Abstract
Background
The purpose of this study was to investigate the associations between clinical factors and cardiac function as measured by pressure–volume loops (PVLs) in a pediatric heart transplant cohort.
Methods
Patients (age < 20 years) who underwent heart transplantation presenting for a clinically indicated catheterization were enrolled. PVLs were recorded using microconductance catheters (CD Leycom®, Zoetermeer, Netherlands). Demographic data, serum B-type natriuretic peptide (BNP), time from transplant, ischemic time, presence of transplant coronary artery disease, donor-specific antibodies, and history of rejection were recorded at the time of catheterization. PVL data included contractility indices: end-systolic elastance and preload recruitable stroke work; ventricular–arterial coupling index; ventricular stiffness constant, Beta; and isovolumic relaxation time constant, tau. Associations between PVL measures and clinical data were investigated using non-parametric statistical tests.
Results
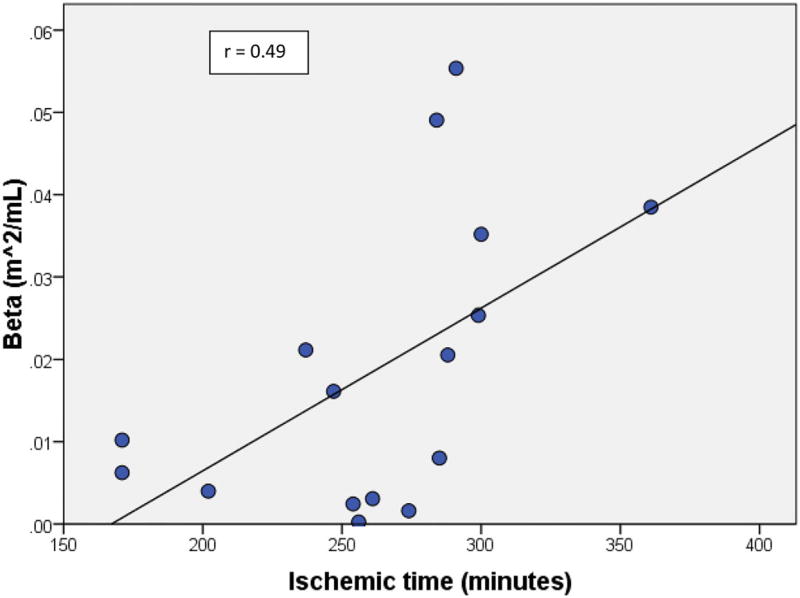
A total of 18 patients were enrolled. Median age was 8.7 years (IQR 5–14 years). There were ten males and eight females. Six patients had a history of rejection and ten had positive donor-specific antibodies. There was no transplant coronary artery disease. Median BNP was 100 pg/mL (IQR 46–140). Time from transplant to PVL obtained during catheterization procedure was 4.1 years (IQR 1.7–7.8 year). No single clinical characteristic was statistically significant when correlated with PVL data. However, longer ischemic time was associated with worse Beta (r = 0.49, p = 0.05).
Conclusions
Our study found that longer ischemic times are associated with increased left ventricular stiffness. No other single clinical variable is associated with cardiac dysfunction as determined by PVL analysis.
Keywords: Heart transplant, Ischemic time, Graft dysfunction, Pediatrics
Introduction
While systolic function may be more easily evaluated by echocardiographic parameters such as ejection fraction, shortening fraction, and ventricular strain, diastolic dysfunction is more difficult to determine non-invasively [1]. Invasive monitoring, such as hemodynamic catheterization, can provide insight into diastolic dysfunction. In addition, the presence of donor-specific antibodies or an elevated B-natriuretic peptide (BNP) may be an early marker of, or indicate a potential for, graft dysfunction [2]. While systolic function of donor hearts may be preserved after transplant, diastolic function often is abnormal months to years later [3, 4]. As donor age increases, diastolic function of the graft is more likely to be impaired [5].
Previous studies have explored the relationship between ischemic time [6–12], gender [13], laboratory markers [14], and outcomes in pediatric heart transplant patients. In this study, we investigated the associations between clinical characteristics and gold standard measures of cardiac function utilizing pressure–volume loops (PVLs) in pediatric heart transplant recipients. We hypothesized that increased or prolonged ischemic time, BNP levels, and length of time since transplant would be associated with elevated markers of systolic and diastolic dysfunction as measured by PVL.
Methods
Our study was approved by the Institutional Review Board at Medical University of South Carolina. Children (age < 21 years) who underwent heart transplantation presenting for a clinically indicated catheterization at the Medical University of South Carolina were recruited prospectively between January and October 2014. Exclusion criteria included the following: (1) medical status for which participation in the study presented more than minimal risk as determined by the attending physician, (2) non-sinus rhythm, and (3) significantly abnormal loading conditions—a significant left to right shunt would adversely affect conductance catheter volume calibration and left ventricular outflow tract obstruction would significantly affect the non-invasive estimation of left ventricular pressure. Parents gave informed consent for all patients < 18 years and those between 13 and 17 years of age gave their assent.
Study Catheterization and PVL Analysis Protocol
All patients underwent general anesthesia as per institutional protocol. All study data were collected following the patient’s diagnostic procedures. A 4-Fr high-fidelity microconductance catheter (CD Leycom®, Netherlands) was placed in the apex of the left ventricle via the femoral approach. The conductance catheter’s micromanometer was calibrated in normal saline for 15 s prior to placement. PVLs were volume calibrated using hypertonic saline to account for parallel conductance. Conductance catheter volumes have been shown to correlate well with cardiac MRI volumes, though they do slightly underestimate absolute volumes [15]. Cardiac output was determined by thermodilution. Conductance electrodes outside of the ventricle were excluded from analysis. All PVL data were recorded in triplicate over 10 s during an expiratory breathhold. Microconductance data were recorded at a sampling rate of 250 Hz. Invasive data were obtained using standard equipment approved for use in human subjects (INCA® intracardiac analyzer; CD Leycom, Netherlands). PVL analysis was performed offline using specialized software (LabChart v8; ADInstruments, Colorado Springs, CO).
PVL Measurements
Contractility indices measured include left ventricular end-systolic elastance (Ees) and ventricular–arterial coupling index (Ea/Ees). Diastolic measures include ventricular stiffness constant (Beta), isovolumic relaxation time (Tau), preload recruitable stroke work (PRSW), and end-diastolic pressure (EDP). Afterload was measured by arterial elastance (Ea). The end-systolic pressure–volume points produced during volume reduction create a linear end-systolic pressure–volume relationship (ESPVR). The slope of this line is end-systolic elastance (Ees) and it was calculated using the iterative regression method [16]. Arterial elastance (Ea) is obtained by dividing the pressure at end systole (Pes) by the stroke volume (SV). Since Ees is a marker of ventricular elastance and Ea is a marker of arterial elastance, the ratio of Ea/Ees is a surrogate for the interaction between the two or ventricular–arterial coupling index. The end-diastolic pressure–volume relationship was obtained via balloon occlusion of the vena cavae and fitted to the equation αeβEDV, where Beta is the chamber stiffness constant, α is the curve-fitting constant, and EDV is the end-diastolic volume [17]. Beta was indexed to body surface area. Increased ventricular stiffness is considered present if Beta > 0.015 mL−1 [18]. The isovolumic relaxation time constant, Tau, a measure of active diastolic relaxation, was obtained via the method of Weiss [19]. Preload recruitable stroke work, a marker of contractility, is measured by linear regression analysis using ventricular stroke work divided by end-diastolic volume [20].
Statistical Analysis
Demographic data, serum B-type natriuretic peptide (BNP) (Abbott assay, Abbott Park, IL), time from transplant, ischemic time, presence of transplant coronary artery disease, donor-specific antibodies, history of rejection, and time from rejection were recorded at the time of catheterization. Normal BNP range in our institution is < 100 pg/mL. Associations between clinical data and PVL measures including Ees, Ea, Ea/Ees, Tau, PRSW, and Beta were investigated using non-parametric statistical tests (Spearman’s rank correlation) using SPSS 16.0 package (SPSS, Chicago, IL, USA). A two-tailed p value ≤ 0.05 was considered statistically significant.
Results
Clinical and Demographic Data
Between January and October 2014, a total of 18 patients who underwent orthotopic heart transplant were enrolled in our study. Median age of the patients was 8.7 years (Interquartile range (IQR) 4.9–13.4). There were ten males and eight females. Six patients had a history of rejection and ten had positive donor-specific antibodies with a median time from rejection to catheterization of 654 days (IQR 331–906 days), although none of the patients were actively being treated for rejection at the time of catheterization. Of those six patients who were treated for rejection, three had antibody-mediated rejection, two had cellular-mediated rejection, and one had mixed. No patient had angiographic evidence of transplant coronary artery disease. Median BNP was 100 pg/mL (IQR 46–140) with 8/18 patients having a BNP of > 100. Time from transplant to catheterization procedure was 4.1 years (IQR 1.7–7.8 years). Median ischemic time was 267 min (IQR 244–288 min) for 16 patients as this value was not available for two patients. Four of the 16 patients had an ischemic time < 240 min. Descriptive statistics are summarized in Table 1.
Table 1.
Descriptive statistics of our patient population with interquartile ranges in parentheses
| Male patients | 10/18 |
| Transplant coronary artery disease | 0/18 |
| History of rejection | 6/18 |
| Median time from rejection to PVL measurement | 654 days (331–906) |
| Median age | 8.7 years (4.9–13.4) |
| Median graft time | 4.1 years (1.7–7.8) |
| Median ischemic time | 267 min (244–288) |
| Median BNP | 100 pg/mL (46–140) |
PVL Data
Median arterial elastance and end-systolic elastance were 2.43 mmHg/mL (IQR 1.71–2.95) and 2.73 mmHg/mL (IQR 2.07–5.08), respectively, with a median ventriculoarterial coupling index of 0.76 (IQR 0.56–1.00). Median indexed Beta was measured at 0.009 m2/mL (0.005–0.033). Median Tau was 28 ms (IQR 24–31). PVL measurements are shown in Table 2 with medians and interquartile ranges reported.
Table 2.
Median PVL measurements with interquartile ranges in parentheses
| Arterial elastance | 2.43 mmHg/mL (1.71–2.95) |
| End-systolic elastance | 2.73 mmHg/mL (2.07–5.08) |
| VA coupling index (Ea/Ees) | 0.76 (0.56–1.00) |
| Beta | 0.009 m2/mL (0.005–0.033) |
| Tau | 28 ms (24–31) |
| PRSW | 52.89 (33.84–73.51) |
Demographic Versus PVL Correlation
No single clinical characteristic (gender, presence of rejection, age, graft time, and BNP) was statistically significant when correlated with PVL data (Ea, Ees, Ea/Ees, Tau, and PRSW) (Table 3). However, longer ischemic time was associated with worse Beta (r = 0.49, p = 0.05) (Fig. 1).
Table 3.
Clinical data correlated with PVL data
| Beta | PRSW | Tau | Ees | Ea/Ees | |
|---|---|---|---|---|---|
| BNP | r = −.31 | r = −.34 | r = .07 | r = .16 | r = −.13 |
| p = .23 | p = .18 | p = .80 | p = .54 | p = .61 | |
| Ischemic time | r = .49 | r = .18 | r = −.28 | r = .49 | r = −.07 |
| p = .05 | p = .51 | p = .30 | p = .06 | p = .81 | |
| Graft time | r = −.25 | r = .01 | r = .35 | r = −.12 | r = −.08 |
| p = .09 | p = .96 | p = .16 | p = .64 | p = .77 | |
| Age | r = −.41 | r = .28 | r = .65 | r = −.52 | r = −.24 |
| p = .09 | p = .27 | p = .01 | p = .03 | p = .34 |
Fig. 1.
Ischemic time is positively correlated with Beta
Discussion
This is one of the first studies to investigate clinical characteristics of pediatric heart transplant patients and gold standard invasively measured pressure–volume loops. We found no correlation between clinical data (age, gender, BNP, graft time, history of rejection) and PVL data (Ea, Ees, Ea/Ees, PRSW, Tau); however, we did find a statistically significant positive correlation between ischemic time and ventricular stiffness (Beta).
Previous studies comparing ischemic times to graft function have had differing conclusions, but this has been mostly in the adult population. Del Rizzo et al. showed that ischemic times longer than 4 h correlated with decreased recipient survival in patients older than 50 years [6]. Russo et al. demonstrated that prolonged ischemic times were more readily tolerated in younger recipients (age < 19 years) and associated with decreased survival in those > 20 years of age [11]. In a retrospective study of 91 pediatric heart transplant patients, Kawauchi et al. concluded that ischemic times greater than 4 h, and in some instances almost 8.5 h, did not result in an increase in frequency of primary graft failure, increase in inotropic support, more days on a ventilator, or evidence of ventricular dysfunction by echocardiography 2 weeks post transplant [7]. Rustad et al. showed that prolonged ischemic times in the adult population were associated with systolic and diastolic graft dysfunction and increasing age was associated with increased diastolic graft dysfunction [5]. While it is fairly common to have diastolic dysfunction immediately after transplant, the presence of diastolic dysfunction, specifically in the right ventricle, greater than 1 year from transplant is associated with increased mortality [21]. In our small cohort of transplant patients, we found that longer ischemic times correlated with an increasing ventricular stiffness (Beta) as measured by invasive PVLs obtained in the catheterization lab. This would suggest that, while systolic function returns to normal after transplant, diastolic function continues to be abnormal even years after transplant. Using the results of this study, more aggressive medical management for ventricular function may be warranted in those patients with longer ischemic times including the use of lusitropic agents (e.g., Milrinone).
Surprisingly, the six patients treated for rejection in our cohort did not have abnormal PVLs or signs of graft dysfunction compared to those without a history of rejection. Of those six, three had moderate to severe rejection—two with Grade 3R cellular-mediated rejection and one with grade 2 antibody-mediated rejection. The median graft time was 2700 days and the median time from the last treatment for rejection was 654 days. This would suggest that treatment for rejection can result in graft recovery as measured by PVL analysis.
With a limited number of donors, wait times on the transplant list are associated with a high mortality, especially among those weighing less than 10–15 kg [22]. Restricting acceptance of donor hearts secondary to borderline or prolonged ischemic times may only serve to increase wait list morbidity and mortality. Further studies are warranted to research if the associations between ventricular stiffness and worse patient outcomes including heart failure symptoms, graft failure, and retransplantation do exist. These data support the finding that the transplant cardiologist is more likely to encounter diastolic dysfunction in patients with prolonged ischemic time, not only in the short term, but in the long term as well.
Limitations
There were limitations in our study. None of the patients had active rejection; however, six of the 18 in the cohort underwent treatment for rejection in the past—for both antibody-mediated as well as cell-mediated rejection. Due to the small sample size of patients in our cohort, and specifically those with a history of rejection, our study did not have the power to detect smaller differences within the population. We had only one data point for PVL analysis and would benefit from studying a larger cohort and collecting serial data points to determine if Beta decreases as the time from transplant increases. In addition, the median ischemic time (267 min) in our study was significantly longer than those of many of the other published studies with only four patients receiving a donor heart with an ischemic time less than 240 min. The longer ischemic times in our study can be partially attributed to the location of our center on the east coast and the distance to large-density populations.
Conclusions
While ischemic time is not necessarily associated with worse outcomes, our study indicates that prolonged ischemic time is associated with increased ventricular stiffness. Future studies need to examine this relationship in a larger population, specifically looking at those patients with active rejection as well as a less skewed distribution of ischemic times. With a larger population, we would be better equipped to address the question—Does abnormal diastolic dysfunction, namely increased ventricular stiffness, have a significant impact on long-term morbidity and mortality in the pediatric heart transplant population?
Abbreviations
- PVL
Pressure–volume loop
- Ees
End-systolic elastance
- Ea
Arterial elastance
- BNP
B-type natriuretic peptide
- EDP
End-diastolic pressure
- PRSW
Preload recruitable stroke work
Footnotes
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest The authors declare that they have no conflict of interest.
Informed Consent Informed consent was obtained from all individual participants included in this study.
References
- 1.Savage A, et al. Evaluation of the myocardial performance index and tissue doppler imaging by comparison to near-simultaneous catheter measurements in pediatric cardiac transplant patients. J Heart Lung Transplant. 2010;29(8):853–858. doi: 10.1016/j.healun.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Maisel AS, et al. Utility of B-natriuretic peptide as a rapid, point-of-care test for screening patients undergoing echocardiography to determine left ventricular dysfunction. Am Heart J. 2001;141(3):367–374. doi: 10.1067/mhj.2001.113215. [DOI] [PubMed] [Google Scholar]
- 3.Hausdorf G, et al. Diastolic function after cardiac and heart-lung transplantation. Br Heart J. 1989;62(2):123–132. doi: 10.1136/hrt.62.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lunze FI, et al. Cardiac allograft function during the first year after transplantation in rejection-free children and young adults. Circ Cardiovasc Imaging. 2012;5(6):756–764. doi: 10.1161/CIRCIMAGING.112.976613. [DOI] [PubMed] [Google Scholar]
- 5.Rustad LA, et al. Heart transplant systolic and diastolic function is impaired by prolonged pretransplant graft ischaemic time and high donor age: an echocardiographic study. Eur J Cardiothorac Surg. 2013;44(2):e97–e104. doi: 10.1093/ejcts/ezt233. [DOI] [PubMed] [Google Scholar]
- 6.Del Rizzo DF, et al. The role of donor age and ischemic time on survival following orthotopic heart transplantation. J Heart Lung Transplant. 1999;18(4):310–319. doi: 10.1016/s1053-2498(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 7.Kawauchi M, et al. Prolonged preservation of human pediatric hearts for transplantation: correlation of ischemic time and subsequent function. J Heart Lung Transplant. 1993;12(1 Pt 1):55–58. [PubMed] [Google Scholar]
- 8.Mitropoulos FA, et al. Outcome of hearts with cold ischemic time greater than 300 minutes. A case-matched study. Eur J Cardiothorac Surg. 2005;28(1):143–148. doi: 10.1016/j.ejcts.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 9.Mullen JC, et al. Extended donor ischemic times and recipient outcome after orthotopic cardiac transplantation. Can J Cardiol. 2001;17(4):421–426. [PubMed] [Google Scholar]
- 10.Pflugfelder PW, et al. Extending cardiac allograft ischemic time and donor age: effect on survival and long-term cardiac function. J Heart Lung Transplant. 1991;10(3):394–400. [PubMed] [Google Scholar]
- 11.Russo MJ, et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2007;133(2):554–559. doi: 10.1016/j.jtcvs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Scheule AM, et al. Duration of graft cold ischemia does not affect outcomes in pediatric heart transplant recipients. Circulation. 2002;106(12 Suppl 1):I163–I167. [PubMed] [Google Scholar]
- 13.Prendergast TW, et al. The role of gender in heart transplantation. Ann Thorac Surg. 1998;65(1):88–94. doi: 10.1016/s0003-4975(97)01105-3. [DOI] [PubMed] [Google Scholar]
- 14.Mehra MR, et al. Usefulness of an elevated B-type natriuretic peptide to predict allograft failure, cardiac allograft vasculopathy, and survival after heart transplantation. Am J Cardiol. 2004;94(4):454–458. doi: 10.1016/j.amjcard.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen JM, et al. Left ventricular volume measurement in mice by conductance catheter: evaluation and optimization of calibration. Am J Physiol Heart Circ Physiol. 2007;293(1):H534–H540. doi: 10.1152/ajpheart.01268.2006. [DOI] [PubMed] [Google Scholar]
- 16.Kass DA, et al. Use of a conductance (volume) catheter and transient inferior vena caval occlusion for rapid determination of pressure-volume relationships in man. Cathet Cardiovasc Diagn. 1988;15(3):192–202. doi: 10.1002/ccd.1810150314. [DOI] [PubMed] [Google Scholar]
- 17.Westermann D, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117(16):2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 18.Sinning D, et al. Increased left ventricular stiffness impairs exercise capacity in patients with heart failure symptoms despite normal left ventricular ejection fraction. Cardiol Res Pract. 2011;2011:692862. doi: 10.4061/2011/692862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest. 1976;58(3):751–760. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glower DD, et al. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71(5):994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- 21.Tallaj JA, et al. Post-heart transplant diastolic dysfunction is a risk factor for mortality. J Am Coll Cardiol. 2007;50(11):1064–1069. doi: 10.1016/j.jacc.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Almond CS, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation. 2009;119(5):717–727. doi: 10.1161/CIRCULATIONAHA.108.815712. [DOI] [PMC free article] [PubMed] [Google Scholar]