Abstract
Cowden syndrome is a rare autosomal dominant disorder characterized by multiple hamartomas of the ectoderm and brain. A 36-year-old Japanese man presented with right facial seizure during sleep and was admitted to our hospital. He showed cobblestoning over the tongue and palmar pitting but no neurological abnormalities while he was not having a seizure. Brain magnetic resonance imaging showed focal cortical dysplasia in the left frontal lobe. Electroencephalography showed sharp waves over the left frontal lesion. A genetic analysis revealed a novel mutation of PTEN. The administration of carbamazepine ended the seizures. This is the first Japanese case of Cowden syndrome with a novel PTEN gene mutation and cortical dysplasia.
Keywords: Cowden syndrome, cortical dysplasia, PTEN, epilepsy
Introduction
Cowden syndrome is a rare disorder caused by autosomal dominant mutations in the phosphatase and tensin homologue on chromosome 10 (PTEN) tumor suppressor gene. Cowden syndrome is characterized by multiple hamartomas, particularly of the skin and gastrointestinal system, and is frequently associated with breast, thyroid, and genitourinary malignancies (1). In a study of 20 patients with Cowden syndrome, 7 (35%) had abnormalities on brain magnetic resonance imaging (MRI), which included dysplastic cerebellar gangliocytomas, meningiomas, and vascular malformations (2). However, cerebral cortical dysplasia is a rare manifestation of Cowden syndrome. We herein report a Japanese patient with Cowden syndrome who had cerebral cortical dysplasia, seizures, and a novel PTEN mutation.
Case Report
A 36-year-old man suffered a right facial seizure during sleep. The seizure awakened him and lasted for a few minutes. Three months later, he experienced another right facial seizure during a nap. He reported that this seizure also lasted for a few minutes, and then he lost consciousness. When he regained consciousness, he found vomitus near his pillow and experienced weakness of his right upper and lower limbs for about half a day. After eight days, he was admitted to our hospital. He had no family history of epilepsy or neurological disorders, but his father died of hepatocellular carcinoma. His birth and growth were normal, and he had no history of epilepsy or febrile convulsions, but he noticed bilateral palmar pitting as a teenager. His general condition was good, and his vital signs were within normal limits. He showed macrocephaly, and a skin examination revealed cobblestoning over the cheek and tongue (Fig. 1) and palmar pitting. He showed no neurological abnormalities during his time without seizures and no cognitive decline, aphasia, or apraxia, and he achieved a full score on the Mini-Mental State Examination. Blood tests for autoantibodies, amino acids, lactate, pyruvate, pituitary hormone, tumor markers, and infectious agents showed no abnormalities. An examination of the cerebrospinal fluid revealed normal pressure, no pleocytosis, a protein level of 30 mg/dL, and an IgG index of 0.370; in addition, myelin basic protein was within normal limits, and oligoclonal bands and culture and cytology were negative.
Figure 1.
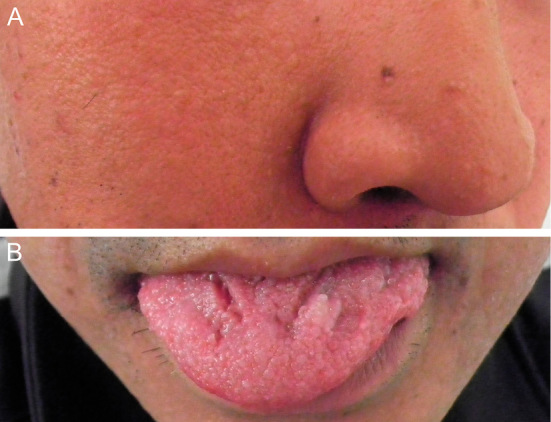
Skin abnormalities in our patient. A skin examination revealed cobblestoning over the cheek (A) and tongue (B).
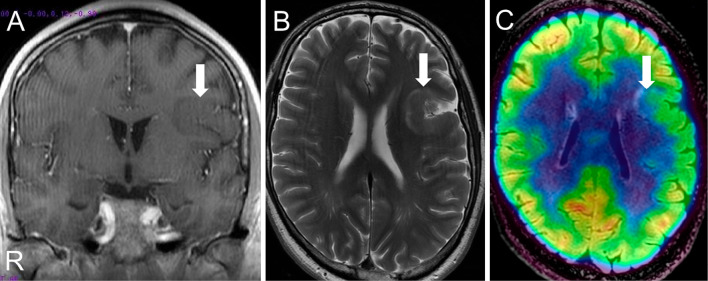
Brain MRI showed left frontal focal cortical dysplasia without contrast enhancement and linear T2 hyperintensities of the subcortical white matter (Fig. 2). Positron Emission Tomography-Computed Tomography showed hypometabolism of cortical dysplasia, and Single photon emission computed tomography showed hypoperfusion in this area (Fig. 2). Electroencephalography during wakefulness and sleep showed sharp waves from the left frontal lesion, along with convulsions during sleep. Sensory evoked potentials (SEPs) showed no cortical delay, laterality, or giant SEPs, and 67Ga scintigraphy showed no abnormal accumulation. A whole-body CT scan showed no hamartomas, even in the thyroid, breast, lung, and liver. Gastrointestinal endoscopy revealed multiple polyps.
Figure 2.
Brain MRI images and PET-CT fusion images. The left frontal lobe showed cortical thickening (A; arrow) and linear subcortical T2 hyperintensities (B; arrow) with hypometabolism in this area (C; arrow).
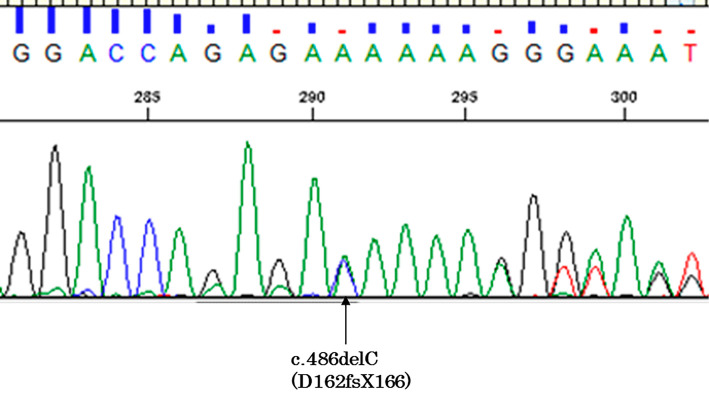
We obtained written informed consent for genetic testing but did not provide genetic counseling because the patient refused it. All nine exons and exon-intron junctions of the PTEN gene were analyzed by polymerase chain reaction direct sequencing. A mutational analysis of PTEN showed a heterozygous deletion in exon 5 (c.486delC, Fig. 3). This mutation generally induces pathogenicity causing Cowden syndrome because of a leading stop codon. The patient was diagnosed with Cowden syndrome with a novel PTEN mutation and secondary epilepsy due to cortical dysplasia. He was administered carbamazepine, and the convulsions during sleep disappeared.
Figure 3.
A mutation analysis of PTEN showed a heterozygous deletion in exon 5 (c.486delC).
Discussion
Cowden syndrome was first described by Lloyd and Dennis in 1963 and is characterized by macrocephaly, mucocutaneous lesions, acral keratosis, papillamatous papules, and a high risk of developing cancer in the breast, thyroid, and endometrium (3). This rare autosomal dominant disease has an estimated prevalence of 1 in 100,000 to 250,000 with genetic identification (4). In 1996, the International Cowden Consortium identified germline PTEN mutations as a cause of Cowden syndrome (5). PTEN is a tumor suppressor gene that encodes a key lipid phosphatase in the phosphoinositide 3-kinase signaling cascade (6) and in cell proliferation and migration (7).
Cowden syndrome represents a late-onset phenotype of the PTEN mutation. Early presentations of PTEN mutations include Lhermitte-Duclos disease (dysplastic gangliocytomas in cerebellum), Bannayan-Riley-Ruvalcaba syndrome, and autism spectrum disorder with macrocephaly (1). An MRI study showed dysplastic gangliocytomas in the cerebellum, meningiomas, and vascular malformations as common abnormalities, occurring in 35% of patients with Cowden syndrome (2). The underlying etiology of these brain hamartomas is currently unknown. Several cases of Cowden syndrome with cortical dysplasia in childhood have been reported (8, 9); however, only one case of adult-onset Cowden syndrome with cortical dysplasia has been published (10).
Recently, PTEN mutations have been found to play an important role in cancer and epilepsy, and PI3/Akt/mTOR inhibitors are being investigated as potential treatments (11). In a mouse model of cortical dysplasia, the PI3/Akt/mTOR inhibitor rapamycin reduced the tumor size and suppressed epileptic discharge and cortical hyperplasia (12). Furthermore, somatic mutations in the mechanistic target of rapamycin (MTOR) gene also cause focal cortical dysplasia (13). This evidence suggests that the PI3/Akt/mTOR pathway is closely related to the development of cortical dysplasia. We do not know why cortical dysplasia was present in our patient, but it may have slowly led to the development of his epilepsy in adulthood.
Cowden syndrome is an important differential diagnosis of adult-onset partial epilepsy with cortical dysplasia, and neurologists must check dermatological lesions, hamartomas, and malignant cancers and conduct a gene analysis in such patients.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Kaori Adachi and Eiji Nanba for performing the genetic analysis.
References
- 1. Blumenthal GM, Dennis PA. PTEN hamartoma tumor syndromes. Eur J Hum Genet 16: 1289-1300, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lok C, Viseux V, Avril MF, et al. . Brain magnetic resonance imaging in patients with Cowden syndrome. Medicine (Baltimore) 84: 129-136, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd KM 2nd, Dennis M. Cowden's disease. A possible new symptom complex with multiple system involvement. Ann Intern Med 58: 136-142, 1963. [DOI] [PubMed] [Google Scholar]
- 4. Farooq A, Walker LJ, Bowling J, Audisio RA. Cowden syndrome. Cancer Treat Rev 36: 577-583, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Nelen MR, Padberg GW, Peeters EA, et al. . Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet 13: 114-116, 1996. [DOI] [PubMed] [Google Scholar]
- 6. Shen WH, Balajee AS, Wang J, et al. . Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128: 157-170, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Orrico A, Galli L, Buoni S, Orsi A, Vonella G, Sorrentino V. Novel PTEN mutations in neurodevelopmental disorders and macrocephaly. Clin Genet 75: 195-198, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Cheung KM, Lam CW, Chan YK, Siu WK, Yong L. Atypical focal cortical dysplasia in a patient with Cowden syndrome. Hong Kong Med J 20: 165-167, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Elia M, Amato C, Bottitta M, et al. . An atypical patient with Cowden syndrome and PTEN gene mutation presenting with cortical malformation and focal epilepsy. Brain Dev 34: 873-876, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Child ND, Cascino GD. Mystery case: Cowden syndrome presenting with partial epilepsy related to focal cortical dysplasia. Neurology 81: e98-e99, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodgers SJ, Ferguson D, Mitchell C, Ooms L. Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases. Biosci Rep 2017(Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen LH, Brewster AL, Clark ME, et al. . mTOR inhibition suppresses established epilepsy in a mouse model of cortical dysplasia. Epilepsia 56: 636-646, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakashima M, Saitsu H, Takei N, et al. . Somatic Mutations in the MTOR gene cause focal cortical dysplasia type IIb. Ann Neurol 78: 375-386, 2015. [DOI] [PubMed] [Google Scholar]