Abstract
Sinonasal disease can contribute to poor asthma control. There are reports that link obesity with an increased prevalence of sinonasal disease, but no studies evaluating the severity of sinonasal disease in obese asthmatics, and how this impacts asthma control. The purpose of the current study was to determine if obesity is associated with increased severity of sinonasal disease, and/or affects response to nasal corticosteroid treatment in asthma.
Methods
This study included 236 adults participating in a 24-week randomized, double-masked, placebo-controlled study of nasal mometasone for the treatment of poorly controlled asthma. Sinonasal disease severity was assessed using validated questionnaires, and compared in participants of differing BMI. Eosinophilic inflammation was assessed using markers in nasal lavage, serum and exhaled nitric oxide. Response to treatment was compared in the different BMI groups.
Results
Obesity had no effect on the severity of sinonasal disease symptoms in asthmatics (Sino-Nasal Outcomes Test 22 score [mean ± SD] 35.4±18.5, 40.2±22.8, 39.1±21.7, p=0.43, in lean, overweight and obese participants), nor on nasal, bronchial or systemic markers of allergic inflammation. Nasal steroids had some limited effects on symptoms, lung function and inflammatory markers in lean participants, but no detectable effect in obese patients.
Conclusions
Obesity does not affect severity of sinonasal disease in patients with asthma; the association of sinonasal disease symptoms with increased asthma severity, and markers of Type 2 inflammation are consistent across all BMI groups. The response of obese patients to nasal corticosteroids requires further study.
Keywords: Obesity, rhinitis/sinusitis, physiology, treatment
INTRODUCTION
Sinonasal disease and asthma share a common pathophysiology, manifestations of a single pathologic process affecting one unified airway [1–3]. Allergen challenge in the nose causes inflammation in the lower airway, while allergen challenge in the lower airway leads to inflammation in the nose [4, 5]. Rhinitis and sinusitis are associated with severe asthma, and in patients with poorly controlled asthma these symptoms have been associated with increased risk of asthma exacerbations [6, 7]. Thus it is thought that uncontrolled sinonasal disease might contribute to poor asthma control.
Much research linking sinonasal disease and asthma was performed before the current obesity epidemic. Obesity is now recognized as a major risk factor for asthma: approximately 250 000 new cases of asthma per year in the United States are related to obesity, and obese asthmatics account for nearly 60% of those with severe/poorly controlled asthma [8, 9]. Increased sinus disease severity has also been associated with obesity in the general population [10], and so it is possible that increased severity of sinonasal disease might be a factor contributing to increased asthma severity in obese asthmatics. Another factor that might contribute to increased severity of airway disease in obesity is reduced response to nasal corticosteroids; obese asthmatics have reduced response to inhaled corticosteroids, though the efficacy of nasal corticosteroids in obesity has not been addressed [11]. It is not known whether the relationship between sinonasal disease and asthma identified in the general population exists in obese patients with asthma. In particular, it is not known whether sinonasal disease in obese asthmatics has increased severity (as has been shown for asthma in obesity) or whether it responds to similar treatments when compared to lean asthmatics.
The purpose of the current study was to investigate the nature of sinonasal disease and markers of Type 2 inflammation in obese patients with asthma. We hypothesized that sinonasal disease severity would be increased in obese patients, contributing to poor asthma control in obesity. We also hypothesized that obese asthmatics with sinonasal disease would have a reduced response to nasal corticosteroid therapy when compared to lean asthmatics.
METHODS
This study included 236 adult asthmatics with chronic sinonasal disease participating in the American Lung Association Airways Clinical Research Centers “Study of Asthma and Nasal Steroids”. Full details of the primary study have been published elsewhere [12]. In brief, this was a 24 week, multicenter, randomized, placebo-controlled trial of a nasal corticosteroid (mometasone) versus placebo for the treatment of sinonasal disease in patients with poorly controlled asthma and chronic sinonasal disease. Sinonasal disease was diagnosed by validated questionnaire [13]. The study was approved by the institutional review boards at all centers, and informed consent was obtained from all participants.
At baseline, participants completed detailed questionnaires regarding asthma, including the Asthma Control Test [14], Asthma Symptom Utility Index [15], and Marks Asthma Quality of Life Questionnaire [16]. Poorly controlled asthma was determined by score on the Asthma Control Test of ≤ 19 [14], and chronic sinonasal disease was identified by questionnaire [13].
Participants performed spirometry, methacholine challenge test (if FEV1> 70% predicted) and measurement of exhaled nitric oxide according to ATS guidelines [17–19]. Allergy skin testing was also performed [12]. Nasal lavage was performed, and tested for eosinophilic cationic protein and CCL-17 [20, 21]. Participants completed questionnaires regarding their sinonasal disease with the SinoNasal Outcomes Test 22 [22], sinonasal questionnaire [13] and sinus symptom questionnaire [23]. Participants were randomized to receive nasal mometasone or placebo treatment for 24 weeks. At the end of 24 weeks they returned for repeat spirometry, methacholine challenge, exhaled nitric oxide test, nasal lavage and completion of asthma and sinus questionnaires.
Data Analysis
Baseline data was summarized by BMI group. Lean was defined as BMI 18.5–24.9 kg/m2, overweight as 25–29.9 kg/m2 and obese as ≥ 30 kg/m2. Analysis of variance and Kruskal-Wallis tests were used to compare normally distributed and non-normally distributed quantitative variables between groups.
We compared baseline symptoms, measures of lung function, and markers of Type 2 inflammation related to asthma and sinonasal disease in adult patients of differing BMI group. We also compared the change in these markers over time between placebo versus mometasone group using mixed models repeated measures analysis of variance. The results for group by visit interaction from these analyses were used to test for significant differences in treatment effects between groups. For all analyses, the significance level was set, a priori, at alpha = 0.05. SAS version 9.2, SAS Institute, Inc., Cary, NC, USA was used for all statistical analysis.
RESULTS
Of the 236 participants in this study 24% were categorized as lean, 26% as overweight and 50% as obese. The study population had a female predominance overall. The obese population had a higher proportion of females and African Americans when compared to the other BMI groups, and was also older than the other BMI groups. There was a low prevalence of Asians in the obese category.
Asthma control and quality of life tended to be a little more severe in the obese groups as measured by the Asthma Control Test (ACT) and Marks Asthma Quality of Life Questionnaire (MAQLQ) respectively, although this did not reach statistical significance, and symptoms of asthma assessed by the ASUI were similar across all three groups (Table 1). Lung function, assessed by FEV1 and FVC were significantly lower in the obese group, and exhaled nitric oxide was also significantly lower in the obese group compared with both the lean and overweight group. The severity of sinonasal disease, as measured by the Sinus Symptom Score and the SNOT 22 questionnaires, showed no significant statistical difference across all three BMI groups, nor were there any differences in markers of Type 2 inflammation in serum or nasal lavage (Table 1).
Table 1.
Demographics of study population
| BMI 18.5–24.9 | BMI 25–29.9 | BMI > 30 | p value | |
|---|---|---|---|---|
| n (%) | 57 (24) | 62 (26) | 117 (50) | |
| Female n (%) | 36 (63) | 38 (62) | 84 (72) | 0.29 |
| Race n (%) | ||||
| White | 37 (65) | 35 (56) | 53 (45) | 0.02 |
| Black | 13 (23) | 18 (29) | 56 (48) | |
| Asian | 3 (5) | 3 (5) | 1 (1) | |
| Other | 4 (7) | 6 (10) | 6 (5) | |
| Ethnicity n (%) | ||||
| Hispanic | 4 (7) | 12 (20) | 21 (18) | 0.13 |
| Age | 36±15 | 42±141 | 42±121 | <0.01 |
| Age of asthma onset | 12.5±13.8 | 16.1±14.5 | 15.5±14.9 | 0.33 |
| Steroids in last 12 months | 21 (37%) | 20 (32%) | 49 (42%) | 0.44 |
| Atopya | 43 (75) | 56 (92) | 98 (82) | 0.06 |
| ACT (range 5–25↑) | 15.8±2.7 | 15.6±3.2 | 14.6±3.6 | 0.06 |
| ASUI (range 0–1↑) | 0.69±0.17 | 0.74±0.18 | 0.69±0.19 | 0.26 |
| MAQLQ (range 1–80↓) | 20.0±12.9 | 21.5±14.6 | 24.8±16.5 | 0.12 |
| FEV1 (% predicted) | 88.1±14.0 | 81.8±17.7 | 79.1±15.8b | < 0.01 |
| FVC (% predicted) | 98.5±14.5 | 93.8±16.1 | 88.2±15.2b | < 0.001 |
| FEV1/FVC (% predicted) | 88.8±10.5 | 86.5±10.5 | 88.7±9.8 | 0.34 |
| Exhaled Nitric Oxide (ppb) | 39.19±32.07 | 41.28±33.79 | 27.04±19.94b,c | <0.01 |
| Sinus Symptom Score (range 1–60↓) | 24.8±12.0 | 26.6±13.8 | 27.4±12.3 | 0.42 |
| SNOT 22 (range 0–120↓) | 35.4±18.5 | 40.2±22.8 | 39.1±21.7 | 0.43 |
| Nasal ECP (ng/ml) | 29.7±32.0 | 25.5±37.4 | 23.3±29.6 | 0.5 |
| Nasal CCL17 (ng/ml) | 22.0±13.4 | 25.4±18.8 | 24.7±21.0 | 0.88 |
| Serum ECP (ng/ml) | 19.3±19.6 | 12.7±12.2 | 17.7±31.2 | 0.32 |
| serum CCL17 (ng/ml) | 365±196 | 341±165 | 361±193 | 0.76 |
↑ higher score indicative of better control, ↓ lower score indicative of better control
Atopy defined as any skin test positive, 233 had skin tests
post hoc testing, p < 0.01 compared with lean group, and
p< 0.05 compared with overweight group (ANOVA followed by Bonferroni correction)
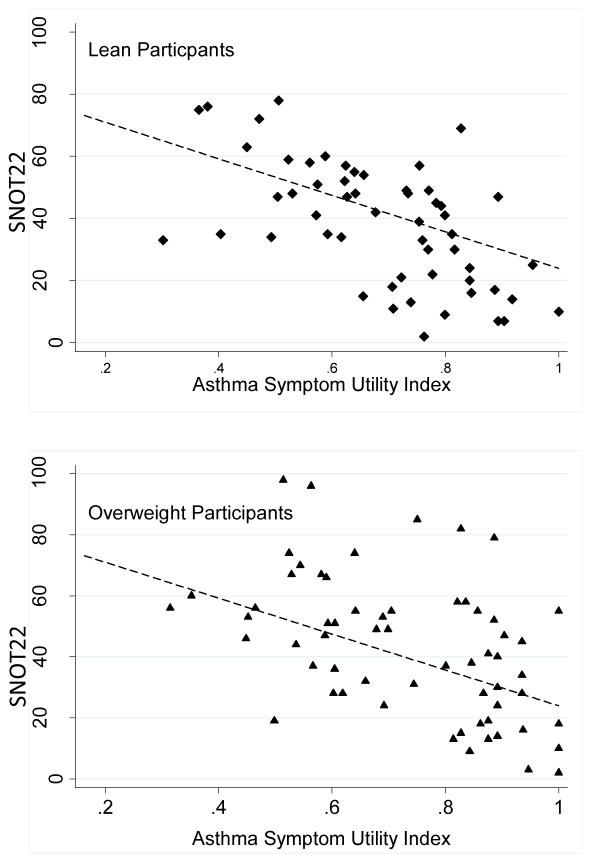
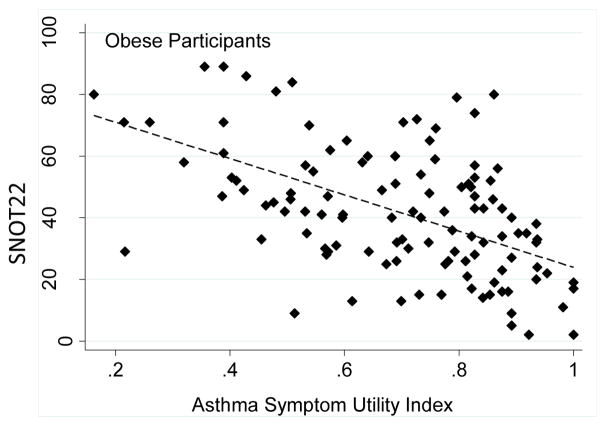
We investigated the relationship between severity of sinonasal symptoms and symptoms of asthma in the different BMI groups. We found that symptoms of asthma and symptoms of sinonasal disease were highly correlated, and there were no differences in this relationship between lean, overweight and obese subjects (Figure 1).
Figure 1.
Relationship between sinus symptoms (measured by the Sino Nasal Outcomes Test-22, SNOT22) and asthma symptoms (measured by the ASUI) in participants of different BMI categories. A. Lean participants, linear slope β coefficient = −0.005, SE = 0.0009, p< 0.001, B. Overweight participants, linear slope β coefficient = −0.004, SE = 0.0009, p< 0.001, C. Obese participants, linear slope coefficient = −0.005, SE 0.0008, p< 0.001. Slope of obese group not significantly different than lean group (p=0.475).
We investigated the efficacy of nasal steroids treatment on asthma and sinus symptoms outcomes in the different BMI groups (Table 2). Overall we found few differences: FVC improved significantly in the lean group treated with nasal mometasone, although this was not related to any improvement in asthma symptoms, as neither the ACT nor the ASUI improved in the lean participants. Lean participants did have a greater improvement in sinus symptoms than the other groups (though this did not quite reach statistical significance). The ASUI improved significantly in the obese group treated with nasal corticosteroids, though the clinical relevance of this is uncertain, given that the lean and overweight groups had a numerically greater improvement in ASUI.
Table 2.
Effect of nasal steroid treatment on symptom scores in different BMI groups
| BMI group | Placebo | Nasal Mometasone | P value |
|---|---|---|---|
| Change from V2 to V5* | Change from V2 to V5* | ||
| FEV1 (% predicted) | |||
| < 25 | 0.82 (−1.22, 2.86) | 1.69 (−1.04, 4.41) | 0.61 |
| 25–29.9 | 1.68 (−2.71, 6.08) | −3.18 (−7.57, 1.22) | 0.12 |
| > 30 | −0.06 (−3.43, 3.30) | −1.99 (−5.11, 1.12) | 0.41 |
| FVC (% predicted) | |||
| < 25 | −0.14 (−1.66, 1.39) | 2.64 (−0.61, 4.68) | 0.03 |
| 25–29.9 | 1.90 (−1.92, 5.72) | −2.61 (−6.43, 1.21) | 0.10 |
| > 30 | −0.79 (−3.81, 2.24) | −2.10 (−4.90, 0.69) | 0.53 |
| ASUI (↑) | |||
| < 25 | 0.09 (0.03, 0.14) | 0.14 (0.07, 0.21) | 0.21 |
| 25–29.9 | 0.04 (−0.02, 0.11) | 0.10 (0.03, 0.16) | 0.25 |
| > 30 | −0.008 (−0.06, 0.04) | 0.07 (0.03, 0.12) | 0.02 |
| ACT (↑) | |||
| < 25 | 3.34 (2.07, 4.61) | 2.93 (1.37, 4.49) | 0.68 |
| 25–29.9 | 2.38 (0.83, 3.92) | 3.96 (2.41, 5.51) | 0.15 |
| > 30 | 1.98 (0.76, 3.19) | 2.14 (1.00, 3.27) | 0.85 |
| SNOT22 (↓) | |||
| < 25 | −4.93 (−10.12, 0.27) | −13.10 (−19.57, −6.63) | 0.053 |
| 25–29.9 | −8.12 (−15.19, −1.05) | −13.90 (−21.18, −6.62) | 0.26 |
| > 30 | −6.25 (−12.37, −0.14) | −10.08 (−15.69, −4.47) | 0.36 |
Numbers shown are mean and SD
↑ higher score indicative of better control, ↓ lower score indicative of better control
V2 is baseline visit, V5 is end of study visit.
We then investigated the effects of nasal steroids on markers of allergic inflammation in the lungs (exhaled nitric oxide), in the serum (serum CCL17 and ECP) and in the nose (nasal CCL17 and ECP) (Table 3). We found that serum CCL17 decreased significantly in lean participants, and nasal CCL17 tended to decrease in overweight participants treated with mometasone versus placebo.
Table 3.
Effect of Treatment on markers of allergic inflammation in different BMI groups.
| BMI group | Placebo | Active treatment | P value |
|---|---|---|---|
| Exhaled NO (ppb) | Change from V2 to V5 | Change from V2 to V5 | |
| <25 | 3.2 (−7.67,14.01) | −4.56 (−14.48, 5.35) | 0.51 |
| 25–29.9 | −5.06 (−20.20, 10.08) | −1.52 (−12.44, 9.39) | 0.35 |
| > 30 | −0.31 (−8.22, 7.61) | 3.13 (−1.24, 7.49) | 0.86 |
| serum CCL17 (ng/ml) | |||
| <25 | −16.75 (−40.0, 6.54) | −60.9 (−89.2, −32.6) | 0.02 |
| 25–29.9 | −20.5 (−35.7, −5.19) | 9.44 (−19.56, 38.4) | 0.25 |
| > 30 | −15.54 (−40.04, 8.97) | −8.41 (−20.41, 3.59) | 0.34 |
| Serum ECP (ng/ml) | |||
| <25 | −1.69 (−3.63, 0.25) | −4.67 (−6.69, −2.65) | 0.81 |
| 25–29.9 | 0.18 (−1.28, 1.65) | −1.97 (−3.68, −0.25) | 0.36 |
| > 30 | −2.00 (−4.64, 0.65) | −1.48 (−2.69, −0.21) | 0.68 |
| Nasal ECP(ng/ml) | |||
| <25 | 11.3 (3.66, 19.0) | 5.65 (−5.52, 16.8) | 0.67 |
| 25–29.9 | −1.26 (−6.65, 4.13) | 5.42 (0.12, 10.72) | 0.92 |
| > 30 | 2.67 (−3.12, 8.53) | −0.22 (−3.93, 3.45) | 0.53 |
| Nasal CCL17 (ng/ml) | |||
| <25 | 0.15 (−2.65,2.95) | 1.89 (0.09,3.70) | 0.25 |
| 25–29.9 | −0.15 (−3.56, 3.26) | −10.57 (−14.74,−6.41) | 0.05 |
| > 30 | −0.81 (− 3.55,1.92) | −2.23 (−4.52,0.05) | 0.49 |
Numbers shown are mean and SD
V2 is baseline visit, V5 is end of study visit.
Discussion
This study showed no significant differences in the severity of sinonasal symptoms or in the markers of Type 2 inflammation across all three BMI groups: more severe asthma symptoms were associated with increased symptoms of sinonasal disease regardless of BMI. This suggests that severity of sinus disease and Type 2 inflammation are unaffected by obesity. We were also interested in the efficacy of nasal corticosteroids across the different BMI groups: there were significant improvements in FVC and serum CCL17 only in the lean group treated with nasal mometasone versus placebo, although this was not related to any improvement in asthma symptoms. Lean participants also had a greater improvement in sinus symptoms when treated with mometasone versus placebo. These data suggest there might be a slight decrease in responsiveness to nasal corticosteroids in obesity, but are far from conclusive, and so this will require further study.
Nationally representative data from the United States show a positive association between obesity and asthma, with the prevalence and the incidence of asthma increasing as the level of obesity increases [24]. Obesity has been associated with a dose-dependent increase in asthma severity, independent of other risk factors such as socio-demographic determinants, physical activity, and dietary patterns [25]. We found some markers of asthma severity were increased in the obese group: there was a significant difference in lung function, with obese participants having the lowest lung function. Using the ACT and the Mark’s AQLQ we found a numeric, though not significant, difference in symptoms and quality of life related to asthma experienced across different BMI groups, with the higher BMI groups reporting worse symptoms and quality of life. One reason that asthma symptoms and quality of life were not significantly different in obese participants in the current study may be related to the fact that the patient population was preselected to have poorly controlled asthma. Indeed, what is perhaps most striking is the very high prevalence of obesity in the study population at 49% (compared to approximately 38% in the U.S. population as a whole [26]). This high prevalence of obesity likely reflects the fact that the majority of patients with severe asthma in the United States are obese: the Tenor study, a study investigating the characteristics of patients with severe asthma in the U.S., found that well over ½ of patients with severe asthma were obese [9]. The very high prevalence of obesity in this study population selected to have poorly controlled asthma are consistent with previous reports suggesting that obesity is associated with severe asthma.
Prospective epidemiologic studies in the general population have provided evidence that strongly connects the development of asthma with a previous history of rhinitis [2, 27]. Furthermore, the likelihood of the development of asthma is much higher in individuals with both perennial and seasonal rhinitis than in individuals with either condition alone [3]. There are strong data linking severe sinonasal disease with severe asthma [27], with severity of sinus disease on CT scan an independent risk factor for asthma exacerbations [28].
A recent report also found that obesity was a risk factor for sinonasal disease. Bhattacharyya et al found that obesity was associated rhinosinusitis [10]: the adjusted odds ratio for acute rhinosinusitis when obesity was present was 1.22 ( P < .001, 95% CI: 1.12–1.33), and the adjusted odds ratio for chronic rhinosinusitis when obesity was present was 1.31 ( P < .001, 95% confidence interval = 1.18–1.45).[10]. We were interested in investigating whether obesity is associated with more severe sinonasal disease in asthma, and if this could be a factor contributing to severe asthma in obesity. Obesity might contribute to both sinonasal disease and asthma through mechanical loading of the chest and excess pharyngeal tissue impairing sinus drainage. When we compared the relationship between severity of sinonasal symptoms and symptoms of asthma across different BMI groups we found a strong and consistent correlation of asthma symptoms with sinus symptoms which did not differ between lean, overweight and obese subjects. Increased severity of sinonasal disease appears to be related to increased severity of asthma, regardless of BMI.
Previous studies have reported that obesity is associated with decreased markers of Type 2 inflammation: there are reports of decreased exhaled nitric oxide in obese asthma (as also found in this study), and also decreased sputum eosinophilia [29, 30]. This has led to conjecture that Type 2 responses are decreased in obesity (indeed, we have found decreased CD4 cell effector function in mouse models and also in obese human subjects [31, 32]), or that the phenotype of Type 2 asthma is less common in the setting of obesity. We were, therefore, interested in comparing markers of eosinophilic inflammation in the different BMI groups. CCL17 (TARC) is a T cell chemokine, and eosinophilic cationic protein is produced by eosinophils: these markers are responsive to glucocorticoid treatment [33–39]. We found that markers of Type 2 inflammation (serum and nasal CCL17 and ECP) were similar across BMI groups, although exhaled nitric oxide was decreased in obese subjects. Recent studies have found that certain Type 2 cytokines (such as IL-25 and IL-5) are actually increased in the sputum of obese asthmatics, and associated with increased mucosal eosinophilia, suggesting perhaps a shift from an adaptive to an innate cause of Type 2 inflammation in obesity [40, 41]. The fact that we only measured markers of eosinophilic inflammation, and they were similar across all BMI groups is consistent with these more recent reports suggesting persistence of Type 2 inflammation across BMI groups (although perhaps mediated through different pathways, which we were not able to address in this study). The fact that exhaled nitric oxide was decreased in the setting of obesity is also consistent with previous reports, and may be related to altered nitric oxide metabolism in the setting of increased oxidative stress in the airway [42].
We were also interested in the response to nasal corticosteroids across the different BMI groups. Epidemiological studies have found decreased responses to inhaled corticosteroids in obese asthmatics [43, 44], though we are not aware of any studies investigating the relationship between response to nasal corticosteroids and obesity. There are a number of mechanisms which might alter response to glucocorticoids in asthma: proinflammatory cytokines, such as IL-1, IL-6, and TNF-α, are increased in many obese individuals, and given that the expression of these same cytokines is altered by glucocorticoids, it is possible that this proinflammatory environment might modify glucocorticoid function in obese patients with asthma (24). Sutherland and colleagues found that obese patients with asthma have reduced molecular responsiveness to glucocorticoids due to reduced induction of the anti-inflammatory signal MKP-1 in response to steroid treatment [45]. Although we found some preliminary evidence of decreased response to nasal glucocorticoids in obese subjects this will require further study.
There are certain limitations to our study. This was a clinical study population, not a population based study, putting limitations on the generalizability of our findings. We did not confirm sinonasal disease by endoscopy or CT, but the questionnaire we used was sensitive and specific for identifying sinonasal disease [13].
Conclusions/key findings
Obesity does not affect severity of sinonasal disease in asthma; severity of sinonasal disease appears related to severity of asthma, regardless of BMI. Nasal steroids might have some decreased efficacy in the setting of obesity, but this will require further study.
Acknowledgments
Supported by grants from the National Heart Blood and Lung Institute (UO1HL089464 and U01 HL089510) and the American Lung Association.
Footnotes
Study drug and placebo provided by Merck (Merck had no role in designing, conducting, or approving the study or analyzing the results).
Declaration of Interest: Dr. Dixon reports consultancy fees from Roche and Vitaeris, a grant from Pfizer, and royalties from Springer. Dr. Wise consultancy fees from AstraZeneca, Merck, GlaxoSmithKline, Boehringer-Ingelheim, Novartis, Pfizer, Mylan, Roche, Janssen, Pulmonx, Spiration, Intermune, MedImmune, Sunovion, and Forest. The rest of the authors declare that they no relevant conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94(6 Pt 1):895–901. [PubMed] [Google Scholar]
- 2.Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc. 1994;15(1):21–5. doi: 10.2500/108854194778816634. [DOI] [PubMed] [Google Scholar]
- 3.Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000;106(5 Suppl):S201–5. doi: 10.1067/mai.2000.110151. [DOI] [PubMed] [Google Scholar]
- 4.Braunstahl GJ, Kleinjan A, Overbeek SE, Prins JB, Hoogsteden HC, Fokkens WJ. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. 2000;161(6):2051–7. doi: 10.1164/ajrccm.161.6.9906121. [DOI] [PubMed] [Google Scholar]
- 5.Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107(3):469–76. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 6.Dixon AE, Kaminsky DA, Holbrook JT, Wise RA, Shade DM, Irvin CG. Allergic rhinitis and sinusitis in asthma: differential effects on symptoms and pulmonary function. Chest. 2006;130(2):429–35. doi: 10.1378/chest.130.2.429. [DOI] [PubMed] [Google Scholar]
- 7.Tay TR, Radhakrishna N, Hore-Lacy F, Smith C, Hoy R, Dabscheck E, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology. 2016 doi: 10.1111/resp.12838. [DOI] [PubMed] [Google Scholar]
- 8.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. American journal of respiratory and critical care medicine. 2007;175(7):661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. The Journal of allergy and clinical immunology. 2014;133(6):1549–56. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya N. Associations between obesity and inflammatory sinonasal disorders. Laryngoscope. 2013;123(8):1840–4. doi: 10.1002/lary.24019. [DOI] [PubMed] [Google Scholar]
- 11.Dixon A. The treatment of asthma in obesity. Expert review of respiratory medicine. 2012;6(3):331–40. doi: 10.1586/ers.12.22. [DOI] [PubMed] [Google Scholar]
- 12.American Lung Association-Asthma Clinical Research Centers’ Writing C. Dixon AE, Castro M, Cohen RI, Gerald LB, Holbrook JT, et al. Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma. The Journal of allergy and clinical immunology. 2015;135(3):701–9. e5. doi: 10.1016/j.jaci.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon AE, Sugar EA, Zinreich SJ, Slavin RG, Corren J, Naclerio RM, et al. Criteria to screen for chronic sinonasal disease. Chest. 2009;136(5):1324–32. doi: 10.1378/chest.08-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. The Journal of allergy and clinical immunology. 2006;117(3):549–56. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 1998;114(4):998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 16.Marks GB, Dunn SM, Woolcock AJ. An evaluation of an asthma quality of life questionnaire as a measure of change in adults with asthma. Journal of clinical epidemiology. 1993;46(10):1103–11. doi: 10.1016/0895-4356(93)90109-e. [DOI] [PubMed] [Google Scholar]
- 17.Standardization of Spirometry, 1994 Update. American Thoracic Society. American journal of respiratory and critical care medicine. 1995;152(3):1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 18.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. American journal of respiratory and critical care medicine. 2011;184(5):602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. American journal of respiratory and critical care medicine. 2000;161(1):309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 20.Yasukochi Y, Nakahara T, Abe T, Kido-Nakahara M, Kohda F, Takeuchi S, et al. Reduction of serum TARC levels in atopic dermatitis by topical anti-inflammatory treatments. Asian Pac J Allergy Immunol. 2014;32(3):240–5. doi: 10.12932/AP0419.32.3.2014. [DOI] [PubMed] [Google Scholar]
- 21.Ventura MT, Murgia N, Montuschi P, Gelardi M, Ciabattoni G, Buquicchio R, et al. Exhaled breath condensate, nasal eosinophil cationic protein level and nasal cytology during immunotherapy for cypress allergy. J Biol Regul Homeost Agents. 2013;27(4):1083–9. [PubMed] [Google Scholar]
- 22.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–54. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 23.Walker FD, White PS. Sinus symptom scores: what is the range in healthy individuals? Clin Otolaryngol Allied Sci. 2000;25(6):482–4. doi: 10.1046/j.1365-2273.2000.00349.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Wang K, Gao X, Paul TK, Cai J, Wang Y. Sex difference in the association between obesity and asthma in U.S. adults: Findings from a national study. Respir Med. 2015;109(8):955–62. doi: 10.1016/j.rmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Barros R, Moreira P, Padrao P, Teixeira VH, Carvalho P, Delgado L, et al. Obesity increases the prevalence and the incidence of asthma and worsens asthma severity. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA: the journal of the American Medical Association. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Togias A. Rhinitis and asthma: evidence for respiratory system integration. The Journal of allergy and clinical immunology. 2003;111(6):1171–83. doi: 10.1067/mai.2003.1592. quiz 84. [DOI] [PubMed] [Google Scholar]
- 28.de Groot JC, Amelink M, de Nijs SB, Plaat R, Reitsma BH, Storm H, et al. Risk factors for frequent severe exacerbations in late-onset eosinophilic asthma. Am J Respir Crit Care Med. 2015;192(7):899–902. doi: 10.1164/rccm.201505-1003LE. [DOI] [PubMed] [Google Scholar]
- 29.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63(5):570–4. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 30.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134(2):317–23. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 31.Ather JL, Chung M, Hoyt LR, Randall MJ, Georgsdottir A, Daphtary NA, et al. Weight Loss Decreases Inherent and Allergic Methacholine Hyperresponsiveness in Mouse Models of Diet-Induced Obese Asthma. American journal of respiratory cell and molecular biology. 2016;55(2):176–87. doi: 10.1165/rcmb.2016-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. The Journal of allergy and clinical immunology. 2011;128(3):508–15. e1–2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlag C, Miehlke S, Heiseke A, Brockow K, Krug A, von Arnim U, et al. Peripheral blood eosinophils and other non-invasive biomarkers can monitor treatment response in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;42(9):1122–30. doi: 10.1111/apt.13386. [DOI] [PubMed] [Google Scholar]
- 34.Claudio E, Tassi I, Wang H, Tang W, Ha HL, Siebenlist U. Cutting Edge: IL-25 Targets Dendritic Cells To Attract IL-9-Producing T Cells in Acute Allergic Lung Inflammation. Journal of immunology. 2015 doi: 10.4049/jimmunol.1500436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terada N, Nomura T, Kim WJ, Otsuka Y, Takahashi R, Kishi H, et al. Expression of C-C chemokine TARC in human nasal mucosa and its regulation by cytokines. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2001;31(12):1923–31. doi: 10.1046/j.1365-2222.2001.01152.x. [DOI] [PubMed] [Google Scholar]
- 36.Choi GS, Kim JH, Shin YS, Ye YM, Kim SH, Park HS. Eosinophil activation and novel mediators in the aspirin-induced nasal response in AERD. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2013;43(7):730–40. doi: 10.1111/cea.12096. [DOI] [PubMed] [Google Scholar]
- 37.Serrano CD, Valero A, Bartra J, Roca-Ferrer J, Munoz-Cano R, Sanchez-Lopez J, et al. Nasal and bronchial inflammation after nasal allergen challenge: assessment using noninvasive methods. J Investig Allergol Clin Immunol. 2012;22(5):351–6. [PubMed] [Google Scholar]
- 38.Bystrom J, Amin K, Bishop-Bailey D. Analysing the eosinophil cationic protein--a clue to the function of the eosinophil granulocyte. Respiratory research. 2011;12:10. doi: 10.1186/1465-9921-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Lorenzo G, Pacor ML, Pellitteri ME, Morici G, Di Gregoli A, Lo Bianco C, et al. Randomized placebo-controlled trial comparing fluticasone aqueous nasal spray in mono-therapy, fluticasone plus cetirizine, fluticasone plus montelukast and cetirizine plus montelukast for seasonal allergic rhinitis. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2004;34(2):259–67. doi: 10.1111/j.1365-2222.2004.01877.x. [DOI] [PubMed] [Google Scholar]
- 40.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. American journal of respiratory and critical care medicine. 2013;188(6):657–63. doi: 10.1164/rccm.201208-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marijsse GS, Seys SF, Schelpe AS, Dilissen E, Goeminne P, Dupont LJ, et al. Obese individuals with asthma preferentially have a high IL-5/IL-17A/IL-25 sputum inflammatory pattern. American journal of respiratory and critical care medicine. 2014;189(10):1284–5. doi: 10.1164/rccm.201311-2011LE. [DOI] [PubMed] [Google Scholar]
- 42.Holguin F, Fitzpatrick A. Obesity, asthma, and oxidative stress. Journal of applied physiology. 2010;108(3):754–9. doi: 10.1152/japplphysiol.00702.2009. [DOI] [PubMed] [Google Scholar]
- 43.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respiratory medicine. 2007;101(11):2240–7. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 44.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. The European respiratory journal. 2006;27(3):495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. American journal of respiratory and critical care medicine. 2008;178(7):682–7. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]