fig. 1C was modified from a previous publication.19
Energy densities (ED) were calculated, as per previous reports,1, 2, 19 for contralateral TMJs in each subject during both jaw closing from laterotrusion and during symmetric jaw closing, via:
| Equation 1 |
| Equation 2 |
Where: W, mechanical work (mJ); Q, TMJ disc cartilage volume under the stress-field (mm3); Ftraction, sum of plowing and frictional forces; f, tractional coefficient; Fnormal, TMJ load; ΔD, distance of stress-field translation (mm); x, aspect ratio=radius of the stress-field/instantaneous TMJ disc thickness; V, velocity of stress-field translation (mm/s); and a, b, c, x0 and V0, constants measured in ex vivo experiments.20
Specifically, dynamic stereometry was used to determine x, ΔD, V, and Q while computer-assisted numerical modeling was used to determine Fnormal for TMJs in each subject. Once data from Imaging Visit #2 and Clinical Visit #2 were collected from each subject, dynamic stereometry was conducted at the University of Zurich and involved the three-dimensional reconstruction of TMJ structures, captured from MR images, and the animation of these structures using the jaw-tracking data via the common head reference system.1, 19 The numerical modeling approach,21–23 applied at the UMKC, required each subject’s three-dimensional craniomandibular anatomy, which was characterized using the CT images made at Clinical Visit #1 (Table 1), and bilateral sagittal effective eminence shapes, which were characterized via dynamic stereometry. These anatomical data were employed in a numerical model with the objective function of minimization of muscle effort, based on previous model-validation experiments,23 to predict the average contralateral Fnormal consequent to 20 N bite-forces applied to the ipsilateral mandibular canine at a range of 324 different angles (between 0–40° in steps of 5,° where vertical is 0,° and 0–350° in steps of 10° in a plane parallel to occlusal plane). These bite-force conditions were chosen to represent a magnitude and directions used during ordinary jaw functions.
For both TMJs in each subject, the variables x, ΔD, V, and Q were calculated for 5 ms intervals during jaw closing for each trial by investigators at UMKC who were blinded to the sex of the subjects. The results for a given TMJ were then averaged for each trial and then the mean and standard deviation of 10 trials per movement were calculated. These data plus the average contralateral Fnormal for the given TMJ and the constants from ex vivo experiments were used in Equations 1 and 2 to determine ED for contralateral TMJs in each subject during jaw closing symmetrically and from laterotrusion.
Differences in ED between jaw closing from laterotrusion compared to symmetric jaw closing were evaluated using a paired t-test, while differences in ED between sexes during jaw closing from laterotrusion were evaluated using a two-group t-test. Multivariate Analysis of Variance (MANOVA) was used to examine differences in x, ΔD, V, and Q (component variables that contribute to ED) between jaw closing movements and between the sexes during jaw closing from laterotrusion, because measurements from the same subject could be correlated. If overall significance was found in the MANOVA, simple effect test was applied for further investigation of differences. Bonferroni correction for multiple comparisons was used to consider significance. All statistical analyses were performed with commercial software (SPSS version 24, IBM SPSS, Chicago, IL) at East Tennessee State University between December 2016–January 2017. Significance was defined by P≤0.05.
Results
One hundred and sixty-one subjects were recruited, however, the following numbers were excluded: 49 had TMJ disc displacement, 26 were unable to comply with the study schedule, 17 had dental problems, 15 had TMJ degenerative disease,14 had jaw-related pain, three had claustrophobia, one was pregnant, one declined to remove piercings for imaging, and one was unable to follow instructions. Seventeen females, average age 29.8 (±7.2) years, and seventeen males, average age 29.7 (±11.1) years, completed the study. On average, data collection from the Imaging and Clinical Visits for each subject occurred within a 3-month period. Complete data for TMJs on both sides (Fig. 2) were provided by all females and 92% of males. That is, two males provided complete data from one TMJ but data from the other side could not be used because of motion during the MR imaging or other artifact during the dynamic stereometry protocols.
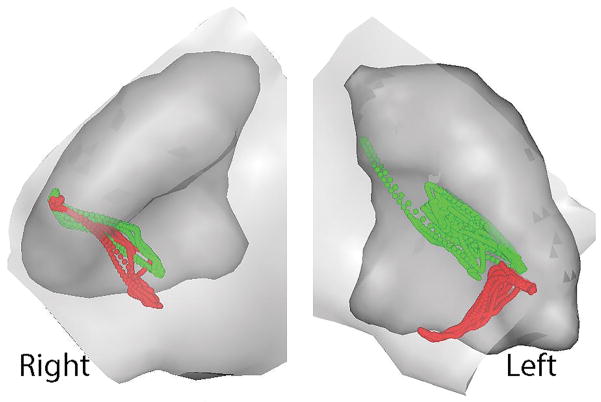
Fig. 2.
Results from dynamic stereometry are illustrated for one subject. Specifically, three-dimensional reconstructions of right and left temporomandibular joints from magnetic resonance images are shown in static superior-anterior views where the disc has been removed for better visualization of the ghosted images of the eminences in light grey over the condyles in shaded darker grey. Also shown superimposed over each condyle are the time-dependent positions of the centroid of the stress-field calculated at 5 ms intervals during jaw closing from laterotrusion (green dots) and during symmetric closing (red dots) as determined from combining the TMJ anatomy and jaw tracking data in three-dimensions. The location of the stress-field centroid for any given time-point was determined by finding the minimum condyle-fossa/eminence distance. The component variables of interest (x, aspect ratio; ΔD, distance of stress-field translation (mm); V, velocity of stress-field translation (mm/s); and Q, TMJ disc cartilage volume under the stress-field (mm3) were calculated for each joint and jaw closing movement at 5 ms intervals.
Overall, mean ED in contralateral TMJs were 26.0 (±33.7) mJ/mm3 during jaw closing from laterotrusion and 7.2 (±9.1) mJ/mm3 during symmetric jaw closing and significantly different (P<0.0001). Specifically, during jaw closing from laterotrusion, mean ED in contralateral TMJs were significantly larger (P=0.036) for females (34.7±44.8 mJ/mm3) than males (17.4±12.0 mJ/mm3).
For both jaw closing from laterotrusion and symmetric jaw closing, mean values for the component variables that contribute to TMJ ED were smaller for females than males but not significantly so for x, ΔD, and V (Tables 2 and 3). However, mean values of Q, were significantly smaller (P≤0.010) for females than males by an average of 1.5-fold, where for jaw closing from laterotrusion these were 119 (±66) and 186 (±107) mm3, respectively, and for symmetric jaw closing these were 121 (±69) and 173 (±89) mm3, respectively (Tables 2 and 3).
Table 2.
Comparison of component variables that contribute to TMJ energy densities between females and males during jaw closure from laterotrusion; where * indicates significant difference.
| Variable | Sex | Mean (Standard Deviation) | P-value |
|---|---|---|---|
| x, aspect ratio | Female | 2.1 (0.8) | 0.222 |
| Male | 2.4 (1.2) | ||
| ΔD, distance of stress-field translation (mm) | Female | 4.4 (3.0) | 0.123 |
| Male | 5.5 (2.8) | ||
| V, velocity of stress-field translation (mm/s) | Female | 4.2 (2.5) | 0.100 |
| Male | 5.2 (2.8) | ||
| Q, cartilage volume under the stress-field (mm3) | Female | 121 (69) | 0.010* |
| Male | 173 (89) |
Table 3.
Comparison of component variables that contribute to TMJ energy densities between females and males during symmetrical jaw closure; where * indicates significant difference.
| Variable | Sex | Mean (Standard Deviation) | P-value |
|---|---|---|---|
| x, aspect ratio | Female | 2.1 (0.9) | 0.107 |
| Male | 2.5 (1.1) | ||
| ΔD, distance of stress-field translation (mm) | Female | 3.0 (1.8) | 0.926 |
| Male | 3.1 (1.9) | ||
| V, velocity of stress-field translation (mm/s) | Female | 3.9 (1.7) | 0.244 |
| Male | 4.5 (2.3) | ||
| Q, cartilage volume under the stress-field (mm3) | Female | 119 (66) | 0.003* |
| Male | 186 (107) |
Within both females and males, mean ED in contralateral TMJs were significantly larger (P≤0.002) during jaw closing from laterotrusion than symmetric jaw closing (Table 4), in females by 3.9-fold and in males by 3.1-fold. Comparison of the component variables that contribute to ED between the two types of jaw closing within females and within males showed that x, V, and Q were relatively similar (Table 4). However, within both females and males, mean ΔD was significantly larger (P≤0.001) during jaw closing from laterotrusion compared to symmetric jaw closing by 1.5-fold and 1.8-fold, respectively (Table 4).
Table 4.
Comparison of variables between jaw closure from laterotrusion (asymmetrical) and symmetrical jaw closure in females and males; where * indicates significant difference.
| Variable | Sex | Jaw Closing | Mean (Standard Deviation) | P-value |
|---|---|---|---|---|
| Energy density (mJ/mm3) | Female | From laterotrusion | 34.7 (44.9) | 0.002* |
| Symmetrical | 8.8 (12.3) | |||
| Male | From laterotrusion | 17.4 (12.0) | <0.0001* | |
| Symmetrical | 5.6 (3.3) | |||
| x, aspect ratio | Female | From laterotrusion | 2.1 (0.8) | 0.800 |
| Symmetrical | 2.1 (0.9) | |||
| Male | From laterotrusion | 2.4 (1.2) | 0.240 | |
| Symmetrical | 2.5 (1.1) | |||
| ΔD, distance of stress-field translation (mm) | Female | From laterotrusion | 4.4 (3.0) | 0.001* |
| Symmetrical | 3.0 (1.8) | |||
| Male | From laterotrusion | 5.5 (2.8) | <0.0001* | |
| Symmetrical | 3.1 (1.9) | |||
| V, velocity of stress-field translation (mm/s) | Female | From laterotrusion | 4.2 (2.5) | 0.347 |
| Symmetrical | 3.9 (1.7) | |||
| Male | From laterotrusion | 5.2 (2.8) | 0.101 | |
| Symmetrical | 4.5 (2.3) | |||
| Q, cartilage volume under the stress-field (mm3) | Female | From laterotrusion | 121 (69) | 0.813 |
| Symmetrical | 119 (68) | |||
| Male | From laterotrusion | 173 (89) | 0.184 | |
| Symmetrical | 186 (107) |
Discussion
The current study demonstrated that ED, the concentration of mechanical work input to the contralateral TMJ disc during the adduction phase of jaw laterotrusion was on average 3.6-fold larger than during symmetric jaw closing. As such, lateral movements of loaded TMJ condyles may be more influential than symmetric jaw movements in the development of mechanical fatigue of the articulating surfaces. The mechanical work imposed on the TMJ articular surfaces is a consequence of plowing tractional forces caused by stress-field translation13 which pressurizes the interstitial fluid phase of the biphasic (fluid, solid) TMJ disc. Stress-field translation moves fluids through the disc which, in turn, provides nutrition and waste disposal.24 Plus, the interstitial fluid pressurization plays an important role in load-carriage in articular cartilages.14, 25, 26 The current study demonstrated that in both sexes, the distance of stress-field translation (ΔD) was significantly larger during jaw closing from laterotrusion than symmetric jaw closing and, thus, accounts for more mechanical work done per jaw closing cycle. Furthermore, the concentration of mechanical work per volume of cartilage was significantly larger in the healthy TMJs of females than males for both jaw closure from laterotrusion and symmetric jaw closing.2 The component variable that accounts for these differences is the significantly smaller TMJ disc cartilage volume under the stress-field (Q) in females compared to males. A consequence of smaller Q is less potential for fluid support within the disc. Repeated jaw-loading functions increase the likelihood of mechanical fatigue of the collagen-proteoglycan matrix of the disc,11 leading to increased tissue porosity, ease of fluid movement out of the tissues, transition of load-carriage from the fluid phase to the solid phase of the TMJ disc14 and escalation of the rate of tissue fatigue failure. Thus, the current results suggest that jaw functions involving closure from laterotrusion compared to a symmetric path and in females compared to males have higher mechanical fatigue liabilities. This is important foundational information for the development of future therapies aimed at reducing “wear and tear” of the jaw joint.
The significantly smaller TMJ disc cartilage volumes under the stress-field (Q) found in healthy females compared to males could be a matter of sex differences in scale, where for the same anatomical part or feature, females have on average smaller dimensions than males. However, sex differences in the kinetics of jaw closing behaviors, as measured via the distances of stress-field translation (ΔD) were not significantly different between females and males in either jaw closing movement. These findings indicate that despite these healthy females having smaller amounts of TMJ disc tissue to support the moving stress-field than the healthy males, the distance that the stress-field traveled during either symmetric or asymmetric jaw closure was similar in both sexes. In a future study with larger sample sizes, the within-sex differences in Q, ΔD, and ED should be tested to investigate if the same relationships exist independent of sex. Furthermore, if there are sex differences in susceptibilities to material failure for the same volume of TMJ disc tissue should be investigated.
The molecular biological perspectives of TMJ loading should also be considered. Mechanical loads, in a dose-dependent manner with respect to magnitude, frequency and duration, inhibit or promote molecular events responsible for cartilage health or destruction, including the synthesis of inflammatory mediators.27–29 Excessive loading and the consequent inflammatory mediators are associated with increased Wnt signaling in the superficial zone of TMJ fibrocartilage and loss of fibrocartilage stem cells in this zone.30 Given these findings, ED may be associated with two parallel mechanisms, classical mechanical fatigue and localized inflammation, which contribute to the pathomechanics of TMJ degeneration. If target therapies toward both mechanisms are needed in order to prevent progressive tissue destruction remains to be determined. It may be possible to control inflammation and Wnt signaling dysregulation, in turn improving the repair capacity of fibrocartilage, thereby enabling resolution of localized tissue damage and facilitating tissue remodeling to reduce ED imposed on the articulating surfaces. Moreover, by learning more about the frequency of jaw use and specific jaw-loading functions in individuals, it may be possible to control behaviors and reduce TMJ ED and, thus, prevent mechanical fatigue of the articulating tissues.
The current project has several limitations including data missing from two TMJs in the male sample. With respect to mechanical fatigue, magnitudes and frequencies of applied ED are likely to determine tissue failure rate. Combined ED and frequency of jaw loading, termed mechanobehavior score, was recently shown to be significantly different in females with and without TMJ disc displacement.19 Frequency of asymmetric and symmetric jaw-loading behaviors was not included in the current project, hence, it is unknown if there are sex differences in these frequencies and in mechanobehavior scores. An additional limitation of this study is the assumption of equal distribution of load over the stress-field, whereas recent results from finite element modeling (FEM) suggest that there are stress-field regional differences of the load-carriage between solid and fluid phases of the disc.14 Future FEM, which uses 3D rendering, such as that produced via dynamic sterometry, may elucidate more detailed distributions of ED within the cartilage, the molecular biological responses, and the local environmental effects on cell metabolism.
Conclusions
Energy densities in contralateral TMJs were significantly larger during jaw closing from laterotrusion compared to symmetric jaw closing, due to significantly larger distances of stress-field translation between the two movements in the TMJs of healthy females and males. During jaw closing from laterotrusion energy densities in contralateral TMJs were significantly larger in healthy females compared to healthy males, due to significantly smaller TMJ disc cartilage volumes under the stress-field in females compared to males.
Acknowledgments
The experiments described were undertaken with the understanding and written consent of each subject according to ethical principles that were independently reviewed and approved by the Institutional Review Boards of the University at Buffalo (#388770-1) and University of Missouri-Kansas City (#13-656). This research was supported by the National Institute of Dental and Craniofacial Research (R01 2DE016417, JN-PI). Michala Marková, Vera Colombo, Stefan Erni, Alessandro Gallo, and Eveline Studer contributed to computer programing and data analysis and management at the University of Zurich.
Footnotes
Conflicts of Interest
Dr. Gallo reports grants from NIH/NIDCR, during the conduct of the study.
Dr. Gonzalez-Stucker reports grants from UMKC/NIH, during the conduct of the study.
Dr. Nickel reports grants from The National Institutes of Health, during the conduct of the study.
Dr. Iwasaki reports grants from National Institutes of Health, during the conduct of the study. Personal fees from Roth Study Club International, outside the submitted work.
The other authors have stated explicitly that there are no conflicts of interest in connection with this article.
Reference list
- 1.Gallo LM, Iwasaki LR, Gonzalez YM, Liu H, Marx DB, Nickel JC. Diagnostic group differences in temporomandibular joint energy densities. Orthod Craniofac Res. 2015;18(Suppl 1):164–169. doi: 10.1111/ocr.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki LR, Gonzalez YM, Liu Y, Liu H, Markova M, Gallo LM, et al. TMJ energy densities in healthy men and women. Osteoarthritis Cartilage. 2017;25:846–849. doi: 10.1016/j.joca.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi C, Kuo J, Bell PD, Yao H. Anisotropic solute diffusion tensor in porcine TMJ discs measured by FRAP with spatial Fourier analysis. Ann Biomed Eng. 2010;38:3398–3408. doi: 10.1007/s10439-010-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Embree MC, Chen M, Pylawka S, Kong D, Iwaoka GM, Kalajzic I, et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat Commun. 2016;7:13073. doi: 10.1038/ncomms13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaul R, O’Brien MH, Dutra E, Lima A, Utreja A, Yadav S. The Effect of Altered Loading on Mandibular Condylar Cartilage. PloS one. 2016;11:e0160121. doi: 10.1371/journal.pone.0160121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Sun Y, He Y, Zhang H, Zheng Y, Yao Y, et al. IL-1beta impedes the chondrogenic differentiation of synovial fluid mesenchymal stem cells in the human temporomandibular joint. Int J Mol Med. 2017;39:317–326. doi: 10.3892/ijmm.2016.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utreja A, Dyment NA, Yadav S, Villa MM, Li Y, Jiang X, et al. Cell and matrix response of temporomandibular cartilage to mechanical loading. Osteoarthritis Cartilage. 2016;24:335–344. doi: 10.1016/j.joca.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.dos Anjos Pontual ML, Freire JS, Barbosa JM, Frazao MA, dos Anjos Pontual A. Evaluation of bone changes in the temporomandibular joint using cone beam CT. Dentomaxillofac Radiol. 2012;41:24–29. doi: 10.1259/dmfr/17815139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K, Wojczynska A, Lee JY. The incidence of osteoarthritic change on computed tomography of Korean temporomandibular disorder patients diagnosed by RDC/TMD; a retrospective study. Acta Odontol Scand. 2016;74:337–342. doi: 10.3109/00016357.2015.1136678. [DOI] [PubMed] [Google Scholar]
- 10.Beatty MW, Hohl RH, Nickel JC, Iwasaki LR, Pidaparti RM. Mode I and Mode III fractures in intermediate zone of full-thickness porcine temporomandibular joint discs. Ann Biomed Eng. 2008;36:801–812. doi: 10.1007/s10439-008-9436-9. [DOI] [PubMed] [Google Scholar]
- 11.Beatty MW, Nickel JC, Iwasaki LR, Leiker M. Mechanical response of the porcine temporomandibular joint disc to an impact event and repeated tensile loading. J Orofac Pain. 2003;17:160–166. [PubMed] [Google Scholar]
- 12.Gallo LM, Chiaravalloti G, Iwasaki LR, Nickel JC, Palla S. Mechanical work during stress-field translation in the human TMJ. J Dent Res. 2006;85:1006–1010. doi: 10.1177/154405910608501106. [DOI] [PubMed] [Google Scholar]
- 13.Guo H, Nickel JC, Iwasaki LR, Spilker RL. An augmented Lagrangian method for sliding contact of soft tissue. J Biomech Eng. 2012;134:084503. doi: 10.1115/1.4007177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Cisewski SE, Wei F, She X, Gonzales TS, Iwasaki LR, et al. Role of interstitial fluid pressurization in tractional force formation on temporomandibular joint disc: A biphasic finite element analysis. Orthod Craniofac Res. doi: 10.1111/ocr.12147. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbs CH, Wickwire NA, Jacobson AP, Lundeen HC, Mahan PE, Lupkiewicz SM. Comparison of typical chewing patterns in normal children and adults. J Am Dent Assoc. 1982;105:33–42. doi: 10.14219/jada.archive.1982.0073. [DOI] [PubMed] [Google Scholar]
- 16.Gallo LM, Nickel JC, Iwasaki LR, Palla S. Stress-field translation in the healthy human temporomandibular joint. J Dent Res. 2000;79:1740–1746. doi: 10.1177/00220345000790100201. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:844–860. doi: 10.1016/j.tripleo.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Orofac Pain. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki LR, Gonzalez YM, Liu Y, Liu H, Markova M, Gallo LM, et al. Mechanobehavioral Scores in Women with and without TMJ Disc Displacement. J Dent Res. 2017 doi: 10.1177/0022034517704375. 22034517704375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nickel J, Spilker R, Iwasaki L, Gonzalez Y, McCall WD, Ohrbach R, et al. Static and dynamic mechanics of the temporomandibular joint: plowing forces, joint load and tissue stress. Orthod Craniofac Res. 2009;12:159–167. doi: 10.1111/j.1601-6343.2009.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasaki LR, Crosby MJ, Gonzalez Y, McCall WD, Marx DB, Ohrbach R, et al. Temporomandibular joint loads in subjects with and without disc displacement. Orthop Rev (Pavia) 2009;1:90–93. doi: 10.4081/or.2009.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasaki LR, Liu H, Gonzalez YM, Marx DB, Nickel JC. Modeling of muscle forces in humans with and without temporomandibular joint disorders. Orthod Craniofac Res. 2015;18(Suppl 1):170–179. doi: 10.1111/ocr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nickel JC, Gonzalez YM, McCall WD, Ohrbach R, Marx DB, Liu H, et al. Muscle organization in individuals with and without pain and joint dysfunction. J Dent Res. 2012;91:568–573. doi: 10.1177/0022034512445909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright GJ, Kuo J, Shi C, Bacro TR, Slate EH, Yao H. Effect of mechanical strain on solute diffusion in human TMJ discs: an electrical conductivity study. Ann Biomed Eng. 2013;41:2349–2357. doi: 10.1007/s10439-013-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ateshian GA. The role of interstitial fluid pressurization in articular cartilage lubrication. J Biomech. 2009;42:1163–1176. doi: 10.1016/j.jbiomech.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman BK, Bonnevie ED, Park M, Zhou Y, Wang L, Burris DL, et al. Role of interstitial fluid pressurization in TMJ lubrication. J Dent Res. 2015;94:85–92. doi: 10.1177/0022034514553626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correro-Shahgaldian MR, Ghayor C, Spencer ND, Weber FE, Gallo LM. A model system of the dynamic loading occurring in synovial joints: the biological effect of plowing on pristine cartilage. Cells Tissues Organs. 2014;199:364–372. doi: 10.1159/000375294. [DOI] [PubMed] [Google Scholar]
- 28.Knobloch TJ, Madhavan S, Nam J, Agarwal S, Jr, Agarwal S. Regulation of chondrocytic gene expression by biomechanical signals. Crit Rev Eukaryot Gene Expr. 2008;18:139–150. doi: 10.1615/critreveukargeneexpr.v18.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schatti OR, Markova M, Torzilli PA, Gallo LM. Mechanical Loading of Cartilage Explants with Compression and Sliding Motion Modulates Gene Expression of Lubricin and Catabolic Enzymes. Cartilage. 2015;6:185–193. doi: 10.1177/1947603515581680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Embree MC, Iwaoka GM, Kong D, Martin BN, Patel RK, Lee AH, et al. Soft tissue ossification and condylar cartilage degeneration following TMJ disc perforation in a rabbit pilot study. Osteoarthritis Cartilage. 2015;23:629–639. doi: 10.1016/j.joca.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]