Abstract
Correlations in activity across disparate brain regions during rest reveal functional networks in the brain. Although previous studies largely agree that there is an age-related decline in the “default mode network,” how age affects other resting-state networks, such as emotion-related networks, is still controversial. Here we used a dual regression approach to investigate age-related alterations in resting-state networks. The results revealed age-related disruptions in functional connectivity in all five identified cognitive networks, namely the default mode network, cognitive-auditory, cognitive-speech (or speech-related somatosensory) and right and left fronto-parietal networks, whereas such age effects were not observed in the three identified emotion networks. In addition, we observed age-related decline in functional connectivity in three visual and three motor/visuospatial networks. Older adults showed greater functional connectivity in regions outside four out of the five identified cognitive networks, consistent with the dedifferentiation effect previously observed in task-based fMRI studies. Both reduced within-network connectivity and increased out-of-network connectivity were correlated with poor cognitive performance, providing potential biomarkers for cognitive aging.
Keywords: aging, resting-state network, functional connectivity, fMRI, dedifferentiation, emotion
1. Introduction
In recent years, resting-state functional magnetic resonance imaging (fMRI) has emerged as a powerful method for investigating age-related changes in large-scale brain networks (Allen et al., 2011; Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Mevel et al., 2013; Tomasi & Volkow, 2012). Unlike traditional fMRI studies wherein subjects perform cognitive tasks, resting-state fMRI requires no specific task. This eliminates variability from individual differences in task compliance and performance, making the data more reproducible and reliable. Critically, resting-state networks closely match task-related functional networks (Laird et al., 2011; Smith et al., 2009), indicating that functional networks utilized during tasks are continuously active at rest. Thus, age-related disruption in resting-state networks should reflect age-related decline in task-related activity, allowing inferences about age-related changes in cognitive function.
Normal aging is typically associated with cognitive decline even in the absence of disease. Although previous research identified multiple resting-state networks in healthy younger and older subjects (Biswal et al., 1995; Fox et al., 2006; Raichle et al., 2001; Smith et al., 2009; Vincent et al., 2008), previous studies on age-related changes in resting-state networks focused on the default mode network (DMN) - the most commonly observed resting-state network. The DMN is associated with internally directed mental states, such as remembering, planning, and social cognition (Buckner & Carroll, 2007; Fransson, 2005; Greicius et al., 2003; Raichle et al., 2001; Vincent et al., 2006). Previous evidence suggests that the DMN-coordinated activity diminishes with age (Allen et al., 2011; Andrews-Hanna et al., 2007; Chan, Park, Savalia, Petersen, & Wig, 2014; Damoiseaux et al., 2008; Esposito et al., 2008; Koch et al., 2010; Mevel et al., 2013), and that the age-related disruption of this network is associated with poor cognitive performance (Damoiseaux et al., 2008; Dong et al., 2012; Mevel et al., 2013).
More recently, researchers began to examine age-related changes in resting-state networks other than the DMN. One study of 603 people aged between 12 and 71 found that the degree of coactivation within networks decreased with age for every component assessed, with no obvious differences between networks in how strongly they were affected by age (Allen et al., 2011). Similarly, another study (Huang et al., 2015) examined people over 50 years old and found age-related decline in several networks including cognitive, auditory, sensorimotor and visual medial networks. In contrast to these findings however, some evidence suggested that not all networks show age-related decline at the same rate (e.g., Meier et al., 2012). One study (Damoiseaux et al., 2008) found that older participants had significantly lower coordinated activity within the DMN but that other networks identified did not show age differences. A more recent study (Chan et al., 2014) using a lifespan sample aged between 20–89 demonstrated that age-related disruption in within-network connectivity was not necessarily homogeneous across multiple networks. Furthermore, another study (Douaud et al., 2014) of 484 participants from age 8 to 85 years revealed that a network of brain regions that develop during late adolescence showed rapid decline in old age, suggesting that some networks are more vulnerable to aging than others.
Unlike the networks associated with cognition, age-related changes in emotion-related networks have received much less focus. Previous research (Onoda, Ishihara, & Yamaguchi, 2012) found that the salience network, which is involved in both cognitive and emotion processing, showed age-related decline, and the disruption of this network was associated with poor cognitive performance. However, another study (Chan et al., 2014) did not find a significant age-related decline within the same network. In addition to these mixed findings, the integrative nature of the salience network (Menon & Uddin, 2010; Onoda et al., 2012; Seeley et al., 2007) makes it hard to determine whether reduced connectivity within this system reflects cognitive impairment, emotional dysfunction or both. Thus, it is important to examine cognitive and emotion networks separately to understand how aging affects the two types of systems.
Previous studies showing age differences in resting state network connectivity (Biswal et al., 2010; Chan et al., 2014; Mowinckel, Espeseth, & Westlye, 2012; Spreng et al., 2016) found age-related reductions in within-network functional connectivity, as well as age-related increases in connectivity between a network and other regions not associated with that network. These results suggest that older adults, compared with younger adults, show dedifferentiation in resting-state brain activity, in addition to reduction in connectivity within networks. Furthermore, these two effects of age might have an opposite relationship with cognitive function; stronger within-network connectivity is typically associated with better cognitive function (Damoiseaux et al., 2008; Dong et al., 2012; Mevel et al., 2013), while increased connectivity outside resting-state networks might be associated with poor cognitive function as it reflects reduced neural specificity. Thus, it is important to separately examine the effects of age on connectivity within vs. outside each resting-state network and their associations with cognitive function.
In the current study, we used a dual-regression analysis approach to examine whether there are age differences associated with multiple resting-state networks. We first analyzed the resting-state data of 80 younger (age range = 18–33) and 80 older individuals (age range = 58–85) using independent component analysis (ICA). The ICA method allowed us to identify functionally discrete networks without a priori regions of interest. The networks identified were matched to previously characterized networks involved in emotion, motor/visuospatial processes, vision and cognition based on large-scale metadata (Laird et al., 2011). Their networks were defined using 8637 functional brain imaging experiments, which contained 69,481 brain activation locations across 31,724 participants whose ages ranged from 1 to 90 years old with the mean of 31.5 years. We then used dual regression analyses to compare individual participant spatial maps associated with each component and examined whether there were age differences (Biswal et al., 2010; Filippini et al., 2009).
We predicted that brain networks involved in cognitive processes would show more age-related decline than networks involved in emotional processes. Previous behavioral research suggests age-related decline in multiple cognitive domains (Park, 2000; Salthouse, 2010) but preserved emotional processing in normal aging (Mather, 2012, 2016). A neuroimaging study on participants aged between 18 and 65 also showed age-related increases in functional connectivity for subcortical and paralimbic structures but decreased functional connectivity for cortical structures (Hampson et al., 2012), suggesting a shift with aging that could favor emotional processes that rely heavily on subcortical and paralimbic brain regions (Kober et al., 2008) over cognitive processes that rely more on dorsal cortical regions. In addition, previous studies examining age-related differences in functional connectivity during emotion-related tasks revealed that younger and older adults showed a similar pattern of functional connectivity involving the amygdala (Nashiro et al., 2013), and that older adults engaged emotional networks more effectively than younger adults (St Jacques et al., 2010), possibly reflecting improved emotion regulation with increasing age. Thus, we hypothesized that the cognitive networks including the DMN (see Table 1 for network categorization) would show robust age-related decline in functional connectivity, whereas other networks, especially those more involved in emotional processes, would be better preserved with age.
Table 1.
Network categorizations.
| Type of Network | Laird | NKI |
|---|---|---|
|
| ||
| Emotion/interoception | ICN 1 | 6 |
| Emotion/interoception | ICN 2 | 15 |
| Emotion/interoception | ICN 3 | – |
| Emotion/interoception | ICN 4 | – |
| Emotion/interoception | ICN 5 | 7 |
| Motor/visuospatial | ICN 6 | – |
| Motor/visuospatial | ICN 7 | 10 |
| Motor/visuospatial | ICN 8 | 8 |
| Motor/visuospatial | ICN 9 | 2 |
| Visual | ICN 10 | 12 |
| Visual | ICN 11 | 1 |
| Visual | ICN 12 | 0 |
| Cognitive (DMN) | ICN 13 | 18 |
| Cognitive (Cerebellum) | ICN 14 | – |
| Cognitive (Right fronto−parietal) | ICN 15 | 4 |
| Cognitive (Cognitive−auditory) | ICN 16 | 16 |
| Cognitive (Cognitive−speech) | ICN 17 | 9 |
| Cognitive (Left fronto−parietal) | ICN 18 | 19 |
Intrinsic connectivity networks (ICNs) were categorized based on BrainMap behavioral taxonomy (see Laird et al., 2011 for more details). Fourteen NKI components were spatially matched with Laird et al.’s ICNs with a mean correlation of r = 0.59 (0.45–0.84). (−) indicates that there were no matches.
Along with age-related decline in functional connectivity within the standard regions associated with each cognitive network, we examined whether there were age-related increases in functional connectivity with regions not associated with that network. In task-related neuroimaging studies, older adults show dedifferentiation of brain activity. That is, they tend to activate a more distributed set of regions that are less specialized for the task they are doing than do younger adults. For instance, when processing faces, places or words, category-specific brain regions that respond selectively to one type of item (e.g., the fusiform face area for faces) become less selective for their preferred category and more likely to activate in response to items from other categories among older adults compared with younger adults (Grady et al., 1992; Park et al., 2004; Park et al., 2012; Voss et al., 2008). Thus, age-related decline in the functional integrity of cognitive networks may also take the form of a less selective set of regions involved in each network.
2. Methods
2.1. Sample
We obtained structural and resting-state data from the Enhanced Nathan Kline Institute (NKI)/Rockland Sample database (http://coins.mrn.org/), which is a collection of neuroimaging data from individuals across the lifespan. The NKI protocol also includes a battery of cognitive, behavioral, and psychiatric assessment. We obtained the data of 80 younger adults over the age of 18 (Mage = 23.13 years; age range: 18–33; 41 males and 39 females) and 80 older individuals (Mage = 67.79; age range: 58–85; 18 males and 62 females), as our primary goal was to examine age-related differences in resting-state functional connectivity, particularly focusing on the comparison between early and late adulthood.
The resting scans were performed on a 3.0-T Siemens MAGNETOM Trio Tim scanner. The imaging parameters were TR=2500 ms, TE=30 ms, slice thickness = 3.0 mm, flip angle = 80°, field of view = 216 mm, slices = 38, voxel size = 3.0 × 3.0 × 3.0 mm, and acquisition time = 5 min. High resolution MPRAGE anatomical images were acquired with the scanning parameters of TR=1900 ms, TE=2.52 ms, slice thickness = 1.0 mm, flip angle = 9°, field of view = 256 mm, and voxel size = 1.0 × 1.0 × 1.0 mm.
2.2. Data Analyses
Data analyses were carried out using FSL tools (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl; Smith et al., 2004). The methods described below were adapted and modified from Biswal et al. (2010).
2.2.1 Image preprocessing
We used FMRIB Software Library (FSL) version 5.0.8. to perform all fMRI pre-processing (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). First, standard image preprocessing was performed on individual data (i.e., motion correction, brain extraction, spatial filtering with full width at half maximum (FWHM) = 6 mm, a high-pass temporal filtering). Noise components were identified and removed using the FMRIB ICA-based Xnoiseifier (FIX v1.06, Griffanti et al., 2014; Salimi-Khorshidi et al., 2014). In addition, we included nine nuisance covariates in each participant’s analysis: six motion parameters, and signal from white matter, cerebrospinal fluid, and whole brain (Biswal et al., 2010). The six motion parameters were generated in the motion-correction step during preprocessing using FSL’s MCFLIRT. The white matter and cerebrospinal fluid were first segmented from each participant’s structural image using FAST in FSL. The resulting segmented images were thresholded at 80% and applied to each participant’s time series. Then, a mean time series was calculated by averaging across time series of all voxels within each mask. The global signal covariate was generated by averaging across the time series of all voxels in the brain. The nine nuisance signals were removed from individual data using fslglm. The denoised functional image of each subject was linearly aligned to their high-resolution brain-extracted structural image and the standard Montreal Neurological Institute (MNI) 2-mm brain using FSL FLIRT. Each structural image was also non-linearly registered to the MNI 2-mm space using FSL FNIRT. The functional images were then warped to the MNI space, using APPLYWARP with the non-linear coefficients obtained by FNIRT. The data were temporally filtered using a high-pass filter (Gaussian-weighted least squares straight-line fitting, with σ = 100.0s).
2.2.2 Independent component analysis
The preprocessed individual data from all 160 individuals were entered into temporal-concatenation group ICA (TC-GICA) to generate group-level components using MELODIC (FSL). Following the methods used by Biswal et al. (2010), the number of components was fixed at 20, and TC-GICA was performed 8 times in order to identify reliable components across multiple ICA runs. A meta-ICA was conducted across the 8 runs to extract the 20 spatially independent components that were consistently identified across the 8 runs. The resulting 20 maps were used as group templates in a dual regression analysis. In parallel with the dual regression analysis, the 20 maps were matched with the 20 task-based networks reported by Laird et al. (2011) through a spatial cross-correlation analysis of the ICA maps between the two datasets. We used thresholded z-statistic images for this analysis.
2.2.3 Dual regression analysis
The preprocessed individual data and the 20 maps from the meta-ICA were used in a dual regression analysis using the FSL dual-regression function, which created subject-specific time series and spatial maps for each of the 20 templates. The individual spatial maps were merged across subjects into single 4D files and between-group differences were tested for statistical significance using voxel-wise nonparametric permutation testing (5000 permutations). All statistical maps were family-wise error (FWE) corrected using p < 0.01, based on the threshold-free cluster enhancement (TFCE) statistic image (Smith & Nichols, 2009).
2.2.4 ROI analysis
Since this study focused on age differences in functional connectivity between cognitive compared to emotion networks, we conducted a 2 (group: younger, older) × 2 (network type: cognition, emotion) mixed ANCOVA on functional connectivity strengths, including sex and intracranial volume (ICV) as covariates. For each network, we extracted mean functional connectivity values for each subject from the subject-specific z-transformed spatial maps of the second stage of the dual regression, and calculated average values across the five identified cognitive networks and across the three identified emotion networks (Table 1).
2.2.5 Behavioral data analysis
Cognitive measures included tests from the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001) and Wechsler Abbreviated Scale of Intelligence (WASI). Participants completed verbal fluency, design fluency, sorting, color-word interference, trail making, word context, tower, proverb and twenty questions (20Q) tests of the D-KEFS, which assess higher-level executive control abilities, as well as verbal and perceptual reasoning tests of the WASI, which measures global cognitive functioning (a total of 11 subtests). Each score was standardized across all participants and averaged to obtain the composite score for each D-KEFS and WASI subtest. For example, there are four scores for verbal fluency of the D-KEFS. For each participant, we standardized each of the four scores and averaged them to calculate a composite score.
2.2.5 Correlations analysis
The brain regions showing age group differences (see 3.2 and 3.3 in the Results section) were thresholded at FWE-corrected p < 0.01 and binarized. For each ROI, we extracted mean functional connectivity values for each participant from the subject-specific z-transformed spatial maps. For each ROI, the functional connectivity values were correlated with the 11 cognitive composite scores using partial correlation analysis including age, sex and ICV as covariates. The final results were corrected for multiple comparisons using the false discovery rate (FDR) approach to control the expected rate of false positives to less than 5% (Benjamini & Hochberg, 1995).
3. Results
3.1. Fourteen resting state networks were identified
Using a data-driven ICA approach, we identified 20 resting state networks from the entire sample. In order to determine which of the 20 components matched previously-identified task-based networks, we compared components between the NKI resting-state and Laird et al. (2011) datasets through spatial cross-correlation of the ICA maps using fslmaths. Of the 20 components generated separately from the two databases, fourteen maps were matched with a mean correlation of r = 0.59 (0.45–0.84). As shown in Table 1 and Figure 1, the fourteen maps were categorized into A) five cognitive, B) three motor/visuospatial, C) three visual and D) three emotion networks, using the behavioral domain categorization from Laird et al. (2011). The remaining maps were either judged to be artifactual, or matched with an NKI component that had already been matched to another Laird et al. network with a higher correlation value (Supplementary Figure 1).
Figure 1.
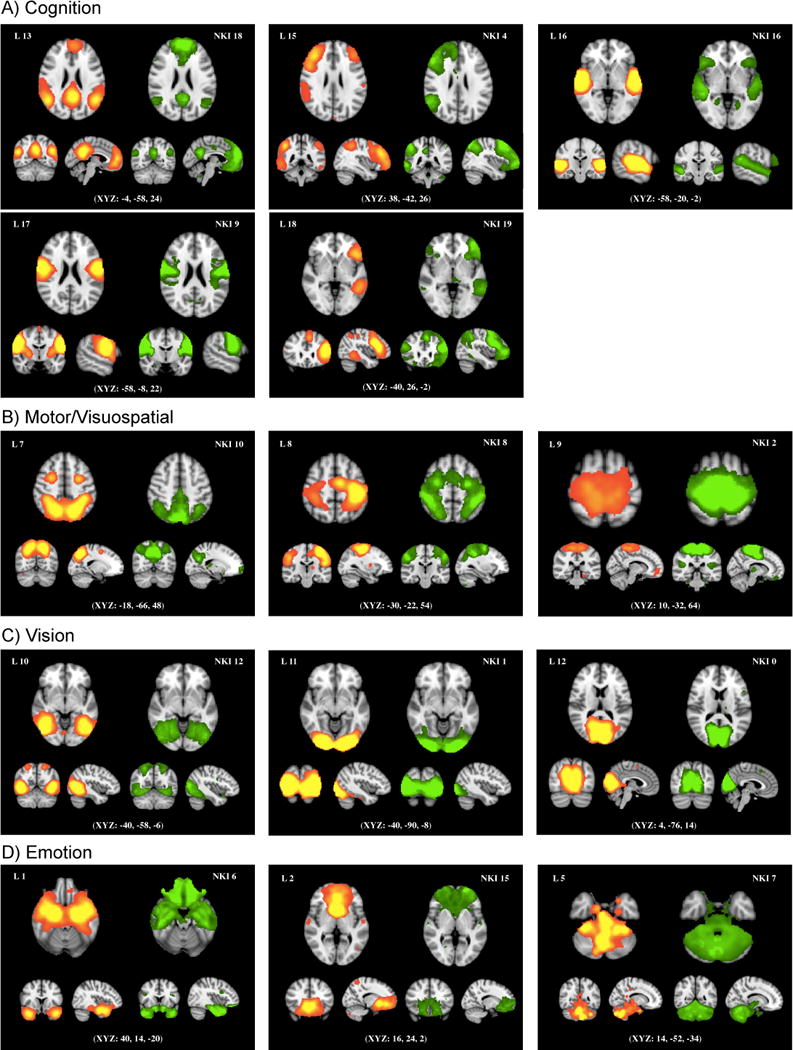
Fourteen well-matched pairs between the 20 NKI maps and the 20 Laird et al.’s (2011) templates. The left side of each pair is Laird et al.’s template with the component number used in their original study (Laird 13, 15, 16, 17, 18 = Divergent Cognitive Networks; Laird 7, 8, 9 = Motor/Visuospatial; Laird 10, 11, 12 = Visual; Laird 1, 2, 5 = Emotion/Interoception). On the right side of each pair is a corresponding network from the NKI database superimposed on the MNI152 standard space template image. Fourteen maps were matched with a mean correlation of r = 0.52 (0.45−0.84). NKI maps were converted to Z statistic images and thresholded at z = 2.3.
3.2. Older adults showed less functional connectivity than younger adults in all networks except for the emotional networks
A dual regression analysis was carried out to investigate age group differences in the resting-state networks. Below we describe each of the five components using the behavioral domain categorization developed by Laird et al. (Laird et al., 2011). In their study, 18 networks were grouped into four categories based on a large-scale meta analysis on behavioral domains and paradigms associated with each network: a) divergent cognitive, b) motor/visuospatial, c) visual and d) emotion networks (Table 1).
All five networks that were categorized as divergent cognitive networks in Laird et al. (2011) had regions in which older adults showed significantly less functional connectivity than did younger adults (Figure 2). NKI 18 (corresponding with Laird 13; Figure 2A) is the DMN, which is strongly associated with remembering, planning, theory of mind and social cognition (Buckner & Carroll, 2007; Fransson, 2005; Greicius et al., 2003; Raichle et al., 2001; Vincent et al., 2006). Consistent with previous findings (Allen et al., 2011; Andrews-Hanna et al., 2007; Esposito et al., 2008; Koch et al., 2010; Mevel et al., 2013), older adults showed significantly less functional connectivity in the anterior part of DMN (i.e., frontal pole). NKI 4 (corresponding with Laird 15; Figure 2A) includes right-lateralized fronto-parietal regions and is involved in multiple cognitive processes including reasoning, attention, inhibition and memory. Within this network, older adults showed significantly less functional connectivity in the right frontal pole, middle and superior frontal gyri than did younger adults. NKI 16 (corresponding with Laird 16; Figure 2A) covers the primary auditory cortices and is associated with audition, music, speech, phonological and oddball discriminations. Although this network is involved in a wide range of tasks (Laird et al., 2011), for the sake of conciseness we call it ‘the cognitive-auditory network’ throughout this paper based on Laired et al.’s findings. Relative to younger adults, older adults showed less functional connectivity in the bilateral inferior frontal and superior temporal gyri and the right supramarginal gyrus within this network. NKI 9 (corresponding with Laird 17; Figure 2A) includes primary sensorimotor cortices and is associated with action and somesthesis involving speech. Within this cognitive-speech network, older adults showed less functional connectivity in the right precentral gyrus, bilateral postcentral gyri and insula. NKI 19 (corresponding with Laird 18; Figure 2A) comprises left-lateralized fronto-parietal regions and is involved in semantic, phonologic and orthographic language tasks as well as working and explicit memory tasks. Older adults had less functional connectivity in bilateral inferior, middle and superior frontal gyri than did younger adults.
Figure 2.
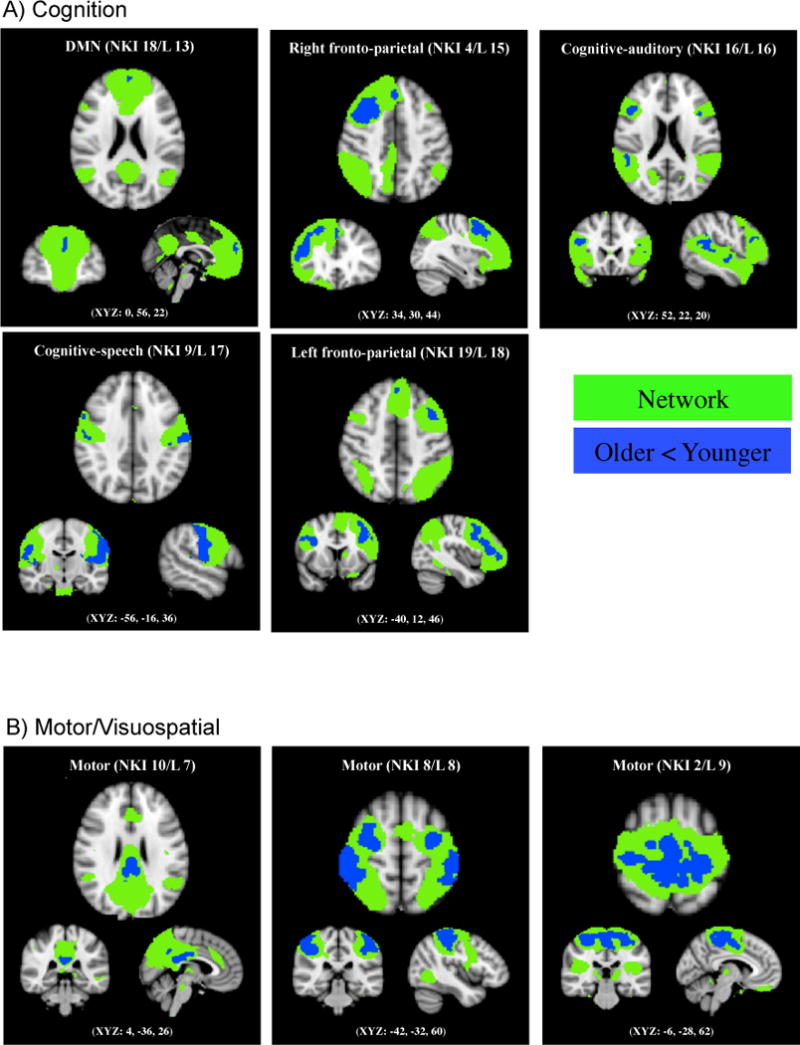
Age-related decline in functional connectivity in eleven resting-state networks. Compared with younger adults, older adults showed reduced functional connectivity in five cognitive, three motor/visuospatial and three visual networks but not in the emotional networks, with p < 0.01, FWE corrected using TFCE technique. The regions highlighted in green represent spatial maps obtained from all participants, and the blue highlights indicate the regions that older adults showed less functional connectivity than did younger adults.
In addition to the five cognitive networks, older adults also showed lower functional connectivity in three motor/visuospatial networks. NKI 10 (corresponding with Laird 7; Figure 2B) includes the posterior parietal and dorsolateral prefrontal cortices and is associated with visuospatial processing and reasoning. In this network, older adults showed significantly less functional connectivity in the posterior cingulate gyrus and precuneus than did younger adults. NKI 8 (corresponding with Laird 8; Figure 2B) is another motor/visuospatial network that includes the primary sensorimotor cortices and plays a role in action and somesthesis. Older adults showed less functional connectivity in the bilateral precentral and postcentral gyri compared with younger adults. NKI 2 (corresponding with Laird 9; Figure 2B) is comprised of the medial superior parietal cortex and is involved in motor execution and learning. Older adults showed less signal in the bilateral precentral and postcentral gyri within this network.
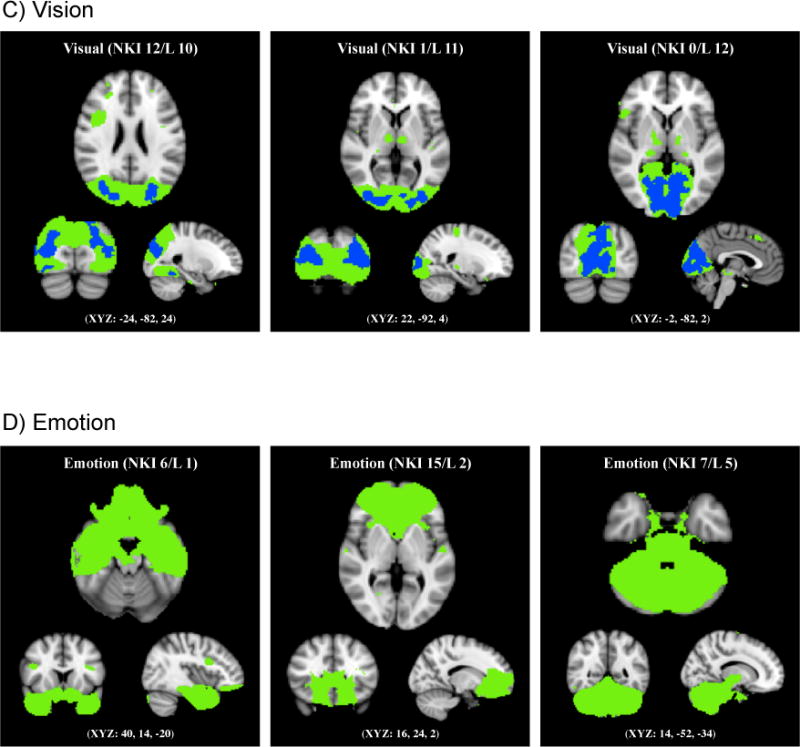
A significant age group difference was also found in three visual networks. NKI 12 (corresponding with Laird 10; Figure 2C) includes the middle temporal visual association area and plays an important role in viewing complex stimuli, action observation, overt picture naming, and visual tracking of moving objects. Older adults showed less functional connectivity in the bilateral lateral occipital and temporal occipital fusiform cortices compared with younger adults. NKI 1 and NKI 0 (corresponding with Laird 11 and 12; Figure 2C) comprise the primary, secondary and tertiary visual cortices. NKI 0 is involved in simple visual processing while NKI 1 is associated with higher level visual processing. Within NKI 0, older adults showed less activity in the bilateral occipital pole and lingual gyri. Within NKI 1, less activity was observed in older adults in the bilateral occipital pole.
Unlike the cognitive, motor/visuospatial and visual networks, none of the networks in the emotion category exhibited age-related decline in functional connectivity in any part of the components.
3.3. Older adults showed greater out-of-network functional connectivity compared with younger adults
Older adults showed greater functional connectivity outside four cognitive networks identified in this study (Figure 3A). These regions included the bilateral superior frontal and precentral gyri and the right occipital pole in connection with DMN, the right lateral occipital cortex in connection with the right fronto-parietal network, the left middle and superior frontal gyri, left superior parietal lobe and posterior cingulate cortex in connection with the cognitive-auditory network, and the right middle frontal gyrus in connection with the left fronto-parietal network.
Figure 3.
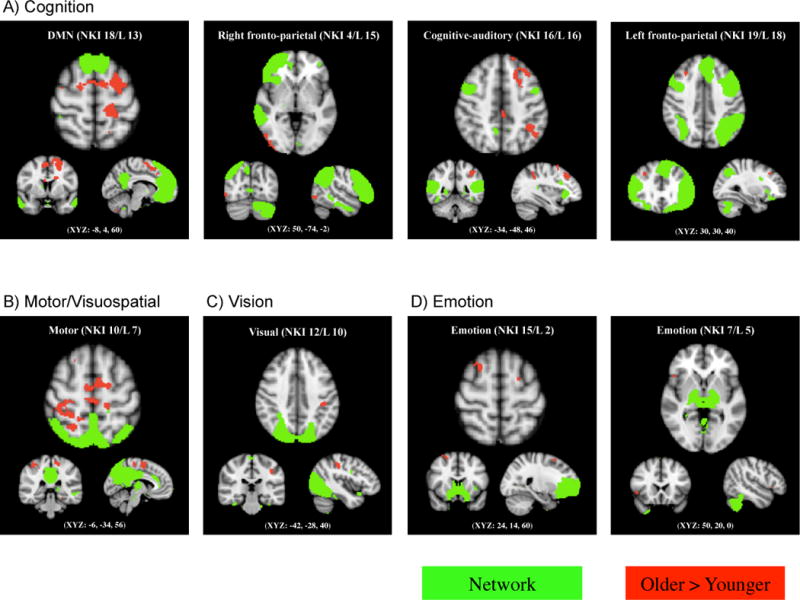
Age-related increase in out-of-network functional connectivity. Four cognitive, one motor, one visual and two emotion networks (shown in green) had regions outside the networks that showed increased functional connectivity in older compared with younger adults (shown in red), with p < 0.01, FWE corrected using TFCE technique.
In addition, older adults showed greater functional connectivity in the bilateral frontal pole, left superior frontal and precentral gyri, and right superior parietal lobe with a motor/visuospatial network (i.e., NKI 10; Figure 3B) as well as in the left postcentral gyrus with a visual network (i.e., NKI 12; Figure 3C). Older adults also showed greater out-of-network connectivity in the bilateral superior frontal gyrus in connection with an emotion network (i.e., NKI 15; Figure 3D) and in the right inferior frontal gyrus in connection with another emotion network (i.e., NKI 7; Figure 3D).
3.4. Age-related difference in cognitive networks were larger than that in emotion networks
Next, we examined the effects of age on functional connectivity across all cognitive networks compared with all emotion networks. The 2 (age group: younger vs. older) × 2 (network type: cognitive vs. emotion) ANCOVA including sex and ICV as covariates revealed that there was a significant interaction, F(1, 156) = 5.51, MSE = 0.08, p = .02, (Figure 4). Post-hoc t-tests indicated that there was a significant age group difference in the mean functional connectivity values across the cognitive networks, t(158) = 6.12, p < .001, but not in those across the emotion networks, t(158) = 1.25, p = .213. As an exploratory post-hoc analysis, we performed the same 2 × 2 ANCOVA including sex and ICV as covariates for the regions showing age group differences outside the cognitive vs. emotion networks (i.e., the mean activation across the red regions in Figure 3A vs. Figure 3D). As expected, there was a main effect of age group, F(1, 156) = 194.94, MSE = 0.45, p < .001, ; however, there were no other significant findings.
Figure 4.
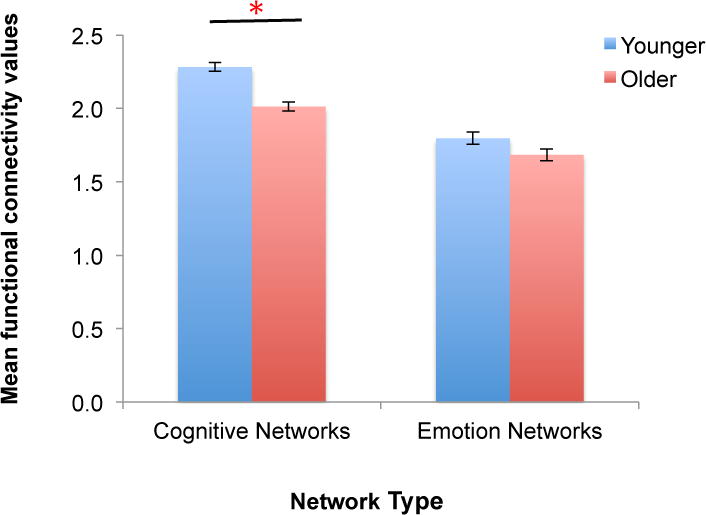
Larger age-group differences in cognitive networks compared to emotion networks. Cognitive network connectivity was calculated by taking an average of functional connectivity values across all five cognitive networks (as shown in green in Figure 1). Similarly, we computed the average functional connectivity values across all three emotion networks. After controlling for sex and ICV, we found a significant age group × network type interaction. This was due to greater cognitive-network connectivity in younger adults compared with older adults with the absence of significant group difference in the emotion networks. The error bars indicate standard errors.
3.5. Greater functional connectivity within cognitive networks was associated with better performance whereas greater out-of-network connectivity was associated with poorer performance
For each network, we conducted partial correlation analyses between each cognitive composite score and functional connectivity in the brain regions showing age-related decline (YA>OA; the blue regions in Figure 2) while controlling for age, sex and ICV. There was a significantly positive correlation between the 20Q score and functional connectivity in the YA>OA regions within DMN (r = 0.27, P < 0.005, q[FDR] = .05; Figure 5A). No other results reached significance after FDR correction. When we repeated the analysis without the ICV covariate, the DMN-20Q correlation remained significant (r = 0.25, P < 0.005, q[FDR] = 0.05).
Figure 5.
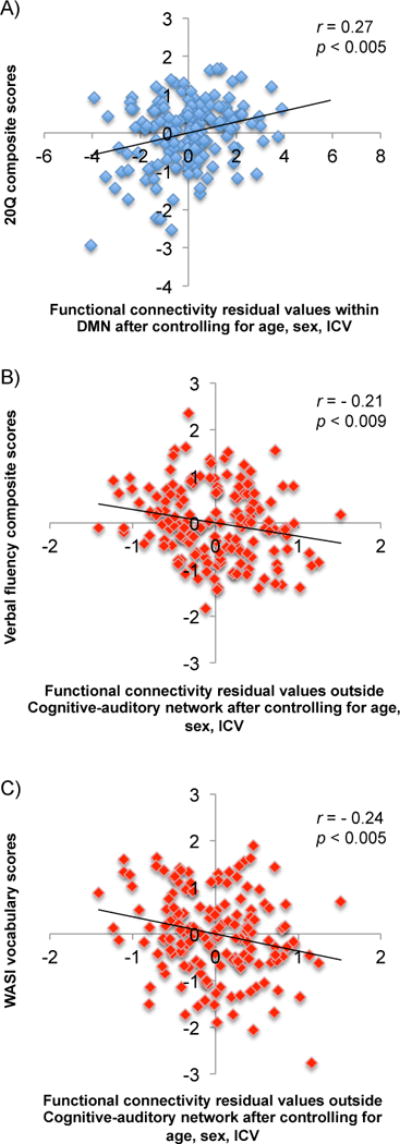
The relationship between cognitive performance and within-network vs. out-of-network functional connectivity. The mean functional connectivity values were obtained for each participant from the regions showing significant age-related decline within DMN (shown in blue in Figure 2) and the regions showing significant age-related increase outside the cognitive-auditory network (shown in red in Figure 2). After controlling for age, sex and ICV, the functional connectivity strengths within DMN were positively correlated with the 20Q composite scores (Pearson r = 0.27, P < 0.005, q[FDR] = .05). The effects of age, sex and ICV were as follows, respectively: β = − 0.27, p = 0.035; β = − 0.08, p = 0.351; β = − 0.22, p = 0.012. In contrast, the functional connectivity strengths outside the cognitive-auditory network were negatively correlated with the verbal fluency and WASI vocabulary scores (r = − 0.21, P < 0.009, q[FDR] = 05; r = − 0.24, P < 0.005, q[FDR] = .05, respectively). The effects of age, sex and ICV were as follows, respectively: β = 0.74, p < 0.001; β = − 0.04, p = 0.612; β = 0.07, p = 0.373.
We also performed partial correlation analyses between each cognitive composite score and the functional connectivity in the regions showing age-related increase (OA>YA; the red regions in Figure 3). After controlling for age, sex and ICV, we found a significantly negative correlation between the verbal fluency score and functional connectivity in the OA>YA regions with the cognitive-auditory network (r = −0.21, P < 0.005, q[FDR] = .05; Figure 5B). In addition, there was a negative correlation between the WASI-verbal score and functional connectivity in the OA>YA regions with the cognitive-auditory network (r = −0.24, P < 0.005, q[FDR] = .05; Figure 5C). No other correlations reached significance after FDR correction. When we analyzed the data without the ICV covariate, the results remained significant except that the negative correlation between the verbal fluency score and functional connectivity in the OA>YA regions with the cognitive-auditory network was only significant with FDR = 0.10 (r = −0.20, P < 0.05, q[FDR] = .10). Overall, our findings suggest that greater out-of-network connectivity was associated with poorer cognitive performance or no performance benefits.
Lastly, we conducted an exploratory post-hoc analysis to examine whether the within-DMN connectivity and outside-cognitive-auditory connectivity contributed to the relationship between age and cognition. We used multiple regression analyses including a cognitive score as the main DV, age as the main IV, and functional connectivity within DMN, sex and ICV as other IVs. The results showed that functional connectivity within DMN did not explain the relationship between age and any of the cognitive scores. We also ran multiple regression analyses for the cognitive-auditory network and found that the positive relationship between age and verbal fluency scores (β=0.279, p=0.009) was partially explained by functional connectivity outside the cognitive-auditory network (β=−0.264, p=0.009). Similarly, the positive relationship between age and WASI vocabulary scores (β =0.497, p<0.001) was partially explained by functional connectivity outside the cognitive-auditory network (β =−0.296, p=0.002). Both results suggest that age-related improvement in verbal skills is in part explained by less functional connectivity outside the cognitive-auditory network.
4. Discussion
This study examined the effects of age on various resting-state networks. Consistent with our hypothesis, older adults showed less functional connectivity in all identified cognitive networks, namely the DMN, cognitive-auditory, cognitive-speech, right and left fronto-parietal networks. In addition, age-related decline was observed in three visual and three motor/visuospatial networks. However, age-related decline was not found in any of the identified emotion networks. Lower functional connectivity within the DMN was correlated with lower cognitive performance. In addition, older adults showed greater functional connectivity in regions outside several networks, and such out-of-network connectivity was associated with poorer cognitive performance or no performance benefits across groups.
Our findings are consistent with previous evidence suggesting age-related disruption in the DMN (Allen et al., 2011; Andrews-Hanna et al., 2007; Chan et al., 2014; Damoiseaux et al., 2008; Esposito et al., 2008; Koch et al., 2010; Mevel et al., 2013). In addition, our observation of age-related decline in most networks associated with cognitive function but not in those associated with emotional function supports the idea that normal aging is less associated with declines in emotional functioning than with declines in cognitive functioning (Mather, 2012, 2016; D. C. Park, 2000; Salthouse, 2010). No age-related decline was observed in any of the identified emotional networks, one of which (NKI 15) includes the anterior cingulate cortex (ACC), the orbitofrontal cortex (OFC) and the ventromedial prefrontal cortex, consistent with previous evidence for structural and functional preservation of these regions with age (Fjell et al., 2009; Nashiro et al., 2013; Salat et al., 2001). The ACC, ventromedial prefrontal cortex and OFC have been implicated in emotion regulation; thus, preserved functional connectivity at rest may be part of the explanation for intact emotion regulation in older adults (together with the preservation of the limbic system, including the amygdala; Mather & Carstensen, 2005; Mather, 2012, 2016; Nashiro et al., 2012; Sakaki et al., 2013; St Jacques et al., 2010). Our findings may provide important implications for cognitive enhancement in older adults. For example, preserved emotional function may be able to facilitate older adults’ cognitive performance, as found in our previous study (Nashiro & Mather, 2011). Previous research also suggest that mindfulness training, which focuses on improving emotional well-being, can enhance executive function (Tang, Yang, Leve, & Harold, 2012). Thus, mindfulness training may be effective for older adults’ cognitive improvement.
Our results were consistent with previous findings on age-related increase on functional connectivity outside networks (Chan et al., 2014; Geerligs, Maurits, Renken, & Lorist, 2014; Song et al., 2014). Our study further demonstrated that this age-related increase was associated with poorer cognitive performance or no performance benefits. We observed that less functional connectivity outside the cognitive-auditory network was associated with better verbal fluency and WASI vocabulary. Although we called it ‘the cognitive-auditory network’ for the sake of conciseness in this paper, Laird et al. (2011) found that this network was involved in a wide range of tasks including those associated with audition and speech. Speech-related tasks may involve verbal skills; thus, our results may suggest that increased functional connectivity outside this network is particularly disadvantageous for cognitive tasks involved in speech or verbal skills. Overall, our findings were in line with previous findings on dedifferentiation in the aging brain, which suggests that as we get older, cortical response becomes less specific to some stimulus categories or tasks (Grady et al., 1992; Park et al., 2004; Park et al., 2012; Voss et al., 2008). Previous studies showed this dedifferentiation effect in the ventral visual cortex during passive viewing of faces and places (Park et al., 2004) and the occipitotemporal cortex and superior parietal cortex during object and spatial tasks (Grady et al., 1992). Here, we observed a similar phenomenon during resting state across different types of networks, consistent with previous findings on reduced segregation of resting state brain networks in aging (Chan et al., 2014).
There are several limitations of this study. Although we found nominally significant correlations between resting-state connectivity strength and cognitive scores, many of them did not reach significance after FDR correction. One possible reason is that functional connectivity strength may represent broader cognitive function than that was measured in the standard cognitive tests in this study. We also need to be cautious about interpreting the absence of age-related decline in the emotion networks. Due to the lack of behavioral measures related to emotion, we were unable to directly test whether functional connectivity strength in the emotion networks is associated with better emotional function. However, an exploratory analysis using the geriatric depression scale (GDS), which was only available in a subset of the older participants (n = 50), suggested that greater functional connectivity in one emotion network (NKI 6) was associated with lower depression (Supplementary material Figure 2). Another limitation was that we failed to find a reliable match for the salience network in our dataset and were unable to examine age-related changes in this network. Future studies should use a larger sample to test the brain-behavior link associated with emotion more thoroughly. In addition, the acquisition time in this study was relatively short (5 min); future studies can be improved by using data with longer acquisition time. Although we had the same number of younger and older adults, it is possible that group-level ICA components were driven more by the younger adults. Future studies with more older adults could improve the selection of networks.
In summary, the key findings of the present study were 1) that older adults showed less functional connectivity than younger adults within all identified resting-state networks except for the emotion networks, and 2) that age-related decline within networks as well as age-related increases in outside-network connectivity were associated with poorer cognitive performance or no performance benefits. The latter finding was consistent with the dedifferentiation hypothesis of aging, suggesting that less specificity in resting state functional connectivity may be an important biomarker for age-related cognitive decline. Future research on resting state as well as task-based brain activity using a lifespan sample may provide critical information about the aging brain and help advance theories of cognitive aging.
Supplementary Material
Supplementary Figure 1. Excluded components. The top image in A–D is an NKI component that was matched with two NKI networks shown at the bottom. The correlation values between NKI and Laird et al.’s networks were shown above the two bottom images. In our analyses, we used the images with red frames as a match, as they had higher correlation values. The networks with grey frames were disregarded in our analyses due to a lower correlation value. Laird 19 and 20 shown in E and F were noise components and were not used in our analyses.
Supplementary Figure 2. The relationship between the geriatric depression scale (GDS) and functional connectivity within an emotion network. An exploratory analysis using a subset of the older participants’ data (n=50) was performed. We ran a multiple regression analysis including GDS as a dependent variable, functional connectivity within an emotion network as a main independent variable (IV), and sex and ICV as other IVs. The results suggested that greater functional connectivity in one emotion network (NKI 6) was associated with lower depression scores (R2[full model]=0.11; p[full model]=0.153; p[partial contribution of functional connectivity]=0.039; p[partial contribution of sex]=0.678; p[partial contribution of ICV]=0.492). When age was included as another independent variable, the result remained significant (R2[full model]=0.11; p[full model]=0.261; p[partial contribution of functional connectivity]=0.049; p[partial contribution of sex]=0.747; p[partial contribution of ICV]=0.501). However, age did not explain this relationship (p[partial contribution of age]=0.808).
Highlights.
Age-related decline was observed in cognitive, visual, and motor/visuospatial resting state networks.
Age-related decline in functional connectivity was not found in emotion networks.
Relative to younger adults, older adults showed increased functional connectivity in regions outside networks.
Reduced within-network connectivity and increased out-of-network connectivity were associated with lower cognitive performance.
Acknowledgments
This work was supported by grants from the National Institute on Aging (R01 AG025340; PI Mather) with additional support from NIH R01 AG041915 and R01 AG040060 (PI Paul Thompson), and P50 AG05142 (PI Helena Chui; Project leader Braskie).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Calhoun VD. A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience. 2011;5:2. doi: 10.3389/fnsys.2011.00002. http://doi.org/10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. http://doi.org/10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. (Series B (Methodological)).Source Journal of the Royal Statistical Society. 1995;57(1):289–300. Retrieved from http://www.jstor.org/stable/2346101. [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Milham MP. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4734–9. doi: 10.1073/pnas.0911855107. http://doi.org/10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. http://doi.org/10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences. 2014;111(46):E4997–E5006. doi: 10.1073/pnas.1415122111. http://doi.org/10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Rombouts SaRB. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. http://doi.org/10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Dong L, Shen Y, Lei X, Luo C, Li Q, Wu W, Li C. The heterogeneity of aging brain: altered functional connectivity in default mode network in older adults during verbal fluency tests. Chinese Medical Journal. 2012;125(4):604–610. [PubMed] [Google Scholar]
- Douaud G, Groves AR, Tamnes CK, Westlye LT, Duff EP, Engvig A, Johansen-Berg H. A common brain network links development, aging, and vulnerability to disease. Proceedings of the National Academy of Sciences. 2014;111(49):17648–17653. doi: 10.1073/pnas.1410378111. http://doi.org/10.1073/pnas.1410378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Aragri A, Pesaresi I, Cirillo S, Tedeschi G, Marciano E, Di Salle F. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magnetic Resonance Imaging. 2008;26(7):905–913. doi: 10.1016/j.mri.2008.01.045. http://doi.org/10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Filippini N, Macintosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex (New York, NY : 1991) 2009;19(9) doi: 10.1093/cercor/bhn232. 2001–12. http://doi.org/10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. http://doi.org/10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping. 2005;26(1):15–29. doi: 10.1002/hbm.20113. http://doi.org/10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Maurits NM, Renken RJ, Lorist MM. Reduced specificity of functional connectivity in the aging brain during task performance. Human Brain Mapping. 2014;35(1):319–330. doi: 10.1002/hbm.22175. http://doi.org/10.1002/hbm.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Haxby JV, Horwitz B, Schapiro MB, Rapoport SI, Ungerleider LG, Herscovitch P. Dissociation of object and spatial vision in human extrastriate cortex: age-related changes in activation of regional cerebral blood flow measured with [(15) o]water and positron emission tomography. Journal of Cognitive Neuroscience. 1992;4(1):23–34. doi: 10.1162/jocn.1992.4.1.23. http://doi.org/10.1162/jocn.1992.4.1.23. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. http://doi.org/10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Smith SM. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage. 2014;95:232–247. doi: 10.1016/j.neuroimage.2014.03.034. http://doi.org/10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Shen X, Scheinost D, Papademetris X, Constable RT. Intrinsic brain connectivity related to age in young and middle aged adults. PloS ONE. 2012;7:9. doi: 10.1371/journal.pone.0044067. http://doi.org/10.1371/journal.pone.0044067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hsieh WJ, Lee PL, Peng LN, Liu LK, Lee WJ, Lin CP. Age-Related Changes in Resting-State Networks of A Large Sample Size of Healthy Elderly. CNS Neuroscience & Therapeutics. 2015;21(10):817–825. doi: 10.1111/cns.12396. http://doi.org/10.1111/cns.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. http://doi.org/10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Buerger K, Bokde aLW, Hampel H, Meindl T. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? NeuroImage. 2010;51(1):280–287. doi: 10.1016/j.neuroimage.2009.12.008. http://doi.org/10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Fox PT. Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience. 2011;23(12):4022–37. doi: 10.1162/jocn_a_00077. http://doi.org/10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. The emotion paradox in the aging brain. Annals of the New York Academy of Sciences. 2012;1251:33–49. doi: 10.1111/j.1749-6632.2012.06471.x. http://doi.org/10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. The Affective Neuroscience of Aging. Annual Review of Psychology. 2016;67(1):213–238. doi: 10.1146/annurev-psych-122414-033540. http://doi.org/10.1146/annurev-psych-122414-033540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. http://doi.org/10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Meier TB, Desphande AS, Vergun S, Nair VA, Song J, Biswal BB, Prabhakaran V. Support vector machine classification and characterization of age-related reorganization of functional brain networks. NeuroImage. 2012;60(1):601–13. doi: 10.1016/j.neuroimage.2011.12.052. http://doi.org/10.1016/j.neuroimage.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. http://doi.org/10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevel K, Landeau B, Fouquet M, La Joie R, Villain N, Mézenge F, Chételat G. Age effect on the default mode network, inner thoughts, and cognitive abilities. Neurobiology of Aging. 2013;34(4):1292–1301. doi: 10.1016/j.neurobiolaging.2012.08.018. http://doi.org/10.1016/j.neurobiolaging.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Mowinckel AM, Espeseth T, Westlye LT. Network-specific effects of age and in-scanner subject motion: a resting-state fMRI study of 238 healthy adults. NeuroImage. 2012;63(3):1364–1373. doi: 10.1016/j.neuroimage.2012.08.004. http://doi.org/10.1016/j.neuroimage.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Nashiro K, Mather M. Effects of Emotional Arousal on Memory Binding in Normal Aging and Alzheimer’s Disease. The American Journal of Psychology. 2011;124(3):301–312. doi: 10.5406/amerjpsyc.124.3.0301. http://doi.org/10.5406/amerjpsyc.124.3.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Mather M. Age differences in brain activity during emotion processing: reflections of age-related decline or increased emotion regulation? Gerontology. 2012;58(2):156–63. doi: 10.1159/000328465. http://doi.org/10.1159/000328465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Nga L, Mather M. Age-related similarities and differences in brain activity underlying reversal learning. Frontiers in Integrative Neuroscience. 2013;7:37. doi: 10.3389/fnint.2013.00037. http://doi.org/10.3389/fnint.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S. Decreased Functional Connectivity by Aging Is Associated with Cognitive Decline. Journal of Cognitive Neuroscience. 2012;24(11):2186–2198. doi: 10.1162/jocn_a_00269. http://doi.org/10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- Park DC. Cognitive Aging: A Primer. Taylor & Francis; 2000. Retrieved from http://books.google.com/books?hl=en&lr=&id=LXJ3SeXoHvoC&pgis=1. [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(35):13091–5. doi: 10.1073/pnas.0405148101. http://doi.org/10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Kennedy KM, Rodrigue KM, Bischof GN, Huang CM, Park DC. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2012;32(6):2154–8. doi: 10.1523/JNEUROSCI.4494-11.2012. http://doi.org/10.1523/JNEUROSCI.4494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. http://doi.org/10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Nga L, Mather M. Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults’ memory. Journal of Cognitive Neuroscience. 2013;25(8):1206–24. doi: 10.1162/jocn_a_00392. http://doi.org/10.1162/jocn_a_00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Archives of Neurology. 2001;58(9):1403–8. doi: 10.1001/archneur.58.9.1403. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11559311. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. http://doi.org/10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. Journal of the International Neuropsychological Society. 2010;16(5):754–760. doi: 10.1017/S1355617710000706. http://doi.org/10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg aF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. http://doi.org/10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. http://doi.org/10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Supplement 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. http://doi.org/10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. http://doi.org/10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Song J, Birn RM, Boly M, Meier TB, Nair VA, Meyerand ME, Prabhakaran V. Age-Related Reorganizational Changes in Modularity and Functional Connectivity of Human Brain Networks. Brain Connectivity. 2014;4(9):662–676. doi: 10.1089/brain.2014.0286. http://doi.org/10.1089/brain.2014.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Viviano JD, Schacter DL, Addis DR, Wong AT, Buckner RL. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiology of Aging. 2016;45:149–160. doi: 10.1016/j.neurobiolaging.2016.05.020. http://doi.org/10.1016/j.neurobiolaging.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques P, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: a network analysis of fMRI data. Neurobiology of Aging. 2010;31(2):315–327. doi: 10.1016/j.neurobiolaging.2008.03.012. http://doi.org/10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Yang L, Leve LD, Harold GT. Improving Executive Function and its Neurobiological Mechanisms through a Mindfulness-Based Intervention: Advances within the Field of Developmental Neuroscience. Child Development Perspectives. 2012;6(4):361–366. doi: 10.1111/j.1750-8606.2012.00250.x. http://doi.org/10.1111/j.1750-8606.2012.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Molecular Psychiatry. 2012;17(5):549–558. doi: 10.1038/mp.2011.81. http://doi.org/10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. http://doi.org/10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. http://doi.org/10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Chaddock L, Prakash RS, Colcombe SJ, Morris KS, Kramer AF. Dedifferentiation in the visual cortex: an fMRI investigation of individual differences in older adults. Brain Research. 2008;1244:121–31. doi: 10.1016/j.brainres.2008.09.051. http://doi.org/10.1016/j.brainres.2008.09.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Excluded components. The top image in A–D is an NKI component that was matched with two NKI networks shown at the bottom. The correlation values between NKI and Laird et al.’s networks were shown above the two bottom images. In our analyses, we used the images with red frames as a match, as they had higher correlation values. The networks with grey frames were disregarded in our analyses due to a lower correlation value. Laird 19 and 20 shown in E and F were noise components and were not used in our analyses.
Supplementary Figure 2. The relationship between the geriatric depression scale (GDS) and functional connectivity within an emotion network. An exploratory analysis using a subset of the older participants’ data (n=50) was performed. We ran a multiple regression analysis including GDS as a dependent variable, functional connectivity within an emotion network as a main independent variable (IV), and sex and ICV as other IVs. The results suggested that greater functional connectivity in one emotion network (NKI 6) was associated with lower depression scores (R2[full model]=0.11; p[full model]=0.153; p[partial contribution of functional connectivity]=0.039; p[partial contribution of sex]=0.678; p[partial contribution of ICV]=0.492). When age was included as another independent variable, the result remained significant (R2[full model]=0.11; p[full model]=0.261; p[partial contribution of functional connectivity]=0.049; p[partial contribution of sex]=0.747; p[partial contribution of ICV]=0.501). However, age did not explain this relationship (p[partial contribution of age]=0.808).


