Abstract
An 80-year-old man presented at our hospital with renal failure. He had been treated with edoxaban, an oral direct factor Xa inhibitor, for deep vein thrombosis for 10 months prior to admission. Although the pulses in his bilateral pedal arteries were palpable, cyanosis was present in the bilateral toes. Laboratory data indicated azotemia and eosinophilia. A skin biopsy confirmed a diagnosis of cholesterol crystal embolism (CCE). Because no invasive vascular procedure was performed, we assumed that CCE was related to edoxaban. To the best of our knowledge, this is the first case report suggesting CCE induced by an Xa inhibitor.
Keywords: acute kidney injury, cholesterol crystal embolism, direct oral anticoagulants, edoxaban, renal failure, Xa inhibitors
Introduction
Cholesterol crystal embolism (CCE) is a systemic disease resulting from the occlusion of the arteries by cholesterol crystals released from atheromatous plaques of the aorta (1). Catheter or surgical manipulation of the aorta is a major cause of CCE, but it can occur spontaneously (2-4), as well as after thrombolysis or anticoagulants (1). Although warfarin is the most common causative anticoagulant, there are no case reports suggesting that any factor Xa inhibitor can cause CCE. There are two case reports in which Xa inhibitors were substituted for warfarin and achieved a complete resolution of CCE (5, 6). It is unclear why Xa inhibitors may be a useful treatment for CCE, and not a cause. In this case report, we describe the first case of CCE with renal failure induced by edoxaban, one of the Xa inhibitors.
Case Report
An 80-year-old man was admitted to our hospital with renal failure and general fatigue. He had a history of hypertension and ischemic stroke with a moderate hemiparetic gait. He also had a history of septic arthritis and deep vein thrombosis (DVT) during his stay in the orthopedic ward at our hospital 11 months prior to this admission. Edoxaban, one of the direct factor Xa inhibitors, was started for the treatment of DVT about 10 months prior to this admission. Although he had an almost completely normal kidney function [estimated glomerular filtration rate (eGFR) ranged from 60 to 110 mL/min/1.73 m2] during his first admission, his kidney function had gradually decreased after discharge, and he was therefore re-admitted with renal failure and general fatigue.
On admission, his blood pressure was 128/49 mmHg, his heart rate was 75 beats per min, and his temperature was 37.1℃. Physical examination revealed no livedo reticularis, but blue-colored bilateral toes (Fig. 1), although the pulses in his bilateral pedal arteries were palpable. Laboratory data indicated azotemia, eosinophilia, iron deficiency anemia, hypoalbuminemia, and elevated C-reactive protein (Table). Chest X-ray revealed right-sided pneumonia and fecal occult blood was positive. We started antibiotics for pneumonia and a proton pump inhibitor for occult gastrointestinal bleeding without performing gastrointestinal endoscopy, as we were concerned about potentially exacerbating the patient's pneumonia.
Figure 1.
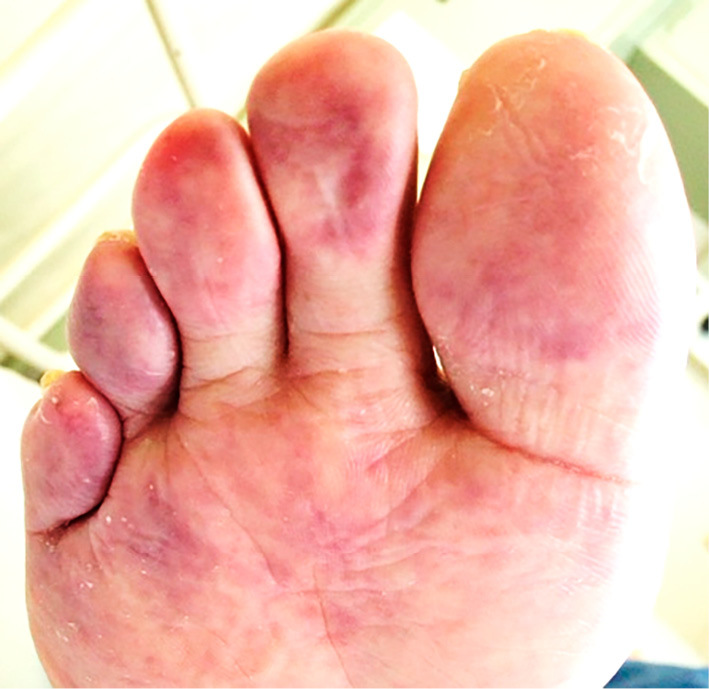
Blue-colored toes of the right foot.
Table.
Laboratory Data on Admission.
| Hematological values | Blood chemical values | Immunological study | ||||||||
| White blood cell count | 15,880 | /μL | Total protein | 6.5 | g/dL | C-reactive protein | 17.55 | mg/dL | ||
| Neutrophil | 73.0 | % | Albumin | 2.9 | g/dL | Complement 3 | 101.1 | mg/dL | ||
| Lymphocyte | 8.0 | % | Total bilirubin | 0.2 | mg/dL | Complement 4 | 31.0 | mg/dL | ||
| Monocyte | 3.0 | % | Aspartate aminotransferase | 40 | U/L | Serum complement titer | 50.1 | U/mL | ||
| Eosinophil | 16.0 | % | Alanine aminotransferase | 19 | U/L | Immunoglobulin G | 2,478.2 | mg/dL | ||
| Red blood cell count | 280×104 | /μL | Lactate dehydrogenase | 241 | U/L | Immunoglobulin A | 415.6 | mg/dL | ||
| Hemoglobin | 7.7 | g/dL | Creatine kinase | 48 | U/L | Immunoglobulin M | 72.4 | mg/dL | ||
| Hematocrit | 22.8 | % | Blood urea nitrogen | 41.4 | mg/dL | MPO-ANCA | <0.5 | U/mL | ||
| MCV | 83.5 | fL | Creatinine | 2.34 | mg/dL | PR3-ANCA | <0.5 | U/mL | ||
| MCHC | 32.0 | g/dL | Uric acid | 6.6 | mg/dL | Coagulation test | ||||
| Platelet count | 26.4×104 | /μL | Sodium | 135 | mEq/L | PT-INR | 1.15 | |||
| Venous blood gas | Potassium | 5.3 | mEq/L | APTT | 33.1 | s | ||||
| pH | 7.314 | Chloride | 110 | mEq/L | Urinary chemistry | |||||
| pCO2 | 29.0 | mmHg | Transferrin saturation | 43 | % | Urinary sodium | 45 | mEq/L | ||
| HCO3 | 14.3 | mmol/L | Ferritin | 75 | ng/mL | Urinary potassium | 25.7 | mEq/L | ||
| Urinalysis | Calcium | 8.8 | mg/dL | Urinary chloride | 42 | mEq/L | ||||
| Protein | (1+) | Phosphate | 3.9 | mg/dL | FENa | 1.0 | % | |||
| Glucose | (−) | Total cholesterol | 183 | mg/dL | FEUN | 36.0 | % | |||
| Occult blood | (±) | Triglyceride | 148 | mg/dL | UP/Cr | 0.68 | g/gCr | |||
| Red blood cell | 1-5 | HPF | HDL-cholesterol | 32.1 | mg/dL | Urinary BMG | 45,027 | µg/L | ||
| White blood cell | 1-5 | HPF | LDL-cholesterol | 121.3 | mg/dL | Urinary NAG | 22.34 | U/L | ||
| Cast | (-) | Glucose | 183 | mg/dL | UNAG/Cr | 29.59 | U/gCr | |||
PTT: activated partial thromboplastin time, BMG: beta 2-microglobulin, FENa: fractional excretion of sodium, FEUN: fractional excretion of urea nitrogen, HDL: high-density lipoprotein, HPF: high-power field, LDL: low-density lipoprotein, MCHC: mean corpuscular hemoglobin concentration, MCV: mean corpuscular volume, NAG: N-acetyl-beta-D-glucosaminidase, PT-INR: international normalized ratio of prothrombin time, UP/Cr: urinary protein/creatinine ratio
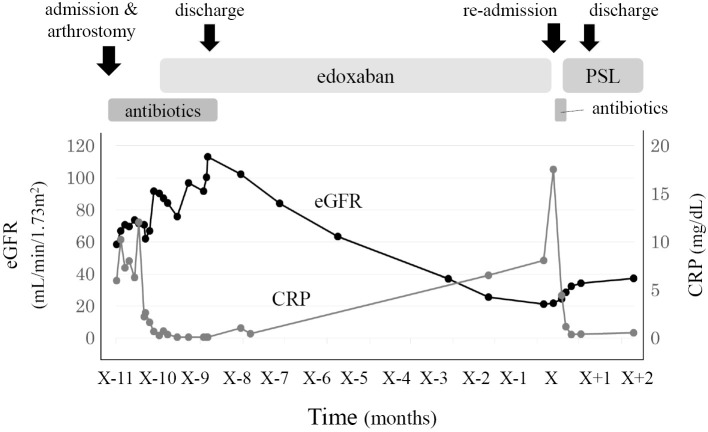
Regarding azotemia, urinary chemistry suggested renal tubular injury. Drugs taken before re-admission were acetaminophen, azilsartan, benidipine, cetirizine, loxoprofen, rebamipide, sennoside, and edoxaban. Although loxoprofen is known to be a cause of renal tubular injury among these particular drugs, loxoprofen was started about 1 month before re-admission. The other drugs, except for edoxaban, were not started during the first admission. Contrast-enhanced computed tomography, which was performed during a previous hospitalization, revealed multiple plaques in the thoracic aorta (Fig. 2). The patient underwent no vascular procedures between the first and second hospitalization. In addition, skin biopsy of the right toe revealed needle-shaped clefts in the lumen of the arterioles, which is a characteristic finding of CCE (Fig. 3). Perivascular and intravascular inflammatory infiltrate including eosinophils indicative of vasculitis syndrome was not identified in the specimen. Because the patient's kidney function continued to decline since edoxaban was started, we diagnosed this case to be CCE caused by edoxaban and stopped the administration of the drug. A low-dose corticosteroid (prednisolone, 15 mg/day) was initiated after recovery from pneumonia with oral antibiotics. Thereafter, his blue-colored toes, azotemia, and eosinophilia gradually improved, and he was later successfully discharged (Fig. 4).
Figure 2.
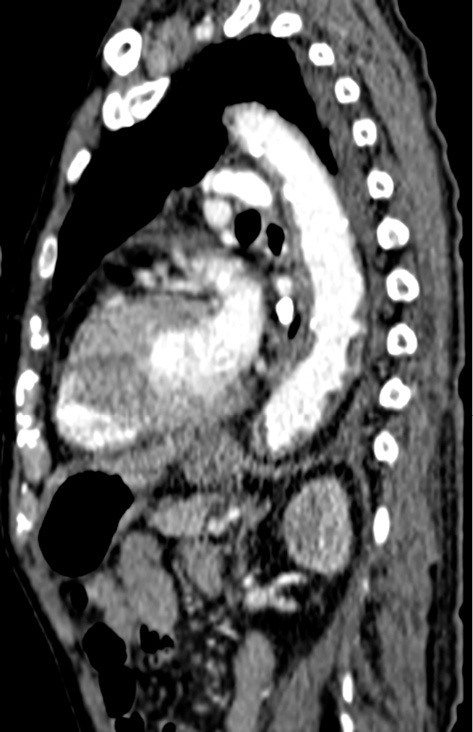
Contrast-enhanced computed tomography, which was performed during a previous hospitalization, revealed multiple plaques in the thoracic aorta.
Figure 3.
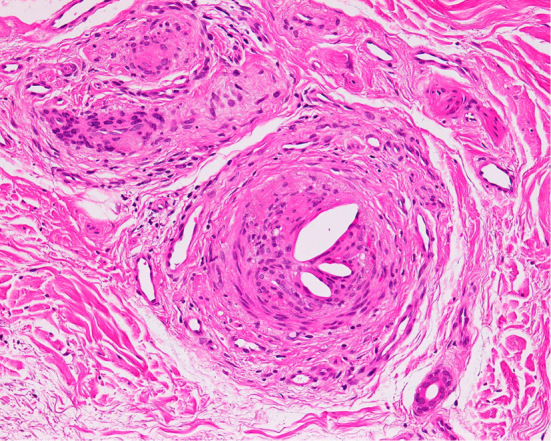
A skin biopsy of the right toe revealed needle-shaped clefts in the lumen of the arterioles (Hematoxylin and Eosin staining; original magnification ×200).
Figure 4.
The patient’s clinical course. The patient’s levels of eGFR (black line) gradually decreased about 1 month after starting edoxaban and gradually recovered after it was stopped and low-dose prednisolone was started. CRP: C-reactive protein, eGFR: estimated glomerular filtration rate, PSL: prednisolone
Discussion
CCE is a systemic disease caused by the occlusion of arteries resulting from the embolization of atheromatous debris including fibrin, platelets, cholesterol crystals, and calcium fragments (1). The atheroemboli typically occlude small arteries of the extremities, brain, eye, kidney, or mesentery. Catheter or surgical manipulation of the aorta is a major cause of CCE, and anticoagulants or thrombolysis might precipitate CCE. Several cases of spontaneous CCE have been reported (2-4). Because invasive vascular procedures were not performed, CCE in this patient was thought to have been induced by anticoagulant therapy for DVT using edoxaban, which is one of the direct factor Xa inhibitors. To the best of our knowledge, there are no reports that Xa inhibitors can cause CCE.
The mechanism behind anticoagulant-induced CCE is not clear; however, several theories have been suggested. One plausible explanation based on an autopsy examination is that anticoagulants lead to CCE by hemorrhaging atherosclerotic plaques, by impairing the healing of ulcerated plaques (7, 8), or by dissolving fibrin clots that stabilized the atheroma in place (9-11).
Factor Xa inhibitors are a relatively new type of anticoagulant, as is the direct thrombin inhibitor dabigatran. Recently, Xa inhibitors and dabigatran have been collectively called DOAC in English (direct oral anticoagulants). DOAC have been reviewed and found to be non-inferior to warfarin for the prevention of ischemic stroke and systemic embolism, and is associated with a reduced incidence of hemorrhagic events (12, 13). There are a number of meta-analyses suggesting that DOAC pose a risk for bleeding comparable with warfarin (14, 15). Further study is therefore needed to clarify the bleeding risk from DOAC (16). There is no doubt, however, that DOAC are associated with a greater risk for bleeding than a placebo, therefore, it is possible that DOAC could be a cause of CCE by hemorrhaging atherosclerotic plaques.
Two case reports suggest that warfarin-induced CCE can be successfully treated using Xa inhibitors; with fondaparinux in one case (5) and with apixaban in the other (6). In these reports, it was noted that warfarin had a direct toxic effect on the capillaries, resulting in vasodilation and increased permeability (17). Although warfarin may pose a greater risk for inducing CCE compared with other anticoagulants (as mentioned in the suggested theory above), Xa inhibitors may have a weak ability to cause CCE indirectly via the bleeding of atherosclerotic plaques. Our findings suggest that edoxaban use is the only possible cause for CCE in the present case.
Regarding the optimal treatment for CCE, azotemia and symptoms are not always reversible even after removing the cause. From a cited case report (5), only six cases improved upon stopping warfarin among 13 reported warfarin-induced CCE cases. Therefore, it is difficult to reach any conclusions through observation by only stopping the suspected causative drug for drug-induced CCE. Although no definitive treatment has yet been established for CCE, the possible benefits of corticosteroids have been reported in a small series of cases (18-20). Therefore, we used corticosteroids for the treatment of CCE in this patient.
Conclusion
CCE can be caused by edoxaban, one of the Xa inhibitors, as well as warfarin and other anticoagulants. Close attention should thus be given to a patient's kidney function and the skin findings of the feet and toes during the use of all types of anticoagulants, including Xa inhibitors.
Author's disclosure of potential Conflicts of Interest (COI).
Kazuhiko Tsuruya: Honoraria, Chugai Pharmaceutical and Kyowa Hakko Kirin; Research funding, Chugai Pharmaceutical, Kyowa Hakko Kirin, Otsuka Pharmaceutical, Takeda Pharmaceutical, Daiichi-Sankyo and Torii Pharmaceutical.
References
- 1. Creager MA, Libby P. Peripheral arterial diseases. In: Heart Disease. 8th edition Braunwald E, Ed. Saunders, Philadelphia, 2008: 1509-1511. [Google Scholar]
- 2. Tan J, Afzal I, Yeadon A, Bird N, Gouldesbrough D, Akbani H. Spontaneous cholesterol embolisation causing acute renal failure. Clin Exp Nephrol 11: 235-237, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Enomae M, Takeda S, Yoshimoto K, Takagawa K. Chronic cholesterol crystal embolism with a spontaneous onset. Intern Med 46: 1123-1126, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Bradley M. Images in clinical medicine. Spontaneous atheroembolism. N Engl J Med 332: 998, 1995. [DOI] [PubMed] [Google Scholar]
- 5. Rindone JP, Mellen CK, Eliason JD. Late onset purple toe syndrome with warfarin successfully treated with fondaparinux. Am J Ther 18: e277-e279, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Cakebread HE, Knight HM, Gajendragadkar PR, Cooper JP. Warfarin-induced purple toe syndrome successfully treated with apixaban. BMJ Case Rep: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hyman BT, Landas SK, Ashman RF, Schelper RL, Robinson RA. Warfarin-related purple toes syndrome and cholesterol microembolization. Am J Med 82: 1233-1237, 1987. [DOI] [PubMed] [Google Scholar]
- 8. Moldveen-Geronimus M, Merriam JC Jr. Cholesterol embolization. From pathological curiosity to clinical entity. Circulation 35: 946-953, 1967. [DOI] [PubMed] [Google Scholar]
- 9. Donohue KG, Saap L, Falanga V. Cholesterol crystal embolization: an atherosclerotic disease with frequent and varied cutaneous manifestations. J Eur Acad Dermatol Venereol 17: 504-511, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Molinos S, Feal C, Gándara A, Morla J, De la Torre C, Rosón E. Cholesterol emboli syndrome: significance of the lesional skin biopsies. Acta Derm Venereol 85: 527-528, 2005. [DOI] [PubMed] [Google Scholar]
- 11. Pennington M, Yeager J, Skelton H, Smith KJ. Cholesterol embolization syndrome: cutaneous histopathological features and the variable onset of symptoms in patients with different risk factors. Br J Dermatol 146: 511-517, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Wassef A, Butcher K. Novel oral anticoagulant management issues for the stroke clinician. Int J Stroke 11: 759-767, 2016. [DOI] [PubMed] [Google Scholar]
- 13. Caldeira D, Rodrigues FB, Barra M, et al. . Non-vitamin K antagonist oral anticoagulants and major bleeding-related fatality in patients with atrial fibrillation and venous thromboembolism: a systematic review and meta-analysis. Heart 101: 1204-1211, 2015. [DOI] [PubMed] [Google Scholar]
- 14. Caldeira D, Canastro M, Barra M, et al. . Risk of substantial intraocular bleeding with novel oral anticoagulants: systematic review and meta-analysis. JAMA Ophthalmol 133: 834-839, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Caldeira D, Barra M, Ferreira A, et al. . Systematic review with meta-analysis: the risk of major gastrointestinal bleeding with non-vitamin K antagonist oral anticoagulants. Aliment Pharmacol Ther 42: 1239-1249, 2015. [DOI] [PubMed] [Google Scholar]
- 16. Sharma M, Cornelius VR, Patel JP, Davies JG, Molokhia M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: systematic review and Meta-Analysis. Circulation 132: 194-204, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feder W, Auerbach R. “Purple toes”: an uncommon sequela of oral coumarin drug therapy. Ann Intern Med 55: 911-917, 1961. [DOI] [PubMed] [Google Scholar]
- 18. Yücel AE, Kart-Köseoglu H, Demirhan B, Ozdemir FN. Cholesterol crystal embolization mimicking vasculitis: success with corticosteroid and cyclophosphamide therapy in two cases. Rheumatol Int 26: 454-460, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Nakayama M, Nagata M, Hirano T, et al. . Low-dose prednisolone ameliorates acute renal failure caused by cholesterol crystal embolism. Clin Nephrol 66: 232-239, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Mann S, Sos TA. Treatment of atheroembolization with corticosteroids. Am J Hypertens 14: 831-834, 2001. [DOI] [PubMed] [Google Scholar]