Abstract
Peripheral nerve injury is a major burden to societies worldwide, however, current therapy options (e.g. autologous nerve grafts) are unable to produce satisfactory outcomes. Many studies have shown that stem cell transplantation holds great potential for peripheral nerve repair, and human neural crest stem cells (NCSCs), which give rise to a variety of tissues in the peripheral nervous system, are particularly promising. NCSCs are one of the best candidates for clinical translation, however, to ensure the viability and quality of NCSCs for research and clinical use, the effect of in vitro cell passaging on therapeutic effects need be evaluated given that passaging is required to expand NCSCs to meet the demands of transplantation in preclinical research and clinical trials. To date, no study has investigated the quality of NCSCs past the 5th passage in vivo. In this study, we employed a multimodal evaluation system to investigate changes in outcomes between transplantation with 5th (p5) and 6th passage (p6) NCSCs in a 15mm rat sciatic nerve injury and repair model. Using CatWalk gait analysis, gastrocnemius muscle index, electrophysiology, immunohistochemistry, and histomorphometric analysis, we showed that p6 NCSCs demonstrated decreased cell survival, Schwann-cell differentiation, axonal growth, and functional outcomes compared to p5 NCSCs (all p<0.05). In conclusion, p6 NCSCs showed significantly reduced therapeutic efficacy compared to p5 NCSCs for peripheral nerve regeneration.
Keywords: human neural crest stem cell, cell passage, peripheral nerve injury, nerve regeneration, gait analysis
Introduction
Peripheral nerve injury is a major burden for society, affecting more than 360,000 patients in the US alone and costing more than $150 billion annually 1–3. Clinicians currently rely on autologous nerve graft techniques to treat patients, however, poor clinical outcomes and limitations of donor tissue make this treatment less than ideal and far from satisfactory 2,4. Regeneration of peripheral nerves after injury is a complicated process which is widely known to involve Schwann cells. However, cell-based therapies using Schwann cells transplantation are not practical due to many factors such as long culturing time, limited number of cells, invasive harvesting techniques, and donor site morbidity 5. Stem cell transplantation features various benefits to peripheral nerve regeneration, and has the unique potential to overcome the limitations of directly using Schwann cells by differentiating into Schwann-like cells that are also able to exert regeneration-promoting effects in vivo. Many basic, pre-clinical, and clinical studies have investigated the possible use of stem cell transplantation for treating peripheral nerve injuries 6–8, and human neural crest stem cells (NCSCs) shows particular promise 9,10. NCSCs, a pluripotent stem cell that can give rise to peripheral nervous tissue in humans, have been shown to boost the rate of axon survivability and enhance recovery by differentiating into Schwann-like cells, facilitating the myelination of axons, exerting neuroprotective effects, and guiding the regrowth of damaged axons 10,11. While NCSCs are generally thought to give rise to the peripheral nervous system tissue, they have also been shown to be able to differentiate into oligodendrite-lineage cells in the central nervous system facilitating regeneration of neurons following brain and spinal cord injuries 12. These exciting features make NCSC transplantation a particularly promising therapy for peripheral nerve injury and possibly many other pathologies of the nervous system.
In order to fully understand the benefits and limitations of NCSC for nerve regeneration, the effect of cell passaging must be investigated. While using low-passage cell cultures for therapy is always preferred, passaging is an inevitable process required to expand the number of cells to sufficient amounts, especially for pre-clinical research and clinical trials 13,14. Cell passaging is known to affect the efficacy of stem cells, and studies have shown that the quality of other stem cells such as mesenchymal stem cells (MSCs) and adipose stem cells (ASCs) decline with passage number, each with different patterns and rate of change 15–18. Thus, knowledge of how many passages a particular strain of stem cells can tolerate before losing therapeutic effects is paramount for managing the trade-off between cell quality and quantity. Previous studies have also shown varying results regarding the effect of passaging on NCSCs, suggesting that the effectiveness of NCSCs decline significantly after anywhere from 3 to 25 passages 19–21. Embryonic Stem Cell (ESC) derived NCSCs feature particularly great advantages for peripheral nerve regeneration especially since ESCs can serve as an unlimited NCSC source 11,20,22, however, only one study to date has reported the effect of passaging on these NCSCs, identifying the 5th passage as the largest efficacious passage number in vitro 22. The effect of passaging on ESC derived NCSCs have not been investigated in vivo or past the 5th passage.
In this study, we evaluated whether there is a significant change in efficacy of ESC-derived NCSC transplantation in vivo following peripheral nerve injury past the 5th passage to investigate whether these cells remain efficacious for functional recovery and nerve regeneration. We employed a multimodal assessment system including histomorphometric, immunohistochemistry, electrophysiology, and behavioral analysis to investigate the impact of NCSC passage number on recovery in a 15mm rat sciatic nerve injury and repair model.
Materials and Methods
Animals, Cells, and Surgical Procedure
All animals were maintained according to NIH guidelines, and experimental protocols were approved by the IACUC of the University of Maryland School of Medicine. Every attempt was made to minimize the total number of animals used and their discomfort and pain. 18 athymic nude rats (Charles River, Wilmington, MA) were used in this study, and were individually housed in controlled temperature, humidity, and light:dark cycles, and all animals had free access to food and water. hNCSCs were derived from human embryonic stem cells (ESCs, H9 line from WiCell, Madison, WI), and provided by Lee group from Johns Hopkins University as previously reported 23. hNCSCs were cultured on Matrigel-coated TCPS for 10 days in neural crest induction medium containing: 50% NSC medium (Neurobasal medium supplemented with NEAA, Glutamax, B27 and 20 ng/ml FGF2), 50% PA6 conditioned medium, 10 μM Rock inhibitor, and 200 μM ascorbic acid. Culture medium was changed every 2 days. For this study, we used 5th and 6th passage NCSCs (p5 and p6, respectively), and cells were passaged using Accutase (Sigma, St Louis, MO).
Our injury and repair model for rat sciatic nerves has been described in our previous studies 6,8. In brief, animals (200–250 g) are anesthetized with isoflurane, and a 15 mm segment of the sciatic nerve was removed and repaired with one of three nerve guide conduit 24 filled with: A) PBS (control), B) 5th passage hNCSC, and C) 6th passage hNCSC (6 rats each). For conduits containing hNCSCs, we injected 2 × 106 hNCSCs medium loaded hydrogel (1:1 mixture of growth medium and Collagen I Rat Tail (Life Technologies, NY)) into the conduits. One hour electrical stimulation (3V, 100μsec and 20Hz pulse) was applied after nerve repair and NCSC transplantation to improve nerve regeneration as previously reported 25. 12 weeks after surgery, rats were euthanized, and nerve and muscle tissue were harvested for evaluation.
CatWalk Gait Analysis
Our previous studies 6,26 have described our methods for using CatWalk (Noldus Information Technology, The Netherlands). Briefly, one week prior to surgery, animals were trained until they were able to make 5 consecutive uninterrupted runs on the CatWalk runway. Prior to euthanization at 12 weeks, the MCMI (max contact mean intensity) and Duty Cycle metrics were recorded. MCMI measures the mean intensity of a paw print at the point where the maximum contact area is detected. Duty Cycle measures the percentage of time a limb is bearing weight during a walk cycle. All recordings were repeated 5 times for each animal both prior to surgery and prior to euthanization. Data are then blindly assessed by trained technicians.
Electrophysiology
Electrophysiological recordings were conducted for all animals at baseline and immediately prior to euthanization 12 weeks after surgery with a Tucker-Davis Technologies system (TDT, Alachua, FL) via a preamplifier. The ground electrode was placed in the tail of the subject. The stimulating needle electrode was placed in the sciatic nerve proximal to the repair. Stimulation were delivered (with 500 μA or 1mA, 100 μs pulse duration, and stimulation frequency of 0.5Hz), and compound motor action potentials (CMAP) and motor evoked potentials (MEP) were recorded using a recording electrode in the sciatic nerve distal to the repair and a recording electrode in the tibialis anterior muscle, respectively. Each stimulation and recording lasted 1 minute and were repeated four times, with 2-minute relaxation periods between each recording. Recordings were done on both the injured and uninjured leg for each animal and the distance between the stimulating electrode and the CMAP recording electrode was kept at 15mm. Peak-to-peak amplitude and latency were then analyzed offline. Peaks were detected with a threshold, which was set to be two or three times the standard deviation above the mean of the signal, based on the signal-to noise ratio 27, and peaks below that threshold were considered noise.
Immunohistochemistry
Cross-sections of mid-parts of nerve conduits were retrieved for immunostaining. Regenerated nerves within the conduits were washed (with PBS), blocked for 1 hour in 5% normal goat serum in 0.2% Triton-X-PBS, and immunostained using Chicken BIII-Tubulin (1:1000, Millipore), rabbit anti-S100 antibody (1:400, Dako), mouse anti-Human NCAM (1:100, Santa Cruz Biotechnology) or mouse anti-Human Nuclei (HuNu, 1:200, EMD Millipore) to detect axons, SCs, and human NCSCs. Then, Alexa 488-conjugated goat anti-Chicken, Alexa 647-conjugated donkey anti-Rabbit, and Alexa 594-conjugated goat anti-Mouse antibodies were used for visualization. Primary antibodies were incubated at 4 °C overnight and secondary antibodies were incubated at room temperature for 1 hour. Stained slides were mounted in Prolong Gold with DAPI (Invitrogen, Carlsbad, CA), and imaged by inverted fluorescent microscope (Nikon Ti-E, Nikon, Kobe, Japan). The number of HuNu positive cells and the proportion of HuNu/S-100 double labeled cells to HuNu positive cells per slide was analyzed by ImageJ software (National Institutes of Health, Bethesda, MD) 28. 6 animals per group (30 μm thickness, 6–8 sections per animal) were included in this analysis.
Histomorphometric Analysis
Nerve conduits were cross-sectioned at 8–10 mm from the proximal end, and processed for toluidine blue staining. Specimen sections were fixed in 4 wt % paraformaldehyde solution overnight, followed by 2 hours of post-fixation with 2% of osmium tetroxide and dehydration prior to mounting in embedding resin. 0.5 μm thick specimens were sectioned on an Ultracut E microtome for semi-thin sections and stained with 1% toluidine blue for light microscopy (Nikon Ti-E inverted microscope, Nikon, Kobe, Japan). Images of the whole nerve cross sections were acquired at 10x. Sets of images representing at least 30% of the nerve cross-sectional area were chosen by systematic random sampling and were acquired at 60x. Myelinated axon density and diameter per section as well as percentage of area occupied by axon fibers were then blindly and quantitatively assessed by ImageJ.
Wet Weight of Gastrocnemius Muscle and H&E muscle staining
Gastrocnemius muscles of both injured and uninjured sides were retrieved and weighed using an electronic scale (Mettler AE 160, USA). The Gastrocnemius Muscle Index (GMI) was blindly calculated as the ratio of the muscle weight between the affected and unaffected sides to evaluate muscle atrophy caused by nerve injury 29. Muscles were then cross-sectioned into 10 μm specimens in a cryostat microtome at –20°C and mounted on glass slides. These specimens were then stained with hemotoxylin-eosin (H&E) using standard protocols to demonstrate muscle fiber morphology to grossly evaluate the effect of denervation and recovery on muscle tissue for the experimental groups.
Statistical Analysis
Data were expressed as the mean ± standard error of the mean (SEM). Statistical significance was evaluated using univariate ANOVAs followed by post hoc paired comparisons using the Least-Significant Difference (LSD) or Student-Newman-Keuls tests when appropriate. Values of P < 0.05 were considered significant. All statistical tests were carried out with statistical software SPSS 20.0 (IBM/SPSS Inc., Chicago, IL, USA).
Results
Gait Analysis showed that p5 hNCSCs led to significantly more recovery compared to p6 hNCSCs
Representative footprints records (Fig. 1A) showed that in the conduit only group, uncoordinated forepaw and hindpaw placement occurred, and that the footprints of the forepaw and hindpaw were frequently mismatched, resulting in an increase in their relative position. The p6 hNCSC group showed slight improvements in rhythmicity, and the group with p5 NCSC showed significant improvements, with more coordinated forepaw and hindpaw footprints. At 12 weeks, MCMI was significantly greater in the p5 group (119.68 ± 6.68) compared to the p6 (70.37 ± 5.86, p<0.001) and control (72.29 ± 3.67, p<0.001) groups (Fig. 1B). There was no significant difference between the p6 group and the control group (p>0.05). The duty cycle metric for p5 group (80.61 ± 3.08%) was significantly greater than the control group (72.38 ± 2.92%, p=0.046). While Duty Cycle for the p5 group was also larger than that of the p6 group (78.74 ± 1.89%), the difference was not statistically significant (p>0.05). Duty cycle was not significantly different between the p6 group and the control group (p>0.05) (Fig. 1C). These results indicate that p5 hNCSCs led to superior functional outcomes compared to p6 hNCSCs and conduit only group.
Figure 1.
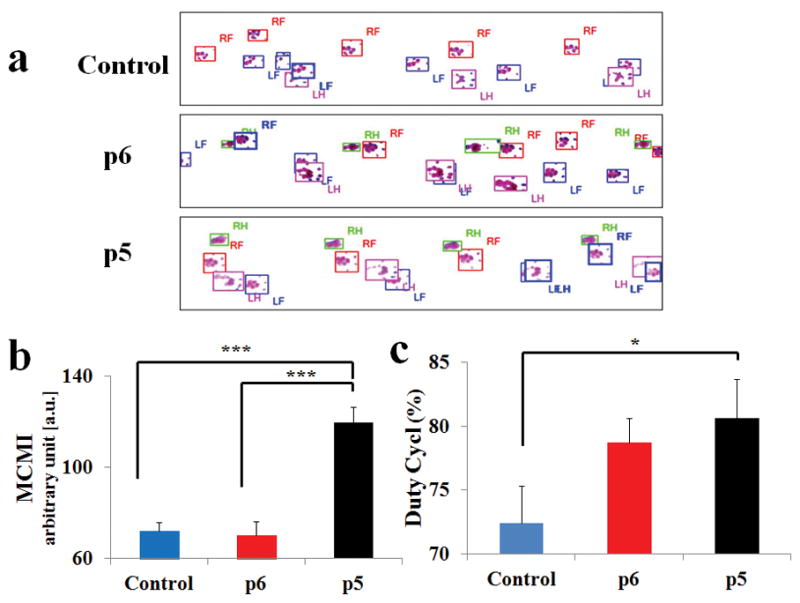
(A) representative footprints measured by the Catwalk system, RF = right forepaw, LF = left forepaw, RH = right hindpaw, and LH = left hindpaw. CatWalk quantitative results for (B) Mean Contact Mean Intensity (MCMI) and (C) Duty Cycle show that p5 was superior to both p6 and control groups. Data were presented as Mean ± SEM. Significance: *p<0.05, ***p<0.001.
Electrophysiological and GMI assessments showed decreased efficacy of p6 NCSCs compared to p5 NCSCs
Electrophysiology studies (Fig. 2A) revealed that the p5 group had a larger number of rats with discernable CMAP signals (3 of 6) than the control (0 of 6) and p6 NCSC (0 of 6) groups. A similar trend was seen with MEP signals, where the p5 group showed a larger number of rats with discernable signals (3 of 6) than the control (0 of 6) and p6 NCSC (0 of 6) groups. Amplitude and latency data were not evaluated due to the small number of rats showing signals.
Figure 2.
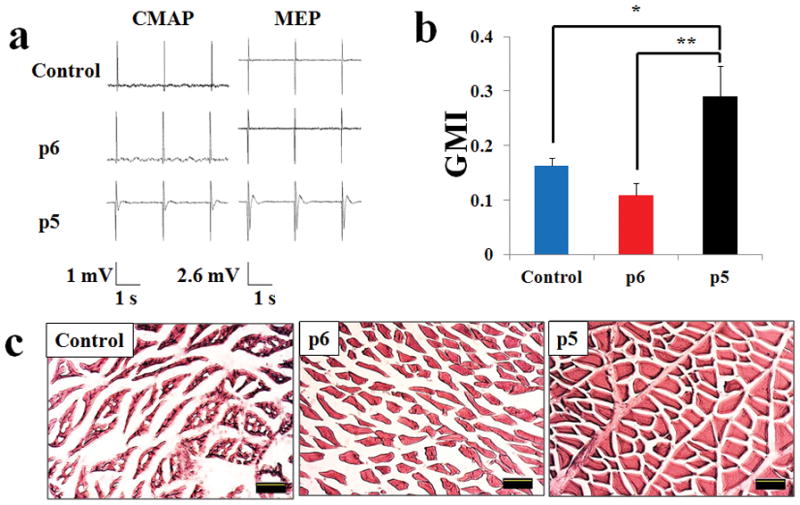
A) Representative electrophysiology data for both compound motor action potential (CMAP) and motor evoked potential (MEP). B) Results for Gastrocnemius Muscle Index (GMI), which show that p5 is superior to both p6 and control. C) Results for H&E staining of gastrocnemius muscle cross sections. Quantitative data presented as Mean ± SEM. Significance: *p<0.05, **p<0.01. Scale bar: 10 μm.
12 weeks after injury and repair, GMI was significantly greater in the p5 group (0.29 ± 0.06%) compared to the p6 (0.11 ± 0.02%, p=0.003) and control (0.16 ± 0.01%, p=0.024) groups (Fig. 2B). There was no significant difference between the p6 group and the control group (p>0.05). H&E staining also revealed that gastrocnemius muscle underwent obviously less fatty infiltration and sarcomere degeneration in both hNCSC transplanted groups than the conduit only group (Fig. 2C), with the p5 group showing significantly healthier muscle fibers showing uniform polygonal morphology than the p6 group. These results suggest that p5 NCSCs resulted in superior nerve conductivity and reduced muscle atrophy compared to p6 NCSCs.
Immunohistochemistry reveals better cell survival and differentiation for p5 hNCSCs compared to p6 hNCSCs
Immunohistochemistry analysis reveals that p5 hNCSCs had superior survivability and differentiation in vivo compared to p6 hNCSCs. Tissue sections were co-stained with S-100 (a Schwann cell marker), human NCAM (a human cell line marker), and tubulin (an axon marker), and more proliferation of cells in the p5 group was observed compared to the p6 group. The conduit only group showed close to no fluorescent signals, indicating that nerve regeneration did not occur to any significant extent. The p5 group showed signals for all three stains, indicating that regeneration has taken place. While the p6 group also showed fluorescent signals for the three antibodies, signals were significantly weaker and sparser when compared with the p5 group (Fig. 3).
Figure 3.
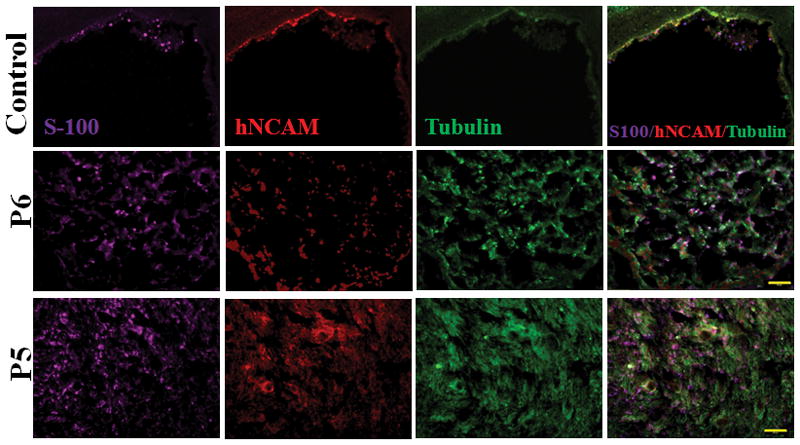
Immunohistochemistry of regenerated nerves stained with S-100 (Schwann Cell Marker), hNCAM (human cell marker), and Tubulin (axon marker) showed that the p5 group clearly displayed stronger and more diffuse signals than the p6 group and control group. Scale bar: 100 μm.
Quantified co-staining tissue sections of HuNu and S100 showed that the survivability of p5 hNCSCs were superior to p6 hNCSCs with a significantly higher HuNu+ cell count in the p5 group (60.20 ± 5.19) compared to the p6 group (16.30 ± 1.13, p<0.001). To evaluate the extent of Schwann cell differentiation of NCSCs, we evaluated the percentage of HuNu+ cells that were co-localized with S-100 stains. Here, p5 NCSCs (63.75 ± 4.76%) were superior compared to p6 NCSCs (28.07 ± 2.99%, p<0.001). (Fig. 4) These results indicate that p5 hNCSCs features superior cell survivability and Schwann-cell differentiating capacity compared to p6 hNCSCs.
Figure 4.
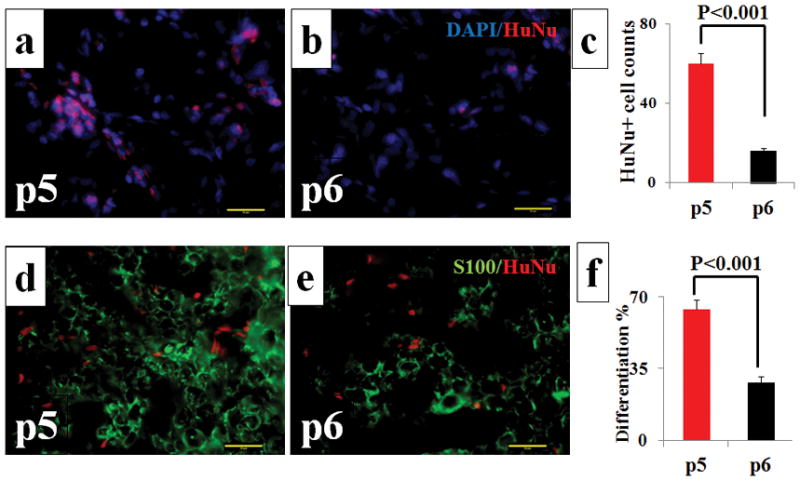
Immunohistochemistry results using HuNu (Red) stain (A and B) and HuNu (Red) / S-100 (Green) double stain (D and E). Quantified analyses show that the number of HuNu-positive cells (C) and the percentage of HuNu-positive cells that were also S-100 positive (F) in p5 were higher than those in p6. Results presented as Mean ± SEM. Scale bar: 20 μm.
Histomorphometric Analysis indicates that p5 hNCSCs lead to more regeneration of myelinated axons comped to p6 hNCSCs
As shown in Fig. 5A, in the p5 group, a significant number of axons stained with toluidine blue were clearly visible under light microscopy. While the p6 hNCSC group also showed some axons, they were much less in number, less diffuse, and more localized in batches compared to the p5 group. The control group also showed a sparse number of axons, again indicating unsuccessful regeneration.
Figure 5.
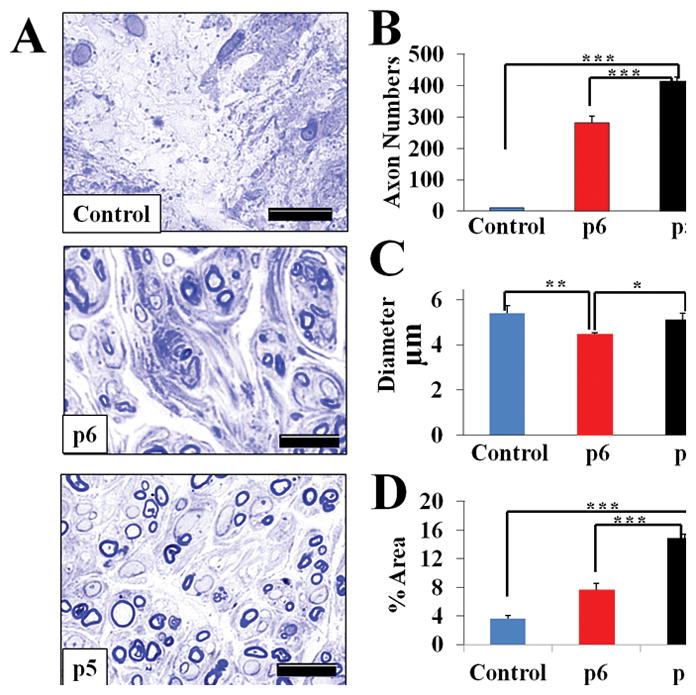
Histomorphometric analysis of the sciatic nerve regeneration. Staining with toluidine blue. (A) Representative images of cross-section of the middle part of sciatic nerve 12 weeks after repair with conduit only and injection of p5 or p6 NCSC (x600 magnification); (B) myelinated axon numbers per section; (C) myelinated axon diameter; and (D) percentage of fascicle area per section. Results presented as Mean ± SEM. Significance: *p<0.05, **p<0.01, ***p<0.001. Scale bar: 10 μm.
Quantitative results (Fig. 5B) showed that the p5 NCSC group had significantly higher cell counts (414.21 ± 21.05) than the control (10.81 ± 0.96, p<0.001) and p6 NCSC (281.13 ± 12.28, p<0.001) groups. The count for p6 NCSC group was significantly greater than the control group (p<0.001). Axon diameter (Fig. 5C), however, showed a different pattern, with the control group showing the highest figures (5.40 ± 0.32 μm), which were significantly larger than the p6 hNCSC group (4.47 ± 0.16 μm, p=0.002). The p5 hNCSC group (5.12 ± 0.08 μm) also showed significantly greater diameter than the p6 hNCSC group (p=0.022). When evaluating the percentage of the cross-sectional area (Fig. 5D) covered by myelinated axons, the p5 hNCSC group (14.88 ± 0.94%) showed significantly higher percentage than the control (3.60 ± 0.47%, p<0.001) and the p6 hNCSC (7.63 ± 0.53%, p<0.001) groups. This percentage was also significantly greater for the p6 hNCSC group compared to the control group (p=0.001) (Fig. 5). These results indicate that while the p6 NCSCs improved axon regeneration, its effects are significantly inferior to that of p5 NCSCs.
Discussion
In this study, we used a multimodal assessment system including immunohistochemistry analysis, histomorphometric analysis, gastrocnemius muscle index, and CatWalk gait analysis, and we found that the in vivo efficacy of 6th passage hNCSC transplantation for peripheral nerve injury is significantly decreased compared to 5th passage hNCSC transplantation. In vitro passaging is a necessary step to expand NCSCs to meet the needs of research and clinical use in the future, and understanding the effect of cell passaging on NCSCs in vivo is crucial. While most prior studies were based on in vitro evaluation only, our validations of long-term contribution, differentiation, and functionality past the 5th passage in an in vivo animal model are of paramount importance to adequately demonstrate the utility of NCSCs in regenerative medicine.
Cell passaging is known to induce modifications on stem cell properties, and in many cases contributes to a decline in efficacy for therapy. It has been widely reported that stem cells senesce and lose proliferation and differential potential with increasing number of passages 30. Studies involving other stem cells, such as MSCs, ASCs, and BMSCs have demonstrated varying results regarding the time course and extent of deterioration of effectiveness with increasing in vitro passage number 15–17. For NCSCs, research has shown that the NCSC population can be clonally amplified and maintained for over 25 passages while retaining the capacity to differentiate into peripheral neurons, smooth muscle cells, and mesenchymal precursor cells 19. However, Cranial NCSC derived from human embryonic stem cells exhibited a very limited self-renewal capacity and spontaneous differentiation occurred at the third passage 21. For ESC-derived NCSCs, which are particularly advantageous since ESCs can serve as an unlimited source of NCSCs, Liu et al. have demonstrated in vitro that NCSCs remain efficacious throughout the first 5 passages, however, the effectiveness of NCSCs past the 5th passage is unknown 20. Here, we compared the effectiveness of 5th and 6th passage hNCSCs in a translational model of nerve injury and repair.
Compared to 5th passage NCSCs, 6th passage NCSCs showed significantly less HuNu-positive cells 12 weeks after injury and repair. Since the transplanted stem cells are the only human-derived cells that were introduced to animals, this result demonstrates that 6th passage hNCSCs were less able to survive and proliferate in vivo compared to 5th passage hNCSCs. Furthermore, the percentage of HuNu-positive cells also showing S-100 (Schwann-cell marker) signals in the nerve section transplanted with 6th passage NCSCs is significantly lower than that in the nerve section transplanted with the 5th passage NCSCs, which demonstrate that 6th passage NCSCs were less able to differentiate into the intended Schwann-cell phenotype. Schwann cells are among the most studied cell types in the nervous system, and is known to play a critical role in peripheral nerve regeneration. The main rationale behind using stem cells for peripheral nerve regeneration is that stem cells have the potential to differentiate into Schwann-like cells that exert similar regeneration-promoting effects as Schwann cells. Thus, the ability of hNCSCs to differentiate into Schwann cells in vitro is likely a major determinant of efficacy, and the reduced ability of 6th passage hNCSCs to survive and differentiate into Schwann cells may be a major factor contributing to the reduction of hNCSCs expected positive influence on nerve regeneration and functional recovery.
Although histomorphometric analysis demonstrated that axon regeneration did take place with 6th passage hNCSC transplantation, and immunohistochemistry analysis revealed that some transplanted cells were able to survive and differentiate, functional outcomes from CatWalk gait analysis and gastrocnemius muscle index showed that 6th passage hNCSC transplantation produced outcomes that were not significantly different from experimental control (empty conduit). These results suggest that while 6th passage hNCSCs may be able to survive and differentiate in vivo to a certain extent, leading to the regeneration of some myelinated axons, they were unable to exert sufficient influence on nerve regeneration to elicit improvements in functional recovery or reverse muscle atrophy due to denervation. This may be due to the number of survived stem cells and the number of differentiated stem cells not meeting the level necessary for therapeutic effect. Another possibility is that 6th passage hNCSCs may have been less effective in treatment due to changes in its ability to produce growth factors that have been shown to contribute to the positive influence on nerve regeneration in previous studies 31–33. While the mechanism by which hNCSCs lose their efficacy with increasing passage number is largely unknown, studies on MSCs have shown that the growth rate, differentiation ability, and growth factors secretion progressively are decreased with additional passages, possibly due to senescence, spontaneous differentiation, or loss of differentiation potential 34–36. Further investigation into the detailed mechanism underlying the deterioration of hNCSCs across cell passages is crucial for a comprehensive understanding of hNCSCs, and will be greatly valuable to pre-clinical research, clinical trials, and therapeutic use.
In conclusion, we investigated the viability and efficacy of 6th passage human hNCSC cells for transplantation following peripheral nerve injury, and found that compared to 5th passage hNCSCs, 6th passage hNCSCs displayed significantly inferior outcomes in terms of cell survival, Schwann-cell differentiation, axon regeneration, and functional recovery. Furthermore, 6th passage hNCSC transplantation did not result in significant improvement over untreated injury in terms of functional recovery and reversal of muscle atrophy due to denervation. Understanding the effect of cell passaging on NCSCs is crucial for pre-clinical research, clinical trials, and future therapeutic use, and the detailed mechanism by which hNCSCs’ beneficial effects deteriorate over time, including the pattern and regulation of growth factors in P5 and P6 NCSCs, awaits further investigation.
Acknowledgments
Supported by Maryland Stem Cell Research Fund (2013-MSCRFE-146-00) (to XJ), and R01HL118084 from NIH (to XJ).
The work was supported by Maryland Stem Cell Research Fund (2013-MSCRFE-146-00) (to XJ), and R01HL118084 from NIH (to XJ).
Footnotes
Compliance with Ethical Standards
Conflict of interest
The authors have declared that there is no conflict of interest.
Portions of this work were previously presented in the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society in Jeju Island, Korea (July 2017) and at the 8th International IEEE Engineering in Medicine and Biology Society (EMBS) Conference on Neural Engineering at Shanghai, China (May 2017).
References
- 1.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang L, Jones S, Jia X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87:381–5. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- 4.Jones S, Eisenberg HM, Jia X. Advances and Future Applications of Augmented Peripheral Nerve Regeneration. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17091494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh S, Midha R. Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurg Focus. 2009;26:E2. doi: 10.3171/FOC.2009.26.2.E2. [DOI] [PubMed] [Google Scholar]
- 6.Lewitus D, Vogelstein RJ, Zhen G, et al. Designing tyrosine-derived polycarbonate polymers for biodegradable regenerative type neural interface capable of neural recording. IEEE Trans Neural Syst Rehabil Eng. 2011;19:204–12. doi: 10.1109/TNSRE.2010.2098047. [DOI] [PubMed] [Google Scholar]
- 7.Eftekhar T, Teimoory N, Miri E, Nikfallah A, Naeimi M, Ghajarzadeh M. Posterior tibial nerve stimulation for treating neurologic bladder in women: a randomized clinical trial. Acta Med Iran. 2014;52:816–21. [PubMed] [Google Scholar]
- 8.Johnson BN, Lancaster KZ, Zhen G, et al. 3D Printed Anatomical Nerve Regeneration Pathways. Adv Funct Mater. 2015;25:6205–17. doi: 10.1002/adfm.201501760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin H, Liu F, Zhang C, et al. Pluripotent hair follicle neural crest stem-cell-derived neurons and schwann cells functionally repair sciatic nerves in rats. Mol Neurobiol. 2009;40:216–23. doi: 10.1007/s12035-009-8082-z. [DOI] [PubMed] [Google Scholar]
- 10.Saadai P, Wang A, Nout YS, et al. Human induced pluripotent stem cell-derived neural crest stem cells integrate into the injured spinal cord in the fetal lamb model of myelomeningocele. J Pediatr Surg. 2013;48:158–63. doi: 10.1016/j.jpedsurg.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Wang A, Tang Z, Park IH, et al. Induced pluripotent stem cells for neural tissue engineering. Biomaterials. 2011;32:5023–32. doi: 10.1016/j.biomaterials.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder E, Rukavina M, Hassani H, et al. Peripheral nervous system progenitors can be reprogrammed to produce myelinating oligodendrocytes and repair brain lesions. J Neurosci. 2011;31:6379–91. doi: 10.1523/JNEUROSCI.0129-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015:35. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan Q, Lui PP, Rui YF. Effect of in vitro passaging on the stem cell-related properties of tendon-derived stem cells-implications in tissue engineering. Stem Cells Dev. 2012;21:790–800. doi: 10.1089/scd.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Wei G, Gu Q, et al. Donor Age and Cell Passage Affect Osteogenic Ability of Rat Bone Marrow Mesenchymal Stem Cells. Cell Biochem Biophys. 2015;72:543–9. doi: 10.1007/s12013-014-0500-9. [DOI] [PubMed] [Google Scholar]
- 16.Requicha JF, Viegas CA, Albuquerque CM, Azevedo JM, Reis RL, Gomes ME. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. 2012;8:1211–22. doi: 10.1007/s12015-012-9397-0. [DOI] [PubMed] [Google Scholar]
- 17.Kretlow JD, Jin YQ, Liu W, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo Surdo J, Bauer SR. Quantitative approaches to detect donor and passage differences in adipogenic potential and clonogenicity in human bone marrow-derived mesenchymal stem cells. Tissue Eng Part C Methods. 2012;18:877–89. doi: 10.1089/ten.tec.2011.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108:19240–5. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q, Spusta SC, Mi R, et al. Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells. Stem Cells Transl Med. 2012;1:266–78. doi: 10.5966/sctm.2011-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Snead ML. Derivation of cranial neural crest-like cells from human embryonic stem cells. Biochem Biophys Res Commun. 2008;376:542–7. doi: 10.1016/j.bbrc.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren YJ, Zhang S, Mi R, et al. Enhanced differentiation of human neural crest stem cells towards the Schwann cell lineage by aligned electrospun fiber matrix. Acta Biomater. 2013;9:7727–36. doi: 10.1016/j.actbio.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 23.Lee G, Kim H, Elkabetz Y, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–75. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 24.Chew SY, Mi R, Hoke A, Leong KW. Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv Funct Mater. 2007;17:1288–96. doi: 10.1002/adfm.200600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–8. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Du J, Zhang Y, Barnes K, Jia X. Establishing a Reliable Gait Evaluation Method for Rodent Studies. J Neurosci Methods. 2017 doi: 10.1016/j.jneumeth.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D, Xiong W, Jia XF, Geocadin RG, Thakor NV. Short- and long-latency somatosensory neuronal responses reveal selective brain injury and effect of hypothermia in global hypoxic ischemia. J Neurophysiol. 2012;107:1164–71. doi: 10.1152/jn.00681.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61:596–602. doi: 10.1227/01.NEU.0000290908.38438.B2. discussion -3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang W, Wang Y, Tang J, et al. Low-intensity pulsed ultrasound treatment improved the rate of autograft peripheral nerve regeneration in rat. Sci Rep. 2016;6:22773. doi: 10.1038/srep22773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crisostomo PR, Wang M, Wairiuko GM, et al. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–80. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 31.Sowa Y, Imura T, Numajiri T, Nishino K, Fushiki S. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev. 2012;21:1852–62. doi: 10.1089/scd.2011.0403. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Wei X, Ma Z, et al. Adipose stromal cells-conditional medium protected glutamate-induced CGNs neuronal death by BDNF. Neurosci Lett. 2009;452:238–40. doi: 10.1016/j.neulet.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Sowa Y, Kishida T, Imura T, et al. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo without Differentiation into Schwann-Like Lineage. Plast Reconstr Surg. 2016;137:318e–30e. doi: 10.1097/01.prs.0000475762.86580.36. [DOI] [PubMed] [Google Scholar]
- 34.Lai Y, Sun Y, Skinner CM, et al. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 2010;19:1095–107. doi: 10.1089/scd.2009.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos F, Andrade PZ, Abecasis MM, et al. Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng Part C Methods. 2011;17:1201–10. doi: 10.1089/ten.tec.2011.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng CP, Sharif AR, Heath DE, et al. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials. 2014;35:4046–57. doi: 10.1016/j.biomaterials.2014.01.081. [DOI] [PubMed] [Google Scholar]


