Abstract
Immunological niches are focal sites of immune activity that can have varying microenvironmental features. Hypoxia is a feature of physiological and pathological immunological niches. The impact of hypoxia on immunity and inflammation can vary depending on the microenvironment and immune processes occurring in a given niche. In physiological immunological niches, such as the bone marrow, lymphoid tissue, placenta and intestinal mucosa, physiological hypoxia controls innate and adaptive immunity by modulating immune cell proliferation, development and effector function, largely via transcriptional changes driven by hypoxia-inducible factor (HIF). By contrast, in pathological immunological niches, such as tumours and chronically inflamed, infected or ischaemic tissues, pathological hypoxia can drive tissue dysfunction and disease development through immune cell dysregulation. Here, we differentiate between the effects of physiological and pathological hypoxia on immune cells and the consequences for immunity and inflammation in different immunological niches. Furthermore, we discuss the possibility of targeting hypoxia-sensitive pathways in immune cells for the treatment of inflammatory disease.
Effective immunity in a healthy individual requires the constant involvement of focal sites of immune activity, which we term physiological immunological niches. Examples of these niches include the bone marrow, placenta, the mucosal surface of the gastrointestinal tract and lymph nodes; at these sites, various aspects of immune cell maturation, development, tolerance and proliferation occur and give rise to immune homeostasis1–3 (FIG. 1). By contrast, pathological immunological niches are sites of tissue pathology where high levels of immune activity are associated with inflammation and tissue dysfunction. Examples of pathological immunological niches include tumours and inflamed, infected or ischaemic tissues4–8 (FIG. 2). Importantly, in some circumstances, chronic inflammation at pathological immunological niches is associated with inflammation-driven carcinogenesis9,10.
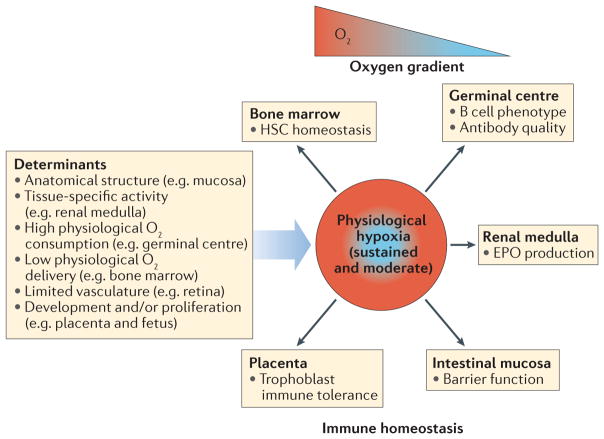
Figure 1. Hypoxia in physiological immunological niches.
The microenvironment of several physiological immunological niches is characterized by consistent and sustained oxygen gradients that lead to areas of stable physiological hypoxia. Depending on the tissue, the determinants of physiological oxygen gradients are varied. In these niches, physiological hypoxia regulates immune homeostasis through the control of resident immune cells. EPO, erythropoietin; HSC, haematopoietic stem cell.
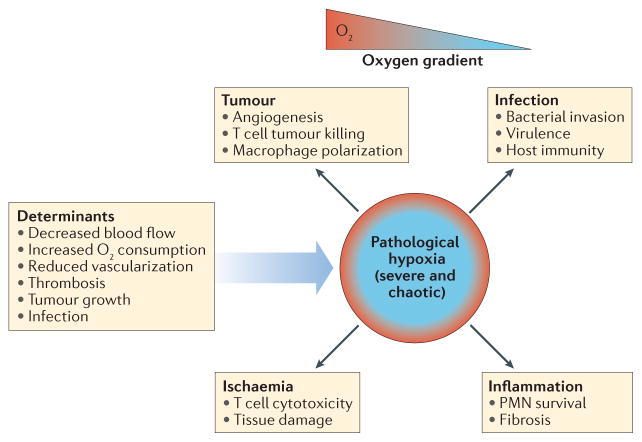
Figure 2. Hypoxia in pathological immunological niches.
The microenvironment of pathological immunological niches is frequently characterized by chaotic and severe oxygen gradients that lead to areas of pathological hypoxia. Depending on the tissue, the determinants of pathological hypoxia are varied. In these niches, pathological hypoxia can drive immune cell dysfunction, which contributes to disease progression. This dysfunction may lead to the promotion of tumour growth, ischaemia, inflammation and a worse outcome in infection. PMN, polymorphonuclear neutrophil.
A range of immunological niches with distinct microenvironmental features can exist in the intermediate states between physiological immune homeostasis and severe pathological inflammation. In order to address the complexity of these niches in a reductionist way, we have selected the opposing ends of the range for comparison, terming them physiological and pathological immunological niches, respectively. Microenvironmental features that can occur in immunological niches play a key role in the regulation of immune cell function and consequently modulate the balance between effective immunity and the development of pathological inflammation. Understanding the nature of immunological niches and how the niche microenvironment affects immune and inflammatory activity may allow the identification of new therapeutic targets for the control of inflammatory disease. One common feature of the immunological niche microenvironment that has a key role in immunity and inflammation in both health and disease is hypoxia, the condition that arises when cellular oxygen demand exceeds supply11–13 (BOXES 1,2; FIGS 1,2).
Box 1. Physiological hypoxia.
Most tissues of the body are provided, through vascular capillaries, with a level of oxygen that exceeds the basal metabolic requirements of the constituent cells. Thus, there are sufficient levels of cellular oxygen to satisfy mitochondrial bioenergetic requirements and an ‘oxygen reserve’, which (by acting as a co-substrate for oxygen-dependent hydroxylases) induces the degradation of hypoxia-inducible factor. However, in some tissues, the normal physiological partial pressure of oxygen (pO2) is comparatively low, which results in regions of ‘physiological hypoxia’. Six examples of tissues where physiological hypoxia occurs are outlined below.
First, physiological oxygen gradients exist in the intestinal mucosa due to the anatomical juxtaposition of the outermost mucosal surface with the oxygen-depleted lumen of the gut and a counter-current oxygen exchange system in the intestinal villi44. Second, the renal medulla experiences physiological hypoxia, probably as a result of a combination of the high oxygen demand that is associated with active ion transport events and the counter-current exchange of oxygen in the medulla140. Third, the limited vascular supply to the bone marrow in normal physiological conditions renders regions of this tissue physiologically hypoxic141. Fourth, the placenta and fetus experience physiological hypoxia due to constant outgrowing of the existing local blood supply as the fetus develops95. Fifth, the limited vasculature of the retina renders it hypoxic in the normal physiological state142. Sixth, physiological hypoxia in the light zone of the germinal centres of lymph nodes occurs as a result of the high level of oxygen consumption associated with dendritic cell–B cell matching52.
Therefore, relative hypoxia is a frequently encountered physiological state in a number of diverse tissues. Physiological oxygen gradients in healthy tissues are generally moderate and stable and activate what can be considered physiologically hypoxic responses. These responses are important in processes that maintain physiologic homeostasis, such as erythropoiesis and physiological angiogenesis. Furthermore, physiological hypoxia is important in the maintenance of innate and adaptive immune cell homeostasis and the control of tissue features such as epithelial barrier function, haematopoietic stem cell quiescence and self-renewal, and antibody production.
Box 2. Inflammation: a metabolic jungle.
Following exposure to inflammatory stimuli such as cytokines or microbial peptides, immune cells undergo cell type-specific changes in metabolic activity, which lead to the microenvironment characteristics that are common in pathological immunological niches. Changes in immune cell metabolism include an increase in oxygen consumption by recruited activated neutrophils, increased rates of glycolysis by activated macrophages and lymphocytes and increased rates of metabolic activity by tissue-resident immune cells, which fuels the increased synthesis of inflammatory mediators. The consequences of these shifts in metabolism for the inflammatory microenvironment include hypoxia, as increased oxygen consumption drives down the local availability of oxygen. Increased rates of glycolysis in macrophages and lymphocytes contribute to a decrease in the microenvironmental pH as a result of increased lactate production. Altered levels of metabolites that control immune cell function (immunometabolites) are also a feature of pathological immunological niches. The altered metabolic environment in turn counter-regulates immune cell function through the activation of hypoxia-inducible factor (HIF) in immune cells, thus creating a regulatory feedforward loop that links immune cell metabolism to inflammation. The cell type specificity of these metabolic changes makes it likely that sites of inflammation consist of heterogenous and complex sub-microenvironments that are characterized by varying levels of oxygen, pH and immunometabolites. Therefore, inflammation gives rise to a heterogenous metabolic microenvironment, which controls the regulation of HIF activation and of other transcriptional regulators of immunity and inflammation.
In mammals, oxygen is the terminal electron acceptor of the electron transport chain (ETC) and as such is essential for oxidative phosphorylation (OXPHOS) and cell survival. In respiring cells, the maintenance of bioenergetic homeostasis requires a constant supply of molecular oxygen14. Hypoxia arises when the oxygen requirements of a cell exceed the vascular supply, and if it is sustained, it can lead to a metabolic crisis that is ultimately lethal to cells. However, hypoxia is a common feature in a number of physiological immunological niches, particularly at sites where cell proliferation and consequently metabolic demand are high. These niches can be considered to be subject to ‘physiological hypoxia’, where a normally structured tissue maintains controlled and consistent oxygen gradients, which regulate pathways that are important for controlling physiological processes3,15,16 (BOX 1; FIG. 1). By contrast, in a number of pathological sites (for example, growing tumours and ischaemic, infected or chronically inflamed tissues), blood supply and metabolic processes can be disrupted, which can lead to the tissue being exposed to unstructured and chaotic oxygen gradients. This exposure can contribute to hypoxia that is typically more severe than that found in physiological immunological niches and that may provoke pathophysiological responses, such as inflammation and cell death, thus contributing to disease development17–20 (BOX 2; FIG. 2). Therefore, hypoxia is a common microenvironmental feature of both physiological and pathological immunological niches. Because of the importance of oxygen for cell survival, it is perhaps not surprising that over the course of evolution, metazoans have evolved mechanisms for sensing oxygen levels in the cellular microenvironment and eliciting appropriate adaptive responses14,21. Adaptation to hypoxia is dependent upon the capacity of a cell to elicit a transcriptional response. In physiological and pathological immunological niches, it is likely that many of the same pathways contribute to the regulation of the immune response by hypoxia. The degree and the temporal and spatial pattern of activation of these pathways is crucial for determining the overall impact of hypoxia on immunity and inflammation. In this Review, we discuss our developing understanding of the central role of hypoxia in the regulation of immune cell function in different immunological niches. We discuss the importance of the temporal and dynamic nature of the hypoxic stimulus in immune cell function and the consequences of this stimulus for immunity and inflammation. Furthermore, we outline the implications of immunological niche hypoxia for the provision of effective immunity in a healthy host or the development of tissue damage during chronic inflammation and immune-related disease.
Transcriptional responses to hypoxia
Hypoxia elicits a profound change in gene transcription. Multiple transcription factors can respond to hypoxia and induce or repress genes, which leads to the initiation of an adaptive transcriptional response22. Chief among these is hypoxia-inducible factor (HIF), which has been termed a ‘master regulator’ of the metazoan adaptive response to hypoxia23–25. HIF is a dimeric transcription factor that comprises an α subunit (HIFα, the stability of which is oxygen-dependent) and a constitutively expressed β-subunit (HIF1β). Under conditions where sufficient oxygen is available to a cell to satisfy and exceed metabolic demand (normoxia), HIFα subunits are synthesized at a high rate but rapidly targeted for ubiquitin-dependent proteasomal degradation by the E3 ubiquitin ligase termed von Hipple–Lindau disease tumour suppressor (pVHL). The degradation of HIFα subunits in normoxia is dependent upon the availability of non-mitochondrial oxygen26.
The oxygen-dependent mechanism by which HIFα is targeted for pVHL-dependent degradation is well understood and has been expertly reviewed elsewhere27,28 (FIG. 3). Briefly, a family of oxygen- dependent dioxygenases termed prolyl hydroxylase domain (PHD) enzymes and an asparagine hydroxylase, termed factor inhibiting HIF (FIH), repress HIFα in normoxia through a combination of targeted degradation and transcriptional repression28. In hypoxia, these hydroxylases are inhibited owing to the lack of available non-mitochondrial oxygen. This inhibition leads to the stabilization and derepression of HIF, which then activates a transcriptional programme that is directed towards adaptation to hypoxia. Interestingly, recent work has identified that HIF stability may also be controlled by the lysosomal degradation pathway in an oxygen-independent manner29–31. Furthermore, it is also now clear that a number of the products of metabolism, including 2-oxoglutarate, 2-hydroxyglutarate, succinate and reactive oxygen species (ROS), can also exert control over the HIF pathway32–36 (FIG. 3). Therefore, in addition to acting as sensors of available oxygen levels, HIF hydroxylases have a wider role as sensors that detect the metabolic status of a cell. There are two major transcriptionally active isoforms of HIF that have been described, termed HIF1 and HIF2, which are involved in the activation of gene expression. These factors contain, along with the HIF1β subunit, either a HIF1α or HIF2α subunit and have distinct but overlapping target gene sets37,38. A third HIFα subunit termed HIF3α has been reported to have both positive and negative effects on hypoxia-dependent gene expression39.
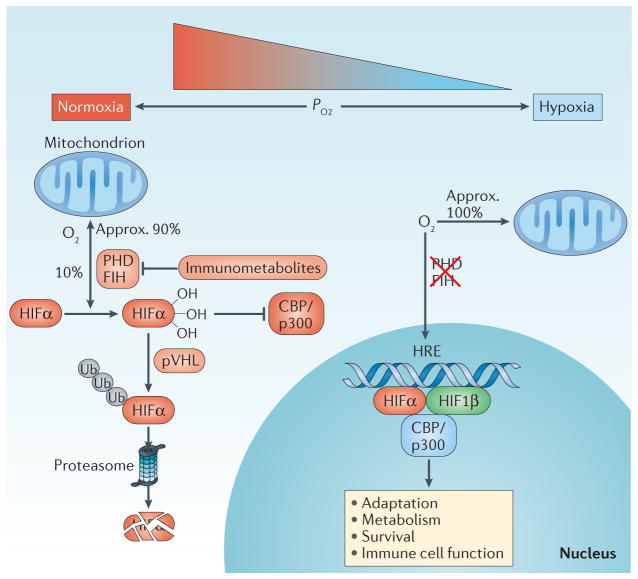
Figure 3. The HIF pathway.
Under conditions of normoxia, the majority of cellular oxygen consumption occurs in the mitochondria during the generation of ATP through oxidative phosphorylation, although some spare non-mitochondrial oxygen is available. Non-mitochondrial oxygen is utilized by hypoxia-inducible factor (HIF) hydroxylase enzymes to hydroxylate HIFα, resulting in its repression through targeted ubiquitylation by the E3 ubiquitin ligase von Hipple–Lindau disease tumour suppressor (pVHL), which results in proteasomal degradation and transcriptional repression by factor inhibiting HIF (FIH) (left side). In hypoxia, the absence of non-mitochondrial oxygen results in hydroxylase inhibition, the derepression of HIF and the activation of an adaptive transcriptional response (right side). CBP, CREB-binding protein; HRE, hypoxia response element; p300, histone acetyltransferase p300; PHD, prolyl hydroxylation domain; pO2, partial pressure of oxygen; Ub, ubiquitin.
HIF1 is ubiquitously expressed, whereas HIF2 shows more tissue-specific expression patterns but is expressed in a number of immune cell subtypes, including macrophages, neutrophils and lymphocytes40. The expression and role of HIF3 in immune cells is unclear. General cellular processes that are regulated by HIF include metabolism (HIF1 promotes glycolysis and represses OXPHOS), angiogenesis (HIF1 and HIF2 promote vascular endothelial growth factor (VEGF)-dependent angiogenesis) and survival (HIF1 and, to a lesser extent, HIF2 have been shown to regulate cell survival41). Furthermore, a number of cell type-specific responses to HIF have been described. For example, HIF promotes the synthesis and secretion of factors that promote a physiological response to hypoxia (such as the HIF2-dependent production of erythropoietin (EPO) by interstitial renal fibroblasts and hepatocytes42). In intestinal epithelial cells, HIF1 induces the expression of an epithelial-specific array of barrier-protective genes, which contribute to gut homeostasis, although HIF2 has been reported to be associated with the promotion of inflammation43–45. Therefore, HIF is a primary regulator of the adaptive transcriptional response to hypoxia. Notably, HIF is self-regulated by several negative feedback loops in tissues; these include the upregulation of the HIF hydroxylases PHD2 and PHD3, and microRNAs, which control HIF expression levels46. Furthermore, not all transcriptional responses to hypoxia are under the control of the HIF pathway, and a range of other transcription factors also display hypoxic sensitivity and can influence the impact of microenvironmental hypoxia on immune cell function22. In this Review, we focus on the specific consequences of HIF activation in immune cells in the context of immunological niches.
HIF modulation of immune cell function
Hypoxia is a common feature of the microenvironment of physiological and pathological immunological niches. These niches can contain resident immune cells, or immune cells may be recruited from the oxygen-rich bloodstream. In either case, cells of the innate and adaptive immune system are commonly exposed to hypoxia, raising the possibility that HIF plays a role in their regulation. Importantly, studies using mouse models in which either HIF1 or HIF2 has been deleted in discrete immune cell subpopulations have indeed shown that HIF is a crucial regulator of innate and adaptive immunity. HIF has cell type-specific roles, which have a profound impact on immune cell gene expression and downstream effector function. The specific functions of HIF in individual immune cell types has been recently reviewed and will be discussed only briefly here (reviewed in REFS 11,12). In neutrophils, HIF has a key role in the regulation of lifespan, antimicrobial peptide production and apoptosis47,48. By contrast, in macrophages, HIF regulates M1 and M2 polarization, motility, bactericidal activity and tumour development48,49. In dendritic cells (DCs), HIF modulates survival, migration, antigen presentation, interferon synthesis and differentiation50,51. Similarly, in T cells, HIF is important for the regulation of not only survival and differentiation but also proliferation and antitumour capacity33. In B cells, HIF also regulates survival, in addition to development and antibody processing52. Finally, in intestinal epithelial cells (a key innate immune cell), HIF modulates ion transport, antimicrobial peptide production and barrier function44,53,54. Although the focus of the current Review is on the role of HIF in regulating immune cell function, it should be noted that other components of the HIF pathway (including both HIF hydroxylases and pVHL) can also regulate immune cell function through HIF-independent mechanisms55–57. Thus, substantial evidence now exists to support a role for HIF as a major regulator of innate and adaptive immune cell function, and HIF should therefore be considered as a central transcriptional regulator of immunity.
The mechanism by which HIF regulates immune cell function is an area of active research; however, some clear themes have recently evolved. A primary mechanism by which HIF regulates immune cell function appears to be through the control of cellular metabolism58. It is known that the metabolic strategy that an immune cell adopts in any given microenvironment can determine the immune effector function of that cell59,60. Thus, oxygen availability, which regulates HIF activity, may have a substantial impact on immune cell function. For example, HIF has been demonstrated to increase the rate of glycolysis through the transcriptional upregulation of glycolytic gene expression61. An increased rate of glycolysis is, in turn, associated with the activation of a number of immune cell types, for example, macrophages, DCs, T cells and B cells58. Immune cell function can also be altered in response to changes in other metabolic pathways influenced by HIF activity, including the tricarboxylic acid cycle, fatty acid oxidation, the pentose phosphate pathway, fatty acid synthesis and amino acid metabolism (the link between immunity and metabolism, termed immunometabolism, has been reviewed in REF. 60).
In summary, immune cells frequently encounter hypoxia in both physiological and pathological states, which leads to the activation of HIF in immunological niches. By altering the metabolic strategy of immune cells, HIF has an important role in controlling effector function and the downstream development of inflammation and/or immunity. Of note, the reverse is also true, as increased immunological activity is associated with increased HIF signalling. The products of inflammation, which include cytokines, chemokines, ROS, nitric oxide and others, influence the activity of the HIF pathway. Therefore, an intimate and complex level of communication between metabolic and immune pathways determines immune cell function, and HIF is integral to this process62 (BOX 3).
Box 3. Hypoxia–inflammation signalling crosstalk.
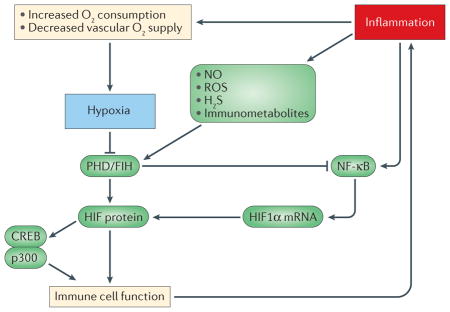
Hypoxia and inflammation are co-incidental events in a diverse range of pathological immunological niches, which include chronically inflamed and ischaemic tissues. In these niches, tissue hypoxia leads to activation of the hypoxia-inducible factor (HIF) pathway, which is a major transcriptional regulator of immune cell effector function (see the figure). The nuclear factor-κB (NF-κB) pathway is also of key importance in the transcriptional regulation of immunity and controls multiple aspects of immune cell function. NF-κB is strongly activated by inflammatory stimuli, such as cytokines or bacterial products, but also demonstrates sensitivity to hypoxia; this sensitivity provides a second mechanism by which hypoxia regulates inflammatory gene expression in pathological immunological niches. Interestingly, the same hydroxylases that regulate HIF also confer hypoxic sensitivity upon the NF-κB pathway through the oxygen-dependent hydroxylation of key components, which include IKKβ143 and ankyrin repeat domain-containing proteins such as p105 (also known as NFKB1) and IκBα144. Furthermore, extensive crosstalk exists between the HIF and NF-κB pathways. For example, increased canonical NF-κB activity that is driven by cytokines or bacterial products promotes the transcription of HIF1α mRNA, thus promoting HIF activity under conditions of chronic inflammation. Other signalling mediators that are elevated by tissue inflammation, which include immunometabolites (for example, S-2-hydroxyglutarate), gasotransmitters (for example, nitric oxide (NO) and H2S) and reactive oxygen species (ROS) (for example, hydrogen peroxide) also regulate HIF activity in immune cells, thereby contributing to the regulation of immunity and inflammation. Crosstalk also exists between HIF and cyclic AMP-responsive element-binding protein (CREB) in the regulation of MHC class I activation145. HIF also regulates the balance between T helper 17 cells and regulatory T cells by binding to the transcription factor forkhead box P3 (FOXP3)146 and the FOXP3 promoter147. Therefore, in pathological niches, there exists an extensive level of crosstalk between hypoxic and inflammatory pathways. FIH, factor inhibiting HIF; p300, histone acetyltransferase p300; PHD, prolyl hydroxylation domain.
Niche features that shape the HIF response
Although it is clear that hypoxia, via HIF signalling, is a key regulator of immune cells, the impact on cell function is cell type-dependent. How the activity of HIF regulates functional immunity and/or inflammation in various immunological niches is probably dependent upon the temporal, spatial and dynamic nature of the hypoxic stimulus and the immune cell types present. Furthermore, other microenvironmental factors also contribute to shaping the HIF response and, consequently, the ultimate immunological outcome. Below, we discuss aspects of the immunological niche microenvironment that shape the nature of the HIF response and thereby influence the impact of hypoxia on immunity.
Similar to all other transcription factors, the dynamics and degree of activation of HIF in a given cell type will determine the impact upon gene expression and, consequently, immune cell effector function. Therefore, the HIF response is a temporally dynamic response with multiple inputs, rather than a binary switch. It is clear that microenvironmental hypoxia regulates the activity of HIF, which in turn modulates immune cell activity. However, the consequences of hypoxia for the immune response in a specific tissue depend on the nature of the hypoxic stimulus and various other niche features. Together, these features will determine the dynamic and temporal profile of the HIF response, which will in turn dictate the genes that are transcribed and the eventual downstream immune cell effector function. With this in mind, a number of key micro-environmental features of physiological and pathological immunological niches are discussed below.
The temporal and spatial nature of the oxygen gradient
Physiological oxygen gradients are typically ordered and sustained. For example, a controlled oxygen gradient is sustained in the intestinal mucosa by the juxtaposition of the rich capillary network of the mucosal vasculature (which supplies the mucosa with oxygen) with the anoxic lumen of the gut. Furthermore, in the light zones of germinal centres (GCs), the consumption of oxygen during lymphocyte–DC matching dictates the controlled oxygen gradient, which in turn controls B cell function52. Despite this, fluctuations in physiological oxygen gradients can occur. For example, the levels of mucosal oxygen increase substantially following the consumption of a meal, when mucosal blood flow is considerably increased. In addition, GC oxygen levels drop during the clonal expansion of B cells, which occurs following the presentation of a receptor-specific antigen52. These changes can be considered physiological fluctuations in tissue oxygen levels.
In contrast to the controlled nature of the hypoxic gradient found in physiological immunological niches, in pathological niches (for example, a growing tumour or a chronically inflamed tissue), oxygen gradients tend to be more chaotic and intermittent. These unstable gradients can result in the unpredictable regulation of oxygen-sensitive transcriptional pathways, such as the HIF pathway63,64. Notably, the range of patterns of hypoxic exposure that may be found in pathological immunological niches is likely wide. For example, in some growing tumours, irregular blood flow may cause an intermittent and variable pattern of hypoxic exposure63. By contrast, during established chronic inflammation such as the mucosal inflammation seen in inflammatory bowel disease (IBD), the pattern of hypoxia may be more uniform and sustained. Notably, intermittent hypoxia tends to promote increased inflammatory responses compared with sustained hypoxia65,66. Therefore, the spatial and temporal nature of the pattern of hypoxia in a particular niche may dictate the degree of HIF activation and consequently the downstream effects on immune cell function.
The degree of hypoxia
Depending upon the balance of oxygen supply and demand in a given immunological niche, the degree of hypoxia to which immune cells are exposed can vary, which results in differing downstream gene expression profiles. For example, in the physiological state, the intestinal mucosa experiences partial pressure of O2 (pO2) values that, although hypoxic, are substantially higher than those experienced in chronically inflamed mucosa19. Furthermore, in a growing tumour, the degree of hypoxia experienced by immune cells is dependent upon the size and structure of the tumour. The degree of hypoxia will determine the degree of HIF activation in immune cells and thus will determine downstream changes in tumour immunity. Furthermore, in severe hypoxia, the lack of available oxygen contributes to a bioenergetic crisis within cells, which in turn can lead to cellular necrosis. This necrosis promotes inflammatory processes in a nonspecific manner through the release of intracellular contents into the surrounding space. In summary, the degree of hypoxia experienced in a given immunological niche will determine the extent to which HIF is activated in immune cells and consequently the downstream effect on immune cell function.
The presence of inflammatory mediators
The presence of cytokines and other immune mediators, such as immunometabolites, in an immunological niche can have a profound modulatory effect upon the profile of the HIF response in immune cells. Immunometabolites are products of metabolism, for example, succinate, 2-oxoglutarate and 2-hydroxyglutarate, which can alter the immune response through HIF33,60,62. In addition to being sensitive to oxygen and metabolite levels within a cell, the HIF pathway can be regulated at the transcriptional level by cytokines or other inflammatory stimuli through the nuclear factor-κB (NF-κB) pathway, which is a pro-inflammatory pathway that is under the control of immunological mediators67,68. The increased presence of inflammatory mediators in chronically inflamed tissues or tumours has been well documented69,70. These inflammatory mediators may impact the nature of the HIF response. Furthermore, the presence of bacterial ligands, such as lipopolysaccharide (LPS), in infected tissues is detected by Toll-like receptors, which can also positively regulate HIF expression at the mRNA level via the NF-κB pathway71. It is possible that the specific profile of cytokines or bacterial products present in an inflamed or infected tissue can dictate the niche-specific impact on HIF expression. Therefore, the presence of inflammatory mediators, including cytokines and bacterial products, can promote inflammation that will substantially impact the level of HIF activity in immune cells and may consequently alter the effector function of these cells.
Oxidative stress regulates HIF activation
In healthy tissues in the physiological state, ROS that are produced by the mitochondrial ETC are neutralized by effective intracellular antioxidant systems, such as the presence of reduced glutathione72. Therefore, in physiological immunological niches, oxidative stress is unlikely to be regularly encountered. However, under stressed, pathological conditions, such as those found in inflamed and infected tissues, the production of ROS is elevated and endogenous antioxidant defence mechanisms may be saturated, leading to oxidative stress. ROS have been demonstrated to modulate the HIF pathway36,73,74. However, the mechanism or mechanisms by which ROS regulate HIF expression and activity are unclear. Therefore, the presence of oxidative stress in the microenvironment may shape the profile of HIF activity in immune cells and therefore impact downstream immune effector function.
Gasotransmitters
Although the HIF pathway appears to have evolved to induce an adaptive response to reduced oxygen levels, it is also clear that other physiological gases with signalling properties (gasotransmitters) can also modulate this pathway. Nitric oxide controls HIF stability, and importantly, the production of this gas increases under inflammatory conditions26. At high concentrations, nitric oxide inhibits HIF hydroxylases, which leads to increased HIF activity, whereas lower concentrations of this gas inhibit mitochondrial oxygen consumption; this inhibition decreases HIF activity as intracellular oxygen is redistributed from the mitochondria to the oxygen-sensing HIF hydroxylases, such as PHDs and FIH26,75 We have recently reported that elevated concentrations of carbon dioxide (CO2), which occur during increased metabolism and dysregulated respiration, can suppress HIF activity by targeting HIF for lysosomal degradation29. Recent work has also identified that hydrogen sulfide (H2S) can also regulate HIF activity76–78. Importantly, all of these physiological gases have been shown to be altered in pathological states, such as chronically inflamed or infected tissues or growing tumours. Therefore, the levels of these physiological gases present in a specific immunological niche will modulate the level of HIF activity and consequently the metabolic and effector sequelae in immune cells that are present.
Hypoxia in physiological immune niches
As outlined above, the combined effects of hypoxia and multiple other microenvironmental features determine the overall impact of the immunological niche microenvironment on HIF activity and downstream immune cell effector function. In turn, these outcomes will dictate the physiological and/or pathological impacts. The impact of hypoxia in immunological niches ranges from beneficial physiological responses, for example, improved intestinal epithelial barrier function, immune cell development and controlled inflammation, to pathological responses, for example, tumour angiogenesis, cell death, tissue damage and bioenergetic crisis. The key point is that the degree of HIF activation in specific immune cell subtypes is dynamically determined by multiple micro-environmental cues, and this activation will determine the immune effector outcome. To emphasize this point, in the next section we focus on the nature of hypoxia and its impact on immune cell function in distinct physiological immunological niches.
Bone marrow
Bone marrow, located in the central cavity of bones, is the primary site of haematopoiesis (blood cell production and differentiation) during adult life and as such plays a pivotal role in supplying the adult body with all types of immune cell. Haematopoiesis depends upon the generation of progenitor cells from a population of pluripotent and self-renewing haematopoietic stem cells (HSCs)79. Under normal physiological conditions, bone marrow is hypoxic80, and this hypoxia may be exacerbated during disease, for example, leukaemia81. The physiological hypoxia in healthy bone marrow has an important role in the maintenance of HSC homeostasis. It regulates cellular quiescence, metabolism, survival, proliferation and differentiation, both directly through the regulation of hypoxia-sensitive transcription factors (for example, HIF in HSCs) and indirectly by stimulating the synthesis and release of soluble factors from other cell types present (for example, adipocytes and endothelial cells)82. The exposure of HSCs to hypoxia or pharmacological hydroxylase inhibition (which activates HIF) promotes HSC quiescence in a HIF-dependent manner83,84. The mechanism by which hypoxia regulates HSC quiescence, self-renewal and proliferation remains an area of considerable investigation; however, some studies indicate that the mechanism involves HIF1-dependent control of metabolism85,86. Furthermore, hypoxia, through the HIF pathway, regulates stem cell development in other niches, including the carotid body87,88. Hypoxia-induced HIF activation also regulates the HSC metabolic strategy through the promotion of glycolysis and the repression of oxidative metabolism89. Removal of HIF1β from HSCs results in a loss of viability in a manner that is dependent upon the loss of HIF-dependent signalling90. However, it should be noted that the role of HIF in HSCs remains controversial, with a different study indicating no role for HIF2α in HSC maintenance91. In summary, while controversial, a number of lines of evidence suggest that physiological hypoxia controls HSC viability and function in bone marrow through the HIF pathway.
Germinal centres
GCs, which are found within secondary lymphoid tissues such as lymph nodes, are the sites in which mature B cells proliferate, differentiate and undergo mutation in antibody-encoding genes, thereby allowing the generation of B cell clones, which express a wide range of antigen-specific receptors. GCs are also the sites at which mature B cells switch their antibody classes and undergo selection during the normal physiological processes of immunity. GCs display physiological oxygen gradients, which are important in the regulation of resident immune cells. The existence of these gradients is exacerbated when B cell populations are rapidly expanding and oxygen consumption is increased52,92. It has recently been demonstrated that the physiological hypoxia experienced in GCs has a role in determining B cell phenotype and behaviour52,92. These studies show that B cell hypoxia in GCs decreases proliferation and alters immunoglobulin class switching, although the impact on class switching remains controversial.
Placenta
The developing placenta is a key site of immunity that provides protection for the developing fetus while preventing the maternal immune system from attacking the semi-allogeneic trophoblasts of the fetus93. As a placenta grows, intra-placental oxygen gradients are established and HIF becomes activated, even under normal physiological conditions94. Interestingly, placental defects are associated with loss or gain of function of HIF in mice95. Two mechanisms that involve HIF activity and provide immune privilege in the placenta are immune invisibility (where fetal cells are invisible to the mother’s immune system) and immune blockade (where the mother’s immune system is blocked in the placenta). The placenta avoids immune attack mostly through trophoblast expression of the non-classical class I histocompatibility antigen HLA-G, a HIF1α-dependent gene product that protects against attack by natural killer cells95,96. Immune blockade in the placenta is provided by hypoxia-dependent regulation of factors such as programmed cell death 1 ligand 1 (PDL1), which inactivates or induces cell death in infiltrating T cells95. Notably, PDL1 expression is under the control of HIF1α97. Therefore, the placenta can also be considered to be a physiological immunological niche, in which physiological hypoxia contributes to immunological control.
It is also known that the fetal microenvironment is relatively hypoxic (compared with adult tissue) and that the immune system plays a key role in fetal development98–100. Little is known about the mechanisms by which fetal hypoxia modulates immunity and thus affects development in this physiological immune niche. However, it is likely that fetal oxygen levels are important in regulating how the immune system impacts development.
Intestinal mucosa
Recent studies in the gastrointestinal mucosa have provided insight into important shifts in metabolism that are associated with inflammation15,19. The intestinal mucosa is situated at the interface of the anaerobic lumen of the gastrointestinal tract and the richly perfused intestinal vascular bed and lamina propria (the connective tissue layer that lies beneath the epithelium). An analysis of counter-current blood flow in the intestine has revealed that oxygen from the arterial supply diffuses to adjacent venules along the crypt–villus axis, resulting in graded hypoxia from the lamina propria towards the intestinal lumen101. These anatomic features result in a steep oxygen gradient across the luminal aspect of the tissue and render intestinal epithelial cells hypoxic, even in the physiological state, which is termed ‘physiological hypoxia’ (REF. 102). Physiological hypoxia in the intestinal mucosa has been tracked using nitroimidazole dyes, a class of reactive compounds that are metabolized dependent on the level of tissue oxygenation102,103. Studies using these compounds in the intestinal mucosa have shown that epithelial cells at the luminal edge of the colon exist normally at a pO2 <20 mmHg (REFS 102,104). These observations were supported by electron paramagnetic resonance oximetry, which was used to image the intestinal mucosa105. Lumenal pO2 was shown to decrease proportionally along the length of the small and large intestine, where measurements in the duodenum, ascending colon and sigmoid colon were determined to be approximately 30, 10 and 3 mmHg, respectively106.
Notably, the intestinal mucosa exists in a constant state of controlled inflammation (even at baseline), due to constant exposure to low levels of antigens from the contents of the lumen. These include components of the intestinal microbiome and non-self peptides derived from digested food particles. This basal immunological activity is the basis of the development of oral tolerance. Therefore, the gastrointestinal mucosa is a physiological immunological niche, in which immune cells are exposed to a combination of hypoxia and immune stimuli, even in the physiological state. Physiological hypoxia at the intestinal mucosal surface regulates innate immunity through the promotion of epithelial cell barrier function and the regulation of resident immune cells15.
Hypoxia in pathological immune niches
Hypoxia can also shape immune responses in pathological environments, such as tumours or inflamed or infected tissue. Next, we discuss the nature of hypoxia and its impact on immune cell function in distinct pathological immunological niches.
Tumours
Tumours are one of the best-characterized pathological immunological niches107. As tumours develop, they outgrow the local blood supply, which can lead to a state of profound intratumoural hypoxia. Initial studies have focused on the induction of HIF-dependent proangiogenic factors, such as VEGF, which in turn induces tumour angiogenesis and promotes tumour development108. Furthermore, the vasculature that is induced in tumours by hypoxia tends to be ‘leaky’ in nature, which provides a route by which tumour cells can escape the primary tumour, enter the bloodstream and establish metastases108. In addition, as outlined above, HIF is a major regulator of the metabolic switch from OXPHOS to glycolysis and, as such, can contribute to adaptive metabolism and tumour cell survival through the promotion of aerobic glycolysis (the Warburg effect; reviewed in REF. 109).
Immune cells are now known to be important in both tumour development and suppression110. Immune cells are recruited to tumours from the oxygen-rich bloodstream and therefore experience a shift to a hypoxic environment. Therapeutic T cell transfer, termed adoptive immunotherapy, is currently an area of intense investigation and has shown encouraging clinical results111. There are multiple possible components that contribute to successful T cell-based tumour immunity, including innate immune cells. For example, HIF activity in myeloid-derived suppressor cell (MDSC) interactions with T cells was demonstrated to have a key role in tumour microenvironment immunity. Although MDSCs from healthy lymphoid tissues and those from tumours share similar markers, they appear to harbour vastly different functions. For example, it was recently demonstrated that tumour-derived MDSCs blocked both antigen-specific and nonspecific T cell responses. By contrast, normal peripheral organ MDSCs repressed only antigen-specific responses. This difference was attributed to HIF1-dependent induction of arginase and nitric oxide synthase in MDSCs within the tumour microenvironment112 Another recent study demonstrated that S-2-hydroxyglutarate, an immunometabolite elevated in cancer, drives HIF-dependent CD8+ T lymphocyte tumour killing capacity33. In summary, hypoxia in the tumour microenvironment regulates immune cell effector function, which in turn plays a role in tumour development.
Inflammation
The hallmarks of acute inflammation include the accumulation of large numbers of neutrophils and fundamental shifts in tissue metabolism19. These studies have suggested an important role for increased oxygen metabolism and the resultant hypoxia, which is termed ‘inflammatory hypoxia’. As such, sites of acute inflammation represent pathological immunological niches. Inflammatory hypoxia results from a combination of oxygen consumption by migrating inflammatory cells (for example, monocytes and neutrophils), local cell proliferation and the consumption of oxygen through activation of oxygenases (for example, oxidases, monooxygenases and dioxygenases) that are expressed in multiple cell types in the inflammatory micro-environment19. The role of hypoxia in acute inflammation can be somewhat tissue-specific. For example, in the lung, neutrophils (the survival of which is increased by hypoxia) may contribute fundamentally to lung injury113. Furthermore, antimicrobial factors that are released by activated neutrophils appear to be highly toxic to pulmonary epithelial cells and can result in bystander tissue damage. The activity of 5 -AMP-activated protein kinase (AMPK) may be central to these mechanisms, as it was recently shown that administration of an AMPK activator (metformin) could protect mice in a model of sepsis-induced lung injury. This protection was thought to be mediated by a mechanism that includes decreased neutrophil accumulation, attenuated HIF1 signalling and decreased epithelial permeability in the lung114.
By contrast, studies in the intestinal mucosa have revealed an essential role for neutrophils and a hypoxic microenvironment in the resolution of inflammation115. Initial studies examined gene expression changes, termed ‘transcriptional imprinting’, in the epithelium by transmigrating neutrophils. This analysis revealed a prominent induction of epithelial HIF target genes elicited by active migration of neutrophils and the consumption of large amounts of local oxygen by neutrophil-derived NADPH oxidase. Ironically, the environment generated by this activity promotes inflammatory resolution. HIF target genes induced within the epithelium were shown to be barrier-protective, and depletion of neutrophils in this setting resulted in exacerbated tissue damage during colitis. These studies were also validated in human patients, where human IBD biopsy samples containing neutrophilic crypt abscesses strongly correlated with expression of the HIF target gene GLUT1. Notably, patients who lack a functional NADPH oxidase (an enzyme that generates superoxide in neutrophils, which is lacking in chronic granulomatous disease) often present with an IBD-like syndrome116. Thus, the acute inflammatory setting, characterized by the accumulation of oxygen-consuming neutrophils, represents a pathological immunological niche that experiences profound levels of hypoxia.
Tissue hypoxia in chronically inflamed tissues can be either pro-inflammatory or anti-inflammatory depending on cell type and context. For example, in IBD, the depth and degree of hypoxia experienced in the intestinal mucosa dramatically increases. This effect may be due to the increased metabolic demands triggered by inflammation and diminished vascular supply resulting from inflammation-induced vascular dysfunction, which can ultimately lead to tissue fibrosis117. Intestinal epithelial cell hypoxia leads to barrier protection through mechanisms that involve multiple HIF target genes, which are expressed in epithelial cells44. Alternatively, in mucosal immune cells, hypoxia can promote immune cell activity and survival through HIF activation. In summary, hypoxia has a complex role in inflammation, but notably, multiple studies have now demonstrated that the net effect of pharmacological HIF activation through hydroxylase inhibition is profoundly anti-inflammatory11.
Bacterial infections
Tissues that have been infected with pathogenic bacteria are frequently found to be hypoxic and demonstrate elevated levels of HIF activity118–120. This increase may be due to a number of factors, including bacterial oxygen consumption, the formation of oxygen-impermeable biofilms and inflammation-associated hypoxia120. For example, in cystic fibrosis patients, Pseudomonas aeruginosa is a common opportunistic pathogen that frequently exists in a hypoxic microenvironment121. Through the regulation of immune cell survival and effector function, hypoxia can have beneficial effects in P. aeruginosa infection122. Interestingly, hypoxia is also important in regulating bacterial behaviour in the microenvironment of an infection. Exposure to hypoxia reduces virulence and infection and increases the development of drug resistance in P. aeruginosa, events that support chronic infection123,124. Recent evidence has also suggested a possible role for bacterial hydroxylases in the regulation of bacterial virulence in P. aeruginosa in hypoxia125. Therefore, at sites of infection, hypoxia can regulate both host immunity and pathogen virulence and consequently impact disease progression.
Ischaemia
Tissue ischaemia occurs when the blood flow to a tissue is disrupted, which causes the tissue to become oxygen-deprived and nutrient-deprived and is associated with some common causes of global morbidity and mortality, such as myocardial infarction and stroke. Inflammation is thought to contribute to the tissue damage that is associated with ischaemic disease20. Ischaemia is also intimately associated with tissue hypoxia. Recent work has suggested a role for HIF in the regulation of inflammatory tissue damage that is associated with ischaemic disease in the brain and kidney126–128.
Targeting the HIF pathway
As described above, microenvironmental hypoxia is a key feature of several immunological niches and may contribute to the development of host immunity and inflammatory disease in a context-dependent manner. Therefore, interfering with the HIF response may be a potential therapeutic approach for the control of immune-related disorders13,129. A number of pharmacological options for interference with the HIF pathway exist. The best developed of these are the hydroxylase inhibitors, which activate the HIF pathway through the inhibition of HIF hydroxylation130. Hydroxylase inhibitors have recently been shown to elevate EPO levels and haematocrit, two markers of positive prognosis in patients suffering from chronic kidney disease-associated anaemia131. Therefore, activating HIF represents an alternative to the administration of recombinant EPO, which can be associated with a number of side effects in some patients. In these studies, hydroxylase inhibitors were well tolerated by patients and proved beneficial in improving outcomes in anaemia131. Other potential pharmacological approaches include HIF inhibitors. Indeed, recent developments have seen the emergence of HIF2α-specific inhibitors, which have shown some efficacy in the treatment of renal cancers132,133. However, the pharmacological effects of HIF2α-specific inhibitors in immunity and inflammation remain largely unknown.
In terms of inflammatory disease, a number of pre-clinical studies have now demonstrated that hydroxylase inhibitors are effective in reducing inflammation11,134 (TABLE 1). This effectiveness has been demonstrated in models of IBD, renal transplant and renal ischaemia. In animal models of pulmonary P. aeruginosa infection, the administration of hydroxylase inhibitors provides protection123. Hydroxylase inhibitors are also protective against sterile sepsis, which has been induced by administering LPS to mice. However, hydroxylase inhibitors exacerbate the lethal effects of bacterial sepsis induced by caecal ligation and puncture, which gives rise to bacterial sepsis135. The impact of specific pharmacological HIF inhibitors on inflammation and immunity in patients is currently unknown but is an area of potentially high therapeutic relevance.
Table 1.
Anti-inflammatory effects of pharmacological hydroxylase inhibition
| Model | Hydroxylase inhibitor | Refs |
|---|---|---|
| DSS-induced colitis | DMOG | 148–150 |
| TNBS-induced colitis | FG4497 | 151 |
| TNFΔARE ileitis | DMOG | 152 |
| Clostridium difficile toxin | DMOG | 153 |
| Intestinal ischaemia–reperfusion | DMOG | 154 |
| Indomethacin | DMOG | 155 |
| TNBS-induced colitis | AKB4924 | 115,156,157 |
| Intestinal radiation injury | DMOG | 158 |
| TNBS-induced colitis | Rosmarinic acid | 159 |
| TNBS- and DSS-induced colitis | TRC160334 | 160 |
| LPS-induced sepsis | DMOG | 135 |
| Ischaemic kidney injury | TRC160334 | 161 |
| Renal transplant | FG4497 | 162 |
| Pseudomonas aeruginosa infection | DMOG | 123 |
| Intestinal fibrosis | DMOG | 163 |
DMOG, dimethyloxalylglycine; DSS, dextran sulfate sodium; LPS, lipopolysaccharide; TNBS, 2,4,6-trinitrobenzenesulfonic acid; TNFΔARE, targeted disruption in the tumour necrosis factor (TNF) AU-rich element.
A key function of the immune system is tumour surveillance and the destruction of transformed cells within the body; this function is carried out predominantly by cytotoxic T cells. Recent evidence suggests that HIF plays a role in the promotion of tumour killing capacity33. In summary, our understanding of the clinical potential of pharmacological interference of the PHD–FIH–HIF pathway is in its infancy but represents an exciting future area of clinical research, especially for immune-related disorders.
Perspective
The recent explosion in available information on the impact of hypoxia and the HIF pathway on immune cell function has identified this pathway as a possible therapeutic target for diseases including chronic inflammation, infection and autoimmunity. The recent use of hydroxylase inhibitors such as Roxadustat (FibroGen, USA), Daprodustat (GSK1278863) and Vadadustat (Akebia Therapeutics, USA) in chronic kidney disease-associated anaemia suggests that these drugs could have a potential use in immune-related disease136–138. Although much of our understanding of the physiology and therapeutic potential of the HIF pathway has been derived from preclinical studies, the information obtained from these clinical trials will give information on the in vivo human pharmacology of reagents that target HIF. However, several factors should be considered to maximize the therapeutic potential of repurposing these drugs for inflammatory disease. First, in order to achieve tissue-specific effects without inducing systemic responses (such as unwanted erythropoiesis), the targeted delivery of drugs to niches should be considered. Recent approaches to this have been used in models of IBD139. Furthermore, the development of specific reagents, including isoform-specific PHD inhibitors or selective HIF1 versus HIF2 inhibitors, will improve the therapeutic potential of interfering with this pathway.
One concern regarding the clinical use of hydroxylase inhibitors is the potential for the promotion of pre-existing tumour growth via the promotion of angiogenesis. However, to date, there is limited evidence for a tumour-promoting role in animal models. By contrast, substantial evidence exists that they effectively raise serum EPO levels, an effect that may contribute to unwanted erythropoiesis in non-anaemic patients. Therefore, it would be attractive to develop targeted delivery systems for the administration of hydroxylase inhibitors to avoid pro-erythropoietic side effects in non-anaemic conditions. In addition to the gastrointestinal drug delivery systems outlined above, there is potential for the topical application of hydroxylase inhibitors to the skin or inhalation delivery systems in the lung; these may represent future delivery systems for organ-specific inflammatory disease. Furthermore, more sophisticated approaches to drug delivery, such as surface antigen-directed delivery systems, may be required to target drugs to specific immune cell subtypes.
In summary, these are exciting times for our rapidly developing understanding of the role of hypoxia in regulating immunity and inflammation in immunological niches. Given the clear evidence that hypoxia-sensitive pathways are key regulators of immune cell function, combined with the evidence for patient tolerance and clinical efficacy of hydroxylase inhibitors, it is likely that the impact of these drugs on inflammatory disease will be an area of important clinical research in the near future.
Acknowledgments
Work from the authors’ laboratories is funded through research grants from Science Foundation Ireland, the European Union and the US National Institutes of Health.
Glossary
- Microenvironmental features
Physiochemical conditions found within a specific niche or tissue.
- Hypoxia
The condition that arises when cellular oxygen demand exceeds supply.
- Electron transport chain (ETC)
Primary eukaryotic system for the reduction of molecular oxygen and the generation of ATP. Located within mitochondria.
- Oxidative phosphorylation (OXPHOS)
The generation of cellular ATP using energy derived from electron transport during aerobic respiration.
- Lysosomal degradation pathway
A mechanism of intracellular protein degradation that involves proteolysis in lysosomal compartments.
- Glycolysis
The utilization of glucose to generate ATP.
- Physiological angiogenesis
The normal growth of blood vessels in healthy tissues.
- Carotid body
Small organelle situated at the bifurcation of the carotid artery responsible for sensing blood oxygen levels and regulating the respiratory rate.
- Semi-allogeneic trophoblasts
Fetal cells that express both maternal and paternal surface antigens.
- Crypt–villus axis
Structure at the mucosal surface of the small intestine.
- Erythropoiesis
The process by which red blood cell production is controlled. Involves the release of erythropoietin from cells of the kidney and liver.
Footnotes
Author contributions
C.T.T. and S.P.C. both contributed to discussions of the content and the writing, review and editing of this manuscript
Competing interests statement
The authors declare competing interests: see Web version for details.
Publishers note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beerman I, Luis TC, Singbrant S, Lo Celso C, Méndez-Ferrer S. The evolving view of the hematopoietic stem cell niche. Exp Hematol. 2017;50:22–26. doi: 10.1016/j.exphem.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah DK, Zúñiga-Pflücker JC. An overview of the intrathymic intricacies of T cell development. J Immunol. 2014;192:4017–4023. doi: 10.4049/jimmunol.1302259. [DOI] [PubMed] [Google Scholar]
- 3.Campbell EL, Kao DJ, Colgan SP. Neutrophils and the inflammatory tissue microenvironment in the mucosa. Immunol Rev. 2016;273:112–120. doi: 10.1111/imr.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin EW, Karakasheva TA, Hicks PD, Bass AJ, Rustgi AK. The tumor microenvironment in esophageal cancer. Oncogene. 2016;35:5337–5349. doi: 10.1038/onc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maru Y. The lung metastatic niche. J Mol Med (Berl ) 2015;93:1185–1192. doi: 10.1007/s00109-015-1355-2. [DOI] [PubMed] [Google Scholar]
- 6.Biswas S, et al. Microenvironmental control of stem cell fate in intestinal homeostasis and disease. J Pathol. 2015;237:135–145. doi: 10.1002/path.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallenbeck JM, Hansson GK, Becker KJ. Immunology of ischemic vascular disease: plaque to attack. Trends Immunol. 2005;26:550–556. doi: 10.1016/j.it.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Gobert AP, Wilson KT. The immune battle against Helicobacter pylori infection: NO offense. Trends Microbiol. 2016;24:366–376. doi: 10.1016/j.tim.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quante M, Wang TC. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda) 2008;23:350–359. doi: 10.1152/physiol.00031.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2012;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor CT, Doherty G, Fallon PG, Cummins EP. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J Clin Invest. 2016;126:3716–3724. doi: 10.1172/JCI84433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummins EP, Keogh CE, Crean D, Taylor CT. The role of HIF in immunity and inflammation. Mol Aspects Med. 2016;47–48:24–34. doi: 10.1016/j.mam.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol. 2013;13:646–653. doi: 10.1016/j.coph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Taylor CT, McElwain JC. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology (Bethesda) 2010;25:272–279. doi: 10.1152/physiol.00029.2010. [DOI] [PubMed] [Google Scholar]
- 15.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C350–C360. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenza GL. The hypoxic tumor microenvironment: a driving force for breast cancer progression. Biochim Biophys Acta. 2016;1863:382–391. doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18:534–543. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colgan SP, Campbell EL, Kominsky DJ. Hypoxia and mucosal inflammation. Annu Rev Pathol. 2016;11:77–100. doi: 10.1146/annurev-pathol-012615-044231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loenarz C, et al. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep. 2011;12:63–70. doi: 10.1038/embor.2010.170. This paper identifies the highly evolutionarily conserved nature of the oxygen-sensing hydroxylase–HIF-dependent pathway in all metazoans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol. 2013;591:2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 26.Taylor CT, Moncada S. Nitric oxide, cytochrome C oxidase, and the cellular response to hypoxia. Arterioscler Thromb Vasc Biol. 2010;30:643–647. doi: 10.1161/ATVBAHA.108.181628. [DOI] [PubMed] [Google Scholar]
- 27.Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 28.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Selfridge AC, et al. Hypercapnia suppresses the HIF-dependent adaptive response to hypoxia. J Biol Chem. 2016;291:11800–11808. doi: 10.1074/jbc.M116.713941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubbi ME, Semenza GL. An essential role for chaperone-mediated autophagy in cell cycle progression. Autophagy. 2015;11:850–851. doi: 10.1080/15548627.2015.1037063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbi ME, Gilkes DM, Hu H, Kshitiz, Ahmed I, Semenza GL. Cyclin-dependent kinases regulate lysosomal degradation of hypoxia-inducible factor 1α to promote cell-cycle progression. Proc Natl Acad Sci USA. 2014;111:E3325–E3334. doi: 10.1073/pnas.1412840111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Intlekofer AM, et al. L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nat Chem Biol. 2017;13:494–500. doi: 10.1038/nchembio.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyrakis PA, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. 2016;540:236–241. doi: 10.1038/nature20165. This study demonstrates that the HIF pathway plays a key role in regulating antitumour activity in cytotoxic T lymphocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koivunen P, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. This study demonstrates that succinate is an immunometabolite that regulates innate immunity through the HIF pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandel NS. Mitochondrial regulation of oxygen sensing. Adv Exp Med Biol. 2010;661:339–354. doi: 10.1007/978-1-60761-500-2_22. [DOI] [PubMed] [Google Scholar]
- 37.Schödel J, Mole DR, Ratcliffe PJ. Pan-genomic binding of hypoxia-inducible transcription factors. Biol Chem. 2013;394:507–517. doi: 10.1515/hsz-2012-0351. [DOI] [PubMed] [Google Scholar]
- 38.Mole DR, et al. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan C. Hypoxia-inducible factor 3 biology: complexities and emerging themes. Am J Physiol Cell Physiol. 2016;310:C260–269. doi: 10.1152/ajpcell.00315.2015. [DOI] [PubMed] [Google Scholar]
- 40.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenihan CR, Taylor CT. The impact of hypoxia on cell death pathways. Biochem Soc Trans. 2013;41:657–663. doi: 10.1042/BST20120345. [DOI] [PubMed] [Google Scholar]
- 42.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27:41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cummins EP, Crean D. Hypoxia and inflammatory bowel disease. Microbes Infect. 2017;19:210–221. doi: 10.1016/j.micinf.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Glover LE, Lee JS, Colgan SP. Oxygen metabolism and barrier regulation in the intestinal mucosa. J Clin Invest. 2016;126:3680–3688. doi: 10.1172/JCI84429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramakrishnan SK, Shah YM. Role of intestinal HIF-2α in health and disease. Annu Rev Physiol. 2016;78:301–325. doi: 10.1146/annurev-physiol-021115-105202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivan M, Kaelin WG., Jr The EGLN-HIF O2-sensing system: multiple inputs and feedbacks. Mol Cell. 2017;66:772–779. doi: 10.1016/j.molcel.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris AJ, Thompson AR, Whyte MK, Walmsley SR. HIF-mediated innate immune responses: cell signaling and therapeutic implications. Hypoxia (Auckl ) 2014;2:47–58. doi: 10.2147/HP.S50269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin N, Simon MC. Hypoxia-inducible factors: key regulators of myeloid cells during inflammation. J Clin Invest. 2016;126:3661–3671. doi: 10.1172/JCI84426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mills E, O’Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24:313–320. doi: 10.1016/j.tcb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Hammami A, Charpentier T, Smans M, Stäger S. IRF-5-mediated inflammation limits CD8+ T Cell expansion by inducing HIF-1α and impairing dendritic cell functions during Leishmania infection. PLoS Pathog. 2015;11:e1004938. doi: 10.1371/journal.ppat.1004938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wobben R, et al. Role of hypoxia inducible factor-1α for interferon synthesis in mouse dendritic cells. Biol Chem. 2013;394:495–505. doi: 10.1515/hsz-2012-0320. [DOI] [PubMed] [Google Scholar]
- 52.Cho SH, et al. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature. 2016;537:234–238. doi: 10.1038/nature19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward JB, et al. Hydroxylase inhibition attenuates colonic epithelial secretory function and ameliorates experimental diarrhea. FASEB J. 2011;25:535–543. doi: 10.1096/fj.10-166983. [DOI] [PubMed] [Google Scholar]
- 54.Kelly CJ, et al. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol. 2013;6:1110–1118. doi: 10.1038/mi.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du J, et al. pVHL negatively regulates antiviral signaling by targeting MAVS for proteasomal degradation. J Immunol. 2015;195:1782–1790. doi: 10.4049/jimmunol.1500588. [DOI] [PubMed] [Google Scholar]
- 56.Stehle F, et al. VHL-dependent alterations in the secretome of renal cell carcinoma: association with immune cell response? Oncotarget. 2015;6:43420–43437. doi: 10.18632/oncotarget.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholz CC, Taylor CT. Hydroxylase-dependent regulation of the NF-κB pathway. Biol Chem. 2013;394:479–493. doi: 10.1515/hsz-2012-0338. [DOI] [PubMed] [Google Scholar]
- 58.Corcoran SE, O’Neill LA. HIF1α and metabolic reprogramming in inflammation. J Clin Invest. 2016;126:3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills EL, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016:16553–16565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. This study identifies the control of glycolytic metabolism by the HIF pathway. [PubMed] [Google Scholar]
- 62.Halligan DN, Murphy SJ, Taylor CT. The hypoxia-inducible factor (HIF) couples immunity with metabolism. Semin Immunol. 2016;28:469–477. doi: 10.1016/j.smim.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Michiels C, Tellier C, Feron O. Cycling hypoxia: a key feature of the tumor microenvironment. Biochim Biophys Acta. 2016;1866:76–86. doi: 10.1016/j.bbcan.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Toffoli S, Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J. 2008;275:2991–3002. doi: 10.1111/j.1742-4658.2008.06454.x. [DOI] [PubMed] [Google Scholar]
- 65.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 66.Taylor CT, Kent BD, Crinion SJ, McNicholas WT, Ryan S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem Biophys Res Commun. 2014;447:660–665. doi: 10.1016/j.bbrc.2014.04.062. [DOI] [PubMed] [Google Scholar]
- 67.D’Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016;283:413–424. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell. 2016;166:288–298. doi: 10.1016/j.cell.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frede S, Stockmann C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pérez S, Taléns-Visconti R, Rius-Pérez S, Finamor I, Sastre J. Redox signaling in the gastrointestinal tract. Free Radic Biol Med. 2017;104:75–103. doi: 10.1016/j.freeradbiomed.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 73.Chandel NS, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 74.Masson N, et al. The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep. 2012;13:251–257. doi: 10.1038/embor.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. This study demonstrates that nitric oxide, a key mediator of inflammation, can regulate HIF stability through the control of intracellular oxygen availability. [DOI] [PubMed] [Google Scholar]
- 76.Lohninger L, et al. Hydrogen sulphide induces HIF-1α and Nrf2 in THP-1 macrophages. Biochimie. 2015;112:187–195. doi: 10.1016/j.biochi.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Flannigan KL, et al. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1α. FASEB J. 2015;29:1591–1602. doi: 10.1096/fj.14-266015. [DOI] [PubMed] [Google Scholar]
- 78.Wu B, Teng H, Yang G, Wu L, Wang R. Hydrogen sulfide inhibits the translational expression of hypoxia-inducible factor-1α. Br J Pharmacol. 2012;167:1492–1505. doi: 10.1111/j.1476-5381.2012.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eliasson P, Jönsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 80.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mortensen BT, et al. Changing bone marrow micro-environment during development of acute myeloid leukaemia in rats. Br J Haematol. 1998;102:458–464. doi: 10.1046/j.1365-2141.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- 82.Morikawa T, Takubo K. Hypoxia regulates the hematopoietic stem cell niche. Pflugers Arch. 2015;468:13–22. doi: 10.1007/s00424-015-1743-z. [DOI] [PubMed] [Google Scholar]
- 83.Forristal CE, et al. Pharmacologic stabilization of HIF-1α increases hematopoietic stem cell quiescence in vivo and accelerates blood recovery after severe irradiation. Blood. 2013;121:759–769. doi: 10.1182/blood-2012-02-408419. [DOI] [PubMed] [Google Scholar]
- 84.Takubo K, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 85.Forristal CE, Levesque JP. Targeting the hypoxia-sensing pathway in clinical hematology. Stem Cells Transl Med. 2014;3:135–140. doi: 10.5966/sctm.2013-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gezer D, Vukovic M, Soga T, Pollard PJ, Kranc KR. Concise review: genetic dissection of hypoxia signaling pathways in normal and leukemic stem cells. Stem Cells. 2014;32:1390–1397. doi: 10.1002/stem.1657. [DOI] [PubMed] [Google Scholar]
- 87.Annese V, Navarro-Guerrero E, Rodríguez-Prieto I, Pardal R. Physiological plasticity of neural-crest-derived stem cells in the adult mammalian carotid body. Cell Rep. 2017;19:471–478. doi: 10.1016/j.celrep.2017.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mazumdar J, et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simsek T, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krock BL, et al. The aryl hydrocarbon receptor nuclear translocator is an essential regulator of murine hematopoietic stem cell viability. Blood. 2015;125:3263–3272. doi: 10.1182/blood-2014-10-607267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guitart AV, et al. Hif-2α is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood. 2013;122:1741–1745. doi: 10.1182/blood-2013-02-484923. [DOI] [PubMed] [Google Scholar]
- 92.Abbott RK, et al. Germinal center hypoxia potentiates immunoglobulin class switch recombination. J Immunol. 2016;197:4014–4020. doi: 10.4049/jimmunol.1601401. References 52 and 92 demonstrate that physiological hypoxia in GCs plays a key role in B cell development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumpel BM, Manoussaka MS. Placental immunology and maternal alloimmune responses. Vox Sang. 2012;102:2–12. doi: 10.1111/j.1423-0410.2011.01533.x. [DOI] [PubMed] [Google Scholar]
- 94.Fryer BH, Simon MC. Hypoxia, HIF and the placenta. Cell Cycle. 2006;5:495–498. doi: 10.4161/cc.5.5.2497. [DOI] [PubMed] [Google Scholar]
- 95.Macklin PS, McAuliffe J, Pugh CW, Yamamoto A. Hypoxia and HIF pathway in cancer and the placenta. Placenta. 2017;56:8–13. doi: 10.1016/j.placenta.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 96.Yaghi L, et al. Hypoxia inducible factor-1 mediates the expression of the immune checkpoint HLA-G in glioma cells through hypoxia response element located in exon 2. Oncotarget. 2016;7:63690–63707. doi: 10.18632/oncotarget.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 98.Webster WS, Abela D. The effect of hypoxia in development. Birth Defects Res C Embryo Today. 2007;81:215–228. doi: 10.1002/bdrc.20102. [DOI] [PubMed] [Google Scholar]
- 99.Filiano AJ, Gadani SP, Kipnis J. Interactions of innate and adaptive immunity in brain development and function. Brain Res. 2015;1617:18–27. doi: 10.1016/j.brainres.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35:299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shepherd AP. Metabolic control of intestinal oxygenation and blood flow. Fed Proc. 1982;41:2084–2089. [PubMed] [Google Scholar]
- 102.Karhausen J, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. This study identifies a protective role for the HIF pathway in intestinal inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evans SM, et al. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 2000;60:2018–2024. [PubMed] [Google Scholar]
- 104.Albenberg L, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–1063. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goda F, et al. In vivo oximetry using EPR and India ink. Magn Reson Med. 1995;33:237–245. doi: 10.1002/mrm.1910330214. [DOI] [PubMed] [Google Scholar]
- 106.He G, et al. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci USA. 1999;96:4586–4591. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol. 2015;42:378–386. doi: 10.1053/j.seminoncol.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Unwith S, Zhao H, Hennah L, Ma D. The potential role of HIF on tumour progression and dissemination. Int J Cancer. 2015;136:2491–2503. doi: 10.1002/ijc.28889. [DOI] [PubMed] [Google Scholar]
- 109.Courtnay R, et al. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep. 2015;42:841–851. doi: 10.1007/s11033-015-3858-x. [DOI] [PubMed] [Google Scholar]
- 110.Miller JF, Sadelain M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell. 2015;27:439–449. doi: 10.1016/j.ccell.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 111.Maus MV, et al. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corzo CA, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Williams AE, Chambers RC. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol. 2014;306:L217–L230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Z, et al. AMP-activated protein kinase and Glycogen Synthase Kinase 3β modulate the severity of sepsis-induced lung injury. Mol Med. 2015;21:937–950. doi: 10.2119/molmed.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Campbell EL, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. This study identifies that the neutrophil oxidative burst is a key cause of mucosal hypoxia in inflammatory disease of the intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang JS, et al. Chronic granulomatous disease caused by a deficiency in p47(phox) mimicking Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:690–695. doi: 10.1016/s1542-3565(04)00292-7. [DOI] [PubMed] [Google Scholar]
- 117.Manresa MC, Godson C, Taylor CT. Hypoxia-sensitive pathways in inflammation-driven fibrosis. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1369–R1380. doi: 10.1152/ajpregu.00349.2014. [DOI] [PubMed] [Google Scholar]
- 118.Devraj G, Beerlage C, Brüne B, Kempf VA. Hypoxia and HIF-1 activation in bacterial infections. Microbes Infect. 2017;19:144–156. doi: 10.1016/j.micinf.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 119.Werth N, et al. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE. 2010;5:e11576. doi: 10.1371/journal.pone.0011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schaffer K, Taylor CT. The impact of hypoxia on bacterial infection. FEBS J. 2015;282:2260–2266. doi: 10.1111/febs.13270. [DOI] [PubMed] [Google Scholar]
- 121.Worlitzsch D, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schaible B, Schaffer K, Taylor CT. Hypoxia, innate immunity and infection in the lung. Respir Physiol Neurobiol. 2010;174:235–243. doi: 10.1016/j.resp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 123.Schaible B, et al. Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLoS ONE. 2013;8:e56491. doi: 10.1371/journal.pone.0056491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schaible B, Taylor CT, Schaffer K. Hypoxia increases antibiotic resistance in Pseudomonas aeruginosa through altering the composition of multidrug efflux pumps. Antimicrob Agents Chemother. 2012;56:2114–2118. doi: 10.1128/AAC.05574-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schaible B, et al. Hypoxia reduces the pathogenicity of Pseudomonas aeruginosa by decreasing the expression of multiple virulence factors. J Infect Dis. 2017;215:1459–1467. doi: 10.1093/infdis/jix139. [DOI] [PubMed] [Google Scholar]
- 126.Koh HS, et al. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat Commun. 2015;6:6340. doi: 10.1038/ncomms7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang J, et al. Hypoxia-Inducible Factor-2α limits natural killer T cell cytotoxicity in renal ischemia/reperfusion injury. J Am Soc Nephrol. 2016;27:92–106. doi: 10.1681/ASN.2014121248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luo L, et al. The role of HIF-1 in up-regulating MICA expression on human renal proximal tubular epithelial cells during hypoxia/reoxygenation. BMC Cell Biol. 2010;11:91. doi: 10.1186/1471-2121-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chan MC, Holt-Martyn JP, Schofield CJ, Ratcliffe PJ. Pharmacological targeting of the HIF hydroxylases—a new field in medicine 1development. Mol Aspects Med. 2016;47–48:54–75. doi: 10.1016/j.mam.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 130.Muchnik E, Kaplan J. HIF prolyl hydroxylase inhibitors for anemia. Expert Opin Investig Drugs. 2011;20:645–656. doi: 10.1517/13543784.2011.566861. [DOI] [PubMed] [Google Scholar]
- 131.Maxwell PH, Eckardt KU. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol. 2016;12:157–168. doi: 10.1038/nrneph.2015.193. [DOI] [PubMed] [Google Scholar]
- 132.Cho H, et al. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature. 2016;539:107–111. doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen W, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539:112–117. doi: 10.1038/nature19796. References 132 and 133 demonstrate that selective targeting of the HIF2 pathway may be of clinical benefit in kidney cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cummins EP, Doherty GA, Taylor CT. Hydroxylases as therapeutic targets in inflammatory bowel disease. Lab Invest. 2013;93:378–383. doi: 10.1038/labinvest.2013.9. [DOI] [PubMed] [Google Scholar]
- 135.Hams E, et al. The hydroxylase inhibitor dimethyloxallyl glycine attenuates endotoxic shock via alternative activation of macrophages and IL-10 production by B1 cells. Shock. 2011;36:295–302. doi: 10.1097/SHK.0b013e318225ad7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Provenzano R, et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol. 2016;11:982–991. doi: 10.2215/CJN.06890615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brigandi RA, et al. A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: 28 day, phase 2A randomized trial. Am J Kidney Dis. 2016;67:861–871. doi: 10.1053/j.ajkd.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 138.Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90:1115–1122. doi: 10.1016/j.kint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 139.Tambuwala MM, et al. Targeted delivery of the hydroxylase inhibitor DMOG provides enhanced efficacy with reduced systemic exposure in a murine model of colitis. J Control Release. 2015;217:221–227. doi: 10.1016/j.jconrel.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 140.Maxwell PH, et al. Sites of erythropoietin production. Kidney Int. 1997;51:393–401. doi: 10.1038/ki.1997.52. [DOI] [PubMed] [Google Scholar]
- 141.Spencer JA, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grimm C, Willmann G. Hypoxia in the eye: a two-sided coin. High Alt Med Biol. 2012:13169–13175. doi: 10.1089/ham.2012.1031. [DOI] [PubMed] [Google Scholar]
- 143.Cummins EP, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cockman ME, et al. Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH) Proc Natl Acad Sci USA. 103:14767–14772. doi: 10.1073/pnas.0606877103. References 143 and 144 demonstrate that components of the NF-κB pathway are targets for hydroxylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ghosh S, Paul A, Sen E. Tumor necrosis factor α-induced hypoxia-inducible factor 1α-β-catenin axis regulates major histocompatibility complex class I gene activation through chromatin remodeling. Mol Cell Biol. 2013;33:2718–2731. doi: 10.1128/MCB.01254-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Clambey ET, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109:E2784–E2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cummins EP, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 149.Hindryckx P, et al. Longitudinal quantification of inflammation in the murine dextran sodium sulfate-induced colitis model using muPET/CT. Inflamm Bowel Dis. 2011;17:2058–2064. doi: 10.1002/ibd.21578. [DOI] [PubMed] [Google Scholar]
- 150.Cosin-Roger J, et al. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat Commun. 2017;8:98. doi: 10.1038/s41467-017-00213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Robinson A, et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. References 148 and 151 demonstrate that pharmacological hydroxylase inhibition is protective in colitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hindryckx P, et al. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J Immunol. 2010;185:6306–6316. doi: 10.4049/jimmunol.1002541. [DOI] [PubMed] [Google Scholar]
- 153.Hirota SA, et al. Hypoxia-inducible factor signaling provides protection in Clostridium difficile-induced intestinal injury. Gastroenterology. 2010;139:259–269. doi: 10.1053/j.gastro.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hart ML, et al. Hypoxia-inducible factor-1 alpha dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Marchbank T, Mahmood A, Harten S, Maxwell PH, Playford RJ. Dimethyloxalyglycine stimulates the early stages of gastrointestinal repair processes through VEGF-dependent mechanisms. Lab Invest. 2011;91:1684–1694. doi: 10.1038/labinvest.2011.129. [DOI] [PubMed] [Google Scholar]
- 156.Keely S, et al. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 2014;7:114–123. doi: 10.1038/mi.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marks E, et al. Oral delivery of prolyl hydroxylase inhibitor: AKB-4924 promotes localized mucosal healing in a mouse model of colitis. Inflamm Bowel Dis. 2015;21:267–275. doi: 10.1097/MIB.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 158.Taniguchi CM, et al. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci Transl Med. 2014;6:236ra64. doi: 10.1126/scitranslmed.3008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jeong S, et al. Lipophilic modification enhances anti-colitic properties of rosmarinic acid by potentiating its HIF-prolyl hydroxylases inhibitory activity. Eur J Pharmacol. 2015;747:114–122. doi: 10.1016/j.ejphar.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 160.Gupta R, et al. Therapeutic treatment with a novel hypoxia-inducible factor hydroxylase inhibitor (TRC160334) ameliorates murine colitis. Clin Exp Gastroenterol. 2014;7:13–23. doi: 10.2147/CEG.S51923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jamadarkhana P, et al. Treatment with a novel hypoxia-inducible factor hydroxylase inhibitor (TRC160334) ameliorates ischemic acute kidney injury. Am J Nephrol. 2012;36:208–218. doi: 10.1159/000341870. [DOI] [PubMed] [Google Scholar]
- 162.Bernhardt WM, et al. Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc Natl Acad Sci USA. 2009;106:21276–21281. doi: 10.1073/pnas.0903978106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Manresa MC, et al. Hydroxylase inhibition regulates inflammation-induced intestinal fibrosis through the suppression of ERK-mediated TGF-β1 signaling [corrected] Am J Physiol Gastrointest Liver Physiol. 2016;311:G1076–G1090. doi: 10.1152/ajpgi.00229.2016. [DOI] [PubMed] [Google Scholar]