Abstract
Background
Chronic itch is a highly debilitating symptom that underlies many medical disorders with no universally effective treatments. Although unique neuronal signaling cascades in the sensory ganglia and spinal cord have been shown to critically promote the pathogenesis of chronic itch, the role of skin-associated cells remains poorly understood.
Objective
We sought to examine the cutaneous mechanisms underlying transient receptor potential vanilloid 4 (TRPV4) -mediated allergic and non-allergic chronic itch.
Method
The expression of TRPV4 in chronic itch and healthy control skin preparations was examined by real-time RT-PCR. Trpv4eGFF mice were used to study the expression and function of TRPV4 in the skin by immunofluorescence staining, calcium imaging, and patch-clamp recordings. Genetic and pharmacological approaches were employed to examine the role and underlying mechanisms of TRPV4 in mouse models of dry skin associated chronic itch and spontaneous scratching associated with SADBE-induced allergic contact dermatitis.
Results
TRPV4 is selectively expressed by dermal macrophages and epidermal keratinocytes in mice. Lineage-specific deletion of TRPV4 in macrophages and keratinocytes reduces allergic and non-allergic chronic itch in mice, respectively. Importantly, TRPV4 expression is significantly elevated in skin biopsies from patients with chronic idiopathic pruritus (CIP) in comparison to skin from healthy control subjects. Moreover, TRPV4-dependent chronic itch requires 5-HT signaling secondary to activation of distinct 5-HT receptors in both allergic and non-allergic chronic itch conditions.
Conclusion
Our study reveals previously unrecognized mechanisms by which TRPV4-expressing epithelial and immune cells in the skin critically and dynamically mediate chronic itch, and unravels novel targets for therapeutics in the setting of chronic itch.
Keywords: TRPV4, chronic itch, macrophage, keratinocyte
Introduction
Chronic itch, a symptom of many primary skin disorders and systemic diseases, is a major medical issue affecting 10–20% of the general population, and has deleterious effects on both quality of life and productivity. 1, 2 Despite decades of research, how chronic itch is generated at the molecular and cellular levels is poorly understood. Recent studies have identified multiple itch-related G-protein coupled receptors (GPCRs) and ion channels in the primary sensory neurons, which enable sensory neurons to detect a variety of pruritogens. 3, 4, 5 However, the upstream pathways, such as the identity of putative receptors that trigger epithelial and immune cells to elicit itch remain unknown. Lack of this critical information has severely limited the development of effective therapies for most types of chronic itch.
TRPV4 is a Ca2+-permeable cation channel in the transient receptor potential vanilloid subfamily (TRPV), and is abundantly expressed in the skin, renal, and urinary bladder epithelia6, 7 (www.biogps.org). TRPV4 is a polymodal sensory transducer that integrates a variety of thermal, mechanical, and chemical stimuli including warmth (27–35°C), hypo-osmotic stimulation, and many inflammatory metabolites. 8 As a result, TRPV4 channels are involved in many physiological and pathological processes. Although it was recently reported that TRPV4 is involved in acute itch elicited by exogenously applied histamine and 5-HT, its precise mechanism in itch induction remains controversial. Indeed, whether TRPV4 predominantly mediates itch indirectly via skin-associated cells or by directly stimulating dorsal root ganglion (DRG) neurons is an active area of investigation. 9, 10 More importantly, the role of TRPV4 in the development of chronic itch remains unexplored.
In the current study, we show that these osmosensitive TRPV4 channels are selectively expressed by skin keratinocytes and dermal macrophages, and that their activation promotes downstream 5-HT signaling, resulting in itch-specific behavioral responses in mouse models of chronic itch. Importantly, TRPV4-expressing macrophages and keratinocytes are differentially involved in the generation of spontaneous itch behaviors in mouse models of allergic and non-allergic chronic itch, respectively. Further, we identified elevated expression of TRPV4 in the skin of patients with chronic idiopathic pruritus (CIP). Collectively, our data demonstrate that TRPV4-expressing cells in the skin are a critical component in the pathogenesis of chronic itch.
Methods
Animals
Adult male and female C57BL/6J mice (Jackson Laboratories), Trpv4eGFP (Mutant Mouse Regional Resource Centers, MMRRC), Trpv4−/−, 11 KitW-sh/W-sh (Jackson Laboratories), Htr7−/− (Jackson Laboratories), Htr2a−/− (a kind gift from Dr Jay Gingrich at the Columbia University) mice were used for the study. The Cre+ and Cre− Pf4Cre;iDTR (Pf4-Cre+ and Pf4-Cre) mice were obtained by crossing the Rosa26iDTR mice (Jackson Laboratories) with the Pf4-Cre mice (Jackson Laboratories). To generate the Trpv4f/f mice, three of the properly targeted ES cell clones were obtained from the KOMP Repository and used for blastocyst injections and one clone led to high contribution chimaeras that produced germline transmitted offspring as assayed by black coat color. This chimera line was then mated to FLPo mice (Jackson Laboratories) to remove the neomycin cassette and generate heterozygous Trpv4f/+ mice, which were crossed with K14CreERT and Cx3cr1CreERT to generate both Cre+ and Cre− Cx3cr1CreERT;Trpv4f/f (Cx3cr1-Cre+ and Cx3cr1-Cre−) and K14CreERT;Trpv4f/f (K14-Cre+ and K14-Cre−) mice, respectively. Toamoxifen-inducible Trpv4 knockdown mice were produced by intraperitoneal administration tamoxifen for five consecutive days at 75mg/kg in 0.2 ml corn oil. Experiments were performed 7 days after the last day of tamoxifen administration. Animals were acclimatized to the experimental room in advance of experiments. All behavioral tests were videotaped from a side angle, and behavioral assessments were done by observers blind to the treatments or genotypes of animals. All the experimental procedures were approved by the Institutional Animal Care and Use Committee of University of Texas Health Science Center at Houston and the Institutional Animal Care and Use Committee at Washington University School of Medicine, and were in accordance with the guidelines provided by the National Institute of Health and the International Association for the Study of Pain. All mice were randomly allocated to different experimental groups by the lab managers who are blinded to the experimental design. All mice were included in the analysis unless it is dead.
Human peripheral blood mononuclear cells and skin biopsies
Human peripheral blood samples were obtained from patients undergoing routine skin cancer surgery in an outpatient dermatology clinic as approved by the Washington University in St. Louis Institutional Review Board (IRB, Protocol 201507042). De-identified human control skin was obtained from non-cancerous, marginal skin from patients (average age of control subjects was 74.5±4.2 years of age) undergoing routine skin cancer surgery under an IRB-exempt protocol. Human chronic idiopathic pruritus (CIP) skin was obtained directly from patients with a firm diagnosis of CIP (average age of CIP patient was 74.3±2.2 years of age) as approved by the Washington University in St. Louis IRB (Protocol 201412117). The numerical rating scale (NRS) itch score for CIP was examined on a scale of 0 to 10 as a peak value during a week before examination. A thorough work-up was performed to establish the diagnosis of CIP by exclusion of other cutaneous and systemic causes of pruritus, including psychiatric and neurologic conditions, dialysis-dependent renal failure, biliary dysfunction, thyroid abnormalities, HIV/AIDS, hepatitis B, and hepatitis C. None of the patients had a history of malignancy. Informed consent was obtained from each subject before all procedures.
Single cell suspensions from mouse and human skin tissues
Fresh mouse ear skin preparations and human skin biopsies were cut and separated using forceps and digested in 0.25 mg/ml Liberase TL (Roche) in DMEM media for 90 minutes at 37°C. Samples were mashed through 70 μm cell strainers and washed with DMEM media supplemented with 5% FBS, 1% L-glutamine (GIBCO), and 1% Pencillin/Streptomycin (GIBCO). Single-cell suspensions were used for subsequent calcium imaging, staining for flow cytometry and cell sorting for RT-PCR.
Mouse and human epidermal keratinocyte culture
The skin keratinocyte culture was prepared according to the previous study. In brief, the skin of newborn mouse pups (P0–P2) or human skin biopsy was removed and placed in a Petri dish containing 2.5% dispase II (Life Technologies, NY) and incubated at 4°C overnight. Epidermis was then separated from subcutaneous tissues. Keratinocytes were dissected by gentle scraping and flushing with culture medium. Harvested cells were plated on coverslips coated with collagen IV and cultured in serum-free, fully supplemented keratinocyte medium CnT-07 (CELLnTEC advanced cell systems, Switzerland) (for mouse keratinocytes) or keratinocyte-SFM medium supplemented with BPE and EGF (Invitrogen, Carlsbad, CA, USA) (for human keratinocytes) under a humidified atmosphere of 5% CO2/95% air at 37°C for 2 days before use.
Isolation and short term culture of mouse DRG neurons
Mouse spinal columns were removed and placed in ice-cold HBSS; neurons were acutely dissociated and maintained as described.13 In brief, laminectomies were performed and bilateral DRG were dissected out. After removal of connective tissues, DRG were transferred to a 1 mL Ca2+/Mg2+-free HBSS containing 2 μL saturated NaIICO3, 0.35 mg 1-cysteine and 20 U papain (Worthington, Lakewood, NJ, USA), and incubated at 37°C for 10 min. The suspension of DRG was centrifuged, the supernatant was removed, 1 mL Ca2+/Mg2+-free HBSS containing 4 mg collagenase type II and 1.25 mg dispase type II (Worthington) was added and incubated at 37°C for 10 min. After digestion, neurons were pelleted, suspended in neurobasal medium containing 2% B-27 supplement, 1% 1-glutamine, 100U·mL−1 penicillin plus 100μg·mL−1 streptomycin, and 50ng·mL−1 nerve growth factor, plated on a 12mm coverslip coated with poly-l-lysine (10 μg·mL−1) and cultured under a humidified atmosphere of 5% CO2/95% air at 37°C for 18–24 hr before use.
Extraction and isolation of mouse platelets
The whole blood was collected into a tube containing 3.8% Na Citrate at a ratio of 9:1 after the anesthesia of mouse. Centrifuge the anti-coagulant whole blood at 100 g for 10 min without brake. Transfer the platelet rich plasma to a new tube and add 1 μM PGE1 and incubate for 5 min. Add another volume of Na Citrate and centrifuge at 400 g for 10 min. Discard the plasma and resuspend platelets in HBSS containing 4 μM Fura2-AM (Life Technologies). Plate the platelets on coverslips coated with poly-l-lysine and incubate at room temperature for 1 hr. The platelets were be used after washed with fresh HBSS for at least 30 min using calcium imaging assay.
Preparation of skin superfusate
Skin biopsies were dissected from the back of mice, chopped into small pieces and incubated in HBSS at 37°C for 30 minutes. The superfusate was collected by centrifugation before we carried out the calcium imaging experiment.
Ca2+ imaging
Fura-2-based ratiometric measurement of [Ca2+]i was performed as described previously. 13 Freshly isolated skin-resident cells, cultured epidermal keratinocytes, cultured DRG neurons and isolated peripheral blood mononuclear cells and platelets were loaded with 4 μM Fura-2 AM (Life Technologies) in culture medium at 37°C for 60 min. Cells were then washed three times and incubated in HBSS at room temperature for 30 min before use. Fluorescence at 340 and 380 nm excitation wavelengths was recorded on an inverted Nikon Ti-E microscope equipped with 340, 360 and 380 nm excitation filter wheels using NIS-Elements imaging software (Nikon Instruments Inc., Melville, NY, USA). Fura-2 ratios (F340/F380) were used to reflect changes in intracellular Ca2+ upon stimulation. Values were obtained from 100–250 cells in time-lapse images from each coverslip. Threshold of activation was defined as 3 standard deviations above the average (~20% above the baseline).
Whole-cell patch-clamp recording
Whole-cell patch-clamp recordings were performed using an EPC 10 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany) or multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA) at room temperature (22–24°C) on the stage of an inverted phase-contrast microscope equipped with a filter set for green fluorescence protein visualization. Pipettes pulled from borosilicate glass (BF 150-86-10; Sutter Instrument Company, Novato, CA, USA) with a Sutter P-97 pipette puller had resistances of 2–4 MΩ when filled with pipette solution containing 140 mM CsCl, 1 mM MgCl2, 0.5 mM EGTA, and 10 mM HEPES with pH 7.3 and 315 mOsm·L−1. Cells were continuously perfused with standard extracellular solution containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose and 10 mM HEPES (pH was adjusted to 7.4 with NaOH, and the osmolarity was adjusted to ≈340 mOsm·L−1 with sucrose). The whole-cell membrane currents were recorded using voltage ramp from −100 to +100 mV for 500 ms at holding potential of 0 mV. Data were acquired using PatchMaster software (HEKA Elektronik) or Clampex 10 (Molecular Devices, Sunnyvale, CA, USA). Currents were filtered at 2 kHz and digitized at 10 kHz. Data were analyzed and plotted using Clampfit 10 (Molecular Devices, Sunnyvale, CA, USA). Values are given as means ± SEM; n represents the number of measurements.
Immunofluorescence
Adult mice, ages 6–16 weeks, were asphyxiated with CO2 and perfused transcardially with 200 ml of phosphate buffered saline (PBS; pH 7.3) followed by 200 ml of fixative (4% paraformaldehyde in 0.1 M PB, pH 7.3, or Zamboni’s fixative [2% paraformaldehyde, 15% (v/v) saturated picric acid, 0.1 M PB, pH 7.3]). The tissues were removed and postfixed overnight at 4°C in the same fixative. All tissues were cryoprotected overnight in 30% sucrose in 0.1 M PB, pH 7.3, frozen in optimal cutting temperature medium (OCT), sectioned with a cryostat at 20 μm, mounted on Superfrost Plus slide, and stored at −20°C. Frozen slides were dried at room temperature for 1 hr and washed three times in PBS with 0.1% Triton X-100 (PBS+TX), blocked for 30 min to 1 hr in PBS+TX containing 10% donkey serum, and incubated overnight at 4°C with primary antibodies diluted in blocking solution (see Table E1 in this article’s Online Repository at www.jacionline.org). The sections were then washed three times in PBS+TX and incubated for 2 hr at room temperature with secondary antibodies conjugated to Alexa-488 fluorochrome (Invitrogen) or Cy3 fluorochromes (Jackson ImmunoResearch, West Grove, PA), and diluted 1:500 in blocking solution. Sections were then washed three times in PBS+TX and mounted in anti-fade medium (Vectashield, Vector Laboratories, Burlingame, CA). All preparations were examined with a Nikon Al Confocal Laser Microscope System. Images were taken and analyzed in NIS-Elements.
Flow cytometry
Isolated ear skin cells from Trpv4eGFP mice were stained with anti-mouse fluorescently conjugated antibodies: CD11b (eBioscience), CD11c (Biolegend), CD3e (eBioscience), B220 (BD Pharmingen), and CCR2 (R&D systems). Samples were acquired on a BD Lsr Fortessa flow cytometer (BD Bioscience) and analyzed using FlowJo software (V10, Tree Star) (see Table E1 in this article’s Online Repository at www.jacionline.org).
Cell sorting and RT-PCR
Isolated GFP+ and GFP− skin cells were sorted using a FACSAriaII (BD Bioscience). Total RNA was extracted from using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA was treated with DNase I (Invitrogen) and the cDNA was synthesized in vitro using ThermoScript® RT-PCR System kit (Invitrogen). PCR conditions: 95°C for 3 min, 40 cycles of 30 s at 95°C, 30 s at 52°C and 60 s at 72°C. The sequences of the primers used are as follows: GFP forward 5′-AAGGGCATCGACTTCAAGG-3′, reverse 5′-TGCTTGTCGGCCATGATATAG-3′; mTRPV4 forward 5′-CCTGCTGGTCACCTACATCA-3′, reverse 5′-CTCAGGAACACAGGGAAGGA-3′; mGAPDH forward 5′-GCACAGTCAAGGCCGAGAAT-3′ reverse 5′-GCCTTCTCCATGGTGGTGAA-3′
Quantitative RT-PCR
Total RNA was extracted from human skin tissue using RNeasy kit (Qiagen) according to manufacturer’s instructions. RNA was treated with DNase I (Invitrogen) and the cDNA was synthesized in vitro using ThermoScript® RT-PCR System kit (Invitrogen). Primer sequences are as follows: hTRPV4 forward 5′-AGAACTTGGGCATCATCAACGAG-3′ reverse 5′-GTTCGAGTTCTTGTTCAGTTCCAC-3′; hTRPV3 forward 5′-GCTGAAGAAGCGCATCTTTGCA -3′ reverse 5′- GTCAGCTTGTGCATGAGGAAG -3′;hGAPDH forward 5′-GTCGGAGTCAACGGATTT G-3′ reverse 5′-TGGGTGGAATCATATTGGAA-3′. Reactions were carried out in a volume of 20 μl per reaction containing 10 μl SYBR Green master mix (2×) (Applied Biosystems), 0.5 μl cDNA, 5 μl 0.4 μM primer mix, and 4.5 μl water using StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA). The generated Ct (cycle threshold) value of human TRPV4 was normalized to its respected Ct value of GAPDH (ΔCt). The value of ΔCt for CIP group was further normalized to that for control group to yield the ΔΔCt. The data were expressed as 2−ΔΔCt.
Conditional platelet depletion
Platelet depletion was accomplished by intraperitoneal injections of DTX (400 ng per mouse) every 48 hr for a total of 3 treatments to the Pf4-Cre+;iDTR mice, 14 and confirmed by counting the number of platelets in the blood obtained from the facial vein. Only animals with platelet counts reduced to less than 15% of the value before DTX administration were used for further experiments.
Mouse model of chronic dry skin itch
In the dry skin model, 15 the rostral back of the mice were treated twice daily with cutaneous application of acetone/ether (1:1) mixture followed by water (AEW). After a 7-d treatment, the mice showed robust spontaneous scratching and the treated skin area exhibited decreased stratum corneum hydration and increased trans-epidermal water loss, which mimic the symptoms of dry skin in patients. Hind paw scratching directed toward the treated area was quantified by recording the number of incidences of scratching bouts for 60 min daily. After the recording, the videotapes were played back, and all behavioral assessments were done by observers blind to the treatments or genotypes of the animals.
Mouse model of allergic contact dermatitis (ACD)
The contact sensitizer squaric acid dibutylester (SADBE) (Tokyo Chemical Co.) was used to elicit contact hypersensitivity in the mouse as a model of allergic contact dermatitis in humans.16 Mice were sensitized by the topical application of 20 μl of 0.5% SADBE in acetone to the shaved abdominal skin once a day for three consecutive days. Five days later, the SADBE-treated group was challenged with a topical application of 20 μl of 0.5% SADBE to the hairy skin of the nape of neck once a day for three consecutive days whereas acetone alone was used as the vehicle control. The scratching behavior with the hind paw was quantified by recording the number of incidences of scratching bouts for 60 min daily.
Statistical Analysis
Values are reported as the mean + standard error of the mean (s.e.m.). Unpaired or paired t-test was used for comparison between two groups or one-way ANOVA and repeated measures tests followed by Tukey-Kramer or Bonferroni post hoc analysis was used for comparison among multiple groups occurring over time. All tests were carried out as two-tailed tests, p < 0.05 was considered statistically significant. All experiments were repeated at least twice unless otherwise noted.
Results
TRPV4 is required for generating spontaneous scratching in mouse models of both allergic and non-allergic chronic itch
TRPV4 has been implicated in both inflammatory pain and acute itch responses elicited by intradermal injections of histamine or 5-HT. 9, 10, 17 Although acute itch may serve protective roles against potential threats and dangers in the environment (e.g., disease-borne insects), chronic itch is universally pathologic and highly debilitating. 5 To investigate whether TRPV4 is involved in chronic itch, we examined the mRNA expression of TRPV4 in pruritic skin from patients with CIP. 18 Classically, patients with CIP present with chronic pruritus secondary to skin barrier dysfunction in the absence of a clinically-defined primary dermatologic disorder. 18 Indeed, TRPV4 mRNA expression was significantly elevated in skin biopsies from patients with CIP in comparison to healthy control skin (Fig 1, A). On the other hand, TRPV3, another channel implicated in itch, was not significantly elevated in the CIP patients. 19, 20 These results suggest that TRPV4 might be involved in the pathogenesis of chronic itch in humans.
Figure 1.
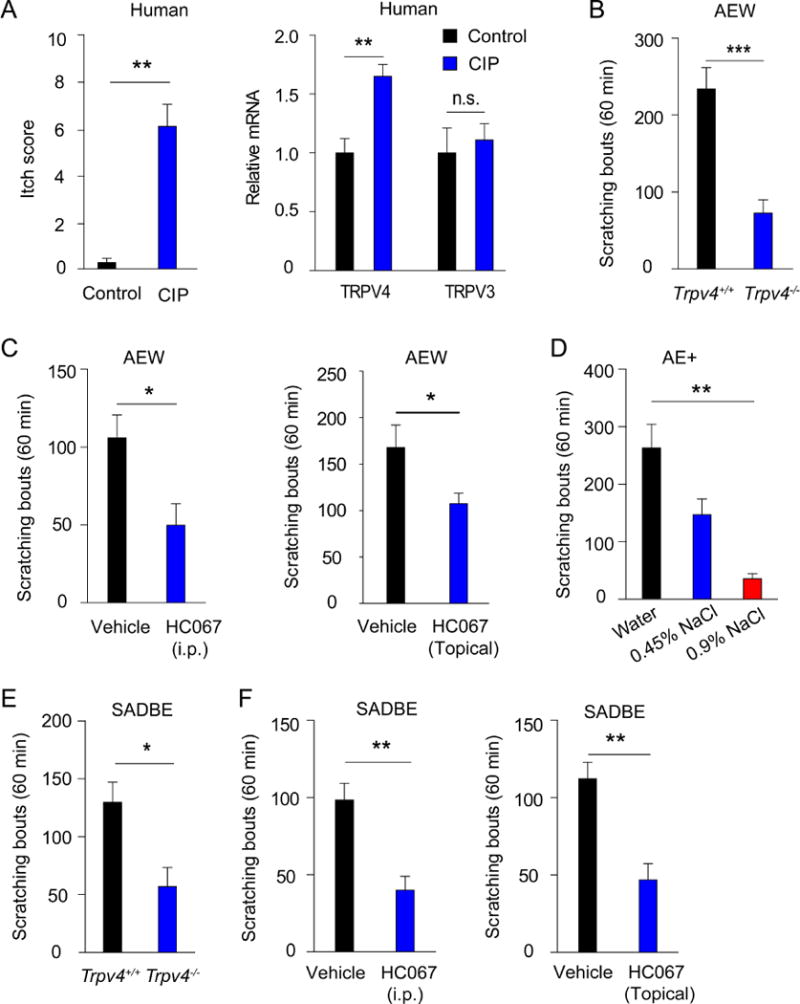
TRPV4 expression is elevated in skin biopsies of CIP patients and TRPV4 function is required for generating mouse models of both allergic and non-allergic chronic itch. A, The bar charts show the averaged itch numerical rating scale (NRS) scores and mRNA transcripts for TRPV4 and TRPV3 in CIP patients and health controls.. *p<0.05, **p<0.01, Student’s t test; n=8. n.s. not significant versus control group. B, Spontaneous scratches in Trpv4+/+ and Trpv4−/− mice after 7 days of AEW treatment. ***p<0.001, Student’s t test; n=7.. C, Spontaneous scratches in Trpv4+/+ mice after 7 days of AEW treatment were reduced by HC067 (20 mg/kg, i.p or topical application) compared with vehicle. *p<0.05, Student’s t test; n=8–9. D, Spontaneous scratches after the treatment with the 1:1 mixture of acetone and ether followed by 0.9% NaCl, 0.45% NaCl, or distilled water, respectively. **p<0.01, ANOVA; n=5–7. E, Spontaneous scratches in Trpv4+/+ and Trpv4−/− mice after SADBE treatment. *p<0.05, Student’s t test; n=6. F, SADBE-induced spontaneous scratches in Trpv4+/+ mice after treatment with HC067 (20 mg/kg, i.p or topical application) or vehicle. *p<0.05, Student’s t test; n=5–6.
Next, we examined the role of TRPV4 in a mouse model of dry skin-associated chronic itch induced by AEW treatment, 3, 21, 22 which produces robust scratching and extensive epidermal hyperplasia in wt mice. 15 We found that the number of spontaneous scratches was markedly reduced in the Trpv4−/− mice at each of the measured time points from day 3 to day 7 after AEW treatment (Fig 1, B, and not shown). This was additionally confirmed by pharmacological inhibition of TRPV4 by HC067, 23 which substantially attenuated spontaneous scratching in the AEW model when applied either systemically or topically (Fig 1, C). AEW-elicited scratching critically depends on the application of distilled water, 2 which is reminiscent of a condition called aquagenic pruritus, in which water exposure induces intense itching, especially in elderly patients. 24, 25 Further, exposure to water is known to exacerbate dry skin and itching in conditions such as chronic hand dermatitis. 26 Since distilled water is extremely hypotonic (≈17 mOsm), 27 we hypothesized that osmotic stress driven by distilled water promotes the scratching response. Indeed, mice treated with distilled water with increased osmolarity at 0.45% saline (141.5 mOsm) displayed significantly reduced scratching, which was further reduced at a concentration of 0.9% saline (283 mOsm) after the AE challenge (Fig 1, D). Consistent with both in vitro and in vivo studies showing that TRPV4 is a molecular sensor for extracellular osmolarity in mammals, 11, 28, 29 these results suggest that TRPV4 is an osmoreceptor in the skin of mice and functionally required to generate dry skin-associated itch, which recapitulates the symptoms of dry skin in humans. 15
We further investigated if TRPV4 is involved in spontaneous scratching associated with ACD using the contact sensitizer SADBE. 16 wt mice receiving SADBE treatment displayed a robust scratching response when compared to mice treated with vehicle control only (not shown). The Trpv4−/− mice displayed a markedly reduced scratching response after SADBE treatment when compared to wt mice (Fig 1, E). Similarly, systemic or topical administration of HC067 significantly reduced spontaneous scratching bouts in wt mice treated with SADBE (Fig 1, F). Combined, these results suggest that TRPV4 contributes to both allergic and non-allergic chronic itch in mice.
Both mouse and human keratinocytes and myeloid cells express functional TRPV4 channels
Although TRPV4 is known to be expressed by both neurons as well as non-neuronal cells, the precise expression pattern of TRPV4 across tissues is controversial. 30, 31 We therefore took a transgenic approach by using BAC transgenic Trpv4eGFP mice 32 and determined the expression of TRPV4-eGFP in the skin. We found that both K14-expressing keratinocytes 33 in the epidermis and F4/80-expressing macrophages in the dermis were TRPV4-eGFP+ (Fig 2, A). RT-PCR analysis using total mRNA isolated from sort-purified eGFP+ and eGFP− cells from mouse skin single-cell suspensions confirmed a correlation between the expression of TRPV4 and eGFP transcripts, further validating the specificity of the BAC transgenic mice (Fig 2, B). Flow cytometry analysis using skin cell suspensions further showed that a subset of TRPV4-eGFP+ cells expressed CD11b and C-C chemokine receptor type 2 (CCR2) as well as low levels of CD11c, which is reminiscent of CCR2/CD11b-positive dermal macrophages 34 (Fig 2, C, and see Fig E1 in this article’s Online Repository at www.jacionline.org). In addition, TRPV4-eGFP+ cells were also immunopositive for CD68, CD206, and CD163, all of which are cell markers commonly used for tissue macrophages35 (see Fig E2, A in this article’s Online Repository at www.jacionline.org).
Figure 2.
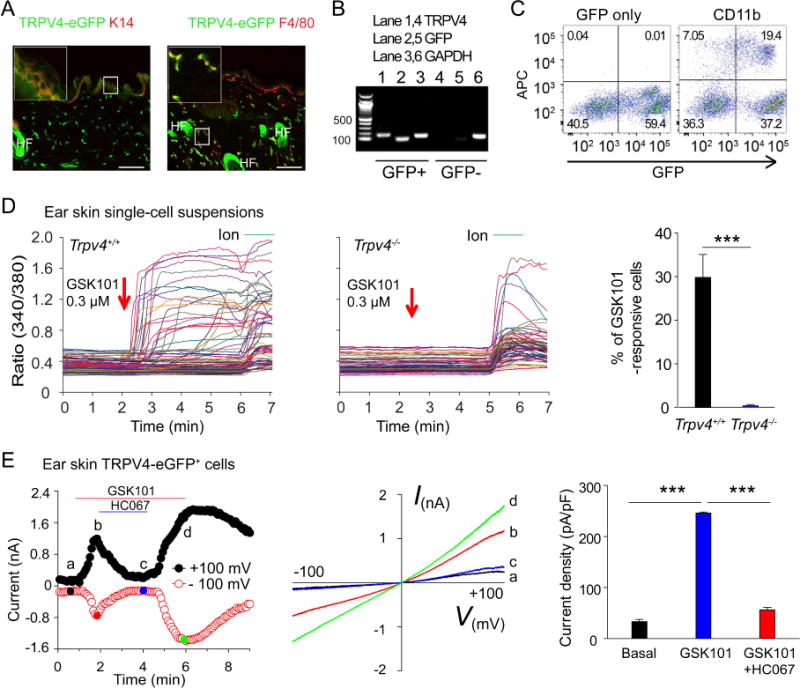
TRPV4 is functionally expressed by mouse skin-resident cells. A, Double labeling reveals the co-localization of TRPV4-eGFP with K14 (left panel) and F4/80 (right panel) in skin sections from the Trpv4eGFP mice. Bar=50 μm. HF: hair follicle. Inset shows the magnification of boxed area. B, RT-PCR shows the correlation between TRPV4 and GFP in sorted GFP+ and GFP− ear skin single-cell suspensions. C, Flow cytometry analysis illustrates that TRPV4-eGFP is expressed in CD11b-positive skin-resident cells. D, Representative traces showing the GSK101-elicited large [Ca2+]i responses in freshly dissociated ear skin single-cell suspensions from Trpv4+/+ (left) but not Trpv4−/− (middle) mice. Arrows indicate the time points of GSK101 applications. Ionomycin (Ion) was used as positive control. Summarized data from in the right showing the percentages of GSK101-responsive skin cells isolated from the Trpv4+/+ and Trpv4−/− mice. ***p<0.001, Student’s t test; n=5–8. E, Time course (left) and representative current-voltage curves (middle) elicited by voltage ramps in the absence or presence of 0.3 μM GSK101 in the TRPV4-eGFP+ myeloid cells from the ear skin single-cell suspensions. HC067 at 5 μM markedly inhibited the GSK101-activated currents. Bar charts in the right illustrate summarized data. ***p<0.001, ANOVA; n=5.
We next assessed the function of TRPV4 in freshly isolated skin resident cells using live cell Ca2+ imaging and patch-clamp recordings. Application of GSK101, a potent and selective TRPV4 agonist 36, induced a robust intracellular Ca2+ ([Ca2+]i) response in a subset of cells in wt but not Trpv4−/− skin single-cell suspensions, which are enriched with resident immune cells (Fig 2, D). 37 Moreover, GSK101 also activated large whole-cell membrane currents with characteristic current–voltage relationship for TRPV4 and [Ca2+]i responses in freshly isolated TRPV4-eGFP+ cells, which was severely attenuated by either GSK219 or HC067, two selective TRPV4 antagonists 23, 38 (Fig 2, E, and see Fig E2, B and C in this article’s Online Repository at www.jacionline.org). Consistent with previous studies showing the presence of functional TRPV4 in mouse keratinocytes 33, we found that GSK101 evoked a large [Ca2+]i response in wt but not TRPV4-deficient mouse primary keratinocytes (see Fig E3 in this article’s Online Repository at www.jacionline.org). Similarly, we also demonstrated that GSK101 elicited robust [Ca2+]i responses in human primary epidermal keratinocytes, freshly isolated human forearm skin single-cell suspensions, and human peripheral blood mononuclear cells, all of which were abolished by GSK219, suggesting that like mice, human skin-resident cells and mononuclear cells also possess functional TRPV4 channels (see Fig E4 in this article’s Online Repository at www.jacionline.org). Combined, these results demonstrate that activation of TRPV4 can lead to functional responses in both keratinocytes and dermal myeloid cells in both mice and human.
TRPV4-expressing macrophages and keratinocytes are differentially involved in allergic and non-allergic chronic itch
Consistent with TRPV4-expressing skin-resident cells being critical to the genesis of chronic itch in mice, we showed that AEW or SADBE treatment produced epidermal hyperplasia with a marked increase in the number of TRPV4-eGFP+ keratinocytes as well as dermal TRPV4-eGFP+ cells moving toward the epidermal-dermal border (Fig 3, A–C). In addition, the TRPV4 mRNA transcripts were also increased in the skin of mice treated with either AEW or SADBE (Fig 3, D). Collectively, these studies demonstrate that TRPV4 expression is not only functional but also enriched in the skin under chronic itch conditions. To further determine the types of TRPV4-expressing cells contributing to chronic itch, we generated TRPV4 flox mice and crossed them to inducible keratinocyte specific K14CreERT and macrophage specific Cx3cr1CreERT mice. After tamoxifen treatment, the AEW-induced spontaneous scratching response was significantly reduced in the K14-Cre+ mice compared with their K14-Cre− littermates (Fig 3, E). However, AEW-induced spontaneous scratching response was not significantly affected in the Cx3cr1-Cre+ mice (Fig 3, F). On the other hand, SADBE-induced spontaneous scratching was significantly attenuated in the Cx3cr1-Cre+ mice but not in the K14-Cre+ mice compared with their respective Cre− littermates (Fig 3, G and H). These studies suggest that TRPV4 function in macrophages and keratinocytes contributes differentially to the pathogenesis of allergic and non-allergic chronic itch in mice.
Figure 3.
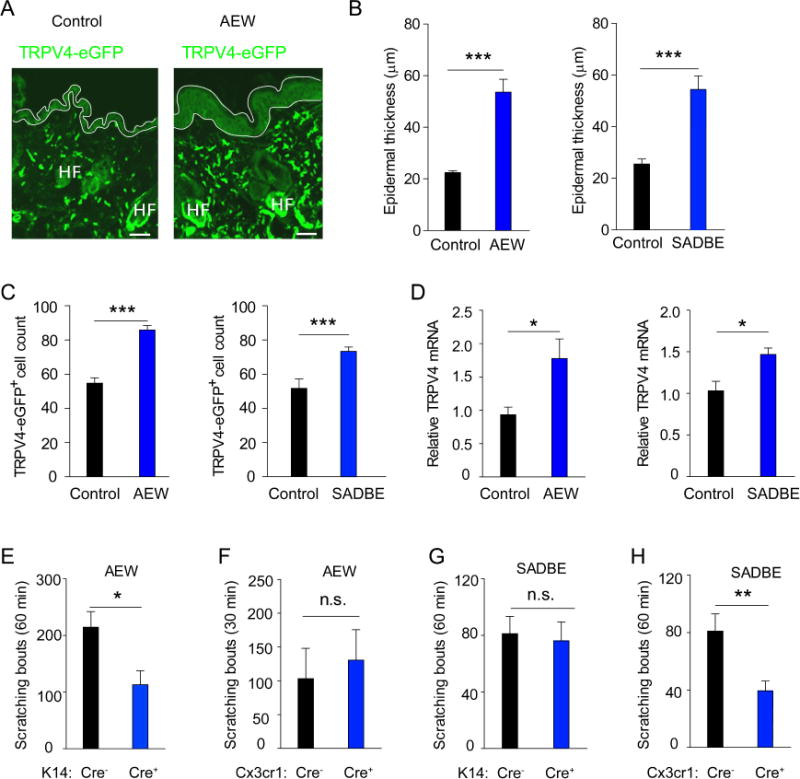
TRPV4 channels expressed by macrophages and keratinocytes contribute differentially to allergic and non-allergic chronic itch. A, Representative images showing the TRPV4-eGFP+ cells in the skin of Trpv4eGFP mice treated with vehicle control and AEW. B, The epidermal thickness was significantly increased by AEW and SADBE treatments compared with their respective vehicle controls. ***p<0.001, Student’s t test; n=6–8. C, The number of TRPV4-eGFP+ dermal macrophages increased significantly following AEW or SADBE treatment. ***p<0.001, Student’s t-test; n=6–8. D, Relative TRPV4 mRNA in the skin of AEW- or SADBE-treated mice, *p<0.05, Student’s f test; n=6–8. E, Spontaneous scratching in the K14-Cre+ and K14-Cre− mice after AEW treatment, *p<0.05, Student’s f test; n=5. F, Spontaneous scratching in the Cx3cr1-Cre+ and Cx3cr1-Cre− mice after AEW treatment. Student’s t test; n=5. n.s. not significant. G, Spontaneous scratching in the K14-Cre+ and K14-Cre− mice after SADBE treatment. Student’s t test; n=8–9. n.s. not significant. H, Spontaneous scratching in the Cx3cr1-Cre+ and Cx3cr1-Cre− mice after SADBE treatment. **p<0.01, Student’s t test; n=9.
5-HT signaling is critically involved in TRPV4-dependent chronic itch
To identify skin-derived chemical mediator(s) of TRPV4-mediated scratching response, we prepared AEW-treated skin superfusates from both Trpv4+/+ and Trpv4−/− mice and applied the superfusates directly to cultured wild-type DRG neurons. As expected, Trpv4+/+ AEW skin superfusate evoked a robust [Ca2+]i response in a subset of DRG neurons. By contrast, the Trpv4−/− AEW skin superfusate activated significantly fewer sensory neurons, suggesting that TRPV4 mediates the release of neuromediators in the AEW-treated skin (Fig 4, A). Interestingly, Trpv4+/+ AEW skin superfusate activated approximately 30% of all 5-HT-responsive DRG neurons, whereas less than 5% of these neurons were activated by Trpv4−/− AEW skin superfusate (Fig 4, B). Therefore, TRPV4 deficiency leads to significantly fewer 5-HT-sensitive sensory neurons responsive to AEW skin superfusates, suggesting that TRPV4 deficiency might result in significantly reduced 5-HT release in the skin in response to AEW treatment.
Figure 4.
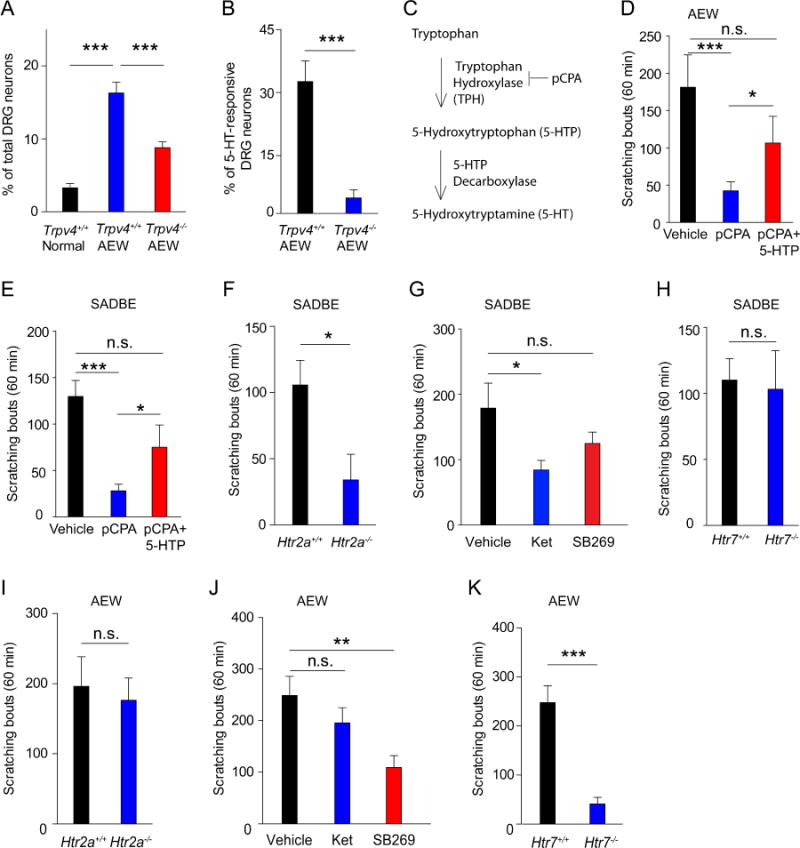
5-HT signaling is required for TRPV4-dependent chronic itch. A, Percentages of DRG neurons responding to skin superfusates from normal Trpv4+/+, AEW Trpv4+/+, and AEW Trpv4−/− mice. ***p<0.001, ANOVA. B, Percentages of 5-HT-responsive DRG neurons responding to the AEW skin superfusates from Trpv4+/+ and Trpv4−/− mice. ***p<0.001, Student’s t test. C, Schematic drawing of the 5-HT synthesis pathway. D and E, Spontaneous itching in AEW- (Fig 4, D) and SADBE- (Fig 4, E) treated mice with pCPA or pCPA+5-HTP. *p<0.05, ***p<0.001, ANOVA; n=8–10. F, Spontaneous scratching in the Htr2a+/+ and Htr2a−/− mice after SADBE treatment. *p<0.05, Student’s t test; n=5. G, Spontaneous scratching in mice treated with vehicle, Ketanserin, or Htr7 antagonist SB269970 (SB269) after SADBE treatment. *p<0.05, ANOVA; n=6. H, Spontaneous scratching in Htr7+/+ and Htr7−/− mice after SADBE treatment. Student’s t test; n=6. I, Spontaneous scratching in Htr2a+/+ and Htr2a−/− mice after AEW treatment. Student’s t test; n=9. J, Spontaneous scratching after AEW treatment in mice treated with vehicle, Htr7 antagonist SB269970 (SB269), or Ketanserin. **p<0.01, ANOVA; n=6–7. K, Spontaneous scratching after AEW treatment in the Htr7+/+ and Htr7−/− mice. ***p<0.001, Student’s t test; n=10–11. n.s. not significant versus vehicle group.
Biosynthesis of 5-HT (Fig 4, C) is catalyzed by the rate-limiting enzyme tryptophan hydroxylase (TPH) and inhibition of TPH activity by a TPH inhibitor p-chlorophenylalanine (pCPA) has been commonly used to investigate the effects of 5-HT depletion on animal behaviors. 39 We thus examined if chemical depletion of 5-HT affected AEW-induced dry skin-associated chronic itch and SADBE-induced chronic allergic itch. The results revealed that administration of pCPA markedly inhibited spontaneous scratching in mice treated with either AEW or SADBE (Fig 4, D and E). To validate that deficiency in 5-HT but not other monoamines mediated the pCPA effect, we concomitantly administered pCPA and 5-hydroxytryptophan (5-HTP), which is converted to 5-HT without the involvement of TPH. Indeed, administration of 5-HTP rescued spontaneous scratching in mice treated with pCPA in both AEW- and SADBE-induced chronic itch models (Fig 4, D and E).
Consistent with the finding that 5-HT is a downstream neuromediator of TRPV4-elicited scratching, administration of ketanserin, a selective antagonist of the 5-HT receptor 2a (Htr2a), which was shown to mediate 5-HT-elicited scratching in mice, 40 or genetic ablation of the Htr2a function, but not inhibition of Htr7 function, significantly reduced spontaneous scratching in mice treated with SADBE (Fig 4, F–H). To our surprise, the number of spontaneous scratches in the AEW-induced chronic dry skin-associated itch was not substantially changed in the Htr2a−/− mice or wt mice treated with ketanserin (Fig 4, I and J). In contrast, the number of spontaneous scratches in the AEW model was substantially reduced by either pharmacological inhibition or genetic ablation of the function of Htr7, which was also shown to mediate 5-HT-induced itch in mice 41 (Fig 4, J and K), suggesting that Htr7 but not Htr2a signaling likely plays a major role in the chronic dry skin-associated itch. Together, these results suggest that both Htr2a and Htr7 are critically involved in TRPV4-dependent chronic itch.
Platelets are required to generate TRPV4-mediated chronic itch
It is well known that mast cell degranulation increases tissue histamine concentration leading to activation of TRPV1-positive pruriceptive sensory neurons and generation of histamine-dependent itch. 4 Besides histamine, mast cells also represent a major source of 5-HT. 42 We therefore examined if TRPV4-eGFP was expressed by mast cells using streptin-avidin-rhodamine. 43 However, there was no co-localization of TRPV4-eGFP with streptin-avidin-rhodamine, suggesting that mast cells are not likely to express TRPV4 (Fig 5, A). In agreement with this finding, spontaneous scratching responses in mice treated with AEW or SADBE were not significantly altered in the mast cell-deficient KitW-sh/W-sh “sash” mice when compared with wt controls (Fig 5, B and C), suggesting that mast cell-derived 5-HT is not a major contributor to TRPV4-dependent chronic itch.
Figure 5.
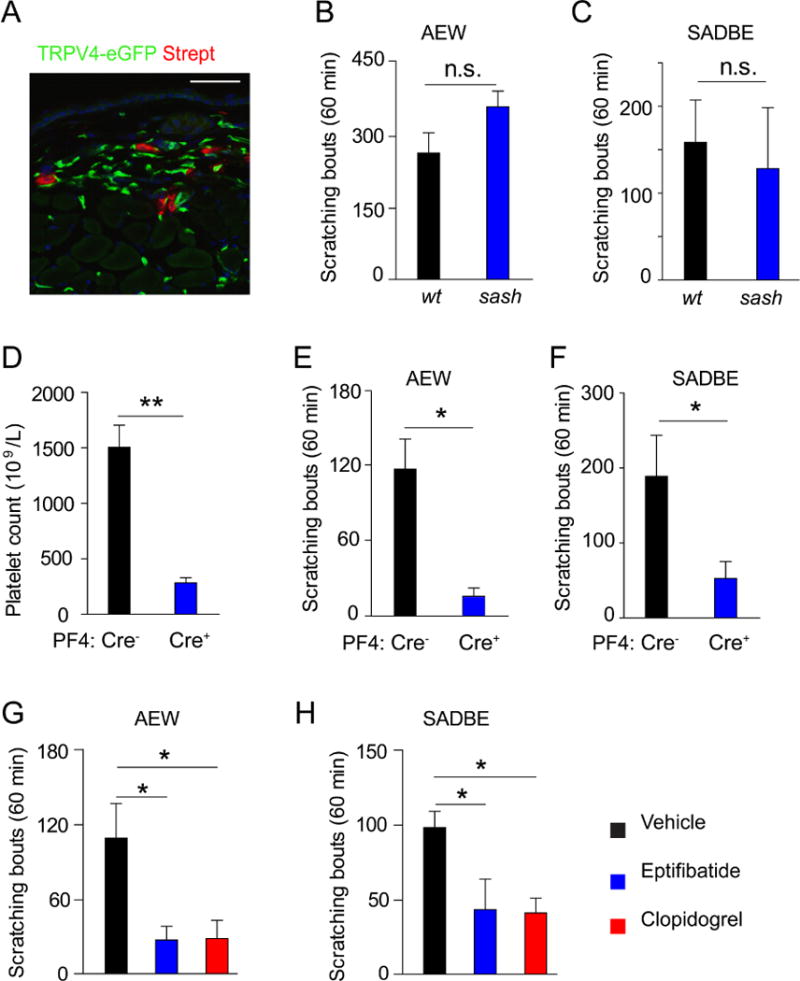
Platelets but not mast cells are required for TRPV4-dependent chronic itch. A, Immunofluorescent staining shows that TRPV4-eGFP was not colocalized with streptavidin, a mast cell marker. Bar=50 μm. B and C, Spontaneous scratching induced by AEW (Fig 5, B) or SADBE (Fig 5, C) treatment was not significantly affected in the sash mice. Student’s t test; n=6 −7. n.s. not significant versus wt control group. D, Platelet count in Pf4-Cre mice and Pf4-Cre+ mice 6 days after the DTX treatment. **p<0.01, Student’s t test; n=7–8. E and F, Spontaneous scratching responses induced by AEW (Fig 5, E) or SADBE (Fig 5, F) in the Pf4-Cre− mice (n=5 for AEW and SADBE) mice and the Pf4-Cre+ mice (n=6 for AEW and SADBE). *p<0.05, Student’s t test. G and H, Spontaneous scratching responses induced by AEW (Fig 5, G) or SADBE (Fig 5, H) in the absence or presence of eptifibatide or clopidogrel. *p<0.05, ANOVA; n=6.
Besides mast cells, platelets are the other major source for 5-HT in the skin. 44 Although it is increasingly understood that platelets have roles in inflammatory and immune processes in addition to their function in hemostasis and thrombosis, the role of platelets in the pathogenesis of chronic itch has not been studied. To investigate if platelets are required for TRPV4-mediated itch we used a conditional platelet depletion model by crossing the Pf4Cre trans genic line with the Cre-dependent Rosa26DTR line (iDTR followed by DTX injections. 14 The number of platelets in peripheral blood of the Pf4-Cre+ mice was markedly reduced after DTX injections compared with DTX-treated Pf4-Cre− littermates but no other blood cells were affected (Fig 5, D, and not shown). 14 DTX-treated Pf4-Cre+ mice displayed comparable thermal and mechanical sensitivities as DTX-treated Pf4-Cre− littermates in the Hargreaves, hot plate, tail immersion, and von Frey filament tests (see Fig E5 in this article’s Online Repository at www.jacionline.org). In addition, Pf4-Cre+ mice had no motor function deficit when compared with DTX-treated Pf4-Cre− littermates as measured by rotarod test (see Fig E5 in this article’s Online Repository at www.jacionline.org), confirming that DTX treatment does not affect acute thermal and mechanical pain sensations. We next tested the responses of Pf4-Cre+ mice and their Pf4-Cre− littermates subjected to treatment with AEW or SADBE and showed that DTX-treated Pf4-Cre+ mice had substantially attenuated spontaneous itch behaviors induced by AEW or SADBE treatment compared with DTX-treated Pf4-Cre littermates (Fig 5, E and F). Together, these results strongly suggest that platelets but not mast cells are required for TRPV4-mediated chronic itch responses.
Multiple pathways are involved in platelet activation, including those activated by adenosine diphosphate (ADP) through the P2Y12 receptor and fibrinogen through the platelet glycoprotein IIb/IIIa receptors. 45 We thus investigated if selective inhibition of the P2Y12 or glycoprotein (GP) IIb/IIIa receptors affects TRPV4-mediated chronic itch responses using the selective P2Y12 receptor antagonist clopidogrel or a selective inhibitor of the GP IIb\IIIa receptors, eptifibatide, both of which are antiplatelet drugs approved by Food and Drug Administration (FDA). Indeed, concomitant application of clopidogrel or eptifibatide severely attenuated AEW-and SADBE-induced spontaneous scratching (Fig 5, G and H). Combined, these results strongly suggest that platelet activation is critical to TRPV4-mediated chronic itch responses and cutaneous platelets are likely the sources of 5-HT release in response to AEW or SADBE treatment.
We finally asked if TRPV4 is functionally expressed by platelets isolated from the peripheral blood of the Trpv4eGFP mice. We did not detect TRPV4-eGFP in platelets. Further, live cell Ca2+ imaging also did not detect [Ca2+]i response elicited by addition of GSK101 although it induced a robust [Ca2+]i response in TRPV4-eGFP+ blood-derived leukocytes (see Fig E6 in this article’s Online Repository at www.jacionline.org). These results suggest that TRPV4 does not directly mediate the activation of the platelets but rather through the release of unknown proinflammatory mediators following activation of the TRPV4-expressing skin-resident cells, which induces activation of platelets to release 5-HT and produce chronic itching.
Discussion
Our findings that TRPV4 function is required for producing spontaneous scratching in dry skin-associated chronic itch and SADBE-induced spontaneous scratching, 15, 16 provide strong evidence for a major role of the TRPV4-expressing skin-resident cells in the pathogenesis of multiple types of chronic itch. We also revealed TRPV4 function in human skin cells and elevated TRPV4 expression in skin biopsies of patients with CIP. Further, we demonstrated that 5-HT is a critical downstream mediator of the TRPV4-mediated spontaneous scratching and distinct 5-HT receptors are required for producing TRPV4-mediated allergic and non-allergic chronic itch. Our results support a model in which the TRPV4-positive skin-resident cells are key signaling components in the pathogenesis of chronic itch.
Our data show that TRPV4 expression is up-regulated by treatment of AEW or SADBE on the skin, as reflected by an increase in the numbers of TRPV4-eGFP+ myeloid cells and keratinocytes as well as increased TRPV4 mRNA transcripts. Moreover, loss of TRPV4 function severely attenuates the spontaneous scratching in both AEW- and SADBE-induced chronic itch models, highlighting the importance of TRPV4 in the pathogenesis of chronic itch and suggesting that cutaneous TRPV4 signaling is critically involved in the pathogenesis of chronic itch in mice. Furthermore, our findings that the osmosensitive TRPV4 expressed by skin-resident cells mediates the scratching response elicited by osmotic stress in the AEW-induced chronic itch model might provide the molecular and cellular basis of aquagenic pruritus of the elderly. We also found a significant increase of TRPV4 expression in CIP patients, further supporting a general role of TRPV4 in mediating chronic itch in both rodents and humans.
Interestingly, by using conditional knockdown of TRPV4 expression from keratinocytes and dermal macrophages we found that TRPV4-expressing keratinocytes are critically involved in AEW-induced dry skin associated chronic itch but not spontaneous scratching in SADBE-induced ACD. Conversely, TRPV4-expressing dermal macrophages are required for generating chronic itch in the SADBE-induced ACD model but not AEW-induced dry skin associated chronic itch. These results are consistent with our findings that osmotic stress mediates AEW-induced spontaneous scratching. Since TRPV4-expressing epidermal keratinocytes are the first skin cells to access water following barrier disruption, which is an important step in the generation of AEW-induced dry skin model, 15 we speculate that activation of TRPV4-mediated signaling in the epidermal keratinocytes by water is largely responsible for AEW-induced chronic itching. Although the mechanism of SADBE in ACD is not fully understood, activation of T cell-mediated immunity is critically involved in the generation of ACD. 46 Besides T cells, it was also reported that there is increased migration of dermal innate immune cells which are important to the sensitization in ACD. 47, 48 Indeed, we detected a marked increase in the number of TRPV4-expressing dermal macrophages after treatment of SADBE. Our results suggest that dermal macrophages might not only contribute to skin inflammation but also chronic itch in SADBE-induced ACD.
Consistent with previous studies showing that 5-HT- but not histamine-elicited responses are sensitized by AEW treatment, 49 we found that chemical depletion of 5-HT by pCPA severely attenuated spontaneous scratching in mice treated with AEW or SADBE. Although dermal mast cells are rich in 5-HT, our results do not support the involvement of mast cells in TRPV4-mediated scratching responses for the following observations: 1) mast cells do not express TRPV4-eGFP; 2) consistent with previous studies, 15 we found that the spontaneous scratching responses induced by AEW or SADBE was not altered by mast cell deficiency. Instead, we showed that DTX-induced depletion of platelets in the Pf4-Cre+ mice severely attenuated spontaneous scratching in mice treated with AEW or SADBE, suggesting that platelet-derived 5-HT is required for TRPV4-mediated chronic itch. In addition, platelets are critical to leukocyte recruitment in chronic skin inflammation through formation of platelet-leukocyte aggregates via P-selectin in peripheral blood and secretion of chemokines at inflamed sites. 50 Furthermore, platelets can also migrate extravascularly and accumulate in inflammatory lesions concomitantly with leukocytes and have been associated with many inflammatory disorders including asthma, arthritis, and inflammatory bowel disease (IBD). 51
Our results showed that Htr2a, one of many 5-HT-responsive GPCRs, is involved in SADBE-induced spontaneous scratching based on our pharmacological inhibition and genetic ablation studies. However, the Htr7 rather than the Htr2a receptor mediates AEW-induced chronic dry skin-associated itch. Although the exact mechanisms underlying the involvement of multiple subtypes of 5-HT receptors in different mouse models of chronic itch remain to be elucidated, our results show that both AEW- and SADBE-induced chronic itch require 5-HT signaling initiated by activation of TRPV4-expressing epithelial and immune cells in the skin, highlighting heterogeneous modules of chronic itch development through distinct TRPV4/5-HT receptor signaling axes. Recognizing the versatility and selectivity of 5-HT receptor signaling in chronic itch might be critical to the development of effective therapies against different forms of chronic itch with distinct etiologies.
In summary, we have shown that the TRPV4-mediated allergic and non-allergic chronic itch involves activation of a series of cellular networks involving TRPV4-expressing keratinocytes, dermal macrophages, platelets, and pruriceptors through paracrine signaling in the skin. Since chronic itch originates in the skin, identification of TRPV4-dependent itch-specific cellular signaling networks in the skin can guide development of selective pharmacological intervention of these chronic itch pathways.
Supplementary Material
Table E1. Primary antibodies used for immunofluorescent staining and flow cytometry.
Figure E1 TRPV4-eGFP is expressed by dermal macrophages but not T or B cells. Flow cytometry analysis using ear skin single-cell suspensions from the Trpv4eGFP mice reveals that TRPV4-eGFP is expressed by a subpopulation of CCR2+ or CD11c+ macrophages but not by the CD3e+ T cells or B220+ B cells. The experiment was repeated 4 times.
Figure E2 Expression of cellular markers for macrophages and dermal macrophages in the skin of Trpv4eGFP mice. A, Double labeling experiments show that CD11b, CD206, CD68, and CD163 were co-expressed with the TRPV4-eGFP+ cells in the skin. Bar=50 μm. B and C, Representative traces (Fig E2, B) and summarized data (Fig E2, C) illustrate that 0.3 μM GSK101 elicited a [Ca2+]i response in freshly isolated TRPV4-eGFP+ ear skin single cell suspensions, which was inhibited by 0.3 μM GSK219 or 5 μM HC067. ***p<0.001, ANOVA; n=11 coverslips for GSK101 and 5 coverslips for GSK219 and HC067.
Figure E3 Functional expression of TRPV4 in primary cultured mouse kereatinocytes. A and B, Representative traces showing the GSK101-elicited [Ca2+]i responses in cultured keratinocytes from Trpv4+/+ (Fig E3, A) and Trpv4−/− (Fig E3, B) mice. C, Summarized data showing the averaged response evoked by GSK101 in cultured keratinocytes from the Trpv4+/+ and Trpv4−/− mice. ***p<0.001, Student’s t test; n=5 coverslips in each group.
Figure E4 GSK101 elicits [Ca2+]i responses in human primary keratinocytes, forearm skin cell suspensions, and human peripheral blood mononuclear cells. A–C, Representative traces showing the GSK101 (0.3 μM)-elicited [Ca2+]i response in the absence (left) and presence (middle) of GSK219 (0.3 μM) in human primary keratinocytes (Fig E4, A), human forearm skin cell suspensions (Fig E4, B), and human peripheral blood mononuclear cells (Fig E4, C). The bar graphs on the right show that GSK219 abolished the GSK101 responses in all cell types tested. *** p<0.001, Student’s t test; n=5 coverslips in each group. Ionomycin (Ion) and ATP were used as positive controls.
Figure E5 Conditional depletion of platelets does not affect thermal and mechanical sensations and motor function in mice. A–E, Paw withdraw latency (Fig E5, A), Hot plate latency (Fig E5, B), Tail withdraw latency (Fig E5, C), Paw withdraw threshold (Fig E5, D), and the latency to fall (Fig E5, E) in the Pf4-Cre− mice (n=5 mice) mice were not significantly different from that of the Pf4-Cre+ mice (n=6 mice). p>0.05, Student’s t test. n.s. not significant versus Pf4-Cre group.
Figure E6 TRPV4 is not functionally expressed by platelets. A, Representative images showing the [Ca2+]i response elicited by 0.3 μM GSK101 and 100 μM ADP. Cell number 1 represents a GFP-positive white blood cell. Cell number 2 represents a GFP-negative white blood cell. Cells number 3 and 4 represent platelets. B, Brightfield and GFP images show that TRPV4-eGFP is present in a white blood cell (cell number 1) but not in platelets. C, Representative traces show that GSK101 elicited a [Ca2+]i response in the GFP+ white blood cell (cell number 1) but not the GFP− white blood cell (cell number 2) or platelets (cells number 3 and 4). ADP serves as a positive control. The same experiment was repeated at least 3 times.
Key Messages.
TRPV4 is expressed predominantly by dermal macrophages in addition to keratinocytes
TRPV4 in keratinocytes and macrophages contribute to spontaneous scratching associated with AEW-induced dry skin and SADBE-induced allergic dermatitis, respectively.
Platelet-derived serotonin is required for TRPV4-mediated itch sensation.
Capsule Summary.
TRPV4 in keratinocytes and macrophages contributes to chronic itch through downstream serotonin signaling pathway, suggesting that TRPV4 may be a novel therapeutic target for the treatment of chronic itch.
Acknowledgments
We thank Drs. M. Suzuki and A. Mizuno for providing the Trpv4 knockout mice and Dr. Zhou-Feng Chen, for the breeder pairs of the Trpv4 knockout mice. We are grateful to Dr. Jay Gingrich for providing the Htr2a knockout mice. We thank Dr. Cristina Strong for providing the primary human epidermal keratinocytes. We also thank Richard Clark, Edgar Walters, and Carmen Dessauer for helpful discussions. This work was supported, in whole or in part, by grants from the National Institutes of Health R01GM101218A, R01DK103901, and the Center for the Study of Itch of the Department of Anesthesiology of Washington University to HH, R01DA019921 to KR, R01EY024704 to QL, and R01DK098401 to RGO.
Abbreviations
- TRPV4
Transient receptor potential vanilloid 4
- GPCRs
G-protein coupled receptors
- DRG
dorsal root ganglion
- CIP
chronic idiopathic pruritus
- AEW
acetone/ether mixture followed by distilled water
- ACD
allergic contact dermatitis
- SADBE
squaric acid dibutylester
- CCR2
C-C chemokine receptor type 2
- TPH
Tryptophan hydroxylase
- pCPA
p-chlorophenylalanine
- 5-HTP
5-hydroxytryptophan
- Htr2a
5-HT receptor 2a
- DTX
Diphtheria toxin
- ADP
Adenosine diphosphate
- GP
Glycoprotein
- FDA
Food and Drug Administration
- IBD
Inflammatory bowel disease
- DMEM
Dulbecco modified Eagle medium
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- Ct
cycle threshold
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statement: The authors declare that they have no competing financial interests.
References
- 1.Matterne U, Strassner T, Apfelbacher CJ, Diepgen TL, Weisshaar E. Measuring the prevalence of chronic itch in the general population: development and validation of a questionnaire for use in large-scale studies. Acta Derm Venereol. 2009;89(3):250–256. doi: 10.2340/00015555-0641. [DOI] [PubMed] [Google Scholar]
- 2.Yosipovitch G. Dry skin and impairment of barrier function associated with itch - new insights. Int J Cosmet Sci. 2004;26(1):1–7. doi: 10.1111/j.0142-5463.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 3.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16(2):174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han SK, Simon MI. Intracellular signaling and the origins of the sensations of itch and pain. Sci Signal. 2011;4(185):pe38. doi: 10.1126/scisignal.2002353. [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Feng J, Liu S, Walters ET, Hu H. Molecular and cellular mechanisms that initiate pain and itch. Cell Mol Life Sci. 2015;72(17):3201–3223. doi: 10.1007/s00018-015-1904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokabe T, Tominaga M. The TRPV4 cation channel: A molecule linking skin temperature and barrier function. Commun Integr Biol. 2010;3(6):619–621. doi: 10.4161/cib.3.6.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamenko M, Zaika O, Boukelmoune N, O’Neil RG, Pochynyuk O. Deciphering physiological role of the mechanosensitive TRPV4 channel in the distal nephron. Am J Physiol Renal Physiol. 2015;308(4):F275–286. doi: 10.1152/ajprenal.00485.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Elias A, Mrkonjic S, Jung C, Pardo-Pastor C, Vicente R, Valverde MA. The TRPV4 channel. Handb Exp Pharmacol. 2014;222:293–319. doi: 10.1007/978-3-642-54215-2_12. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama T, Ivanov M, Nagamine M, Davoodi A, Carstens MI, Ikoma A, et al. Involvement of TRPV4 in Serotonin-Evoked Scratching. J Invest Dermatol. 2016;136(1):154–160. doi: 10.1038/JID.2015.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Fang Q, Wang Z, Zhang JY, MacLeod AS, Hall RP, et al. Transient receptor potential vanilloid 4 ion channel functions as a pruriceptor in epidermal keratinocytes to evoke histaminergic itch. J Biol Chem. 2016;291(19):10252–10262. doi: 10.1074/jbc.M116.716464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278(25):22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Stewart R, Berdeaux R, Hu H. Tonic inhibition of TRPV3 by Mg2+ in mouse epidermal keratinocytes. J Invest Dermatol. 2012;132(9):2158–2165. doi: 10.1038/jid.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin S, Luo J, Qian A, Du J, Yang Q, Zhou S, et al. Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. J Clin Invest. 2013;123(9):3941–3951. doi: 10.1172/JCI66413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wuescher LM, Takashima A, Worth RG. A novel conditional platelet depletion mouse model reveals the importance of platelets in protection against Staphylococcus aureus bacteremia. J Thromb Haemost. 2015;13(2):303–313. doi: 10.1111/jth.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88(3):285–292. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- 16.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, et al. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain. 2014;137(Pt 4):1039–1050. doi: 10.1093/brain/awu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118(1–2):70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Xu AZ, Tripathi SV, Kau AL, Schaffer A, Kim BS. Immune dysregulation underlies a subset of patients with chronic idiopathic pruritus. J Am Acad Dermatol. 2016;74(5):1017–1020. doi: 10.1016/j.jaad.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinhoff M, Biro T. A TR(I)P to pruritus research: role of TRPV3 in inflammation and itch. J Invest Dermatol. 2009;129(3):531–535. doi: 10.1038/jid.2008.440. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90(3):558–564. doi: 10.1016/j.ajhg.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155(2):285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) J Invest Dermatol. 2012;132(7):1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 2010;107(44):19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73–86. doi: 10.2165/00128071-200910020-00001. [DOI] [PubMed] [Google Scholar]
- 25.Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310(22):2443–2450. doi: 10.1001/jama.2013.282023. [DOI] [PubMed] [Google Scholar]
- 26.Suman M, Reddy BS. Pattern of contact sensitivity in Indian patients with hand eczema. J Dermatol. 2003;30(9):649–654. doi: 10.1111/j.1346-8138.2003.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 27.Vergnolle N, Cenac N, Altier C, Cellars L, Chapman K, Zamponi GW, et al. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br J Pharmacol. 2010;159(5):1161–1173. doi: 10.1111/j.1476-5381.2009.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100(23):13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lechner SG, Markworth S, Poole K, Smith ES, Lapatsina L, Frahm S, et al. The molecular and cellular identity of peripheral osmoreceptors. Neuron. 2011;69(2):332–344. doi: 10.1016/j.neuron.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 30.Ryskamp DA, Witkovsky P, Barabas P, Huang W, Koehler C, Akimov NP, et al. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci. 2011;31(19):7089–7101. doi: 10.1523/JNEUROSCI.0359-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander R, Kerby A, Aubdool AA, Power AR, Grover S, Gentry C, et al. 4alpha-phorbol 12,13-didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4. Br J Pharmacol. 2013;168(3):761–772. doi: 10.1111/j.1476-5381.2012.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 33.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279(20):21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- 34.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39(5):925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Gros E, Novak N. Cutaneous dendritic cells in allergic inflammation. Clin Exp Allergy. 2012;42(8):1161–1175. doi: 10.1111/j.1365-2222.2012.03964.x. [DOI] [PubMed] [Google Scholar]
- 36.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1 -piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther. 2008;326(2):432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 37.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34(6):973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med. 2012;4(159):159ra148. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- 39.Roberts AC. The importance of serotonin for orbitofrontal function. Biol Psychiatry. 2011;69(12):1185–1191. doi: 10.1016/j.biopsych.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 40.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morita T, McClain SP, Batia LM, Pellegrino M, Wilson SR, Kienzler MA, et al. HTR7 Mediates Serotonergic Acute and Chronic Itch. Neuron. 2015;87(1):124–138. doi: 10.1016/j.neuron.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nordlind K, Azmitia EC, Slominski A. The skin as a mirror of the soul: exploring the possible roles of serotonin. Exp Dermatol. 2008;17(4):301–311. doi: 10.1111/j.1600-0625.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee MG, Dong X, Liu Q, Patel KN, Choi OH, Vonakis B, et al. Agonists of the MAS-related gene (Mrgs) orphan receptors as novel mediators of mast cell-sensory nerve interactions. J Immunol. 2008;180(4):2251–2255. doi: 10.4049/jimmunol.180.4.2251. [DOI] [PubMed] [Google Scholar]
- 44.Geba GP, Ptak W, Anderson GM, Paliwal V, Ratzlaff RE, Levin J, et al. Delayed-type hypersensitivity in mast cell-deficient mice: dependence on platelets for expression of contact sensitivity. J Immunol. 1996;157(2):557–565. [PubMed] [Google Scholar]
- 45.Lombardi F, De Chaumont C, Shields DC, Moran N. Platelet signalling networks: pathway perturbation demonstrates differential sensitivity of ADP secretion and fibrinogen binding. Platelets. 2012;23(1):17–25. doi: 10.3109/09537104.2011.594190. [DOI] [PubMed] [Google Scholar]
- 46.Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33(3):300–304. doi: 10.1016/j.clindermatol.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Chun KH, Imai Y, Higashi N, Irimura T. Migration of dermal cells expressing a macrophage C-type lectin during the sensitization phase of delayed-type hypersensitivity. J Leukoc Biol. 2000;68(4):471–478. [PubMed] [Google Scholar]
- 48.Sato K, Imai Y, Irimura T. Contribution of dermal macrophage trafficking in the sensitization phase of contact hypersensitivity. J Immunol. 1998;161(12):6835–6844. [PubMed] [Google Scholar]
- 49.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151(2):378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamagawa-Mineoka R. Important roles of platelets as immune cells in the skin. J Dermatol Sci. 2015;77(2):93–101. doi: 10.1016/j.jdermsci.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 51.McNicol A, Israels SJ. Beyond hemostasis: the role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targets. 2008;8(2):99–117. doi: 10.2174/187152908784533739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table E1. Primary antibodies used for immunofluorescent staining and flow cytometry.
Figure E1 TRPV4-eGFP is expressed by dermal macrophages but not T or B cells. Flow cytometry analysis using ear skin single-cell suspensions from the Trpv4eGFP mice reveals that TRPV4-eGFP is expressed by a subpopulation of CCR2+ or CD11c+ macrophages but not by the CD3e+ T cells or B220+ B cells. The experiment was repeated 4 times.
Figure E2 Expression of cellular markers for macrophages and dermal macrophages in the skin of Trpv4eGFP mice. A, Double labeling experiments show that CD11b, CD206, CD68, and CD163 were co-expressed with the TRPV4-eGFP+ cells in the skin. Bar=50 μm. B and C, Representative traces (Fig E2, B) and summarized data (Fig E2, C) illustrate that 0.3 μM GSK101 elicited a [Ca2+]i response in freshly isolated TRPV4-eGFP+ ear skin single cell suspensions, which was inhibited by 0.3 μM GSK219 or 5 μM HC067. ***p<0.001, ANOVA; n=11 coverslips for GSK101 and 5 coverslips for GSK219 and HC067.
Figure E3 Functional expression of TRPV4 in primary cultured mouse kereatinocytes. A and B, Representative traces showing the GSK101-elicited [Ca2+]i responses in cultured keratinocytes from Trpv4+/+ (Fig E3, A) and Trpv4−/− (Fig E3, B) mice. C, Summarized data showing the averaged response evoked by GSK101 in cultured keratinocytes from the Trpv4+/+ and Trpv4−/− mice. ***p<0.001, Student’s t test; n=5 coverslips in each group.
Figure E4 GSK101 elicits [Ca2+]i responses in human primary keratinocytes, forearm skin cell suspensions, and human peripheral blood mononuclear cells. A–C, Representative traces showing the GSK101 (0.3 μM)-elicited [Ca2+]i response in the absence (left) and presence (middle) of GSK219 (0.3 μM) in human primary keratinocytes (Fig E4, A), human forearm skin cell suspensions (Fig E4, B), and human peripheral blood mononuclear cells (Fig E4, C). The bar graphs on the right show that GSK219 abolished the GSK101 responses in all cell types tested. *** p<0.001, Student’s t test; n=5 coverslips in each group. Ionomycin (Ion) and ATP were used as positive controls.
Figure E5 Conditional depletion of platelets does not affect thermal and mechanical sensations and motor function in mice. A–E, Paw withdraw latency (Fig E5, A), Hot plate latency (Fig E5, B), Tail withdraw latency (Fig E5, C), Paw withdraw threshold (Fig E5, D), and the latency to fall (Fig E5, E) in the Pf4-Cre− mice (n=5 mice) mice were not significantly different from that of the Pf4-Cre+ mice (n=6 mice). p>0.05, Student’s t test. n.s. not significant versus Pf4-Cre group.
Figure E6 TRPV4 is not functionally expressed by platelets. A, Representative images showing the [Ca2+]i response elicited by 0.3 μM GSK101 and 100 μM ADP. Cell number 1 represents a GFP-positive white blood cell. Cell number 2 represents a GFP-negative white blood cell. Cells number 3 and 4 represent platelets. B, Brightfield and GFP images show that TRPV4-eGFP is present in a white blood cell (cell number 1) but not in platelets. C, Representative traces show that GSK101 elicited a [Ca2+]i response in the GFP+ white blood cell (cell number 1) but not the GFP− white blood cell (cell number 2) or platelets (cells number 3 and 4). ADP serves as a positive control. The same experiment was repeated at least 3 times.


