Abstract
Background/Objectives
Humans carrying the genetic risk variant C at the circadian CLOCK (Circadian Locomotor Output Cycles Kaput) 3111T/C have been shown to have more difficulties to achieve desired weight loss than TT carriers. We tested the hypothesis that the daily rhythm of autonomic nervous function differs in CLOCK 3111C carriers, leading to reduced effectiveness in weight control.
Subjects/Methods
We recruited 40 overweight/obese Caucasian women (BMI>25), 20 carrying CLOCK 3111C (CC and TC) and 20 non-carriers with matched age and BMI who participated in a dietary obesity treatment program of up to 30 weeks. Following the treatment, ambulatory electrocardiography was continuously monitored for up to 3.5 days when subjects underwent their normal daily activities. To assess autonomic function, heart rate variability analysis (HRV) was performed hourly to obtain mean (mean RR) and standard deviation of normal-to-normal heartbeat intervals (SDNN), and two parasympathetic measures, namely, proportion of differences between adjacent NN intervals that are >50 msec (pNN50), and high-frequency (HF: 0.15–0.4 Hz) power.
Results
In the TT carriers, all tested HRV indices showed significant daily rhythms (all P values <0.0001) with lower mean RR, SDNN, pNN50, and HF during the daytime as compared to the nighttime. The amplitudes of these rhythms except for SDNN were reduced significantly in the C carriers (mean RR: ~19.7%, P=0.001; pNN50: 58.1%, P=0.001; and HF: 41.1%, P=0.001). In addition, subjects with less weight loss during the treatment program had smaller amplitudes in the rhythms of mean RR (P<0.0001), pNN50 (P = 0.007) and HF (P=0.003). Furthermore, the rhythmicity-weight-loss associations were much stronger in the C carriers as compared to the TT carriers (mean RR: P =0.028, pNN50: P=0.0002; HF: P =0.015).
Conclusions
The daily rhythm of parasympathetic modulation may play a role in the influence of the CLOCK variation on body weight control.
Keywords: Circadian rhythm, obesity, autonomic nervous system, heart rate variability, genetics
Introduction
Humans carrying the genetic variant C at the circadian gene CLOCK (Circadian Locomotor Output Cycles Kaput) 3111T/C are more likely to be obese, exhibit greater difficulties in controlling body weight than non-carriers (TT) and have a worse prognosis in dietary weight loss treatments 1–9. Among the many clock genes and other metabolic genes examined in our ONTIME study (registered at clinicaltrials.gov as NCT02829619), the CLOCK 3111C Single-Nucleotide Polymorphism (SNP; rs1801260) is the only SNP that is independently associated with total weight loss and weight loss evolution in a dietary program, i.e., C carriers lost ~23% less during the same weight loss program than T carriers 2. In addition, the effect of this SNP on obesity or obesity-related-metabolic alterations has also been demonstrated and replicated in several populations with different environment and genetic background 4–8,10. Because the CLOCK 3111C SNP is present in 46% of the U.S. population 1, understanding the mechanisms underlying the obesity risk associated with this genetic variation is of great relevance to public health.
Many physiological processes and functions, including motor activity and autonomic control, display daily rhythms that are in synchrony to the day-night cycle 11. These rhythms are generated and/or orchestrated by the circadian system that is composed of a large number of cell-autonomous clocks in the brain and in peripheral organs and tissues 11. The molecular basis for the circadian clocks consists of a family of proteins that function together in a transcriptional–translational feedback loop with the CLOCK protein as a key transcription factor of this loop 12. A recent study showed that the CLOCK 3111C SNP resulted in higher expression of CLOCK and also increased the expression of PER2— a transcriptional target of CLOCK 13, suggesting a functional role of the genetic variant in the circadian control and, thus, altered circadian/daily rhythms of behavior and physiology in human C carriers.
The circadian control is important for metabolism and body weight control 14,15. Perturbing the circadian control, as occurs in shift workers with sleep deprivation and/or irregular sleep-wake schedule, is associated with increased adiposity 16 and increases the risk of obesity and diabetes 17,18. In terms of the underlying mechanisms, it has been proposed that autonomic nervous system (ANS) is essential for the pathway through which the circadian clocks regulate metabolism 15. This proposed theory is based on two established links: (i) The autonomic nervous system (ANS) affects energy balance via its influences on eating behavior and energy storage/consumption 19. It is generally believed that low sympathetic activity and high parasympathetic activity reduce energy expenditure and predispose onset of obesity (MONA LISA Hypothesis: ‘most obesities known are low in sympathetic activity’)20. The hypothesis is supported by many studies on the innervation of adipose tissue by autonomic nerves, showing that the sympathetic nervous system is catabolic while the parasympathetic nervous system is anabolic 21. (ii) The central circadian clock at the hypothalamus (Suprachiasmatic nucleus: SCN) has specific neural projections to control the sympathetic/parasympathetic balance in ANS activity 22. In humans ANS activity and its responses to behavior-related stress are modulated by the circadian system, displaying endogenous circadian variations 23–26.
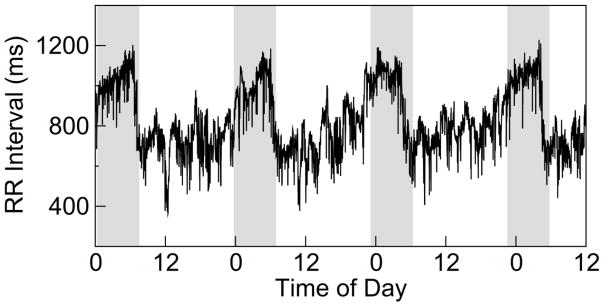
Based on these findings, we hypothesize that humans carrying risk C allele have reduced daily ANS activity rhythm due to differences in the circadian control and that the reduction is more pronounced in those individuals with more difficulty achieving desired weight loss. To test the hypotheses, we studied 20 women carrying the risk allele CLOCK 3111C allele (CC and TC) and 20 women TT carriers who participated in and completed a dietary obesity treatment program of up to ~30 weeks. We collected continuous electrocardiography (ECG) recordings up to 3.5 days during their normal daily activity and obtained heartbeat intervals (Fig. 1). Using heart rate variability (HRV) analysis 27 —an established noninvasive technique to assess ANS activity from heartbeat intervals, we examined the daily rhythm of autonomic function and its relation with weight loss during the obesity treatment.
Fig. 1.
A representative heartbeat recording of a TT carrier. The grey shaded areas indicate subject’s habitual sleep episodes.
Materials and Methods
Subjects
We recruited 40 overweight/obese Caucasian women (BMI>25) including 20 C risk allele carriers (including 3 CC carriers and 17 TC carriers) and 20 TT carriers (Table 1). The C carriers were selected from the database of the clinics of Murcia (Spain). From the previously established database, we arranged the list of the female carriers of the C allele (n=119) in ascending order according to their code numbers. We then randomly divided them in two groups (i.e., potential participants and non-participants) using a computer-executed software (http://www.randomization.com). Those women classified as participants were contacted by phone until 20 of them accepted to participate in the study. The TT carriers (n=20) were selected as controls to match for age, obesity parameters, energy intake and expenditure (Table 1) and menopausal status (32% were postmenopausal). Participants receiving thermogenic, lipogenic, or sleep drugs, or melatonin or those diagnosed with insomnia, cognitive disorders, diabetes mellitus, chronic renal failure, hepatic diseases or cancer were excluded from the study. The written informed consent was obtained before subjects were included in the study. Subjects attended outpatient obesity clinics in the city of Murcia, located in southeastern Spain. All procedures were in accordance with good clinical practice and patient data were approved by the Ethical Committee of the University of Murcia.
Table 1.
General characteristics of the study population. Data are presented as mean ± SD. NS indicates P>0.1. * Two subjects (one for each of C and TT carriers) did not complete the weight-loss program and, thus, were excluded for the analysis related to weight loss.
| CLOCK 3111T/C | |||
|---|---|---|---|
| Risk carriers(TC+CC) | Non-risk carriers(TT) | Group difference | |
| n=20 (17TC+3CC) | n=20 | ||
| Obesity parameters | Mean± SD | Mean± SD | P value |
| Age (y) | 42.0±9.7 | 46.5±11.04 | NS |
| Height (m) | 1.63±0.06 | 1.63±0.05 | NS |
| Weight (kg) | 74.87±8.83 | 76.32±12.00 | NS |
| BMI(kg/m2) | 28.14±2.41 | 28.66±4.41 | NS |
| Waist (cm) | 89.10±9.55 | 91.60±10.80 | NS |
| Hip (cm) | 105.65±8.72 | 110.25±10.50 | NS |
| Body fat (%) | 35.03±3.72 | 34.81±6.26 | NS |
| Glucose (mg/dl) | 84.80±20.47 | 87.11±10.16 | NS |
| Insulin (μUI/mL) | 10.27±13.99 | 6.03±3.16 | NS |
| Total Tryclicerydes (mg/dl) | 104.35±43.25 | 87.00±28.90 | NS |
| Total cholesterol (mg/dl) | 198.10±24.67 | 194.83±26.08 | NS |
| Cholesterol HDL (mg/dl) | 57.80±12.99 | 54.33±14.36 | NS |
| Cholesterol LDL (mg/dl) | 119.45±18.65 | 123.03±26.90 | NS |
| Leptin (ng/mL) | 21.14±9.65 | 20.34±9.32 | NS |
| adiponectin (ng/mL) | 74.60±26.62 | 78.70±25.24 | NS |
| IL6 (μg/mL) | 24.79±5.90 | 24.81±6.18 | NS |
| Total energy intake (kcal/day) | 1980±767 | 1893±601 | NS |
| Physical Activity Test (METs) | 3614±2802 | 2842±2886 | NS |
| Sleep duration (hours) | 7.8±0.6 | 7.4±0.8 | NS |
| Bedtime | 0:36±55(min) | 0:21±47(min) | NS |
| Wake-up Time | 8:18±46(min) | 7:47±38(min) | 0.028 |
| Breakfast Time | 9:30±64(min) | 8:35±48(min) | 0.004 |
| Lunch Time | 14:51±29(min) | 14:20±23(min) | NS |
| Dinner Time | 21:32±32(min) | 21:25±24(min) | NS |
| Intervention Duration (weeks) | 24±21 | 22±16 | NS |
| Total weight loss (Kg) | 6.3±3.2 | 9.8±5.1 | 0.045* |
| Medication use (Total) | 9 | 9 | NS |
| Antihypertensives | 2 | 2 | NS |
| Lipid lowering drug | 1 | 1 | NS |
| Antidiabetics | 0 | 1 | NS |
| Antidepressives | 3 | 4 | NS |
| Anxiolytics | 2 | 3 | NS |
| Thyroid hormone | 3 | 0 | 0.036 |
| Anticonceptives | 1 | 3 | NS |
Procedure
All 40 subjects participated in a dietary obesity treatment program of up to ~30 weeks. The duration of the program varied depending on the weight-loss goal. Dietetic treatment was based on the principles of the Mediterranean diet and the distribution of the macronutrient components adhered to the recommendations of the Spanish Society of Community Nutrition 28. Subjects attended a 60-minute group therapy session once per week. Certified nutritionists led the program sessions. Details of the weight loss program are described in the Supplementary Materials and at ClinicalTrials.gov (NCT02829619). After the treatment program, subjects underwent a field study of up to 4 days for the assessment of daily rhythm of cardiac activity (see below).
DNA isolation and clock genotyping
DNA was isolated from blood samples using standard procedures (Qiagen, Valencia, CA, USA). We performed genotyping of the CLOCK 3111T/C SNP using a TaqMan assay with allele-specific probes on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) according to the standardized laboratory protocols previously used for this genetic variant 1. DNA samples are deposited in the database of the Institute of Health Carlos III belonging to the Ministry of Economy and Competitiveness (Registration number: C0003626).
Obesity related parameters
Total body fat was measured by bioelectrical impedance, using TANITA TBF-300 (Tanita Corporation of America, Arlington Heights, IL, USA) equipment. Subjects were requested not to drink liquids during the 2 h before the measurement. Blood for biochemical determinations were collected in the morning after overnight fast. All the parameters were collected before the start of the weight loss program. Height and weight of each subject were obtained at the same time of the day in each clinical visit.
Ambulatory study and data acquisition
During a visit in the outpatient obesity clinics, research staff showed consented subjects the operation of a Holter monitor (MyECG E3-80, Mircostar Company, Taipei; PhysiolGuard, Taipei) and the standard procedure for data collection of 4-lead ECG (i.e., placing/removing electrodes and starting/stopping a recording). After the visit, subjects brought the device home, together with written instructions. During the ambulatory study phase of ~3.5 days, subjects continuously wore the electrodes and the device except when: (i) showering; and (ii) the battery of the device was being charged, which occurred only once on the second day of the study. ECG waveforms were continuously recorded at 1000 Hz, and data were saved directly to a memory card within the device. For all 40 participants, the ECG recordings were collected during the same period that was between three to five months after the dietary obesity treatment program. All the ECG data collected by MG and CB were first de-identified and then were provided to MT, HY, KH who performed the heart rate variability analysis and statistical analysis.
Heart rate variability analysis
R waves in the ECG were identified with a QRS wave detector based on amplitude and the first derivative of the ECG waveform 29. Identified R waves were visually scanned by a trained technician to ensure that only normal R waves were included for further analysis (see Supplementary Materials for details). To assess autonomic nervous system activity, we performed heart rate variability analysis in both time and frequency domains according to published standards 27. In the time domain analysis, mean and SD of normal-to-normal (SDNN) beat intervals were calculated, along with the percentage of differences between adjacent normal-to-normal intervals that were >50 ms (pNN50) as a parasympathetic activity measure. In the frequency domain analysis, power spectra of RR intervals were calculated in non-overlapped 10-min windows by fast Fourier transform of 3.41-Hz cubic spline resampled data. The high frequency (HF) power at 0.15–0.40 Hz was calculated as another measure of parasympathetic activity. Data were re-sampled into hourly bins for subsequent analyses.
Statistical analysis
C carriers were pooled for analyses because T/C carriers had obesity related characteristics similar to those of C/C subjects 7. Three statistical models were performed (Online Tables 1, 2 and 3). To assess daily rhythms of HRV variables and the effects of the genotype on the rhythms, we performed cosinor analyses using mixed models 25. In the first models, daily rhythms were estimated using a 24-h sinusoidal and a 12-h harmonic: Yi = μ + a1f1(ti) + b1g1(ti) + a2f2(ti) + b2g2(ti) + εi, where Yi is the ith data point of a HRV variable at time ti, μ is the mean (MESOR), f1(ti)=cos(2πti/24) and g1(ti)=sin(2πti/24) represent the cosine and sine components of the fundamental 24-hr rhythm, and f2(ti)=cos(2πti/12) and g2(ti)=sin(2πti/12) describe the 12-hr harmonic. In the models, HRV variables are included as outcome measures (a log transform was applied on HF to ensure a normal distribution); the four sinusoidal functions, genotype-group (TC/CC and TT), and their interactions as fixed factors; and subject as a random effect on the MESOR/intercept. For those HRV variables with significant interactions between genotypes and the sinusoidal functions, we further performed a bootstrap analysis to determine whether the interactions were due to the group differences in amplitude, in acrophase or both (see Supplementary Materials). To explore the associations between HRV daily rhythms and weight loss, we used similar cosinor mixed models in which HRV indices are included as outcome variables, f1(ti), g1(ti) , weight loss and their interactions as independent variables, and subject as a random effect (Online Table 2). In the third model (Online Table 3), the genotypic group and its interaction with f1(ti), g1(ti) was added to model 2 to access the effects of genotype on the association of weight loss. Two subjects (one C carrier and one TT carrier) did not complete the weight-loss program and, thus, were excluded for the analysis related to weight loss. In all the models, the potential effect of use of medication (yes or no) was also accounted for. All the analyses were performed using JMP 11 pro (SAS Institute Inc, North Carolina). The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Results
Participant characteristics
The duration of the weight loss treatment was not significantly different between two groups of selected subjects (24±21 weeks for C carriers and 22±16 weeks for non-carriers; P>0.7). The efficacy of treatment was based on mean values of weight loss. C carriers lost less weight (~3.5kg) as compared to non-carriers despite that all the obesity related parameters were similar between the two groups at baseline (Table 1). Habitual total energy intake, physical activity values, and sleep duration were also similar between the two genotype-groups. The only group differences were the times of wake-up and breakfast, i.e., the C carriers woke up ~31 minutes later (P<0.05) and ate their breakfasts ~55 minutes later in the morning (P<0.01).
Daily rhythm in heart rate variability
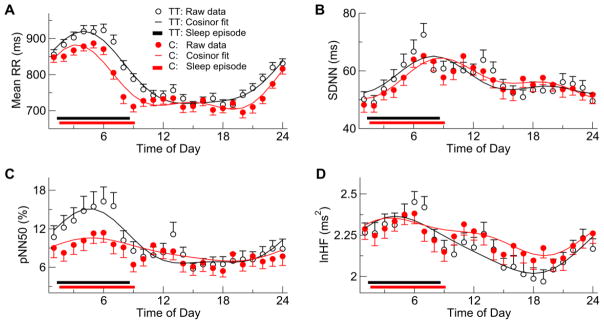
All tested HRV indices showed significant daily variations (Fig. 2). Mean normal-to-normal heart beat interval (mean RR) was larger at ~0:00–8:00AM with an evident peak in the early morning hours between 3-6AM, and became smaller during the daytime and early evening hours (Fig. 2A). SDNN was lowest at around the bedtime (0:00–1:00AM), increased across the sleep period, and gradually decreased after wake-up and throughout the daytime and evening (Fig. 2B). Consistently, the parasympathetic activity indices including pNN50 and HF showed lower values during the daytime and higher values during the nighttime (Fig. 2C, 2D). Note that the log transform of HF (lnHF) was used to ensure a normal distribution. Cosinor mixed models confirmed the daily HRV variations and revealed significant 24-h sinusoidal components in all test HRV indices (all P values <0.0001). Additionally, all measures except for SDNN showed significant 12-h harmonics, indicating that daily HRV rhythms did not follow a simple sinusoidal form.
Fig. 2.
Daily pattern of heart rate variability (HRV). Shown are (means±SE) group averages of mean RR, SDNN (a marker for overall HRV), pNN50 (a time-domain marker of cardiac vagal modulation), and HF (a frequency-domain marker of cardiac vagal modulation). The log transform of HF (lnHF) was used to HF to ensure a normal distribution. Solid curves are the fits based on cosinor mixed models.
Reduced daily HRV rhythm in C carriers
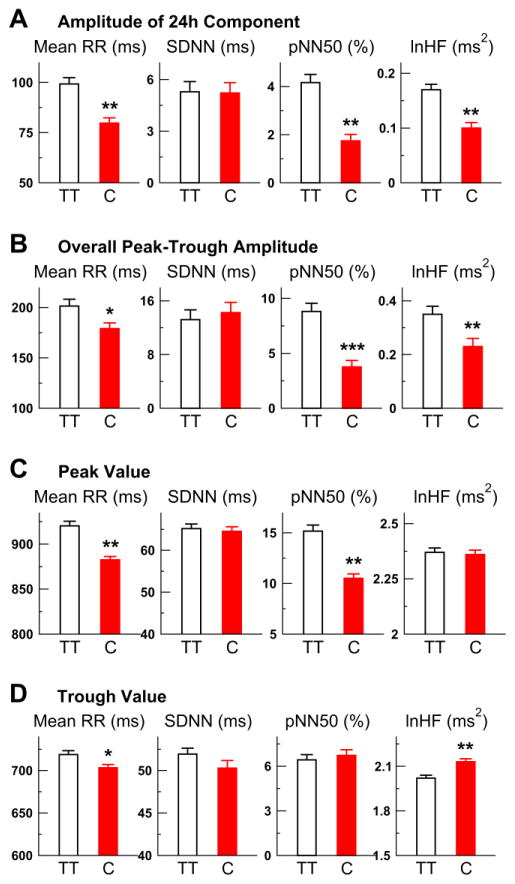
For all tested HRV variables, there were significant group differences in the 24-h components (P<0.05 for the interaction term: group x 24-h components) (Fig. 2). Based on the bootstrap analysis (see Supplementary Materials), such group differences were characterized by reduced amplitudes of 24-h sinusoidal components in the risk carriers except for SDNN, i.e., reduction was ~19.7% for mean RR (P=0.001), 58.1% for pNN50 (P=0.001), and 41.1% for HF (P=0.001) (Fig. 3A; Table 2). In the 24-h SDNN component, the amplitude was similar between groups while the acrophase was significantly delayed about ~1.9 hours in the C carriers (Table 2). The group differences in these daily HRV rhythms were independent of the effects of medication.
Fig. 3.
Effect of the genetic variant CLOCK 3111T/C on the amplitude of daily heart rate variability rhythm. The rhythms of HRV indices (mean RR, SDNN, pNN50, HF) were estimated using 24-h sinusoids and 12-h harmonics. The log transform of HF (lnHF) was used to HF to ensure a normal distribution. (A–D) Group averages (SE) of the amplitudes/coefficients of the 24-h components (A), the peak-trough amplitudes of the overall fits (B), the peak values (C) and the trough values (D). Significant group differences are indicated by * (p<0.05), ** (p<0.01), or *** (p<0.0001).
Table 2.
Parameter estimates of daily/circadian profiles of heart rate variability measures. Results were obtained using bootstrap. Amplitudes in 24-h and 12-h components are peak to mesor values.
| Group T (n=20) | Group C (n=20) | Group difference | |
|---|---|---|---|
| mean ± SD | mean ± SD | P-value | |
| Mean RR (msec) | |||
| 24-h component | |||
| Amplitude | 99.24 ± 3.15 | 79.73 ± 2.62 | 0.001 |
| Acrophase(h) | 3.83 ± 0.13 | 3.51 ± 0.14 | NS |
| 12-h harmonic | |||
| Amplitude | 23.99 ± 3.18 | 35.96 ± 2.80 | 0.005 |
| Acrophase(h) | 4.13 ± 0.27 | 3.19 ± 0.14 | 0.003 |
| Overall | |||
| Peak-trough amplitude | 201.50 ± 6.78 | 179.07 ± 5.71 | 0.012 |
| Peak time(h) | 3.98 ± 0.17 | 3.30 ± 0.13 | 0.002 |
| Trough time(h) | 14.62 ± 1.14 | 18.68 ± 1.67 | 0.045 |
| Peak value | 920.21 ± 5.17 | 882.46 ± 3.73 | 0.001 |
| Trough value | 718.70 ± 4.61 | 703.19 ± 3.87 | 0.011 |
| SDNN (msec) | |||
| 24-h component | |||
| Amplitude | 5.29 ± 0.59 | 5.22 ± 0.6 | NS |
| Acrophase(h) | 8.33 ± 0.42 | 10.32 ± 0.44 | 0.001 |
| Overall | |||
| Peak-trough amplitude | 13.20 ± 1.46 | 14.25 ± 1.52 | NS |
| Peak time(h) | 7.98 ± 0.31 | 8.74 ± 0.34 | NS |
| Trough time(h) | 22.84 ± 3.05 | 24.95 ± 0.37 | NS |
| Peak value | 65.18 ± 1.10 | 64.53 ± 1.09 | NS |
| Trough value | 51.93 ± 0.70 | 50.28 ± 0.89 | NS |
| pNN50 (%) | |||
| 24-h component | |||
| Amplitude | 4.16 ± 0.34 | 1.74 ± 0.27 | 0.001 |
| Acrophase(h) | 4.5 ± 0.26 | 5.52 ± 0.6 | NS |
| 12-h harmonic | |||
| Amplitude | 1.36 ± 0.31 | 0.43 ± 0.21 | 0.014 |
| Acrophase(h) | 4.64 ± 0.48 | 3.68 ± 1.96 | NS |
| Overall | |||
| Peak-trough amplitude | 8.81 ± 0.75 | 3.77 ± 0.58 | 0.0001 |
| Peak time(h) | 4.58 ± 0.31 | 4.85 ± 0.89 | NS |
| Trough time(h) | 15.99 ± 2.70 | 18.56 ± 1.59 | NS |
| Peak value | 15.17 ± 0.60 | 10.50 ± 0.44 | 0.001 |
| Trough value | 6.43 ± 0.35 | 6.73 ± 0.38 | NS |
| HF | |||
| 24-h component | |||
| Amplitude | 0.17 ± 0.01 | 0.10 ± 0.01 | 0.001 |
| Acrophase(h) | 4.93 ± 0.26 | 5.83 ± 0.50 | NS |
| 12-h harmonic | |||
| Amplitude | 0.03 ± 0.01 | 0.04 ± 0.01 | NS |
| Acrophase(h) | 2.34 ± 1.14 | 1.95 ± 0.64 | NS |
| Overall | |||
| Peak-trough amplitude | 0.35 ± 0.03 | 0.23 ± 0.03 | 0.002 |
| Peak time(h) | 4.06 ± 0.46 | 3.64 ± 0.76 | NS |
| Trough time(h) | 18.00 ± 0.57 | 19.08 ± 0.50 | NS |
| Peak value | 2.37 ± 0.02 | 2.36 ± 0.02 | NS |
| Trough value | 2.02 ± 0.02 | 2.13 ± 0.02 | 0.001 |
The similar group differences were also observed in the overall fitted daily rhythms (i.e., combining the 24-h and 12-h components). The bootstrap analysis showed that the C carriers had smaller peak-to-trough amplitudes in mean RR (11.1% smaller) and parasympathetic activity rhythms (pNN50: 57.2% smaller, HF: 34.2% smaller) as compared to TT (Fig. 3B). The bootstrap analysis also revealed that the C carriers had smaller peak and trough values for mean RR; a smaller peak value but a similar trough value for pNN50; and a larger trough value but a similar peak value for HF (Figs. 3C and 3D). The seemingly discrepancy between the results of pNN50 and HF may be due to the different sensitivities of the two parasympathetic measures at different levels of vagal tone, i.e., HF may be not sensitive at relatively high levels of vagal tone (e.g., during sleep) due to a saturation effect 30 while pNN50 may be not sensitive at relatively low levels of vagal tone 31 (see Supplementary Materials for details). In addition, the C carriers had an advanced peak (i.e., ~0.68 h earlier) and a delayed trough (i.e., ~4.04 h delayed) in the overall fitted rhythm of mean RR (Table 2). No significant group differences in the peak and trough locations were observed for the fitted rhythms of the parasympathetic indices (i.e., pNN50 and HF).
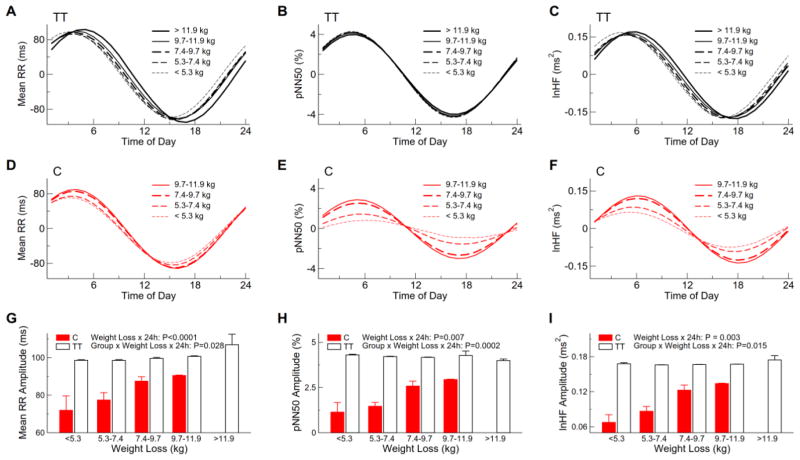
Interactive effects of CLOCK 3111 T/C SNP and daily parasympathetic activity rhythm on total weight loss
We further examined whether the amplitudes of 24-h HRV rhythm were associated with weight loss When combining subjects in both groups, subjects who lost less weight had smaller amplitudes of 24-h sinusoidal components in the daily rhythms of mean RR (P<0.0001), pNN50 (P=0.007) and HF (P=0.003). No such association was found for SDNN (P>0.3). These associations between the 24-h HRV components and weight loss were different between two groups (P= 0.028 for mean RR; P=0.0002 for pNN50; and P=0.015 for HF) (Fig. 4). These rhythmicity-weight-loss associations were highly significant within the C carriers (P = 0.0001, P<0.0001 and P=0.002 for mean RR, pNN50, and HF, respectively) but became less or not significant within the TT carriers (P=0.0001, P>0.7 and P=0.014 for mean RR, pNN50 and HF, respectively). For instance, the predicted amplitude of the 24-h component in the daily pNN50 (or lnHF) rhythm was ~2.9% (or 0.13) in the 3 C carriers with weight loss of 9.7 Kg and was reduced to ~1.14% (or 0.07) in the 5 C carriers with weight loss <5.3 Kg; and the predicted amplitude of the 24-h component in the daily pNN50 (or lnHF) rhythm was similar between the 8 non-carriers with weight loss 9.7 Kg and the 5 non-carriers subject with weight loss <5.3 Kg (Figs. 4H, 4I).
Fig. 4.
Effect of the genetic variant CLOCK 3111T/C on the association between weight loss and daily heart rate variability rhythm. To demonstrate the weight-rhythm association, the 24-h components in the daily rhythms of heart rate variability indices (A–F) and the corresponding amplitudes (G–I) in five categories of weight loss (i.e., <5.3kg, 5.3–7.4 kg, 7.4–9.7 kg, 9.7–11.9kg, and >11.9 kg) were shown. Note that there were no C carriers in the category of >11.9 kg. Results were based on mixed models with weight loss (a continuous variable), group, coefficients of 24-h components, and their interactions (weigh loss x 24-h coefficients) as the fixed effects and subject as a random effect.
Discussion
This study shows for the first time that the 24-h profile of heart rate variability in women differs across CLOCK 3111T/C genetic variants. As compared to TT carriers, risk allele C carriers had a reduction of 34–57% in the daily rhythm amplitude of parasympathetic activity as estimated by pNN50 and HF power. The reduction was caused by reduced parasympathetic activity during the nighttime when vagal tone is supposed to be high (indicated by pNN50) and increased parasympathetic activity during the daytime when vagal tone is supposed to be low (as indicated by HF). Consistently, the daily rhythm amplitude of mean RR was also reduced with decreased values during the nighttime and increased values during the daytime. More interestingly, the amplitude of the parasympathetic rhythm was associated with the weight loss during the obesity treatment. It is accepted that the autonomic nervous system plays an important role in obesity and obesity-related metabolic alterations 32,33. The observed increase in parasympathetic activity during the daytime in C carriers is consistent with the MONA LISA hypothesis 20, suggesting reduced energy expenditure that can explain less weight loss as observed in these individuals.
One of the main problems in weight loss treatments is the dramatic inter-individual variability in response to the treatment 34. It is proposed that the elucidation of the genetic component in prognosis of weight management could assist in development of more effective and individually tailored therapeutic strategies 35. A previous study in 1495 overweight/obese subjects showed that carriers of the risk allele C had several behavioral and metabolic alterations that could be implicated in their weight loss difficulties such as shorter sleep duration, higher plasma ghrelin concentrations (the "hunger hormone"), delayed breakfast time, evening preference and less compliance with a Mediterranean Diet pattern, as compared with TT homozygotes 7. The current study suggests the alteration of autonomic function as a common mechanism underlying weight control difficulties and possibly other metabolic alterations in C carriers. Our results highlight the importance to examine the 24-h pattern of autonomic function together with the genetic background in order to predict the individual outcome of obesity treatment based on low-energy diets.
Though the current study is focused on the understanding of the difficulty to achieve desired weight loss in C carriers, our results may also have implications on risk for obesity in these individuals. Indeed, many studies showed the effect of this SNP on obesity or obesity-related-metabolic alterations 4–8,10. For instance, our previous study showed that C carriers weight 3 kg more (87 kg vs 84 kg ) than T carriers after controlling for other factors such as age gender and clinical center1. However, no significant associations of the SNP with BMI or waist circumference were reported in the recent studies from the GIANT consortium 36,37. The seemingly inconsistent results may be caused by the different threshold of the p value for a significance level used in the GWAS studies (i.e., due to more associations were tested in the GWAS studies). It is worth noting that weight loss and obesity are different outcomes such that different behaviors and genetic factors may be accounting for them. Currently there have been no GWAS studies investigating weight loss as a result of dietary treatments.
A concern on previous studies of the link between autonomic function and obesity is that timing of assessment is seldom considered. In chronobiology, it is established that autonomic function displays 24-h variations with higher sympathetic activity and lower parasympathetic activity during the daytime compared with nighttime 38. Due to the daily variations, a certain level of ANS activity can be physiological at one time point but might be pathophysiological at a different time point of the day 39. There is evidence that changes in these daily variations in autonomic function may be implicated in the risk for obesity-related pathologies 40. Here we found that daily parasympathetic activity variations are implicated in the response to an obesity treatment, especially in women carrying CLOCK 3111C.
The mechanisms underlying the effects of the genetic variant on daily variations of ANS activities are yet to be determined. One possibility is that daily rhythms in human physiology are produced by the daily behavioral cycles. Specifically, scheduled behaviors such as meal times, food intake, and physical activity levels may differ in C carriers during the daytime, leading to different ANS activities. We accounted for these potential behavioral effects in the current study by selecting the C carriers and non-carriers with matched physical activity levels as well as many other external factors including energy intake and sleep duration. The only group differences were that the C carriers woke up and ate their breakfasts later in the morning. These differences may explain the delayed peak of the overall heart rate variability (SDNN) but could not explain the reduced daily amplitudes of mean RR and parasympathetic indices in C carriers.
The observed reduction in parasympathetic activity in C carriers during the night might be associated with reduced sleep quality. This sleep hypothesis may explain the difficulty of weight control in the C carriers because decreased sleep quality leads to increased hunger and appetite, decreased concentrations of the satiety hormone leptin, increased concentrations of the hunger hormone ghrelin when caloric intake is controlled, and increased weight gain when caloric intake is ad libitum 38. To explore this possibility, we examined previously collected Polysomnography-based sleep measures of the 40 participants, including arousals, obstructive apnea events, and desaturation events. We found no significant group differences (all P values >0.5) 4. Note that these sleep measures were collected before the current study. Thus, simultaneous assessments of sleep and autonomic function are required to formally confirm or refute the link between autonomic function and sleep dynamics in risk C carriers.
Alternatively, daily rhythms of behavior and physiology can be contributed by the central circadian clock which prepares the body for the anticipated environmental and behavioral cycles 11. In mammals, the central circadian clock is the hypothalamic suprachiasmatic nucleus (SCN), which generates self-sustained oscillations with a period of ~24 h and controls the circadian rhythms of core physiological variables such as heart rate, body temperature and motor activity 11. A slight change in the circadian clock can have significant influences on behavior and physiology. For instance, the intrinsic period of the circadian clock is associated with chronotype (i.e., a longer period >24 h for evening-types and a shorter period <24h for morning-types). Future studies using more sophisticated circadian protocols are necessary to determine whether the intrinsic properties of the circadian clock may be different in C carriers, contributing to reduced parasympathetic activity directly or indirectly via its interaction with sleep-wake cycles.
Study limitations
Because daily activity and the circadian rhythm were not monitored/controlled simultaneously, we could not determine their separate contributions to the altered daily HRV rhythm of in C carriers. To better control the effects of activity and posture on autonomic nervous system activity, it is ideal to monitor activity and posture simultaneously with the ANS activity. In addition, the sample size in this study was relatively small. We did power calculation, and found that 40 subjects allowed the identification of the reported key effects with power > 80%, e.g., group differences in the amplitudes of daily HRV rhythms: >98% for both pNN50 and HF; the associations between the amplitudes and weight loss: >84% for pNN50 and HF. HRV allows only reliable assessment of parasympathetic activity. Whether daily sympathetic activity rhythm differs in C carriers are worth further investigating. Furthermore, the HRV assessment was performed only once after the weight-loss intervention such that the ability of the HRV daily rhythm to predict the effectiveness of achieving desired weight loss is to be determined. Thus, future studies with better design and large sample sizes are warranted to confirm the findings in this pilot study.
Conclusions
The daily rhythm of vagal modulation is reduced in women carrying CLOCK 3111C as compared to non-carriers. The reduction negatively correlates with the effectiveness of achieving desired weight loss in these C carriers. These findings suggest that altered daily ANS activity may underlie the effect of genetic variant of CLOCK 3111T/C on body weight control.
Supplementary Material
Acknowledgments
We thank the research volunteers for their participation. We thank Yu-Hsin Tzeng for the help of ECG preprocessing, and Tommy To for the help with the figures. This study was supported by Spanish Ministry of Economy and Competitiveness (SAF2014-52480) (to M.G.), by National Science Council (NCU) of Taiwan grants 104-3115-E-008-001-, 103-2321-B-008-003-, 103-2221-E-008-006-MY3 (to M.T.L.), and by joint foundation of CGH and NCU grants CNJRF-101CGH-NCU-A4 and VGHUST103-G1-3-3 (to M.T.L). K.H. was supported by NIH grants R00-HL102241, P01AG009975, and R01AG048108. F.A.J.L.S. was supported in part by NIH grants R01 DK099512, R01 HL118601, and R01 DK102696.
Footnotes
Conflict of Interest: The authors declare no conflict of interest
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
References
- 1.Garaulet M, Lee Y-C, Shen J, Parnell LD, Arnett DK, Tsai MY, et al. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am J Clin Nutr. 2009;90(6):1466–75. doi: 10.3945/ajcn.2009.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garaulet M, Corbalán MD, Madrid JA, Morales E, Baraza JC, Lee YC, et al. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int J Obes 2005. 2010;34:516–523. doi: 10.1038/ijo.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovaninni NP, Fuly JT, Moraes LI, Coutinho TN, Trarbach EB, de Jorge AAL, et al. Study of the association between 3111T/C polymorphism of the CLOCK gene and the presence of overweight in schoolchildren. J Pediatr (Rio J) 2014;90:500–505. doi: 10.1016/j.jped.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Bandín C, Martinez-Nicolas A, Ordovás JM, Ros Lucas JA, Castell P, Silvente T, et al. Differences in circadian rhythmicity in CLOCK 3111T/C genetic variants in moderate obese women as assessed by thermometry, actimetry and body position. Int J Obes. 2005–2013;37:1044–1050. doi: 10.1038/ijo.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galbete C, Contreras R, Martínez JA, Martínez-González MÁ, Guillén-Grima F, Marti A. Physical activity and sex modulate obesity risk linked to 3111T/C gene variant of the CLOCK gene in an elderly population: the SUN Project. Chronobiol Int. 2012;29:1397–1404. doi: 10.3109/07420528.2012.728657. [DOI] [PubMed] [Google Scholar]
- 6.Garaulet M, Esteban Tardido A, Lee Y-C, Smith CE, Parnell LD, Ordovás JM. SIRT1 and CLOCK 3111T> C combined genotype is associated with evening preference and weight loss resistance in a behavioral therapy treatment for obesity. Int J Obes. 2005–2012;36:1436–1441. doi: 10.1038/ijo.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garaulet M, Sánchez-Moreno C, Smith CE, Lee Y-C, Nicolás F, Ordovás JM. Ghrelin, sleep reduction and evening preference: relationships to CLOCK 3111 T/C SNP and weight loss. PloS One. 2011;6:e17435. doi: 10.1371/journal.pone.0017435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteleone P, Tortorella A, Docimo L, Maldonato MN, Canestrelli B, De Luca L, et al. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: association with higher body mass index. Neurosci Lett. 2008;435:30–33. doi: 10.1016/j.neulet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Lozano T, Vidal J, de Hollanda A, Canteras M, Garaulet M, Izquierdo-Pulido M. Evening-chronotype associates with obesity in severe obese subjects: interaction with CLOCK 3111T/C. Int J Obes. 2005–2016 doi: 10.1038/ijo.2016.116. [DOI] [PubMed] [Google Scholar]
- 10.Tsuzaki K, Kotani K, Sano Y, Fujiwara S, Takahashi K, Sakane N. The association of the Clock 3111 T/C SNP with lipids and lipoproteins including small dense low-density lipoprotein: results from the Mima study. BMC Med Genet. 2010;11:150. doi: 10.1186/1471-2350-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 12.Mohawk JA, Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 2011;34:349–358. doi: 10.1016/j.tins.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozburn AR, Purohit K, Parekh PK, Kaplan GN, Falcon E, Mukherjee S, et al. Functional Implications of the CLOCK 3111T/C Single-Nucleotide Polymorphism. Front Psychiatry. 2016;7:67. doi: 10.3389/fpsyt.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalsbeek A, la Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab. 2014;3:372–383. doi: 10.1016/j.molmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol. 2009;20:127–134. doi: 10.1097/MOL.0b013e3283292399. [DOI] [PubMed] [Google Scholar]
- 17.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messina G, De Luca V, Viggiano A, Ascione A, Iannaccone T, Chieffi S, et al. Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol Res Int. 2013;2013:639280. doi: 10.1155/2013/639280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray GA, York DA. The MONA LISA hypothesis in the time of leptin. Recent Prog Horm Res. 1998;53:95-117-118. [PubMed] [Google Scholar]
- 21.Romijn JA, Fliers E. Sympathetic and parasympathetic innervation of adipose tissue: metabolic implications. Curr Opin Clin Nutr Metab Care. 2005;8:440–444. doi: 10.1097/01.mco.0000172586.09762.55. [DOI] [PubMed] [Google Scholar]
- 22.Kalsbeek A, Palm IF, La Fleur SE, Scheer Fa JL, Perreau-Lenz S, Ruiter M, et al. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 23.Shea SA, Hilton MF, Hu K, Scheer FAJL. Existence of an Endogenous Circadian Blood Pressure Rhythm in Humans That Peaks in the Evening. Circ Res. 2011;108:980–U207. doi: 10.1161/CIRCRESAHA.110.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheer FAJL, Michelson AD, Frelinger AL, Evoniuk H, Kelly EE, McCarthy M, et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PloS One. 2011;6:e24549. doi: 10.1371/journal.pone.0024549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu K, Scheer FAJL, Laker M, Smales C, Shea SA. Endogenous Circadian Rhythm in Vasovagal Response to Head-Up Tilt. Circulation. 2011;123:961–U85. doi: 10.1161/CIRCULATIONAHA.110.943019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheer FAJL, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010;107:20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 28.Serra-Majem L, Aranceta J SENC Working Group on Nutritional Objectives for the Spanish Population. Spanish Society of Community Nutrition. Nutritional objectives for the Spanish population. Consensus from the Spanish Society of Community Nutrition. Public Health Nutr. 2001;4:1409–1413. doi: 10.1079/phn2001229. [DOI] [PubMed] [Google Scholar]
- 29.Friesen GM, Jannett TC, Jadallah MA, Yates SL, Quint SR, Nagle HT. A comparison of the noise sensitivity of nine QRS detection algorithms. IEEE Trans Biomed Eng. 1990;37:85–98. doi: 10.1109/10.43620. [DOI] [PubMed] [Google Scholar]
- 30.Chapleau MW, Sabharwal R. Methods of assessing vagus nerve activity and reflexes. Heart Fail Rev. 2011;16:109–127. doi: 10.1007/s10741-010-9174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mietus JE, Peng C-K, Henry I, Goldsmith RL, Goldberger AL. The pNNx files: reexamining a widely used heart rate variability measure. Heart Br Card Soc. 2002;88:378–380. doi: 10.1136/heart.88.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licht CMM, Vreeburg SA, van Reedt Dortland AKB, Giltay EJ, Hoogendijk WJG, DeRijk RH, et al. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J Clin Endocrinol Metab. 2010;95:2458–2466. doi: 10.1210/jc.2009-2801. [DOI] [PubMed] [Google Scholar]
- 33.Chang C-J, Yang Y-C, Lu F-H, Lin T-S, Chen J-J, Yeh T-L, et al. Altered cardiac autonomic function may precede insulin resistance in metabolic syndrome. Am J Med. 2010;123:432–438. doi: 10.1016/j.amjmed.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Bandín C, Martinez-Nicolas A, Ordovás JM, Madrid JA, Garaulet M. Circadian rhythmicity as a predictor of weight-loss effectiveness. Int J Obes. 2005–2014;38:1083–1088. doi: 10.1038/ijo.2013.211. [DOI] [PubMed] [Google Scholar]
- 35.Delahanty LM, Pan Q, Jablonski KA, Watson KE, McCaffery JM, Shuldiner A, et al. Genetic predictors of weight loss and weight regain after intensive lifestyle modification, metformin treatment, or standard care in the Diabetes Prevention Program. Diabetes Care. 2012;35:363–366. doi: 10.2337/dc11-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G, et al. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81:537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- 39.Kreier F, Yilmaz A, Kalsbeek A, Romijn JA, Sauerwein HP, Fliers E, et al. Hypothesis: shifting the equilibrium from activity to food leads to autonomic unbalance and the metabolic syndrome. Diabetes. 2003;52:2652–2656. doi: 10.2337/diabetes.52.11.2652. [DOI] [PubMed] [Google Scholar]
- 40.Buijs RM, Escobar C, Swaab DF. The circadian system and the balance of the autonomic nervous system. Handb Clin Neurol. 2013;117:173–191. doi: 10.1016/B978-0-444-53491-0.00015-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.