Abstract
Aim
To examine data from the Australian HIV Observational Database (AHOD) to, firstly, describe the incidence of chronic kidney disease (CKD) and rate of loss of renal function in HIV-infected individuals living in Australia, and then to examine the risk factors contributing to CKD in this population.
Methods
AHOD patients over 18 years of age were eligible if they had at least two serum creatinine measurements from 1 April 2008 until 31 March 2016 and an initial estimated glomerular filtration rate (eGFR) greater than 60mL/min/1.73m3. Cox proportional hazards models were used to assess risk factors for CKD, which included key patient demographic data and antiretroviral therapy (ART) exposure.
Results
Of 1924 patients included in the analysis between April 2008 and March 2016, 81 (4.2%) developed CKD (confirmed eGFR of less than 60mL/min/1.73m3 through two consecutive eGFR measurements at least three months apart). Of the examined risk factors, baseline age, baseline eGFR, and the route of HIV acquisition were statistically significant predictors of development of CKD. ART exposure, viral hepatitis co-infection, high viral load and low CD4 lymphocyte count were not found to be significant risk factors for CKD.
Conclusion
This is the first study to investigate the risk factors for development of CKD amongst Australian HIV-infected patients using cohort data. It highlights the need for awareness of renal risk factors, particularly amongst older patients or in those with pre-existing renal dysfunction. Further research is required to explore the discrepancy between patients who have acquired HIV through different means of exposure.
Keywords: AIDS-associated Nephropathy, HIV, HIV Infections, Kidney Diseases, Renal Insufficiency, Chronic
Introduction
Modern antiretroviral therapy (ART) has led to a significant improvement in survival amongst human immunodeficiency virus (HIV)-infected individuals.1 As life expectancy in this group has risen to almost match that of the general population,2 non-infectious comorbidities, including chronic kidney disease (CKD) and traditional risk factors for CKD, such as diabetes and hypertension, have emerged as important challenges in the long-term management of these patients.3,4
Despite advances in HIV treatment, the rates of acute kidney injury and CKD in HIV-infected adults exceed those of the general population.5–7 HIV-infected patients are exposed to a number of unique factors that are thought to put them at additional risk of kidney disease. Medication-related nephrotoxicity is a frequently encountered clinical problem. Tenofovir disoproxil fumarate (TDF), ritonavir-boosted atazanavir (ATV/r) and lopinavir-boosted ritonavir (LPV/r) are commonly implicated in patients developing new renal dysfunction whilst on treatment.8,9 Hepatitis C co-infection has also been reported as a risk factor for kidney disease.10 In this patient group, CKD is an important consideration due to its association with increased morbidity and mortality as well as cardiovascular disease.11 The rates of HIV-infected patients requiring dialysis or kidney transplantation are also on the rise.12
The purpose of this study was to examine data from the Australian HIV Observational Database (AHOD) to, firstly, describe the incidence of CKD and rate of loss of renal function in HIV-infected individuals living in Australia, and then to examine the risk factors contributing to CKD in this population.
Methods
Study population
AHOD is a prospective cohort study which was established in 1999. In brief, AHOD collects data from the routine clinical care of over 4000 HIV-positive patients at 30 treatment sites across Australia and New Zealand. This includes demographic information, ART, CD4 lymphocyte (CD4+) counts, viral load, other laboratory values, AIDS events and deaths.13
AHOD patients were eligible for this study if they were older than 18 years of age and had at least two (for rates) or three (for predictor analysis) serum creatinine measurements from 1 April 2008 (the start of prospective creatinine data collection in AHOD) until 31 March 2016 and an initial estimated glomerular filtration rate (eGFR) greater than 60mL/min/1.73m3 (predictor analysis only). Ethics approvals were obtained from the UNSW Sydney Human Research Ethics Committee and institutional review boards at participating sites, as well as written informed consent from all patients.
Definitions
For the predictor analysis, follow-up duration was from baseline, defined as the time of the first serum creatinine measurement after 1 April 2008, until development of CKD, defined as a confirmed eGFR of less than 60mL/min/1.73m3 through two consecutive eGFR measurements at least three months apart, or until the last creatinine measurement during follow-up.
For the calculation of rates, baseline for each period was defined as 1 April of the first year of the two-year-time window and patients with at least two creatinine measurements in each two-year-period were counted.
The CKD-EPI formula was used to calculate eGFR.14 If more than one serum creatinine measurement was taken within 28 days, the mean value and date across this period was used.
Baseline weight (for BMI), CD4+ counts and HIV viral load measurements were the closest within 180 days prior to baseline; if none were available, a value at a maximum of 30 days after baseline was taken. All variables were measured up to baseline only, except for hepatitis B and C and smoking status, which were reported for the whole study period (due to the way they are coded in AHOD), and time-updated variables.
Statistical methods
The rate of CKD was determined by patients with confirmed eGFR measurements less than 60mL/min/1.73m3 divided by total patients eligible for each period, stratified by key baseline characteristics. Profile likelihood confidence intervals were calculated.
Cox proportional hazards models were used to assess risk factors for CKD. Risk factors assessed include key patient demographic data and ART exposure. We assumed a linear association between antiretroviral exposure and chronic kidney disease as seen in the D:A:D study.15 Variables with a p-value of 0.1 or less in the univariate models were assessed in a multivariate model. Variables with p-values less than 0.05 in the multivariate model were determined as independent risk factors.
Subgroup analysis was performed on patients aged 50 and over to further examine the effect of age on CKD risk factors.
Results
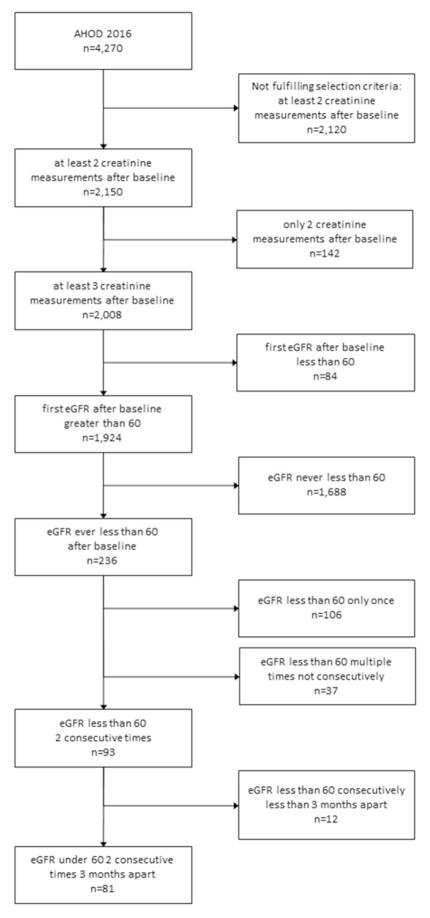
Of 4,270 AHOD patients, 2,150 (50.3%) were eligible for at least one of the above analyses. Eighty-one patients, or 4.2%, developed CKD as per the definition above, out of 1924 who had at least 3 creatinine measurements after baseline and had initial eGFR greater than 60mL/min/1.73m3 (Figure 1).
Figure 1.
Analysis eligibility diagram
Abbreviations: AHOD, Australian HIV Observational Database, eGFR, estimated glomerular filtration rate
Table 1 shows baseline characteristics for 1,924 patients included in the predictor analysis. There were significant differences between those developing CKD and those who did not in a number of variables. There was a greater male predominance in the CKD group (97.5%) compared to those who did not develop CKD (90.5%). The median age of CKD developers was 57, compared to the median age of 45 in non-developers. The median baseline eGFR of CKD developers was 73mL/min/1.73m3, while for non-CKD developers it was 101mL/min/1.73m3. Those who developed CKD began ART earlier and had longer exposure to ART compared to those who did not (median of 10 years of treatment versus 5).
Table 1. Patient characteristics at baseline.
Baseline was defined as the time of the first serum creatinine measurement after 1 April 2008. Chronic kidney disease (CKD) was defined as a confirmed eGFR of less than 60mL/min/1.73m3 through two consecutive eGFR measurements at least three months apart.
| non-CKD-developers | CKD-developers | P | |
|---|---|---|---|
| Total patients | 1843 | 81 | |
| Sex | |||
| male | 1667 (90.5%) | 79 (97.5%) | 0.031 |
| Age | |||
| median (IQR) | 45 (38–52) | 57 (51–64) | <0.001∘ |
| ATSI | |||
| n | 44 (3.0%) | 1 (1.6%) | 1.0000* |
| HIV exposure category | |||
| homosexual contact | 1292 (71.7%) | 54 (69.2%) | 0.064* |
| heterosexual contact | 383 (21.3%) | 13 (16.7%) | |
| IDU | 76 (4.2%) | 5 (6.4%) | |
| other | 51 (2.8%) | 6 (7.7%) | |
| BMI | |||
| n | 768 | 35 | |
| median (IQR) | 24.5 (22.2–26.8) | 24.6 (22.0–27.6) | 0.899∘ |
| eGFR | |||
| median (IQR) | 101 (89–111) | 73 (65–82) | <0.001 |
| ever smoked | |||
| n | 485 (53.3%) | 28 (65.1%) | 0.236 |
| HBV ever | |||
| n | 64 (4.1%) | 5 (6.9%) | 0.229* |
| HCV ever | |||
| n | 167 (10.2%) | 10 (13.5%) | 0.493 |
| CD4+ (cells/mm3) | |||
| n | 1782 | 77 | |
| median (IQR) | 520 (350–720) | 480 (285–670) | 0.176∘ |
| CD4+ nadir (cells/mm3) | |||
| n | 1562 | 81 | |
| median (IQR) | 224 (110–357) | 157 (54–250) | 0.000∘ |
| HIV viral load | |||
| n | 1740 | 75 | |
| undetectable (<50 copies/ml) | 1112 (63.9%) | 56 (74.7%) | 0.065 |
| median (IQR) | 49 (40–1145) | 49 (39–58) | 0.093∘ |
| off treatment | |||
| off | 45 (2.4%) | 0 (0.0%) | 0.259* |
| Treatment start era | |||
| pre-cART (prior to 1996) | 260 (14.6%) | 24 (29.6%) | <0.001 |
| early cART (1996–2007) | 753 (42.1%) | 42 (51.9%) | |
| late cART (after 2007) | 774 (43.3%) | 15 (18.5%) | |
| Year of treatment start | |||
| median | 2004 (1997–2010) | 1999 (1996–2005) | <0.001∘ |
| Years on treatment | |||
| median (IQR) | 5 (0–10) | 10 (4–13) | 0.000∘ |
| Tenofovir Disoproxil Fumarate exposure | |||
| never | 913 (49.5%) | 26 (32.1%) | <0.001 |
| less than 2 years | 420 (22.8%) | 15 (18.5%)) | |
| 2 years or more | 510 (27.7%) | 40 (49.4%) | |
| Indinavir exposure | |||
| never | 1508 (81.8%) | 49 (60.5%) | <0.001 |
| less than 2 years | 168 (9.1%) | 12 (14.8%)) | |
| 2 years or more | 167 (9.1%) | 20 (24.7%) | |
| Ritonavir-boosted Atazanavir exposure | |||
| never | 1538 (83.5%) | 62 (76.5%) | 0.222 |
| less than 2 years | 147 (8.0%) | 8 (9.9%)) | |
| 2 years or more | 158 (8.6%) | 11 (13.6%) | |
| other Ritonavir-boosted PI exposure | |||
| never | 1398 (75.9%) | 45 (55.6%) | <0.001 |
| less than 2 years | 202 (11.0%) | 16 (19.8%)) | |
| 2 years or more | 243 (13.2%) | 20 (24.7%) | |
Fisher’s exact test
Mann-Whitney test
Abbreviations: ATSI, Aboriginal and Torres Straight Islanders; BMI, body mass index; cART, combination antiretroviral therapy; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; IDU, injecting drug use; IQR, interquartile range; PI, protease inhibitor;
The crude rates of occurrence of confirmed eGFR measurements in two-year periods are shown in Table 2. The overall incidence rate increased from 3.5% in the initial period (2008/09) through each two-year period to 6.0% in the final period (2014/15). The rate in each period for patients under the age of 45 years was significantly lower than that of those over 60 years, ranging from 0.2% to 1.2% and 15.3% to 18.2% respectively.
Table 2. Rate of recording a confirmed eGFR measurement below 60mL/min/1.73m3.
Crude rates shown per 2-year periods. Not all n add up to the total number of patients because of missing values.
| 2008/09 | 2010/11 | 2012/13 | 2014/15 | |
|---|---|---|---|---|
|
| ||||
| Category | rate (CI; n) | rate (CI; n) | rate (CI; n) | rate (CI; n) |
| Overall | 3.5 (2.6–4.7; 1195) | 3.8 (2.9–5.0; 1347) | 4.9 (3.9–6.1; 1527) | 6.0 (4.9–7.2; 1524) |
|
| ||||
| Sex | ||||
| male | 3.4 (2.5–4.6; 1131) | 4.6 (3.5–5.7; 1406) | 6.1 (5.0–7.3; 1669) | 6.3 (5.1–7.7; 1368) |
| female | 4.7 (1.2–11.7; 64) | 3.6 (1.1–8.2; 110) | 2.4 (0.8–5.5; 166) | 3.2 (1.2–6.8; 156) |
|
| ||||
| Age at baseline | ||||
| ≤45 | 1.2 (0.5–2.4; 502) | 0.7 (0.2–1.7; 554) | 0.3 (0.1–1.0; 587) | 0.2 (0.0–0.9; 502) |
| 45–60 | 2.4 (1.3–3.9; 547) | 3.1 (1.9–4.7; 604) | 4.2 (2.9–5.9; 687) | 4.9 (3.4–6.6; 720) |
| >60 | 15.7 (10.5–22.2; 146) | 15.3 (10.7–20.9; 189) | 17.4 (13.1–22.4; 253) | 18.2 (14.1–22.8; 302) |
|
| ||||
| ATSI | ||||
| no | 3.5 (2.5–4.7; 1033) | 3.9 (2.9–5.2; 1093) | 5.1 (3.9–6.4; 1161) | 5.8 (4.5–7.2; 1164) |
| yes | 12.5 (2.2–33.8; 16) | 10.0 (1.8–27.8; 20) | 13.3 (4.3–18.3; 30) | 10.3 (3.3–22.2; 39) |
Abbreviations: ATSI, Aboriginal and Torres Straight Islanders; CI, confidence interval;
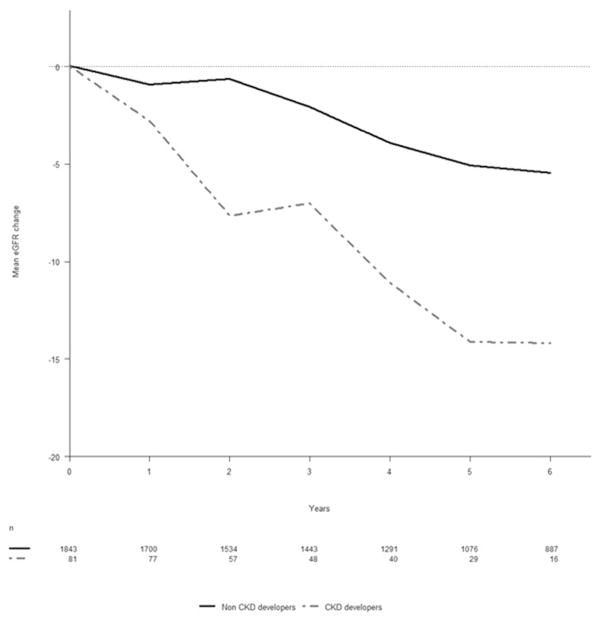
Figure 2 shows the mean change in eGFR over time of those who developed CKD compared to those who did not. A reduction in eGFR over time was observed in both groups, but those who developed CKD demonstrated a much more rapid loss of renal function.
Figure 2.
Mean change in eGFR over time
Baseline was defined as the time of the first serum creatinine measurement after 1 April 2008. Chronic kidney disease (CKD) was defined as a confirmed eGFR of less than 60mL/min/1.73m3 through two consecutive eGFR measurements at least three months apart.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate
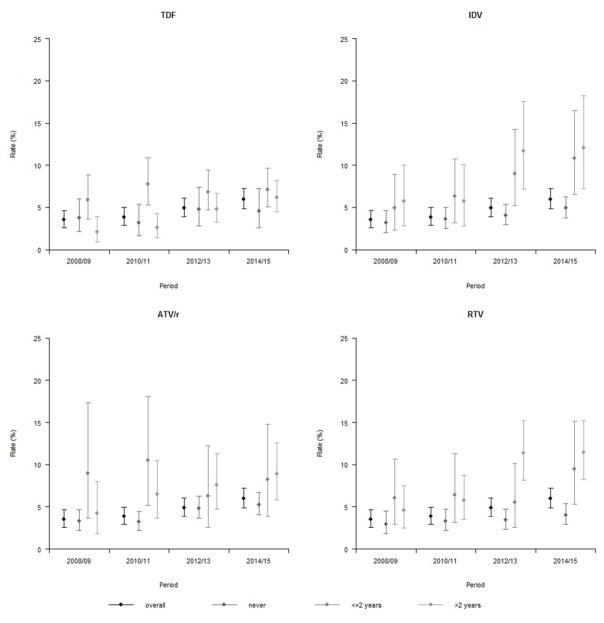
Patients never exposed to TDF, indinavir (IDV), ATV/r or other ritonavir-boosted protease inhibitors (RTV) consistently had a lower rate of a confirmed eGFR less than 60mL/min/1.73m3 compared to patients exposed for two years or less. However, subpopulations exposed to treatment for more than two years did not necessarily have higher rates of CKD compared to those exposed to treatment for two years or less (Figure 3).
Figure 3.
Rate of recording a confirmed eGFR measurement below 60mL/min/1.73m3
Crude rates by time of exposure to Tenofovir Disoproxil Fumarate (TDF), Indinavir (IDV), Ritonavir boosted Atazanavir (ATV/r), or other Ritonavir boosted PI (RTV) at the beginning of each period.
Abbreviations: ATV/r, Ritonavir boosted Atazanavir; IDV, Indinavir; RTV, other Ritonavir boosted PI; TDF, Tenofovir Disoproxil Fumarate
Of the factors associated with CKD in univariate models (sex, age, eGFR, HIV exposure category, baseline CD4+, nadir CD4+, treatment era, year of cART start, TDF, IDV, and RTV exposure) only baseline age and eGFR, and HIV exposure category remained significant in a multivariable model (Table 3).
Table 3. Factors associated with developing chronic kidney disease.
Chronic kidney disease (CKD) was defined as a confirmed eGFR of less than 60mL/min/1.73m3 through two consecutive eGFR measurements at least three months apart.
| CKD | Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No | Yes | HR (CI) | P | P overall | aHR (CI) | P | P overall | |
| Overall | 1843 | 81 | ||||||
|
| ||||||||
| Sex | ||||||||
| male | 1667 | 79 | 1 | 1 | ||||
| female | 176 | 2 | 0.30 (0.08–1.24) | 0.097 | 0.56 (0.12–2.51) | 0.447 | ||
|
| ||||||||
| Age at baseline | ||||||||
| 1 year | 1843 | 81 | 1.11 (1.09–1.13) | 0.000 | 1.06 (1.03–1.09) | 0.000 | ||
| 10 years | 2.81 (2.28–3.46) | 0.000 | 1.81 (1.38–2.38) | 0.000 | ||||
|
| ||||||||
| HIV exposure category | ||||||||
| homosexual contact | 1292 | 54 | 1 | 1 | ||||
| heterosexual contact | 383 | 13 | 0.91 (0.48–1.62) | 0.769 | 0.039 | 0.90 (0.44–1.84) | 0.777 | 0.005 |
| IDU | 76 | 5 | 1.62 (0.65–4.05) | 0.303 | 4.50 (1.67–12.1) | 0.003 | ||
| other | 51 | 6 | 3.08 (1.32–7.16) | 0.009 | 2.42 (0.96–6.10) | 0.060 | ||
|
| ||||||||
| eGFR at baseline (mL/min/1.73m3) | ||||||||
| 1 Unit | 1843 | 81 | 0.90 (0.89–0.92) | 0.000 | 0.92 (0.90–0.93) | 0.000 | ||
| 10 Units | 0.37 (0.31–0.44) | 0.000 | 0.41 (0.34–0.50) | 0.000 | ||||
|
| ||||||||
| baseline CD4+ (cells/mm3) | ||||||||
| 10 cells | 1859 | 77 | 0.99 (0.98–1.00) | 0.043 | 0.99 (0.98–1.01) | 0.223 | ||
| 100 cells | 0.92 (0.84–1.00) | 0.043 | 0.93 (0.84–1.04) | 0.223 | ||||
|
| ||||||||
| nadir CD4+ (cells/mm3) | ||||||||
| 10 cells | 1643 | 81 | 0.97 (0.96–0.99) | 0.000 | 0.99 (0.97–1.01) | 0.242 | ||
| 100 cells | 0.74 (0.64–0.86) | 0.000 | 0.89 (0.73–1.08) | 0.242 | ||||
|
| ||||||||
| Treatment start era | ||||||||
| late cART (after 2007) | 774 | 15 | 1 | 1 | ||||
| pre-cART (prior to 1996) | 260 | 24 | 3.10 (1.62–5.92) | 0.001 | 0.003 | 2.82 (0.72–11.1) | 0.137 | 0.164 |
| early cART (1996–2007) | 753 | 42 | 2.06 (1.14–3.73) | 0.017 | 1.37 (0.50–3.74) | 0.545 | ||
|
| ||||||||
| Year of cART start | ||||||||
| 1 year | 1785 | 81 | 0.94 (0.91–0.98) | 0.004 | 1.04 (0.94–1.14) | 0.467 | ||
|
| ||||||||
| Years on treatment at baseline | ||||||||
| 1 year | 1843 | 81 | 1.08 (1.04–1.13) | 0.000 | 0.96 (0.87–1.06) | 0.436 | ||
| 5 years | 1.49 (1.23–1.82) | 0.000 | 0.82 (0.50–1.35) | 0.436 | ||||
|
| ||||||||
| TDF exposure at baseline | ||||||||
| 1 year | 1843 | 81 | 1.19 (1.09–1.30) | 0.000 | 1.08 (0.96–1.20) | 0.205 | ||
| 5 years | 2.36 (1.51–3.70) | 0.000 | 1.44 (0.82–2.53) | 0.205 | ||||
|
| ||||||||
| IDV exposure at baseline | ||||||||
| 1 year | 1843 | 81 | 1.18 (1.07–1.29) | 0.001 | 1.03 (0.90–1.17) | 0.687 | ||
| 5 years | 2.24 (1.41–3.55) | 0.001 | 1.15 (0.59–2.21) | 0.687 | ||||
|
| ||||||||
| other RTV-boosted PI exposure at baseline | ||||||||
| 1 year | 1843 | 81 | 1.10 (1.01–1.20) | 0.028 | 1.06 (0.94–1.20) | 0.356 | ||
| 5 years | 1.63 (1.05–2.53) | 0.028 | 1.34 (0.72–2.51) | 0.356 | ||||
The following variables did not reach a p-value of 0.1 in a univariate model: ATSI, BMI, smoking, HBV, HCV, time-updated CD4+ counts, HIV viral load at base line and time-updated, ATV/r exposure at baseline
Abbreviations: ATSI, Aboriginal and Torres Straight Islanders; BMI, body mass index; cART, combination antiretroviral therapy; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; (a)HR, (adjusted) hazard ratio; IDU, injecting drug use; IQR, interquartile range; PI, protease inhibitor;
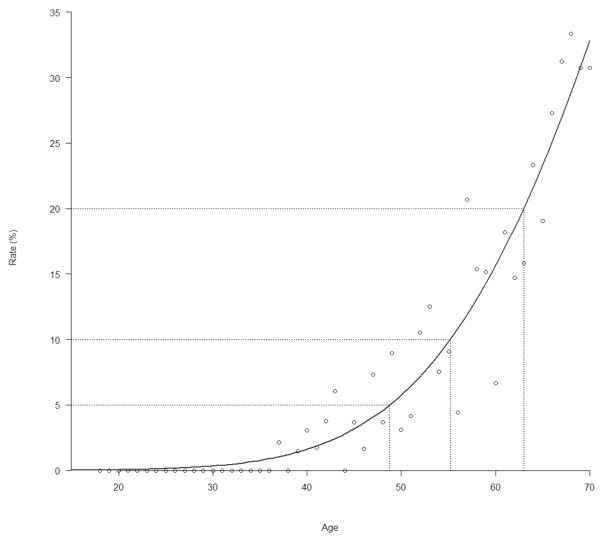
Figure 4 further highlights the effect of age by showing that the rate of ever dropping below eGFR 60mL/min/1.73m3 dramatically increases with age. Subgroup analysis found that in those aged over 50 years, nadir CD4+ count and viral load were statistically significant additional risk factors for developing confirmed CKD (Supplementary Table A.1).
Figure 4.
Rate of eGFR ever dropping below 60mL/min/1.73m3 by age
The dots represent the observed rates and the red line is the estimated curve showing the relation between age and decrease in kidney function.
Discussion
Of the 1924 patients included for analysis between April 2008 and March 2016, 81 (4.2%) developed CKD. As expected, the overall incidence rate increased over time as patients in the cohort became older and were exposed to potential risk factors for longer. Of the examined risk factors, only baseline age, baseline eGFR and HIV exposure category were statistically significant across all patients included for analysis.
The highest risk of CKD in our cohort was seen in those who were aged 60 or over at baseline, with the crude incidence rate of CKD in these individuals being well above 15% during each 2-year period for the entire duration of the study. Our findings are consistent with general population data in Australia which demonstrate the highest risk of renal disease in this age group.16 The rate of ever dropping below eGFR of 60mL/min/1.73m3 dramatically increased with age, highlighting the importance of this risk factor in both temporary as well as permanent decline in renal function.
A lower baseline eGFR was associated with a higher risk of developing CKD. A higher baseline eGFR would conceivably indicate the presence of more renal reserve to provide protection against insults such as acute illness, nephrotoxins, and development of traditional risk factors such as diabetes and hypertension. Caution must be exercised when prescribing for patients with lower eGFR, and modifiable risk factors should be actively screened for and aggressively managed.
Patients exposed to HIV infection via IDU were at significantly higher risk of CKD than those exposed to HIV via sexual contact (hazard ratio 4.50 compared to homosexual contact). Those in the “other” category, which most commonly included those acquiring HIV through bisexual or unspecified contact, or receipt of blood products, also had a higher risk overall. The reasons for the discrepancy between groups are unable to be further explored in this cohort analysis, but may relate to the different medical comorbidities and socio-economic statuses of patients in each of these categories as well as barriers to accessing preventive medicine.17
Exposure to TDF, IDV or ATV/r, as well as duration of exposure, were associated with CKD in univariate models, but did not remain statistically significant after adjusting for other factors in multivariable analyses. Although ART as a risk factor for CKD has been described previously,15, 18, 19 our findings reflect those from a number of studies which were unable to demonstrate a definite association.9,20–22 Clinical interventions not captured in this cohort potentially explain the difficulty in establishing this link. Results were almost certainly influenced by clinicians appropriately ceasing nephrotoxic medications upon noticing declining renal function, as well as selection bias with high risk patients receiving potentially less nephrotoxic treatment regimens. These clinical decisions may explain our finding that the group exposed to TDF for the longest duration had a reduced rate of CKD compared those never exposed in some of the time periods examined. Confounders such as well-documented interaction between TDF and ATV resulting in higher serum concentration of TDF and lower concentration of ATV,23, 24 as well as the use of prophylactic co-trimoxazole amongst patients with low CD4+ counts may also play a role. The lack of a clear association in this study between ART and CKD does not negate the need for ongoing caution and vigilance when using these drugs.
Low CD4+ count and high viral load have been described in the literature as risk factors for CKD. HIV-associated nephropathy (HIVAN) was previously a leading cause of CKD in the HIV-infected population and typically affected young patients, particularly those of African descent, with advanced AIDS.25 In our study, neither of these variables were associated with CKD across the board after adjusting for other factors. This may be explained by high rates of ART uptake across the cohort and low prevalence of patients of African descent in the AHOD population.26 Interestingly, analysis of those aged over 50 years in our study showed low CD4+ count and high viral load to be significant risk factors for CKD. These may simply be markers of overall poor health of individuals not responding to or taking treatment who are at increased susceptibility of CKD due to age and immunocompromised state, or alternatively may be a signal towards a second distinct group of patients who may be at risk of HIVAN. The previously mentioned association between HIVAN and ethnicity may partly reflect engagement in care as well as genetic predisposition. Since the AHOD cohort is generally well-engaged with healthcare, there are relatively few with unchecked viraemia and many will have received some management of traditional risk factors. The effect on the kidneys of low CD4+ count and high viral load may therefore take longer to manifest as CKD, subsequently becoming apparent only in the older patients in our cohort.
Co-infection with hepatitis B and C were also not significant risk factors in our study. This may be explained by the impact of early detection through routine screening practices and more efficacious treatment options for these conditions in the modern era.
Limitations
Our analysis was limited by the inability of the AHOD study to account for some traditional risk factors. For example, blood glucose was an AHOD field, but not HbA1c or history of diabetes. Diagnoses of hypertension and proteinuria were similarly not included, and the overall recording rate of smoking was low. Our scope to perform certain analyses, such as examining renal function before and after cessation of TDF, was limited by the relatively small sample sizes.
Secondly, AHOD data is likely not fully representative of the broader HIV-positive population in Australia. While AHOD represents a notable proportion of the Australian population on treatment (>12%)27, 28 and we believe that our data is largely representative of people living with HIV in care in Australia, deductions to the entirety of the HIV-positive population should be made with caution. In particular, the sample size of Aboriginal and Torres Strait Islander patients was small (45 patients included for analysis, with only one developing CKD). The burden of CKD in the HIV-positive Indigenous population was therefore unable to be explored.
Conclusion
This is the first study to investigate the CKD risk factors of HIV-infected individuals living in Australia using cohort data. We demonstrated that age and baseline renal function are significant risk factors for CKD in this population, mirroring trends in the general non-HIV infected population. Further research is needed to explore the reasons for patients exposed to HIV through IDU and “other means” having a significantly higher risk of CKD than those exposed through homosexual or heterosexual contact, so that clinical care and support can be tailored for these groups.
Our findings highlight that in the constantly evolving modern HIV landscape where effective therapy exists and survival is expected to continue to improve, merely achieving control of viral replication can no longer be considered optimal clinical care. These data confirm the burden of CKD amongst this patient group, particularly amongst the older demographic which is expected to dramatically grow in the years to come. More studies are needed to assess clinical risk factors for renal disease and whether early intervention may prevent an explosion in the number of people in this population requiring dialysis or kidney transplantation.
Supplementary Material
References
- 1.Hogg R, Lima V, Sterne JA, Grabar, et al. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLos One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown TT, Cole SR, Li X, et al. Antiretroviral Therapy and the Prevalence and Incidence of Diabetes Mellitus in the Multicenter AIDS Cohort Study. Arch Intern Med. 2005;165(10):1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 4.Seaberg EC, Muñoz A, Lu M, Detels R, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19(9):953–60. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 5.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O’Hare AM. Racial Differences in End-Stage Renal Disease Rates in HIV Infection versus Diabetes. J Am Soc Nephrol. 2007;18(11):2968–74. doi: 10.1681/ASN.2007040402. [DOI] [PubMed] [Google Scholar]
- 6.Wyatt CM, Arons RR, Klotman PE, Klotman ME. Acute renal failure in hospitalized patients with HIV: risk factors and impact on in-hospital mortality. AIDS. 2006;20(4):561–5. doi: 10.1097/01.aids.0000210610.52836.07. [DOI] [PubMed] [Google Scholar]
- 7.Medapalli RK, Parikh CR, Gordon K, et al. Comorbid diabetes and the risk of progressive chronic kidney disease in HIV-infected adults: data from the Veterans Aging Cohort Study. J Acquir Immune Defic Syndr. 1999;60(4):393–9. doi: 10.1097/QAI.0b013e31825b70d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morlat P, Vivot A, Vandenhende M-A, et al. Role of Traditional Risk Factors and Antiretroviral Drugs in the Incidence of Chronic Kidney Disease, ANRS CO3 Aquitaine Cohort, France, 2004–2012. PLoS ONE [Internet] 2013;8(6) doi: 10.1371/journal.pone.0066223. [cited 2017 Feb 1]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3680439/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalayjian RC, Lau B, Mechekano RN, et al. Risk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine care. AIDS. 2012;26(15):1907–15. doi: 10.1097/QAD.0b013e328357f5ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters L, Grint D, Lundgren JD, et al. Hepatitis C virus viremia increases the incidence of chronic kidney disease in HIV-infected patients. AIDS. 2012;26(15):1917–26. doi: 10.1097/QAD.0b013e3283574e71. [DOI] [PubMed] [Google Scholar]
- 11.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 12.Lucas GM, Ross MJ, Stock PG, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(9):e96–138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petoumenos K. Australian HIV Observational Database. The role of observational data in monitoring trends in antiretroviral treatment and HIV disease stage: results from the Australian HIV observational database. J Clin Virol. 2003;26(2):209–22. doi: 10.1016/s1386-6532(02)00119-1. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207(9):1359–69. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green F, Ryan C. An overview of chronic kidney disease in Australia, 2009 [Internet] Australian Institute of Health and Welfare. 2009:36. [cited 2017 Feb 1] Available from: http://www.aihw.gov.au/publication-detail/?id=6442468245.
- 17.Coffin PO, Jin H, Huriaux E, Mirzazadeh A, Raymond HF. Trends in use of health care and HIV prevention services for persons who inject drugs in San Francisco: results from National HIV Behavioral Surveillance 2005–2012. Drug Alcohol Depend. 2015;146:45–51. doi: 10.1016/j.drugalcdep.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24(11):1667–78. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 19.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867–75. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansi L, Hughes A, Bhagani S, et al. Clinical epidemiology of HIV-associated end-stage renal failure in the UK. AIDS. 2009;23(18):2517–21. doi: 10.1097/QAD.0b013e3283320e12. [DOI] [PubMed] [Google Scholar]
- 21.Ryom L, Kirk O, Lundgren JD, et al. Advanced chronic kidney disease, end-stage renal disease and renal death among HIV-positive individuals in Europe. HIV Med. 2013;14(8):503–8. doi: 10.1111/hiv.12038. [DOI] [PubMed] [Google Scholar]
- 22.Ryom L, Mocroft A, Kirk O, et al. Predictors of advanced chronic kidney disease and end-stage renal disease in HIV-positive persons. AIDS. 2014;28(2):187–99. doi: 10.1097/QAD.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 23.Taburet A, Piketty C, Chazallon C, et al. Interactions between Atazanavir-Ritonavir and Tenofovir in Heavily Pretreated Human Immunodeficiency Virus-Infected Patients. Antimicrob Agents Chemother. 2004;48(6):2091–2096. doi: 10.1128/AAC.48.6.2091-2096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaul S, Bassi K, Damle B, et al. Pharmacokinetic evaluation of the combination of atazanavir, enteric coated didanosine and tenofovir disoproxil fumarate for a once daily antiretroviral regimen. Presented at: 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; IL, USA. 13–17 September 2003. [Google Scholar]
- 25.Wyatt CM, Klotman PE, D’Agati VD. HIV-Associated Nephropathy: Clinical Presentation, Pathology, and Epidemiology in the Era of Antiretroviral Therapy. Semin Nephrol. 2008;28(6):513–22. doi: 10.1016/j.semnephrol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilley DM, Griggs E, Hoy J, et al. Treatment and disease outcomes of migrants from low- and middle-income countries in the Australian HIV Observational Database cohort. AIDS Care. 2015;27(11):1410–7. doi: 10.1080/09540121.2015.1113227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Kirby Institute. Australian HIV Observational Database Annual Report 2016. 1. Vol. 16. The Kirby Institute UNSW; Australia, Sydney NSW: 2016. [Internet] [cited 2017 June 22]. Available from: https://kirby.unsw.edu.au/sites/default/files/AHOD_Report-2016.pdf. [Google Scholar]
- 28.The Kirby Institute. Annual Surveillance Report of HIV, viral hepatitis, STIs 2016. The Kirby Institute, UNSW; Australia, Sydney NSW: 2016. [Internet] [cited 2017 June 22]. Available from: https://kirby.unsw.edu.au/sites/default/files/kirby/report/SERP_2016-Annual-Surveillance-Report_UPD170116.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.