Abstract
Esophageal lichen planus (ELP) is rare and only about 80 cases have been reported in the literature. An 85-year-old woman presented with dysphagia and odynophagia. Endoscopy revealed a severe stricture in the proximal esophagus. Oral examinations at two years after the first endoscopy revealed erosions around the gingiva, and an examination of biopsy specimens taken from the site of erosion led to a diagnosis of oral lichen planus. Esophageal endoscopy was performed again, and biopsy specimens showed spongiosis and necrotic keratinocytes in the epithelium (civatte bodies). The patient was diagnosed with ELP and was treated with systemic corticosteroids, which resulted in clinical relief.
Keywords: esophageal lichen planus, systemic corticosteroids, civatte body
Introduction
Lichen planus (LP) is an idiopathic disorder that generally affects middle-aged patients with clinical manifestations on the skin, mucous membranes, genitalia, hair, and nails (1). Proposed etiologies include a reaction to medication, hepatitis C or other viral infections, bacteria such as Helicobacter pylori, or autoimmune processes (2). Esophageal LP (ELP) is a rare and under-recognized disorder; its diagnosis is usually delayed. There is no standardized management. Since 1982, only approximately 80 cases have been described worldwide (3). ELP can cause stricture, ulceration, and squamous cell carcinoma. It is important to take great precautions to rule out ELP, especially in patients with dysphagia. We herein describe a case of ELP in a patient who presented with dysphagia and was treated with systemic corticosteroids, which resulted in clinical relief.
Case Report
An 85-year-old woman with dysphagia, odynophagia, and chest discomfort for a period of one month was referred to our hospital. Her comorbidities included hypertension and hyperlipidemia, and she had been using a calcium channel antagonist (Azelnidipine), an angiotensin II receptor blocker (Losartan), a diuretic (Hydrochlorothiazide), a proton pump inhibitor (Lansoprazole) and a statin (Atorvastatin). The laboratory findings showed hypoalbuminemia and hypoproteinemia. A serological test was positive for anti-hepatitis C virus (HCV) antibodies. Endoscopy revealed a severe stricture in the proximal esophagus and the esophageal mucosa was easily exfoliated at removal (Kobner phenomenon) ( Fig. 1, 2). Biopsy specimens taken from the stenotic site showed non-specific inflammation. Although endoscopic balloon dilatation using a CRE balloon dilator (Boston Scientific, Boston, USA: 10-12 mm) was performed, the effect was slight and transient. Consequently, it was repeated four times over a two-year period. At the final session, triamcinolone acetate (Bristol-Myers Squibb, Anagni, Italy) was injected, but this failed to prevent re-stenosis. Although pemphigus vulgaris was suspected based on the finding of mucosal exfoliation, autoantibodies against desmoglein 1 and desmoglein 3 were not detected. An oral examination revealed erosions around the gingiva (Fig. 3), and dense lymphatic infiltration in the epithelium was found in the biopsy specimens taken from the site of erosion, leading to a diagnosis of oral LP (Fig. 4). Esophageal endoscopy was performed again (Fig. 5) and the biopsy specimens showed spongiosis and necrotic keratinocytes (civatte bodies) (Fig. 6). Although the submucosa was not taken for the evaluation of lichenoid inflammation, the histological findings were considered to be consistent with ELP. Systemic corticosteroid treatment was started at a dose of 20 mg daily. The patient's symptoms improved within 1 week, and the oral corticosteroid was tapered by 5 mg every 2 weeks until it reached a dose of 5 mg daily. An esophageal examination at 3 months after the initiation of corticosteroid treatment indicated endoscopic and histological improvement (Fig. 7, 8). Her clinical remission has remained for 2 years.
Figure 1.
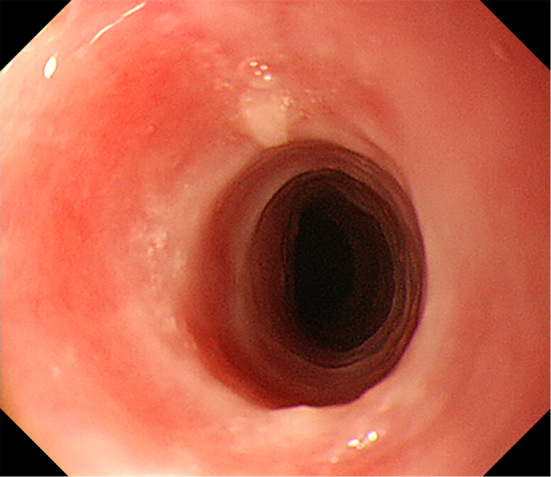
An endoscopic image of the stricture with erosion in the proximal esophagus.
Figure 2.
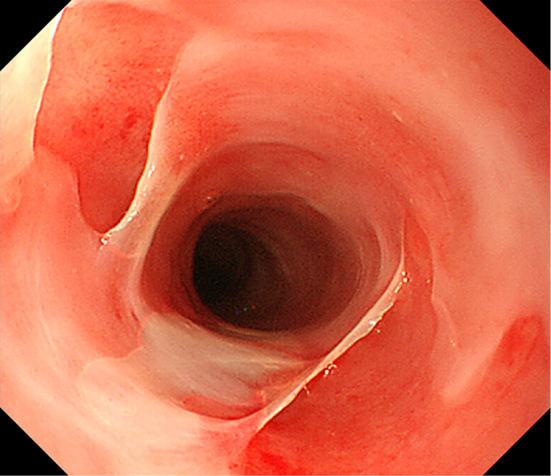
An endoscopic image showing the Kobner phenomenon.
Figure 3.
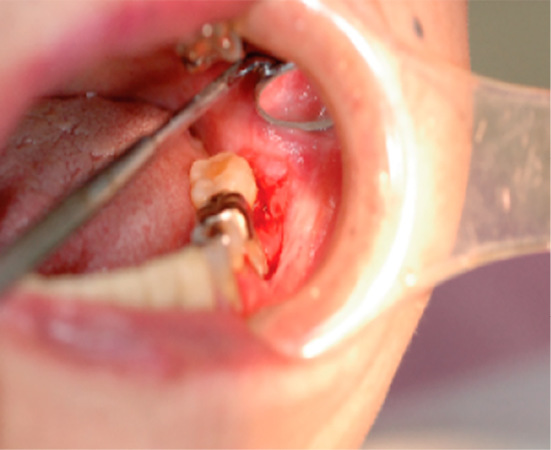
An image of oral erosion around the gingiva.
Figure 4.
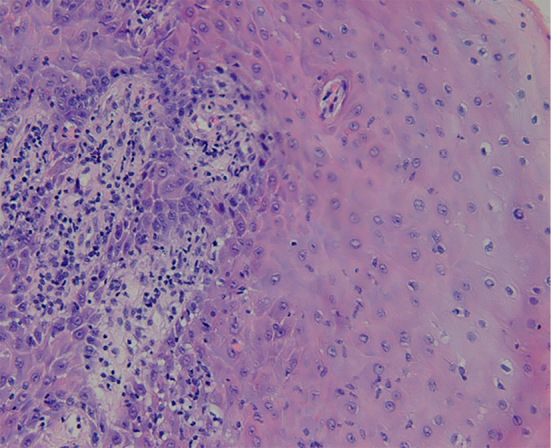
A biopsy specimen taken from the sites of erosion shows dense lymphatic infiltration in the lamina propria.
Figure 5.
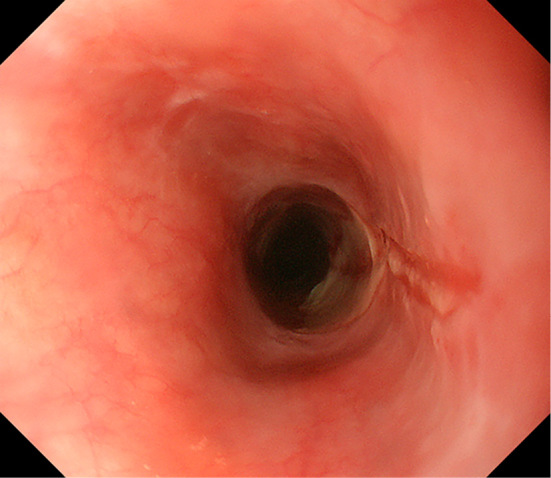
An endoscopic image of the stricture with an ulcerated and friable mucosa in the proximal esophagus.
Figure 6.
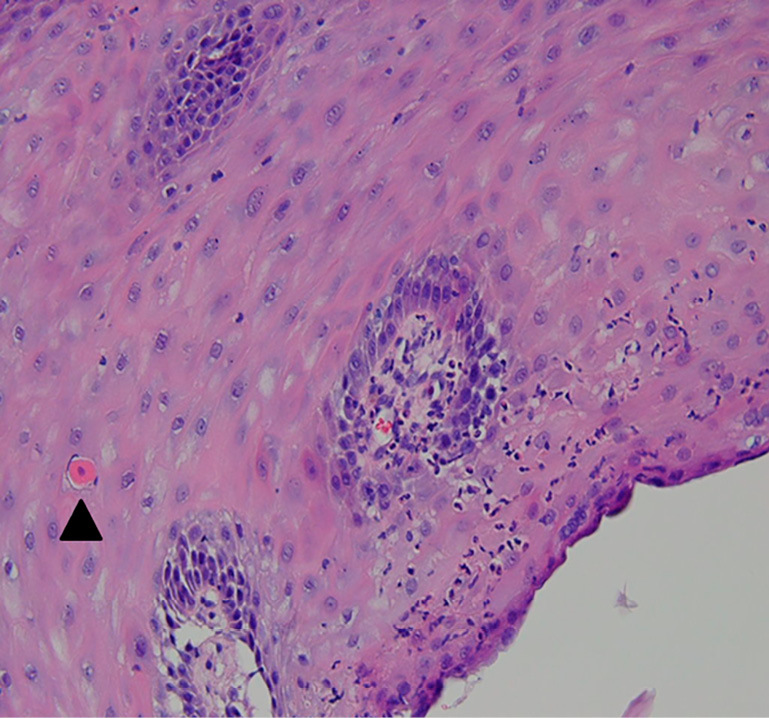
Biopsy specimens showing inflammatory infiltration involving the superficial lamina propria, spongiosis in the epithelium, and necrotic keratinocytes (civatte bodies, arrowhead).
Figure 7.
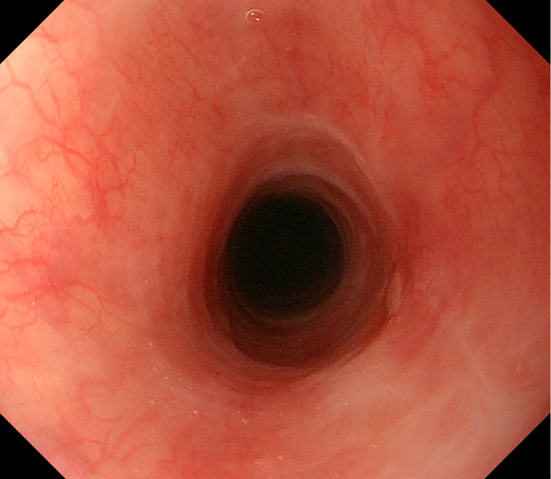
An endoscopic image of the improved esophageal mucosa after 3 months of corticosteroids treatment.
Figure 8.
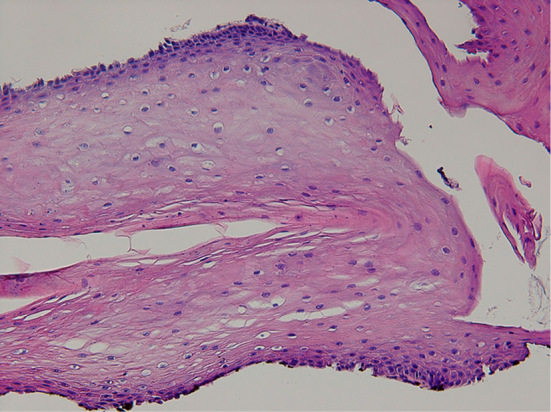
Biopsy specimens showing improved inflammatory infiltration in the epithelium.
Discussion
LP is an idiopathic inflammatory disorder that involves the skin, scalp, nails, and mucous membranes (1). Oral LP is a relatively frequent inflammatory mucocutaneous disease of middle-aged individuals, affecting approximately 1.27% (0.96% of men and 1.57% of women) of the world population (4). ELP accounts for approximately 1% of oral LP (5). Fox et al. reported that among the 72 patients with ELP, 87% of the patients were female and that oral LP was present in 89% (6). Dysphagia was present in 81% and odynophagia was present in 24%. In the Mayo Clinic study, 4 patients (67%) had stricture; in all cases, the stricture was located in the proximal esophagus (7). The proximal or mid-esophagus is the most common location for the esophageal lesions of LP. The proximal esophagus is affected in 90% of cases with or without distal involvement (8).
Esophagogastroduodenoscopy plays a role in the diagnosis of ELP. Possible endoscopic findings include pseudomembranes, a friable and inflamed mucosa, submucosal papules, lacy white plaques, erosion, stricture, and other abnormalities (9, 10). The differential diagnosis of ELP includes reflux esophagitis, eosinophilic esophagitis, viral esophagitis, and bullous disorders such as pemphigus vulgaris and bullous pemphigoid. ELP typically affects the upper and mid-esophagus, sparing the esophagogastric junction, (in contrast to reflux esophagitis) (11). Biopsies are necessary to differentiate ELP from other disorders. The most indicative characteristics of ELP include infiltration of the lymphohistiocytic interface and dyskeratotic cells (Civatte bodies) (12). ELP should be suspected when the following characteristics are present: 1) the patient is a middle-aged or older woman; 2) other erosive mucosal lesions are present; 3) the proximal esophagus is involved; and 4) a histologic examination reveals band-like or lichenoid lymphoid infiltration involving the superficial lamina propria and basal epithelium, with the presence of civatte bodies (13). In addition to histological findings of civatte bodies, the lack of bullous lesions in the skin, anti-desmoglein antibody negativity, and the history of oral LP allowed for the exclusion of bullous disorders; however specific immunofluorescent staining was not performed in the present case. Lymphocytic esophagitis is a recently established entity that is associated with severe dysphagia and which is characterized by high numbers of intraepithelial lymphocytes (14, 15). Although it was differentiated from ELP by the absence of marked intraepithelial lymphocyte infiltration in our case, further study on lymphocytic esophagitis is needed because the details of the pathology, clinical symptoms, and endoscopic findings of lymphocytic esophagitis remain unclear.
To the best of our knowledge, there is no standard treatment for ELP. Historically, systemic corticosteroids have been considered the first-line treatment with a response rate of up to 74% based on multiple reports (10). However, the relapse rate after steroid withdrawal can be as high as 85% (16). Recently, fluticasone propionate (220 μg twice daily, topically) was shown to result in clinical and endoscopic improvement (3, 10, 17, 18). This treatment has the advantage of being associated with fewer side effects in comparison to systematic corticosteroid treatment. In the present case, we recommended switching from oral corticosteroids to swallowed fluticasone propionate; however, the patient refused this recommendation because swallowed fluticasone was not covered by Japanese insurance. In isolated cases, patients have been successfully treated with oral tacrolimus (19), alitretinoin (20), and rituximab (21).
Endoscopic balloon dilation is another possible option in patients who remain symptomatic despite medical management (3). A recent report noted that the placement of removable fully-covered metal esophageal stent was an option when standard medical therapy and endoscopic dilatation fails in a patient undergoing steroid treatment (22).
ELP may represent a premalignant disease because esophageal squamous cell carcinomas (SCCs) arising from ELP have been described (9, 23, 24). Schwartz et al. suggested the use of high-magnification chromoendoscopy to differentiate areas of hyperplasia, or low-grade dysplasia, from areas of severe dysplasia and SCC (23). Narrow-band imaging (NBI) may also be useful for screening for esophageal SCC in patients with ELP as well as head and neck cancer (25). In our case, only squamous intraepithelial neoplasia was found by NBI endoscopy and iodine-chromoendoscopy. Because the esophageal SCCs developed more than 6 years after the diagnosis of ELP, periodic surveillance endoscopy should be performed (23, 24).
In our case, ELP was first diagnosed after the oral LP was found. There are some reasons why the diagnosis of ELP was not made at the first endoscopy. First, ELP is rare and only approximately 80 cases have been reported in the literature. Second, the specimen taken from the esophagus at the first endoscopy might have been small and insufficient to make a diagnosis of ELP. Finally, clinical information is important for the pathologist because ELP is an under-recognized disorder.
In conclusion, we presented a rare case of ELP in a patient who was successfully treated with systemic corticosteroids. Since ELP is an under-recognized disorder that may be mistaken for reflux or candida esophagitis, gastroenterologists should pay attention to not only the skin but also the oral cavity as this can be helpful in making a correct diagnosis. In patients with ELP strictures most commonly occur in the proximal esophagus. Corticosteroids can achieve effective relief from the symptoms.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Sharma A, Białynicki-Birula R, Schwartz RA, et al. Lichen planus: an update and review. Cutis 90: 17-23, 2012. [PubMed] [Google Scholar]
- 2. Nielsen JA1, Law RM, Fiman KH, et al. Esophageal lichen planus: a case report and review of the literature. World J Gastroenterol 19: 2278-2281, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franco DL, Islam SR, Lam-Himlin DM, et al. Presentation, diagnosis, and management of esophageal lichen planus: a series of six cases. Case Rep Gastroenterol 9: 253-260, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med 37: 447-453, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88: 431-436, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol 65: 175-183, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Harewood GC, Murray JA, Cameron AJ. Esophageal lichen planus: the Mayo Clinic experience. Dis Esophagus 12: 309-311, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci World J 2014: 742826, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chryssostalis A, Gaudric M, Terris B, et al. Esophageal lichen planus: a series of eight cases including a patient with esophageal verrucous carcinoma. A case series. Endoscopy 40: 764-768, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Ynson ML, Forouhar F, Vaziri H. Case report and review of esophageal lichen planus treated with fluticasone. World J Gastroenterol 19: 1652-1656, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams TR, Haider-Shah H. Diffuse esophageal stricture secondary to esophageal lichen planus. Abdom Imaging 30: 355-357, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Quispel R, van Boxel OS, Schipper ME, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy 41: 187-193, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Chandan VS, Murray JA, Abraham SC. Esophageal lichen planus. Arch Pathol Lab Med 132: 1026-1029, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Rubio CA, Sjödahl K, Lagergren J. Lymphocytic esophagitis: a histologic subset of chronic esophagitis. Am J Clin Pathol 125: 432-437, 2006. [PubMed] [Google Scholar]
- 15. Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut 61: 1108-1114, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Westbrook R, Riley S. Esophageal lichen planus: case report and literature review. Dysphagia 23: 331-334, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Donnellan F, Swan MP, May GR, et al. Fluticasone propionate for treatment of esophageal lichen planus. A case series. Dis Esophagus 24: 211-214, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Hou JK, Qureshi W. Swallowed fluticasone for the treatment of esophageal lichen planus. Gastrointest Endosc 74: 708-709, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Keate RF, Williams JW, Connolly SM. Lichen planus esophagitis: report of three patients treated with oral tacrolimus or intraesophageal corticosteroid injections or both. Dis Esophagus 16: 47-53, 2003. [DOI] [PubMed] [Google Scholar]
- 20. Kolios AG, Marques Maggio E, Gubler C, et al. Oral, esophageal and cutaneous lichen ruber planus controlled with alitretinoin: case report and review of the literature. Dermatology 226: 302-310, 2013. [DOI] [PubMed] [Google Scholar]
- 21. Parmentier L, Bron BA, Prins C, et al. Mucocutaneous lichen planus with esophageal involvement: successful treatment with an anti-CD20 monoclonal antibody. Arch Dermatol 144: 1427-1430, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Stein P, Brun A, Zaidi H, et al. Successful treatment of a persistent esophageal lichen planus stricture with a fully covered metal stent. ACG Case Rep J 3: 98-100, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz MP, Sigurdsson V, Vreuls W, et al. Two siblings with lichen planus and squamous cell carcinoma of the oesophagus. Eur J Gastroenterol Hepatol 18: 1111-1115, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Zvidi I, Geller A, Gal E, et al. Malignant transformation of esophageal lichen planus. Isr Med Assoc J 14: 395-396, 2012. [PubMed] [Google Scholar]
- 25. Takenaka R, Kawahara Y, Okada H, et al. Narrow-band imaging provides reliable screening for esophageal malignancy in patients with head and neck cancers. Am J Gastroenterol 104: 2942-2948, 2009. [DOI] [PubMed] [Google Scholar]


