Abstract
We describe a case of amiodarone-induced thyrotoxicosis (AIT) with cardiopulmonary arrest (CPA) in a 49-year-old woman. The patient had been treated with amiodarone for non-sustained ventricular tachycardia. Two weeks prior to her admission, she developed thyrotoxicosis and prednisolone (PSL, 30 mg daily) was administered with the continuation of amiodarone. However, she was admitted to our hospital for CPA. We performed total thyroidectomy to control her thyrotoxicosis and the pathological findings were consistent with type 2 AIT. She gradually improved and was discharged on day 84. This case demonstrates the importance of considering immediate total thyroidectomy for patients with uncontrollable AIT.
Keywords: amiodarone-induced thyrotoxicosis, hyperthyroxinemia, cardiopulmonary arrest, prednisolone, thyroidectomy
Introduction
Amiodarone, a widely used antiarrhythmic drug, is known to cause thyroid dysfunction, such as amiodarone-induced thyrotoxicosis (AIT) and amiodarone-induced hypothyroidism (AIH) through its pharmacological and toxic actions (1, 2). AIT represents a diagnostic and therapeutic challenge, while AIH does not cause critical problems in most cases. AIT is classified as type 1 (iodine-induced hyperthyroidism), type 2 (destructive thyroiditis), and mixed type (types 1 and 2) (3). In Japan, where the iodine intake is sufficient, almost all cases of AIT are type 2 and are mainly treated with prednisolone (PSL) (4). However, some cases of type 2 AIT are reported to be resistant to oral PSL therapy (5, 6). Moreover, patients who are treated with amiodarone usually have severe cardiac abnormalities and amiodarone withdrawal is often difficult. In such cases, immediate total thyroidectomy should be considered as a second-line therapy (3) because prolonged thyrotoxicosis has the potential to cause cardiac damage and muscle weakness (7). We herein report a case of uncontrolled type 2 AIT with cardiopulmonary arrest (CPA) which ultimately required total thyroidectomy.
Case Report
A 49-year-old woman was referred to our hospital for thyrotoxicosis. Her significant medical history included severe hypertrophic cardiomyopathy and non-sustained ventricular tachycardia (NSVT). She had been treated with amiodarone (100 mg per day) and an implantable cardioverter-defibrillator (ICD) for 2 years prior to her presentation. She had no personal or family history of thyroid disease. After the initiation of amiodarone treatment, her free thyroxine (FT4), free triiodothyronine (FT3) and thyroid-stimulating hormone (TSH) levels were measured at 3-month intervals. The levels remained normal for the first 2 years of the therapy; however, her most recent test showed hyperthyroxinemia with elevated FT4 (>7.8 ng/dL) and FT3 (19.2 pg/mL) levels, suppressed TSH levels (<0.005 μIU/mL), and undetectable autoimmune thyroid antibody levels, including anti-thyroglobulin, anti-thyroperoxidase, and anti-TSH receptor antibodies. She complained of a 3-month history of increasing fatigue, loss of appetite, and 7 kg of unintentional weight loss. Type 2 AIT was highly suspected based on her clinical course and the laboratory findings. Amiodarone was continued because her NSVT was difficult to manage with other antiarrhythmic agents. The administration of PSL [30 mg per day, orally (0.5 mg/kg)] was therefore initiated with the continuation of amiodarone. However, 2 weeks later, she was admitted to the intensive care unit of our hospital because of an episode of CPA.
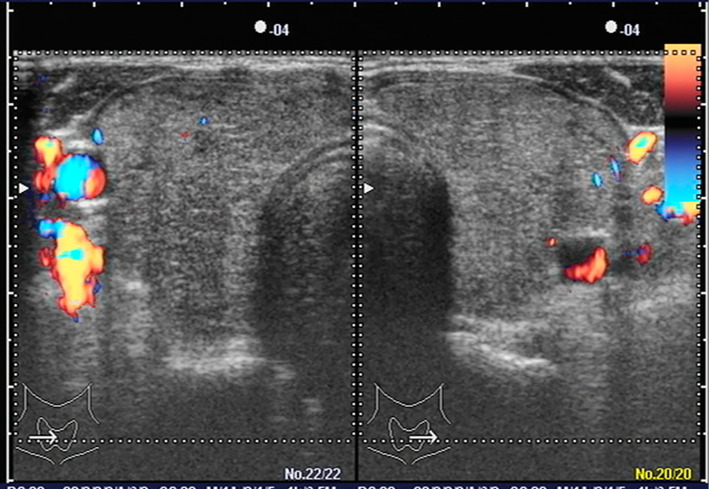
On admission, her height and body weight were 165 cm and 40 kg, respectively. Her Glasgow Coma Scale (GCS) was E1V1M1, her systolic blood pressure was 80 mmHg, and her pulse rate was 100 beats/min. She had a small firm grade 2 goiter (WHO classification). Her ICD recording showed ventricular fibrillation (VF), which was treated by direct current cardioversion (35 J). Table shows the results of the laboratory tests. She had severe thyrotoxicosis, despite the PSL treatment. Her potassium level was 5.6 mEq/L and her amiodarone concentration was 134.1 ng/mL. Moreover, the patient had a high carbon dioxide level (139 mmHg), diaphragmatic paralysis, and respiratory muscle weakness, which required mechanical ventilation. Echocardiography showed diffuse hypokinesis (estimated ejection fraction, 40%) and no asynergy. A thyroid ultrasound showed diffuse goiter [thyroid volume calculated by the ellipsoid formula (8), 43.5 cm3] and color flow Doppler sonography (CFDS) revealed a lack of intraparenchymal vascularity (Fig. 1).
Table.
Laboratory Findings.
| Blood samples | Thyroid function | |||||||||
| WBC | 1.93×104 | /μL | TP | 6.7 | g/dL | TSH | <0.005 | μIU/mL | ||
| Hb | 12.7 | g/dL | Alb | 3.7 | g/dL | Free T4 | >7.8 | ng/dL | ||
| Plt | 28.4×104 | /μL | Na | 138 | mEq/L | Free T3 | 19.2 | pg/mL | ||
| AST | 141 | IU/L | K | 5.6 | mEq/L | Tyroglobulin | 173.4 | ng/mL | ||
| ALT | 97 | IU/L | P | 3.9 | mg/dL | TRAb (3rd) | <0.4 | IU/L | ||
| ALP | 435 | IU/L | Ca | 9.8 | mg/dL | TPOAb | <0.3 | IU/L | ||
| LDH | 326 | IU/L | Glucose | 227 | mg/dL | TgAb | 1.6 | IU/L | ||
| CK | 55 | IU/L | BNP | 208.1 | pg/mL | |||||
| BUN | 46.3 | mg/dL | KL-6 | 156 | IU/mL | |||||
| Cr | 0.68 | mg/dL | CRP | 0.03 | mg/dL | |||||
WBC: white blood cell count, Hb: hemoglobin, Plt: platelet, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, TP: total protein, P: phosphorus, BNP: brain natriuretic peptide, KL-6: sialylated carbohydrate antigen: Krebs von den Lungen-6, CRP: C-reactive protein, TSH: thyroid-stimulating hormone, T4: thyroxine, T3: triiodothyronine, TRAb anti-TSH receptor antibody, TPOAb: anti-thyroperoxidase antibody, TgAb: anti-thyroglobulin antibody
Figure 1.
Thyroid ultrasound. Right lobe, 2.2 (wide) ×2.7 (depth) ×7.5 (length) cm; left lobe, 2.1×2.4×7.4 cm; Isthmus, 0.36×3.1 cm. The thyroid volume, as calculated by the ellipsoid formula, was 43.5 cm3. Color flow Doppler sonography revealed no intraparenchymal vascularity.
Other reasons for thyrotoxicosis, such as subacute thyroiditis, toxic multinodular goiter, were less likely than AIT because she had used amiodarone for 2 years, and because she had no signs of inflammation (Table), and no signs of nodules in her thyroid (Fig. 1). In addition to the regional characteristics of iodine sufficiency in Japan, her autoimmune thyroid antibody negativity and the CFDS findings strengthened the possibility of type 2 rather than type 1 AIT.
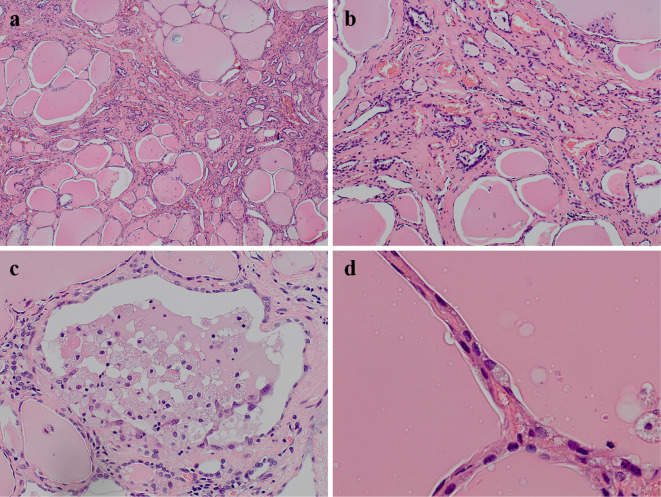
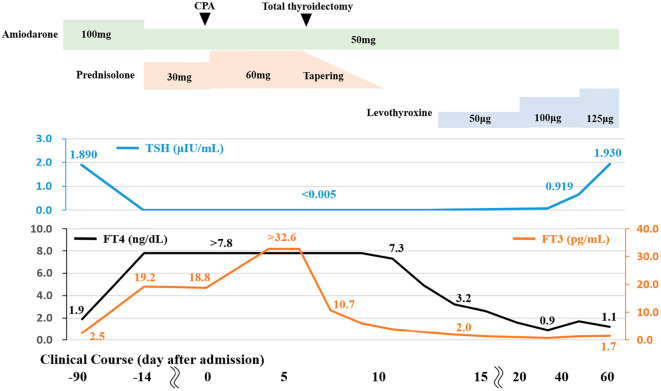
Although the PSL dose was increased to 60 mg (1.0 mg/kg) daily, she remained unconscious and her thyroid function tests continued to show hyperthyroxinemia (FT4>7.8 ng/dL, FT3>32.6 pg/mL, and TSH <0.005 μIU/mL). Thus, total thyroidectomy was performed on day 7 after admission. Histologically, most of the thyroid follicles were filled with colloid and lined with flattened follicular epithelial cells. We occasionally found atrophic follicles with fibrosis (Fig. 2a and b) and disrupted follicles with the infiltration of macrophages (Fig. 2c). Cytoplasmic vacuolization, deposits of lipofuscin, and the detachment of follicular epithelial cells were observed in these damaged follicles (Fig. 2d). The lymphocytic infiltration was slight. After total thyroidectomy, her thyroid hormone levels gradually decreased. The PSL dose was tapered and levothyroxine was initiated to maintain a euthyroid status on day 12 after admission. Amiodarone (50 mg daily) was continued to control her NSVT. Her unconsciousness, diaphragmatic paralysis, and respiratory muscle weakness gradually improved and she was removed from the mechanical ventilator on day 14. Following rehabilitation, the patient was finally discharged on the 84th day after admission. Treatment with levothyroxine was continued at a dose of 125 μg. Her clinical course is shown in Fig. 3.
Figure 2.
The pathological findings of the thyroid. Atrophic follicles with interstitial fibrosis [a and b: Hematoxylin and Eosin (H&E) staining]. Disrupted follicles with detachment of the follicular epithelium and the macrophage infiltration (c: H&E staining). Cytoplasmic vacuolization and lipofuscin in flattened follicular epithelial cells (d: H&E staining).
Figure 3.
The clinical course. Two weeks after the administration of prednisolone (PSL) for amiodarone-induced thyrotoxicosis, she had an episode of cardiopulmonary arrest (CPA). Total thyroidectomy was performed on day 7 to treat her uncontrolled hyperthyroxinemia. After resection, her thyroid hormone levels gradually decreased. The PSL dose was tapered and levothyroxine was started. Amiodarone was continued to control her non-sustained ventricular tachycardia (NSVT). After rehabilitation, she finally discharged on day 84.
Discussion
Amiodarone is a benzofuranic iodine-rich antiarrhythmic drug (class III) that causes thyroid dysfunction in 15-20% of patients (3). Amiodarone contains 75 mg iodine in each 200 mg tablet and releases approximately 10% of the iodine as free iodine each day (1, 9), which affects the thyroid hormone metabolism. The prevalence of AIT is 2% and 12% in iodine-sufficient and iodine-deficient areas, respectively (10). It can also develop after the withdrawal of amiodarone because the drug has a long half-life of approximately 100 days (2). The immediate detection and treatment of AIT are necessary because prolonged thyrotoxicosis causes dangerous and critical situations for patients with cardiac abnormalities.
AIT has two different subtypes: type 1 (a form of iodine-induced hyperthyroidism) and type 2 (a drug-induced destructive thyroiditis) (3). It is important to clarify these two types because they require different therapeutic approaches; antithyroid drugs for type 1 and PSL for type 2 (11). We diagnosed the present case as having type 2 AIT based on the following characteristics: 1) she did not have underlying thyroid disease and was negative for thyroid antibodies; 2) CFDS showed the absence of hypervascularity; and 3) she was living in Japan, where the iodine intake is sufficient and almost all reported AIT cases are type 2 (3). The long duration of amiodarone intake and the increased FT4/FT3 ratio also supported this diagnosis; however, we did not perform a radioactive iodine intake scan or evaluate other parameters such as the serum interleukin-6 level (5, 10). We introduced oral PSL as the first-line treatment for type 2 AIT (12), but her thyrotoxicosis was difficult to control with high-dose PSL therapy. A previous study reported that the serum thyroid hormone levels and thyroid volume might predict the response time to PSL in type 2 AIT patients (13). Our patient had high FT4 levels and diffuse goiter, which implied that it would take a significant period of time to achieve a euthyroid status; however, a rapid restoration was needed due to her severe cardiac disease. Thus, we performed total thyroidectomy (14), which led to the improvement of her condition. The pathological findings of the current case were consistent with those of previous reports of amiodarone-induced destructive thyroiditis (15, 16). The destruction of the thyroid follicles, as shown in Fig. 2, would have caused the excessive release of colloid, and may have led to the elevated thyroid hormone levels and uncontrollable hyperthyroidism in the present case.
AIT cases with CPA have rarely been described (5). We assumed that her episode of CPA was caused by both cardiac and respiratory dysfunction. Her uncontrollable prolonged thyrotoxicosis might have damaged her cardiac function, which ultimately led to the short-term VF (17, 18). Moreover, the patient had elevated carbon dioxide levels, transient diaphragmatic paralysis, and respiratory muscle weakness. Thyrotoxicosis is well known to cause acute-onset muscle weakness, not only in the distal muscles but also in the proximal and respiratory muscle, through an extracellular shift of intracellular potassium through the increased activity of the Na+/K+ ATP pump (19), which represents a life-threatening condition (20, 21). Although the present case did not show hypokalaemia or hypophosphatemia, such electrolyte abnormalities are not necessary to induce myopathy in patients with thyrotoxicosis (7). It is also possible that her emaciation exacerbated her muscle weakness.
In conclusion, we experienced a rare case of type 2 AIT in a Japanese patient with CPA who was completely cured after total thyroidectomy. This case demonstrated the importance of detecting thyroid abnormalities in patients with a history of amiodarone use and of treating them as soon as possible because prolonged thyrotoxicosis might cause CPA. Total thyroidectomy should be considered when patients require the immediate restoration of euthyroidism because of severe conditions.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med 118: 706-714, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev 22: 240-254, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab 95: 2529-2535, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Uchida T, Kasai T. Prevalence of amiodarone-induced thyrotoxicosis and associated risk factors in Japanese patients. 2014: 534904, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta AN, Vallera RD, Tate CR, Sager RA, Welch BJ. Total thyroidectomy for medically refractory amiodarone-induced thyrotoxicosis. Bayl Univ Med Cent 21: 382-385, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hashimoto K, Ota M, Irie T, et al. A case of type 2 amiodarone-induced thyrotoxicosis that underwent total thyroidectomy under high-dose steroid administration. Case Rep Endocrinol 2015: 416145, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin SH. Thyrotoxic periodic paralysis. Mayo Clin Proc 80: 99-105, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Trimboli P, Ruggieri M, Fumarola A, et al. A mathematical formula to estimate in vivo thyroid volume from two-dimensional ultrasonography. Thyroid 18: 879-882, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Kennedy RL, Griffiths H, Gray TA. Amiodarone and the thyroid. Clin Chem 35: 1882-1887, 1989. [PubMed] [Google Scholar]
- 10. Bogazzi F, Tomisti L, Bartalena L, Aghini-Lombardi F, Martino E. Amiodarone and the thyroid: a 2012 update. J Endocrinol Invest 35: 340-348, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Eskes SA, Endert E, Fliers E, et al. Treatment of amiodarone-induced thyrotoxicosis type 2: a randomized clinical trial. J Clin Endocrinol Metab 97: 499-506, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Bogazzi F, Tomisti L, Rossi G, et al. Glucocorticoids are preferable to thionamides as first-line treatment for amiodarone-induced thyrotoxicosis due to destructive thyroiditis: a matched retrospective cohort study. J Clin Endocrinol Metab 94: 3757-3762, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Bogazzi F, Bartalena L, Tomisti L, et al. Glucocorticoid response in amiodarone-induced thyrotoxicosis resulting from destructive thyroiditis is predicted by thyroid volume and serum free thyroid hormone concentrations. J Clin Endocrinol Metab 92: 556-562, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Gough J, Gough IR. Total thyroidectomy for amiodarone-associated thyrotoxicosis in patients with severe cardiac disease. World J Surg 30: 1957-1961, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Smyrk TC, Goellner JR, Brennan MD, Carney JA. Pathology of the thyroid in amiodarone-associated thyrotoxicosis. Am J Surg Pathol 11: 197-204, 1987. [DOI] [PubMed] [Google Scholar]
- 16. Nakazawa T, Murata S, Kondo T, et al. Histopathology of the thyroid in amiodarone-induced hypothyroidism. Pathol Int 58: 55-58, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Ando T, Henmi T, Haruta D, et al. Graves' disease complicated by ventricular fibrillation in three men who were smokers. Thyroid 21: 1021-1025, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Brooks MJ, Pattison DA, Teo EP, Price S, Gurvitch R. Amiodarone-induced destructive thyroiditis associated with coronary artery vasospasm and recurrent ventricular fibrillation. Eur Thyroid J 2: 65-67, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kung AW. Clinical review: thyrotoxic periodic paralysis: a diagnostic challenge. J Clin Endocrinol Metab 91: 2490-2495, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Abbasi B, Sharif Z, Sprabery LR. Hypokalemic thyrotoxic periodic paralysis with thyrotoxic psychosis and hypercapnic respiratory failure. Am J Med Sci 340: 147-153, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Wu CZ, Wu YK, Lin JD, Kuo SW. Thyrotoxic periodic paralysis complicated by acute hypercapnic respiratory failure and ventricular tachycardia. Thyroid 18: 1321-1324, 2008. [DOI] [PubMed] [Google Scholar]