Abstract
Objectives:
This report reviews the carcinogenicity of multi-walled carbon nanotubes (MWCNTs) in experimental animals, concentrating on MWNT-7, a straight fibrous MWCNT.
Methods:
MWCNTs were administered to mice and rats by intraperitoneal injection, intrascrotal injection, subcutaneous injection, intratracheal instillation and inhalation.
Results:
Intraperitoneal injection of MWNT-7 induced peritoneal mesothelioma in mice and rats. Intrascrotal injection induced peritoneal mesothelioma in rats. Intratracheal instillation of MWCNT-N (another straight fibrous MWCNT) induced both lung carcinoma and pleural mesothelioma in rats. In the whole body inhalation studies, in mice MWNT-7 promoted methylcholanthrene-initiated lung carcinogenesis. In rats, inhalation of MWNT-7 induced lung carcinoma and lung burdens of MWNT-7 increased with increasing concentration of airborne MWNT-7 and increasing duration of exposure.
Conclusions:
Straight, fibrous MWCNTs exerted carcinogenicity in experimental animals. Phagocytosis of MWCNT fibers by macrophages was very likely to be a principle factor in MWCNT lung carcinogenesis. Using no-observed-adverse-effect level-based approach, we calculated that the occupational exposure limit (OEL) of MWNT-7 for cancer protection is 0.15 μg/m3 for a human worker. Further studies on the effects of the shape and size of MWCNT fibers and mode of action on the carcinogenicity are required.
Keywords: Inhalation exposure, Lung burden, Lung carcinogenicity, Mesothelioma, Multi-walled carbon nanotube, MWCNT
1. Introduction
Nanotechnology is one of the most important fields of technology, at present. The exceptional physico-chemical properties of nanomaterials give them a wide range of industrial and commercial applications. Advances in nanotechnology are continually being made and use of nanomaterials to improve product performance has tremendous potential in the near future. However, due to the rapid pace of nanotechnology development, comprehensive risk assessment of the majority of nanomaterials has not been carried out. There are central concerns that nanomaterials may harbor nanomaterial-specific toxicities in humans.
Among the 11 kinds of industrial nanomaterials for which Organisation for Economic Co-operation and Development (OECD)1) has compiled dossiers, carbon nanotubes (CNTs) are one of the most widely used; the use of CNTs have increased rapidly in recent years and their use is expected to increase even more in the near future. CNTs are cylinder structures composed of rolled up one atom-thick sheets of graphite with single, double, and multiple layers of graphite sheets used to generate single-walled nanotubes, double-walled nanotubes, and multi-walled nanotubes, respectively. Generally CNTs are less than 100 nm in diameter, but micrometers in length. CNTs are classified into 2 types, single-walled carbon nanotubes and multi-walled carbon nanotubes (MWCNTs), with double-walled nanotubes being classified as MWCNTs. MWCNTs are classified into two subtypes, straight and tangled. The straight type of MWCNTs is fibrous and firm, and the tangled type is thin enough to bend and self-assemble into tangled aggregates.
Humans can be exposed to CNTs through inhalation and dermal exposure, and potentially through contaminated food and water. In particular, there is the potential of exposure to airborne CNTs at the workplace. It is a central concern that exposure to MWCNTs might cause asbestos-like lung diseases, such as lung cancer, lung fibrosis, pleural plaque, and pleural mesothelioma in humans. The straight type of MWCNTs is firm fiber with high aspect ratios and may persist in tissues for prolonged periods of time, these MWCNTs share properties with asbestos. The Stanton and Pott hypothesis2,3) states that biopersistent fibers of defined ranges of diameter and length cause cancer irrespective of their physicochemical nature, simply because they are fibers. This is known as the fiber paradigm and is an important consideration for safety assessment, particularly in the carcinogenicity of MWCNTs. Indeed, Takagi et al.4) first reported that peritoneal mesotheliomas were induced by intraperitoneal injection of one type of fibrous straight MWCNTs (Mitsui MWCNT-7, known as MWNT-7) to p53 heterozygous mice. Subsequently, several studies reported mesotheliomas developed in mice or rats by intraperitoneal or intrascrotal injection of this MWCNT5-8). Moreover, Poland et al.9) reported that intraperitoneal injection of long, straight type MWCNT fibers or asbestos in mice, but not short or tangled MWCNTs or short asbestos fibers, resulted in inflammation and the formation of granulomatous lesions in the peritoneal cavity: in this study, mice were killed 24 hours and 7 days after administration of MWCNT fibers. These results indicate that exposure to MWCNTs can result in asbestos-like pathologic changes in experimental animals and strongly suggest that exposure to MWCNTs may result in asbestos-like harmful effects, such as lung carcinoma or pleural mesothelioma, in humans.
Generally carcinogens in humans have been identified by epidemiological data. However, there are no epidemiological data on exposure to MWCNTs and carcinogenicity in humans, possibly due to the long latency period of cancers in humans and the relatively short period of time that MWCNTs have been in production, approximately 15 years. Nevertheless, based on theoretical considerations such as the Stanton and Pott hypothesis2,3) and the harmful effect of MWCNT exposure in experimental animals, the Ministry of Health, Labour and Welfare of Japan was concerned about adverse health effects in humans exposed to MWCNTs. Accordingly, it was decided to further examine carcinogenicity of these nanomaterials in rodents. Consequently, the Japan Bioassay Research Center (JBRC) became involved in examining the carcinogenicity of airborne MWCNTs in rats. There are four important issues to be considered regarding studies of the carcinogenicity of airborne MWCNTs: (1) development of an aerosol MWCNT generator for inhalation exposure of rats to airborne MWCNTs; (2) decision of the highest concentration of inhaled MWCNT in the carcinogenicity study; (3) development of a sensitive method to determine the retention and distribution of inhaled MWCNT in target organs; (4) performance of the inhalation study under OECD carcinogenicity testing guidelines. This review summarizes results of the carcinogenicity studies of MMCNTs in experimental animals carried out at JBRC and other laboratories.
2. Carcinogenicity of MWCNTs
1) Carcinogenicity of MWCNTs by Inhalation Exposure
There are two methods of exposure of rats and mice to test materials in inhalation studies: whole body and nose-only inhalation exposures10). The former allows free movement of the animals, resulting in minimal stress, while the later severely restricts movement by placing animals in narrow tubes, resulting in more severe stress. In addition, long continuous exposure to MWCNTs may itself increase stress to the animals. Overall, whole body inhalation exposure results in less stress to the animal and is consequently recommended as the optimal exposure method for carcinogenicity studies of air borne materials using rats and mice. This is especially true for long term studies in which the stress caused by restraining the test animal, added to the stress caused by exposure to MWCNTs, could become a confounding factor in the study.
There are two systems for generation of MWCNTs for inhalation exposure studies. One is a dry powder system and the other is the liquid suspension system. The former can generate well-dispersed dry MWCNTs, which is similar to human inhalation exposure. The later disperses MWCNTs in suspension with water or saline, which could have an impact on the surface characteristics of the MWCNT fibers.
A) Methods for Preparing MWCNTs for Inhalation Carcinogenicity Studies
MWCNTs are very fine and light, and human exposure is most likely to be by inhalation. It is therefore essential to conduct animal inhalation studies using dry air. There are, however, difficulties due to technical limitations in generating MWCNT aerosols suitable for toxicological studies in animals. The major problem is that MWCNTs do not retain their original single form at high concentrations owing to agglomeration. Therefore, an aerosol generator that is able to separate agglomerates from small structures and single fibers is required in order to provide single fiber MWCNTs at various exposure concentrations to the inhalation chamber. In addition, the generator must allow aerosols to be consistently introduced into the inhalation chamber throughout long exposure periods.
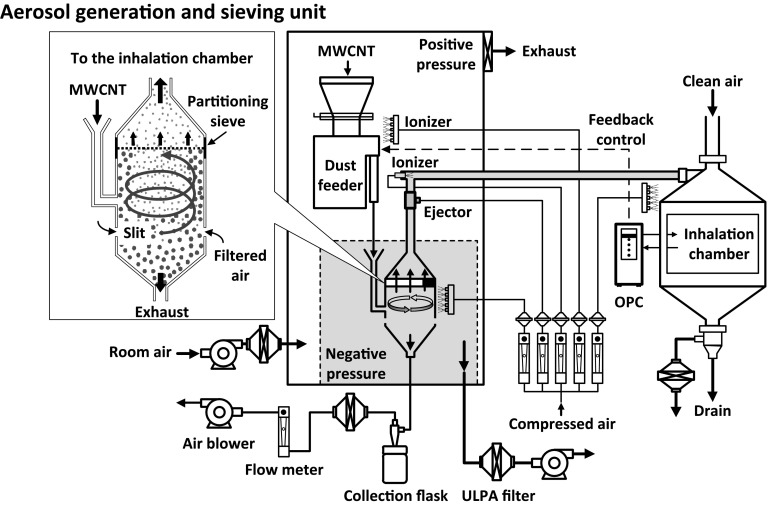
Because there were no acceptable aerosol generators for two-year whole body inhalation exposure carcinogenicity studies of MWCNT in rats at the start of the study, the JBRC first had to develop a new system of MWCNT fiber generation11-13). The layout of the aerosol generation and exposure system, which is a dry powder system, is illustrated in Fig. 111). The cyclone sieve method (our aerosol generation method) was used to generate MWCNT aerosols: in our experiments described in this review we used MWNT-7 purchased from Hodogaya Chemical, Co. Ltd (Tokyo, Japan). The aerosol generator is composed of an aerosol generation and sieving unit, a concentration control unit, and a data-recording device. The aerosol generation and sieving unit consists of ionizers, a cylindrical container with a partitioning sieve, and a solenoid impactor. A dust feeder unit supplies bulk MWNT-7 to the aerosol generator. The aerosol generator dry-aerosolizes the MWNT-7 with an upward spiraling airstream by introducing high speed air from slits at the bottom of the generator. The particles were classified by gravitational and centrifugal forces, limiting particle size with a sieve (pore size, 53 μm), to provide the inhalation chamber with size-limited MWNT-7 aerosols. Chamber atmosphere samples are taken from the animals' breathing zone. Particle concentrations of MWNT-7 in the chambers were monitored in real-time with an optical particle controller (OPC). The mass-concentrations of MWNT-7 were determined gravimetrically by collecting aerosols on Teflon-binder filters for each target exposure concentration. The mass per particle (the K value) was calculated based on the particle concentration data (particles/m3) collected by the OPC and the gravimetric results (mass/m3). Using this K-value, which is determined every 2 weeks, particle concentrations were converted into mass-concentrations for each group during the exposure periods. The chamber MWNT-7 concentrations were held constant by controlling the movement of the dust feeder using a feedback loop from the OPC: when the chamber concentration rose above the upper limit of a designated concentration range, the dust feeder stops supplying bulk MWNT-7 to the aerosol generator. When the chamber concentration fell below the lower limit, the dust feeder resumed supplying bulk MWNT-7 to the aerosol generator. The size distribution of the fibers was ascertained using a micro-orifice uniform deposit cascade impactor, and the morphology of the fibers examined by scanning electron microscopy (SEM).
Fig. 1.
Aerosol generation and inhalation exposure system. MWNT-7 aerosol is generated by the cyclone sieve method and sent to the inhalation chamber.
McKinney et al14) developed a computer controlled acoustic aerosol generation system for dry MWCNT powders, used for acute and carcinogenicity studies in mice15,16). Bulk MWCNT was placed on flexible latex diaphragms in a cylindrical acrylic chamber, and a loudspeaker driven by a computer-generated analog signal placed below the bottom diaphragm of the cylinder. The exposure chamber was monitored for concentration and particle size distribution. The MWCNT was aerosolized and dispersed in a respirable size range. Mass concentration was tightly controlled and maintained stably by a feedback system over the exposure period. In the Sargent et al. study16), the mass median aerodynamic diameter was 1.59 μm with a geometric standard deviation of 1.69, and the count mode aerodynamic diameter was about 400 nm. The shapes generated by this system were similar to those found in a MWCNT manufacturing plant.
For other aerosol generators previously developed for toxicity studies of MWCNTs: see Fujitani et al. 200917), Kim et al. 201218), Ma-Hock et al. 200719), Mitchell et al. 200720), Myojo et al. 200921), Pauluhn and Thiel 200722), Ryman-Rasmussen et al. 200923), and Taquahashi et al. 201324).
B) Setting the Highest Concentration and the Range of MWCNTs in Inhalation Carcinogenicity Studies
There are two requirements for setting the highest concentration of inhaled MWCNTs in inhalation carcinogenicity studies. One is determining the maximum tolerated dose (MTD) : The MTD is the highest concentration that does not alter normal longevity of animals from toxic effects other than carcinogenicity and causes no more than a 10% weight decrement compared to concurrent controls. The other requirement is that the highest concentration does not overload the respiratory physiology of the test animal. Therefore, subchronic inhalation toxicity studies were necessary to decide the highest concentration of the MWCNT aerosol to be used in the inhalation carcinogenicity study.
The sub-maximum concentrations of MWCNT used in carcinogenicity studies also need to be determined. The middle and low concentration levels should be set to confirm concentration-dependence of potential carcinogenic effects. The overall range of MWCNT concentrations used should be selected to allow the identification of target organs, demonstrate a well-defined concentration-response and clear proof of carcinogenicity in animals for extrapolation in humans, and contribute to a meaningful evaluation of the results.; If the material is not carcinogenic, the range of concentrations used should convincingly demonstrate lack of carcinogenicity in the experimental animals used in the study. Therefore, preliminary studies are necessary to determine the range, as well as the MTD, of MWCNT aerosol concentrations to be used in long-term carcinogenicity studies.
In our inhalation carcinogenicity studies of MWNT-7 in rats, we planned an initial 2-week inhalation toxicity study, followed by a 13-week inhalation toxicity study to decide the MWNT-7 aerosol concentrations for use in our 2-year inhalation carcinogenicity study. In the 2-week inhalation toxicity study, 6-week-old male and female F344 rats were exposed to MWNT-7 at concentrations of 0, 0.2, 1, and 5 mg/m3 for 6 h/d, 5 d/wk using the whole body exposure system described above (cyclone sieve system using dry air)13). One-half of the animals were necropsied at the end of the 2-week exposure period and the remaining animals were necropsied 4 weeks after end of the exposure period. Granulomatous and inflammatory changes were observed until end of the 4-week recovery period, but inflammatory changes had largely regressed. The no-observed-adverse-effect level (NOAEL) was 0.2 mg/m3.
In our 13-week inhalation toxicity study, 6-week-old male and female F344 rats were exposed by inhalation to MWNT-7 using the same exposure conditions as in the 2-week inhalation toxicity study12). There were no significant differences in the body weights of the exposed animal compared to the controls. Lung burdens at concentrations of 0.2, 1, and 5 mg/m3 after 13 weeks were 3.23, 21.2, and 120.3 μg/left lung in males and 2.30, 13.7, and 80.3 μg/left lung in females, showing a concentration-dependent increase in lung burden. The lung weights of both sexes increased 1.2 fold in the 1 mg/m3 group and 1.3 fold in the 5 mg/m3 group compared to the control. In the bronchoalveolar lavage fluid (BALF), inflammatory changes were observed in all three exposed groups of both sexes and were concentration-dependent. However, there were no differences in the number of alveolar macrophages among the groups. Alveolar macrophages had a wide range of sizes, had swelling with foamy cytoplasm, and were found to have phagocytosed MWCNT fibers in all exposed rats. Histopathologically, granulomatous lesions were induced at concentrations of 1 and 5 mg/m3 in females and in all exposed male groups. Focal fibrosis in the alveolar wall was observed in both sexes at 1 and 5 mg/m3. The low-observed-adverse-effect level was 0.2 mg/m3. Based on the overall results of this study, we concluded that 2 mg/m3 is at or below the MTD and would not cause excessive overload of the respiratory physiology of the rats used for the 2-year inhalation carcinogenicity study. Therefore, 0.2 and 0.02 mg/m3 would be appropriate middle and low concentrations.
The National Institute for Occupational Safety and Health (NIOSH), USA investigated the pulmonary toxicity of inhaled MWNT-7 in mice, to determine appropriate doses for use in their 2-stage (initiation/promotion) lung carcinogenesis study of mice exposed to MWNT-7 by inhalation15). Male mice were exposed to MWNT-7 aerosol at a concentration of 10 mg/m3, 5 h/d for 2, 4, 8, or 12 days using the whole-body inhalation exposure system described in McKinney et al14). The MWNT-7 lung burden was linearly related to exposure duration, ranging from 6.6 to 30.6 μg/lung after 2 and 12 days of exposure. MWNT-7 was observed in the bronchioles and proximal alveolar region and also observed at the pleural wall. Histopathologic examination of the lung showed bronchiolocentric inflammation, bronchiolar epithelial hyperplasia and hypertrophy, fibrosis, and rare pleural penetration of MWNT-7.
Other subchronic inhalation studies of MWCNT to examine the general toxicity of inhaled MWCNT in rats were carried out by Ma-Hock et al.25) and Pauluhn26).
C) Carcinogenicity Studies of Inhalation Exposure to MWCNT
We carried out a 2-year inhalation carcinogenicity study of MWNT-7 of rats27) using the dry aerosol generation and exposure system (cyclone sieve method) described above11). Based on the results of our 13-week inhalation toxicity study12), male and female F344 rats, 6-week-old at the commencement of exposure, were exposed to MWNT-7 at concentrations of 0, 0.02, 0.2, and 2 mg/m3 for 6 h/d, 5 d/wk and 104 weeks. We used two lots of bulk MWNT-7: lot No. 071223 fibers had an average width of 83.8 nm and length of 5.2 μm, and lot No. 080126 fibers had an average width of 90.7 nm and length of 5.7 μm. The MWNT-7 contained iron, chromium, and nickel at 4,400, 48, and 17 ppm, respectively28). The MWNT-7 aerosols to which the rats were exposed were maintained at the target concentrations and the MWNT-7 fibers were clearly dispersed (Figure 2, 3a). There were no differences of survival rates between the control and exposed groups of either sex. No growth retardation was found in any of the male or female groups during the experiment, and the relative body weights of the 0, 0.02, 0.2, and 2 mg/m3-exposed animals at the end of the experiment were similar among the male groups and among the female groups. Table 1 shows induction of tumors in the lung of rats. Exposure to MWNT-7 induced significant increases with concentration-dependent manner of bronchiolo-alveolar carcinoma, total carcinomas, and total adenomas and/or carcinoma in both male and female rats. In the groups exposed to 0.2 mg/m3, only males had a significant increase in lung tumor incidence, indicating a gender difference in the lung carcinogenicity of inhaled MWNT-7. Carcinomas were mainly bronchiolo-alveolar carcinomas. In addition, adenosquamous carcinoma, adenocarcinoma and squamous cell carcinoma, which very seldom develop spontaneously, were induced. Notably, the MWNT-7-induced bronchiolo-alveolar carcinomas were often accompanied by proliferative fibrous connective tissue, which was not present in the spontaneous bronchiolo-alveolar carcinoma that developed in the control rat. Moreover, pre-neoplastic, bronchiolo-alveolar hyperplasia and atypical hyperplasia were significantly increased in the lungs of both sexes. Development of this pre-neoplastic lesion was more striking in males than in females. Alveolar fibrosis and granulomatous changes were also significantly increased in the lungs of both sexes.
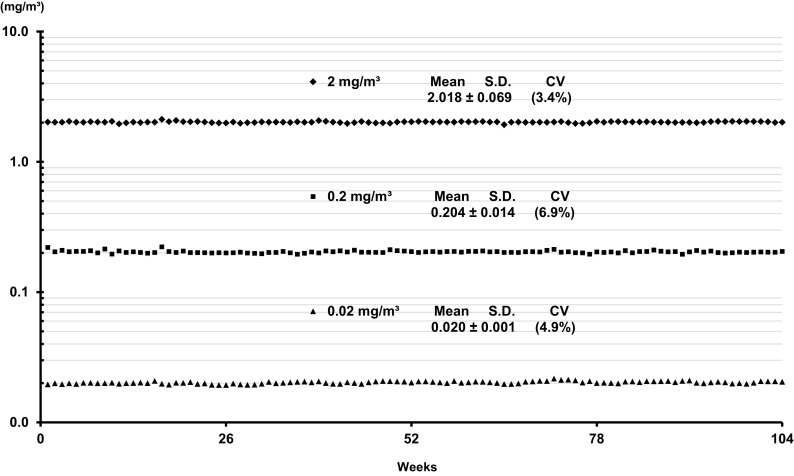
Fig. 2.
MWNT-7 concentrations in the inhalation chambers throughout the 104 week experimental period. MWNT-7 aerosols were constantly generated for each concentration group during the exposure period (6 h/day, 5 days/week, 104 weeks).
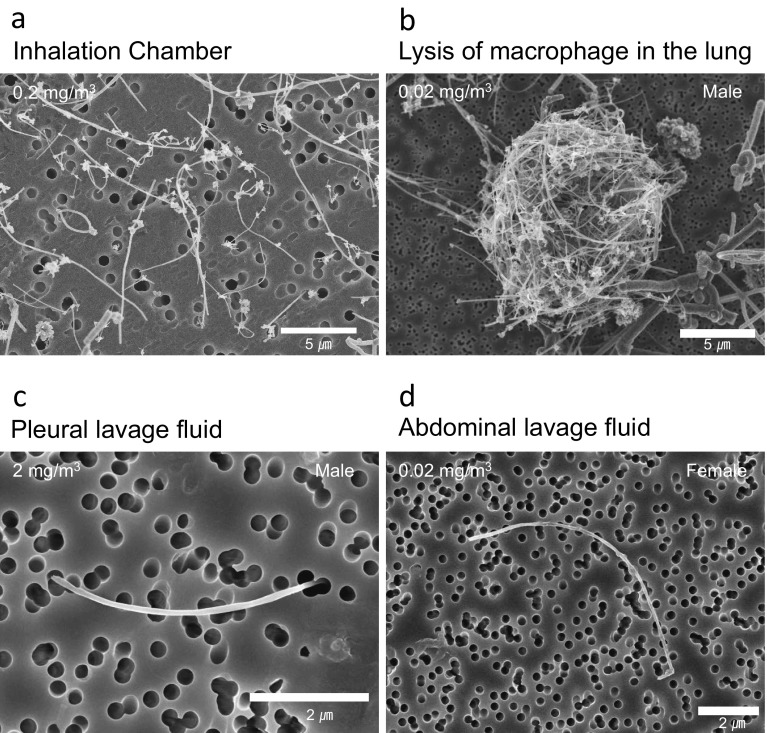
Fig. 3.
Scanning electron microscope (SEM) images of MWNT-7 fibers. a: MWNT-7 fibers at a concentration of 0.2 mg/m3 in the inhalation chamber. MWNT-7 fibers were well dispersed. b: MWNT-7 fibers after digestion of an alveolar macrophage. MWNT-7 fibers form a cocoon-like mass. c: A representative MWNT-7 fiber in the pleural lavage fluid. The MWNT-7 fiber is single, long, and straight. d: A representative MWNT-7 fiber in the peritoneal cavity. The MWNT-7 fiber is single, long, and straight.
Table 1.
Incidences of Tumor in the Lung of Rats Exposed to MWNT-7 for 104 Weeks
| Male | Peto's test | Female | Peto's test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group (mg/m3) | 0 | 0.02 | 0.2 | 2 | 0 | 0.02 | 0.2 | 2 | ||||
| Number of animals examined | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | ||||
| Lung | ||||||||||||
| Bronchiolo-alveolar adenoma | 1 | 1 | 7* | 5 | 3 | 1 | 4 | 3 | ||||
| Bronchiolo-alveolar carcinoma | 1 | 1 | 8* | 10** | ↑↑ | 0 | 1 | 0 | 5** | ↑↑ | ||
| Adenosquamous carcinoma | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | ||||
| Adenocarcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||||
| Squamous carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||||
| Total carcinoma | 1 | 1 | 8* | 11** | ↑↑ | 0 | 1 | 0 | 8** | ↑↑ | ||
| Total adenoma and/or carcinoma | 2 | 2 | 13** | 16** | ↑↑ | 3 | 2 | 4 | 11* | ↑↑ | ||
| Values indicate number of animals bearing tumors. Significant difference; *: p≦0.05, **: p≦0.01 by Fisher Exact Test, ↑: p≦0.05, ↑↑: p≦0.01 by Peto's Test | ||||||||||||
On the other hand, there was no induction of MWNT-7-related tumors in other tissues, including pleural and peritoneal mesotheliomas, although MWNT-7 fibers were translocated to the pleural and peritoneal cavities. In male rats, but not in females exposed to 2 mg/m3 MWNT-7, there was a significant increase in the incidences of simple mesothelial hyperplasia in the parietal pleura and focal fibrosis in the parietal pleura side of the diaphragm.
NIOSH16) examined the activity of inhaled MWNT-7 in initiation/promotion carcinogenesis study of lung carcinogenesis in mice. Six-week-old male B6C3F1 mice received a single intraperitoneal injection of an initiator, methylcholanthrene (MCA), at a dose of 0 or 10 μg/g bw. One week later, mice were exposed to 5 mg/m3 MWNT-7 or filtered air (air controls) for 5 h/d for 15 days, using the whole body inhalation system described by McKinney et al14). At 17 months post-exposure, mice were euthanized and examined for tumor formation. The lung tumor incidence in the MCA only group was 51.9% and the number of tumors per animal was 0.81, while mice injected with MCA followed by inhalation exposure to MWNT-7 group had a lung tumor incidence of 90.5% with a multiplicity of 2.9. Lung tumor incidence in mice injected with vehicle and then exposed to MWNT-7 was not different from the air controls. The incidences of bronchiolo-alveolar adenocarcinomas in the lung were 22% in the MCA only group and 62% in the MCA-MWNT-7 group. These results indicate that inhalation of MWNT-7 promoted lung carcinogenesis, but did not act as a complete carcinogen. This supports the possibility that inhaled MWNT-7 could also be carcinogenic in mice, if the mice are exposed to MWNT-7 for a longer duration. It is possible that not only the amount of airborne MWNT-7, but also the exposure duration has an important role in lung carcinogenesis. Moreover, mice are less sensitive than rats to lung carcinogenesis from inhaled particles, including fibers29,30).
In summary, lung carcinoma, but not pleural mesothelioma was induced in male and female rats in a 2-year carcinogenicity study of exposure to MWNT-7 by inhalation. Therefore, there is clear evidence of MWNT-7 lung carcinogenicity in male and female rats exposed to MWNT-7 aerosol by inhalation. Gender difference was observed in the lung carcinogenicity of MWNT-7 in rats, with males being more susceptible than females to MWNT-7. Exposure to airborne MWNT-7 for 15-days promoted lung carcinogenesis initiated by MCA in mice.
Central issues to be resolved in experimental carcinogenesis of airborne MWNT-7 include, how the lung burden of straight fibrous MWNT-7 contributes to carcinogenesis; how the number of fibers correlate with lung carcinogenesis and carcinogenic threshold; what sizes of straight type MWCNTs fibers with physical properties similar to asbestos exert carcinogenicity; and why inhalation of airborne MWNT-7 did not cause development of pleural mesothelioma. For hazard identification, it is important to determine what shapes and what sizes of MWCNTs, including both straight fibrous types and tangled types, are carcinogenic in experimental animals exposed to MWCNT fibers by inhalation9,31).
2) Carcinogenicity Studies of Intratracheally Instilled MWCNTs
Suzui et al.32) found that intratracheal instillation of one of the straight fibrous types of MWCNTs was carcinogenic in rats. They used MWCNT-N (Nikkiso Tokyo, Japan) with an original size of 4.2 μm in length and 30-80 nm in diameter. Male F344 rats, 6-week-old at the commencement of the study, were administered MWCNT-N at doses of 0 (control) or 125 μg/rat in 0.5 ml saline containing 0.5% Pluronic F68, which prevents aggregation of MWCNT-N. The rats were administered MWCNT-N 8 times over 2 weeks (total dose, 1 mg/rat). Before administration, the MWCNT-N was fractionated through a sieve with a pore size of 25 μm. Fiber lengths in the unfiltered MWCNT-N group, the flow-through MWCNT-N group, and the retained MWCNT-N group were 4.2, 2.6, >2.6 μm, respectively, and the diameters were 30-80 nm. After the two week administration period, the rats were observed without any further treatment until week 109. Pleural mesotheliomas and lung tumors were observed in the 3 treated groups; there was no significant difference in tumor development between the groups. The total combined incidences of mesotheliomas and the total combined incidences of lung tumors (bronchiolo-alveolar adenomas and/or carcinomas) in the three groups were 6/38 (15.8%, p<0.05) and 14/38 (36.8%, p<0.001) respectively, compared to the control (0/28 and 0/28). All mesotheliomas were located in the mediastinal space, but the primary site could not be determined because of the advanced stage of the tumor. While there were no differences in lung tumor and mesothelioma development between the three MWCNT-N-treated groups, the authors noted that both unfiltered and flow-through fractions induced mesothelioma, while the retained fraction did not. Interestingly, in the rats administered unfiltered MWCNT-N, the size of the fibers in the lung tissue tended to be smaller than in the administered preparation. They speculated that one possibility was that the longer MWCNT did not transfer to the alveoli as efficiently as shorter MWCNT31). Translocation of MWCNT-N to the pleural cavity after intratracheal instillation was observed in the pleural lavage fluid lavage (quantity not reported)33,34). Thus, intratracheal instillation of MWCNT-N induced lung adenomas and carcinomas, and pleural mesotheliomas.
In contrast, Nakanishi et al.35) reported that intratracheally instilled MWCNT (N) (Nikkiso, Japan) was not carcinogenic in rats. The MWCNT (N) had a diameter of 52 nm and a length of 2.87 μm in water. The rats were instilled with MWCNT (N) at doses of 0, 0.67 and 3.3 mg/kg bw (perhaps once). After 2 years, no tumorigenesis was observed in the lung.
3) Carcinogenicity Studies of Intraperitoneally Injected MWCNTs
The surface of the peritoneal cavity is covered by a mesothelium similar to that of pleural cavity and has been recognized as a surrogate for mesothelioma of the pleural cavity in asbestos fiber studies36). When MWCNTs are injected into the intraperitoneal cavity of animals, the fibers are able to immediately interact with mesothelial cells. Consequently, intraperitoneal injection method may be a good screening assay for detection of mesotheliomagenicity (carcinogenicity of the mesothelium) of MWCNTs.
Takagi et al.4) found that in p53 heterozygous mice, which have increased susceptibility to carcinogenesis, intraperitoneal injection of MWNT-7 induced mesothelioma. 9 to 11 week-old male mice were given a single intraperitoneal injection of 3 mg MWNT-7 (corresponding to 1×109 fibers) suspended in Triton X-100 and necropsied at 25 weeks after injection. The average diameter of the fibers was about 100 nm and 27.5% of the fibers were longer than 5 μm. The incidence of peritoneal mesothelioma was 14/16 (87.5%), (the control, 0%). Next, they8) examined the dose-response of the carcinogenic effect of MWNT-7. p53 heterozygous mice were given single intraperitoneal injections of 0, 3, 30, 300 μg (0, 1×106, 1×107, 1×108 fibers) and then followed for 1 year. The incidence of mesothelioma increased in a dose-dependent manner (0/20, 5/20, 17/20, 19/20). The time of mesothelioma onset was apparently independent of the dose.
Mesothelium lines the scrotum and testes, forming the scrotal cavity, and the scrotal cavity is connected freely with the peritoneal cavity in rats. In male Fischer 344 rats mesotheliomas spontaneously develop in the scrotal mesothelium and spread into the peritoneal cavity. Therefore, in order to increase the sensitivity of assays reported by Takagi et al.4), who used p53 heterozygous mice and a very high dose of MWNT-7, Sakamoto et al.7) administered MWNT-7 by intrascrotal injection to intact rats. The 12-week-old male F344 rats received a single injection of MWNT-7 at a dose of 1 mg/kg bw (more than 100-fold lower than the dose used in the initial study reported by Takagi et al.4)) and were followed for 52 weeks. The incidence of peritoneal mesothelioma was 85.7%, (the control, 0%). Mesotheliomas were invasive to adjacent organs and frequently metastasized into the pleura.
As noted above, MWCNTs are classified into two subtypes, straight, which is fibrous, firm and tangled, which is thin and self-assembles into tangled aggregates. Nagai et al.5), administered both subtypes to rats by intraperitoneal injection: male 6-week-old rats were injected with 1 ml of 0.5 or 5 mg/ml of non-aggregated straight MWCNTs (diameter, ~50 nm and ~150 nm) or tangled MWCNTs (diameter, ~2-20 nm) twice with one week interval between injections and followed without any treatment for about 1 year. Both straight types of MWCNT induced mesotheliomas, however, the thinner diameter straight MWCNT (diameter, ~50 nm), which had high crystallinity, were markedly more carcinogenic than the thicker diameter straight MWCNTs (diameter, ~150 nm). The tangled MWCNT (diameter, ~2-20 nm) was not carcinogenic. The authors suggested that control of the diameter of MWCNTs is an important factor that contributes to the potential hazard in human health. Nagai et al.37) also reported that a high dose, 10 mg/rat of a tangled form of MWCNT (15 nm in diameter) did not induce mesothelioma after intraperitoneal injection in rats, which were followed for up to 3 years after injection. This observation strengthens the previous finding that the rigidity, diameter, length, and surface properties of MWCNTs are important factors in the induction of mesothelioma in vivo.
Rittinghausen et al.6) examined potency of mesothelioma induction by 4 different MWCNT fibers using intraperitoneal injection with 1×109 or 5×109 WHO carbon nanotubes in Wistar rats. The medium length and diameter of the 4 MWCNT fibers were as follows: A (straight type), 8.57 μm and 85 nm; B (straight type), 9.30 μm and 62 nm; C (straight type), 10.24 μm and 40 nm; D (mostly curved type), 7.91 μm and 37 nm. The highest frequencies and earliest appearances of mesothelioma induction after the injections were in the rats administered MWCNTs A and B, which are a little thicker than those of MWCNTs C and D. In rats administered MWCNT C, which is thin and long, the earliest appearances of tumors were slightly later than in the A and B groups, and tumors appeared last in rats administered MWCNT D, which were thin and curved. The authors concluded that in addition to aspect ratio, curvature seems to be an important parameter influencing the carcinogenicity of MWCNTs.
Muller et al.38) reported the absence of carcinogenicity of a MWCNT fiber in a 2-year bioassay of intraperitoneally injected rats. They used MWCNT with or without structural defects, both were 11.3 nm in diameter and about 0.7 μm in length. Wistar rats were injected intraperitoneally with a single dose of MWCNT with defects (2 or 20 mg/animal) and MWCNT without defects (20 mg/animal) and then followed without treatment for 2 years. The MWCNTs, both with and without defects, did not induce mesothelioma. Thus, short MWCNTs (less than 1 μm average) did not induce mesothelioma. Interestingly, however, the MWCNT with structural defects were genotoxic39,40).
In summary, intraperitoneal injection is a good screening assay to detect mesothelial carcinogenicity of MWCNTs in rats and mice. Straight, needle type MWCNTs induce mesothelioma in animals after intraperitoneal injection, and long, straight needle type MWCNTs were stronger carcinogens than short type MWCNTs. On the other hand, tangled type MWCNTs do not seem to induce formation of mesothelioma in vivo, although further studies are required. Although these types of screening assays are not relevant to human risk assessment, the data from these screening assays show harmful effects of MWCNTs.
4) Carcinogenicity Studies of Subcutaneously Injected MWCNTs
The use of MWCNTs in clinical applications is of wide interest and for such applications the carcinogenicity of MWCNTs were examined using subcutaneous injection in rasH2 mice, which are highly susceptible to carcinogenesis41). The male, 6-week-old, mice were administered MWCNT (average diameter, 100 nm; average length, 10 μm) at a dose of approximately 75 mg/kg by a single subcutaneous injection and then followed for 26 weeks. There were no tumors in either the skin or subcutaneous tissue at the injected site or other organs, indicating that subcutaneous injection of MWCNT was not carcinogenic in this study. The amount of MWCNT in the tissue was not measured.
3. Lung Burden and Distribution of Inhaled MWCNTs
1) Measurement of MWCNTs retained in target organs
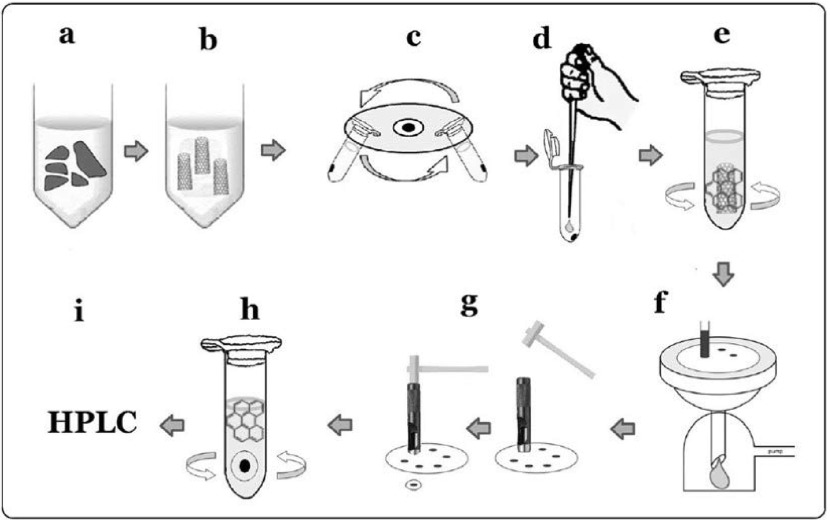
Inhaled MWCNT fibers are excluded from the lung by the mucociliary escalator of the tracheobronchial epithelium, and they also translocated from the lung to the lymph node42) and many other organs including the brain43) and the pleural cavity. Measurement of the retention of MWCNT in organs, including the lung, is important for the interpretation of toxicity due to MWCNT inhalation. Sequential measurements of the lung burden during inhalation exposure are necessary to determine the relationship between the aerosol dynamics of the deposited MWCNT and the lesions induced in the lung. At present, there are few analytical methods for measuring MWCNT in the lung and other organs15,44,45). However, these methods seem to be unable to measure very small amounts of MWCNTs in tissues (limit levels: 1-2 μg). Therefore, we established a more sensitive method for measuring MWCNT fibers that used a hybrid marker for nanotube analysis10,46,47). The method involved coupling and adsorption of a marker, benzo[ghi]perylene (B (g) P) to nanotubes, followed by their desorption and measurement by means of high performance liquid chromatography (limit levels: 0.1 μg) (Fig. 4). The lung tissue was digested by incubation with Clean99 K-200 (C99) at room temperature overnight (Figure 4a, 4b), and then centrifuged at 12,000 rpm for 10 minutes (Fig. 4c), and the supernatant was discarded. The pellet was re-suspended in 1 ml of TW solution (9.6% phosphate-buffered saline containing 0.1% Tween 80), centrifuged at 12,000 rpm for 10 minutes, and the supernatant was discarded. The washed pellet was then re-suspended in 200 μl of concentrated sulfuric acid to remove the organic content (Fig. 4d). One ml TW solution was stirred into the acid solution, and the solution was then sonicated for 10 seconds. 0.125 μg/ml B (g) P and 50 μl acetonitrile were added to the sonicated solution, and the solution stirred for 15 minutes (Fig. 4e) to allow adsorption of the B (g) P onto the MWNT-7 fibers. The mixture was then passed through a nuclepore membrane filter (Fig. 4f). The regions of the filter containing the MWNT-7 were punched out in 8 mm-diameter circular disks (Fig. 4g) and soaked in 1 ml acetonitrile with stirring, to remove the attached B (g) P from the MWNT-7 (Fig. 4h). The solution was then filtered for HPLC analysis (Fig. 4i). Chromatography was performed using an Acuity UPLC system coupled to a fluorescence detector. Eluates were analyzed quantitatively by monitoring at fluorescent wavelengths of 294 nm for excitation and 410 nm for emission with 5 μl aliquots of extract injected onto a C18 column. The mobile phases were acetonitrile : methanol : distilled water = 75 : 20 : 5. An eluent flow rate of 0.5 ml/min was used for all analyses. A calibration curve was also prepared to convert the HPLC results to MWNT-7 fiber weight. MWNT-7 was suspended in C99 as a colloidal dispersant and subjected to ultrasonication for 30 minutes with an ultrasonic homogenizer. A 10-mg sample of MWCNT was added to 40 ml of TW solution and sonicated for 30 minutes; the solution was diluted to 1 μg/ml with C99 and used to prepare calibration standards of 0.2, 0.4, 0.6 and 0.8 μg/ml MWNT-7 in C99; and the calibration standards were then treated in exactly the same manner as described above for the tissue samples to create a calibration curve.
Fig. 4.
Lung burden measurement method of MWNT-7. A defining feature of the method is the use of a hybrid marker, benzo[ghi]perylene, for coupling and adsorption onto the MWNT-7 fiber.
Other analytical methods reported currently are carbon analysis44,45) and spectrophotometry15). However, as noted above, these methods cannot measure very small amounts of MWCNTs in the lung and other tissues.
Morphological examination by scanning electron microscopy (SEM), which can be carried out routinely, is needed for the measurement of the number of MWCNT fibers retained in the lung and other organs. SEM examination is also used to examine the shape of MWCNTs; although transmission electron microscopy (TEM) can also be utilized for examining the shape of MWCNT fibers.
2) Lung burden of MWNT-7 in Our Inhalation Carcinogenicity Study
Lung burden is related to the MWCNT exposure concentrations and the overall exposure duration. This is due to deposition of MWCNTs, combined with removal of inhaled MWCNTs from the lung by the mucociliary escalator in the tracheobronchial epithelium and translocation to lung-associated lymph nodes through bronchus-associated lymphoid tissue (BALT) by MWCNT-phagocytosed macrophages. Histopathologically, deposition sites in the lung of MWCNTs are mostly in macrophages, granulomatous lesions, sites of alveolar fibrosis, and BALT, and also non-phagocytosed MWCNTs were observed in the alveolar space.
In our 13-week inhalation toxicity study of MWNT-7 in rats, performed before the commencement of the 2-year inhalation carcinogenicity study, lung burden, induction of fibrosis and granulomatous change in the lungs of males and females increased in a concentration-dependent manner12).
In the 2-year inhalation carcinogenicity study, the MWNT-7 lung burdens of rats that were euthanized or died before the end of the 104-week experimental period (exposure period longer than about 70 weeks) were related to the concentrations and overall exposure time27). Finally, the amount of MWNT-7 retained in the lungs of the rats that survived to the end of the 2-year inhalation carcinogenicity study also increased in a concentration-dependent manner (Table 2), and fibrosis in the alveolar wall and granulomatous lesions correlated with MWNT-7 lung burden. Taken together, it is clear that the lung burden in rats exposed to MWNT-7 was dependent on the concentration of airborne MWNT-7 and the duration of exposure. Table 2 shows the amount of MWNT-7 retained in the lungs of the rats that survived to the end of the 2-year experimental period. The amounts of retained MWNT-7 increased in a concentration-dependent manner of MWNT-7 exposure and were similar between males and females. Curves of lung burden measured at several points during exposure period showed linearity in each concentration of MWNT-7 exposure. Notably in males, induction of lung carcinomas increased in amount-dependent manner of MWNT-7 lung burden. It is highly likely that MWNT-7 lung burden has a crucial role in lung carcinogenesis in males. As females were less susceptible to the carcinogenic effects of MWNT-7, the lung burden-dependent effect of MWNT-7 was not seen in females exposed to MWNT-7; however, results observed in the female rats were consistent with the premise that amounts of MWNT-7 in the lung burden have a crucial role in lung carcinogenesis.
Table 2.
Incidence of lung carcinoma and lung burden in rats exposed to MWNT-7 for 104 weeks
| Concentration(mg/m3) | Lung carcinoma incidence (%) | Lung burden | ||||
|---|---|---|---|---|---|---|
| Amount of MWNT-7 (µg/g of bw) | Number of MWNT-7 (No/lung) | |||||
| Male | Female | Male | Female | Male | Female | |
| Number of MWNT-7: 1µg=9.03×106 (calculated by SEM) Significant difference; *: p≦0.05 ** : p≦0.01 by Fisher Exact test | ||||||
| 0 | 2 | 0 | - | - | - | - |
| 0.02 | 2 | 2 | 0.029±0.008 | 0.034±0.008 | 0.89×109 | 0.07×109 |
| 0.2 | 16* | 0 | 0.434±0.056 | 0.453±0.056 | 1.38×109 | 1.07×109 |
| 2 | 22** | 16** | 4.954±0.420 | 4.712±0.201 | 16.2×109 | 10.8×109 |
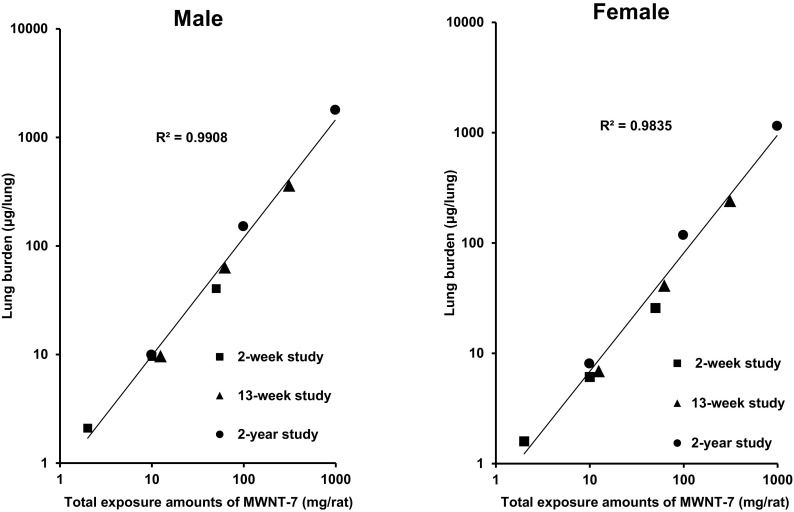
Fig. 5 shows summarized results of lung burden measured in male and female rats at week 2, week 13, and week 104 of 3 inhalation exposure studies. Rats were exposed to MWNT-7 concentrations of 0.2, 1, and 5 mg/m3 in the 2-week and 13-week studies, and 0.02, 0.2, and 2 mg/m3 in the 2-year study. The amount of MWNT-7 in the lung increased linearly with concentration in both males and females at weeks 2, 13 and 104, and the lung burden of MWNT-7 in each study was similar. Thus, the results indicated that the highest MWCNT concentrations used in the subacute, subchronic and chronic inhalation studies did not overload the respiratory physiology of the rats.
Fig. 5.
Summarized results of lung burden measured in male and female of rats exposed to 3 concentrations at week 2, week 13, and week 104 of inhalation exposure studies. Concentrations: 0.2, 1, and 5 mg/m3 in 2-week and 13-week studies, and 0.02, 0.2, and 2 mg/m3 in 2-year study.
The number of MWNT-7 fibers in the lungs of each concentration group were calculated by lung burden and SEM analysis of MWNT-7 fibers (Table 2). The numbers of MWNT-7 increased in a concentration-dependent manner and the numbers needed for inducing carcinomas were calculated to be over 1×109 per lung in males and about 10×109 per lung in females. Thus, large numbers of MWCNTs are needed to exert lung carcinogenicity in rats. Interestingly, Rittinghausen et al.6) reported intraperitoneal injection of 1×109 or 5×109 MWCNT fibers in male rats induced peritoneal mesothelioma incidences of 40% and 100%. This result also indicates large numbers of MWCNT fibers have a crucial role in the carcinogenesis in the pleural cavity. Indeed, MWNT-7 fibers in the pleural lavage fluid were observed in a concentration-dependent manner in our 2-year inhalation carcinogenicity study. However, the fiber numbers were about 1.5×103 and about 1×103 in males and females of the 2 mg/m3 exposed group, too low to induce mesothelioma.
As shown in Table 2, the gender difference in susceptibility to MWNT-7 induced carcinogenicity was not due to different the amounts of MWCNT fibers in the lungs of males and females, and was therefore caused by some other physiological difference in the male and female lungs. One possibility is that males are more susceptible to fiber-induced toxicity in general. The fact that the incidence of spontaneous lung carcinomas in males was higher than in females suggests that the gender difference in the induction of carcinomas by MWNT-7 is most likely due to the basic susceptibility to lung tumor development in males, rather than to carcinogenesis related to MWNT-7.
Single fibers and bundles of fibers were found in the alveolar space, indicating the possibility of direct MWNT-7 mediated cellular damage. However, most fibers were found phagocytosed by macrophages, which would result in reactive oxygen species (ROS) formation and oxidative stress. In all three studies, the 2- and 13-week studies and the 2-year study, SEM findings also showed large aggregates of MWNT-7 in alveolar macrophages12,13,27). Fig. 3b shows an SEM photograph of a macrophage with a cocoon-like mass of MWNT-7 fibers. Importantly, these cocoon-like masses were not seen in the MWNT-7 aerosols in the inhalation chamber, and consequently, were formed by single macrophages or a series of single macrophages phagocytosing multiple fibers. It is possible that confining numerous fibers to a single macrophage would result in generation of ROS, although it might be useful for clearance of the fibers from the lung.
3) Lung burden of MWNT-7 in a Mouse Inhalation Exposure Study
Before commencement of the initiation/promotion carcinogenesis study by Sargent et al. 201416), Porter et al.15) measured the lung burden of mice exposed to airborne MWNT-7 at a concentration of 10 mg/m3 (5h/d) by whole body inhalation for 2 or 12 days. The lung burden was 6.6 μg/lung and 30.6 μg/lung after 2 days and 12 days exposure, respectively. Thus, lung burden significantly increased within the duration of exposure. The distribution of MWNT-7 was 24% in the airways and 76% in the alveolar region, which was distributed 14.6% in the air space, 11.9% in the tissue, and 49.2% in macrophages. This MWNT-7 induced pulmonary inflammation with development of pulmonary fibrosis. Rare pleural penetration of MWNT-7 was also observed.
4) Lung burden of MWCNT-N after Intratracheal Instillation
Rats were administered MWCNT-N at a total dose of 1 mg/rat (8 administrations of 125 μg/rat) during the initial 2 weeks of the experiment by intratracheal instillation and then observed up to 109 weeks, as described above32). At the end of experimental week 109, the MWCNT remaining in the lungs of the rats administered unfiltered MWCNT-N, the flow-through fraction, or the retained fraction was 25.4, 48.0, or 26.3%, respectively, as measured 24 hours after the final instillation. Lung carcinoma and/or mesothelioma were induced in all treated groups.
In conclusion, the lung burden of MWNT-7 increased with concentration during exposure of rats and mice to airborne MWNT-7. In our inhalation carcinogenicity study, rats were exposed to 0.02, 0.2, or 2 mg/m3 MWNT-7 for 2 years27), while in the NIOSH initiation/promotion carcinogenesis study, mice were exposed to 5 mg/m3 MWNT-7 for 15 days and then followed without any treatment for 17 months16). Therefore, the amounts of MWNT-7 retained in the mouse lung in the NIOSH study should be much less than in the rat lung in our study. In rats, MWNT-7 induced lung carcinomas, but not in mice, although it promoted MCA-initiated lung carcinogenesis. It is possible that if the mice were exposed to a higher concentration of MWNT-7 or exposed for a longer period of time, MWNT-7 alone may have induced lung carcinomas in the mice.
Inhalation exposure to MWNT-7 induced lung carcinoma but not pleural mesothelioma in rats, however, MWCNT-N administered by intratracheal instillation induced not only lung carcinoma but also pleural mesothelioma in rats. Both these MWCNTs are straight, needle types, although length of the MWCNT-N is a little shorter than that of MWNT-7. However, it seems unlikely that this difference in length could account for the difference in malignant tumor induction. On the other hand, intratracheal instillation induces a bolus effect in the lung, because of the rapid speed of administration of large amounts of MWCNTs, while inhalation exposure does not cause this effect.
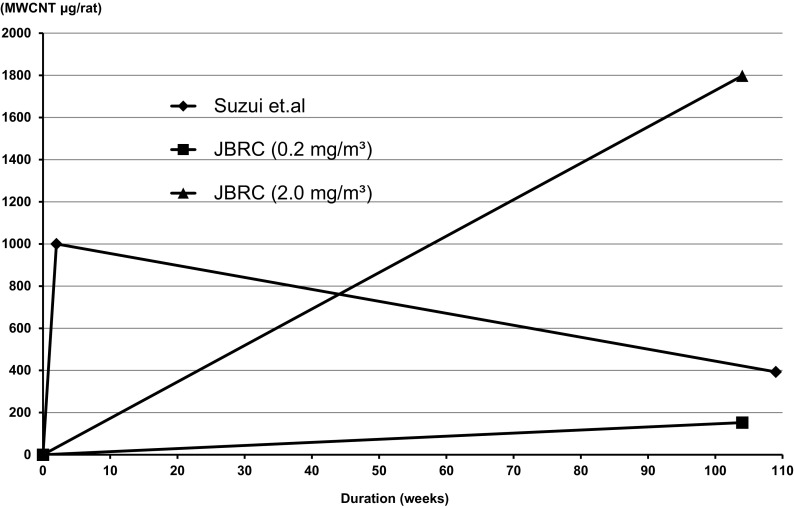
Fig. 6 illustrates the area under the curve (AUC) of the lung burden of MWCNTs (MWNT-7 and MWCNT-N), which is critically dependent on duration, in male rats of the carcinogenicity studies using inhalation exposure of MWNT-7 and intratracheal instillation of MWNT-7. Notably, inhalation of 0.2 mg/m3 and 2 mg/m3 resulted in lung carcinogenicity, while intratracheal instillation resulted in carcinogenicity in both the lung and pleural cavity.
Fig. 6.
Comparison of the area under the lung burden curves in the MWNT-7 carcinogenicity study of rats by inhalation exposure and MWCNT-N carcinogenicity study of rats by intratracheal administration. The shapes of the areas under the lung burden curves are clearly different.
Although this figure is not wholly accurate because of the lack of data points, it illustrates a basic difference in the two exposure methods. The curve for inhalation exposure of MWNT-7 increases linearly with period of exposure, but the curve for intratracheal instillation of MWCNT-N is maximal at the end of the exposure period and then decreases gradually during the course of the experimental period. The AUC is likely to be in the order of inhalation exposure to 2.0 mg/m3 > intratracheal instillation > inhalation exposure to 0.2 mg/m3. Interestingly, the AUC data of the 0.2 mg/m3 group is small and very useful for relevance of MWCNT cancer risk assessment to humans. The different shapes of the AUCs highlight the differences in amount and duration of the lung burden of the MWCNTs, and may explain the difference in the development of mesothelioma by inhalation exposure and intratracheal instillation. With intratracheal instillation, the amount of MWCNT-N in the lung is initially very high and continues at a high level for most, or all, of the experimental period. As discussed above, MWNT-7, and probably MWCNT-N, must be present in large amounts to exert carcinogenicity in rodents. Intratracheal instillation introduces large amounts into the lung at the start of the experiment, allowing the process of carcinogenesis to proceed for virtually the entire experiment period. Introduction of large amount of fibers into the lung at the beginning of the experiment also allows for a longer period of time for higher numbers of fibers to be translocated into the pleural cavity and exert a carcinogenic effect on the mesothelium. During inhalation exposure, translocation of MWCNTs to the pleural cavity may be small and slow. Indeed, in our inhalation carcinogenicity study, the amount of MWNT-7 fibers in the pleural cavity were very low compared to that of the lung.
4. Extrapulmonary Translocation of MWCNTs
1) Translocation of MWCNTs into the Pleural Cavity
Pleural mesothelioma is one of the most topic lesions in humans exposed to asbestos. Asbestos fibers penetrate into the pleural cavity from the alveoli and deposit in the pleural cavity36).
An important issue is translocation of inhaled MWCNTs into pleural cavity. Ryman-Rasmussen et al.23) examined whether an MWCNT (length 0.5 to 50 μm) reached the subpleural tissue in mice after inhalation exposure. Mice were exposed to aerosolized MWCNT of 1 or 30 mg/m3 for 6 hours by nose-only inhalation and then observed for up to 14 weeks. MWCNT fibers reached the subpleural tissue of the lung and the subpleural fibrosis score increased in a time dependent manner.
In another study, male mice were exposed to MWNT-7 aerosol at a concentration of 5 mg/m3, 5 h/d for 12 days by whole-body inhalation exposure48). Fibrotic responses following inhalation exposure to MWNT-7 were observed. Penetration of the visceral pleura by MWNT-7 fibers were observed.
Another group treated male F344 rats with 0.5 ml of 500 μg/ml suspensions of MWNT-7 or MWCNT-N five times over a 9-day period by intratracheal instillation33). The total dose of the MWCNTs was 1.25 mg/rat. Six hours after the last administration, the rats were necropsied. MWCNT-N fibers were found in the pleural cavity lavage, mostly in macrophages. Hyperplastic mesothelial proliferation was observed in the visceral mesothelium and their proliferating cell nuclear antigen indices were approximately 10-fold that of the vehicle control. However, mesothelial cell proliferation was not observed in the parietal pleura. In addition, they examined the pleural translocation of large MWCNT-N (8 μm in length, 50 nm in diameter) and small MWCNT-N (3 μm in length, 15 nm in diameter), which forms cotton-like aggregates: the MWCNT fibers were administered by intratracheal instillation once every 2 weeks for 24 weeks. The large MWCNT-N, but not the small MWCNT-N translocated into the pleural cavity, deposited in the parietal pleura, and induced fibrosis and patchy parietal mesothelial cell proliferation34).
In our MWNT-7 inhalation carcinogenicity study of rats27), the number of fibers in the pleural cavity of the 2 mg/m3 group was about 1,500 and 800 fibers in males and females. These values are 106 fold lower than those of the fibers in the lung that induced lung carcinomas. Histologically, simple mesothelial hyperplasia and focal fibrosis, but not mesothelioma was observed in the parietal pleura of both sexes. The considerable difference in fiber numbers between the lung and the pleura likely accounts for difference in these changes. SEM examination revealed that the MWNT-7 fibers in the pleural cavity were individual, long, straight, and not aggregated (Fig. 3c).
In summary, inhaled and intratracheally instilled MWCNTs translocate from the lung to the pleural cavity. Inhalation of MWNT-7 by rats did not induce pleural mesothelioma in our 2-year inhalation carcinogenicity study. Pleural mesotheliomas, however, were induced in rats administered MWCNT-N by intratracheal instillation. While the amount of MWCNT-N retained in the pleural cavity was not measured, the evidence suggests that large amounts of intratracheally instilled MWCNT translocated to the pleural cavity, beginning very shortly after administration, resulting in induction of pleural mesothelioma. There is some evidence that MWCNT fibers were able to penetrate the visceral pleura49), however, the route of translocation of inhaled MWCNT from the lung alveoli into the pleural cavity remain an important, but unanswered question.
2) Translocation of MWCNT into the Peritoneal Cavity
In our inhalation carcinogenicity study27), the number of MWNT-7 fibers in abdominal lavage fluid were counted under SEM. The numbers of MWNT-7 fibers in the peritoneal cavity were about 2,400 and 3,300 fibers in male and female of rats. MWNT-7 fibers in the peritoneal cavity were individual, long, straight, and not aggregated (Fig. 3d). Interestingly, the lengths of these MWNT-7 fibers were somewhat longer than those found in the pleural cavity. We speculate that possibly MWCNT fibers translocate to the peritoneal cavity through lymphatic flow, perhaps through retrograde lymphatic translocation of fiber-phagocytosed macrophages. However, histopathological changes were not observed in the serosa of the abdominal organs and tissues. This agrees with the premise that large amounts of MWCNTs are needed to exert a carcinogenic effect in rodents, and that the number of MWNT-7 fibers that were translocated to the peritoneal cavity, especially when compared to the total area of the serosal surface in the peritoneal cavity, were far below the threshold required to induce a mesothelial hyperplastic response.
3) Translocation to the Lymph Nodes
In our 2-year inhalation carcinogenicity study, the deposition of MWNT-7 in the mediastinal lymph nodes was concentration-dependent in both male and female rats27). In a rat-subchronic toxicity study using intratracheal instillation, Aiso et al.42) also observed translocation of MWNT-7 from the lung to the mediastinal lymph nodes. In this study, it was also demonstrated that MWNT-7 translocated to the parathymic lymph nodes. Deposition of MWNT-7 in the lymph nodes increased over time, and MWCNT-laden macrophages were observed in bronchus-associated lymphoid tissue. In rats exposed by inhalation to another type of MWCNT, Baytube, it was found that the MWCNT fibers were deposited in BALT and increased the paracortical cellularity of these lymph nodes26).
4) Translocation to Other Organs and Tissue
In our 2-year inhalation carcinogenicity study, single or aggregated MWNT-7 fibers were present in the nasal cavity, larynx, trachea, spleen, liver, kidney, olfactory bulb, and brain without any histopathological changes, including inflammation27). The same findings were observed in mice exposed to inhaled MWNT-7 at a dose of 5 mg/m3, 5 h/d for 12 days49). Interestingly, accumulation of MWCNTs and an inflammatory response were observed in the liver, spleen, and lung 28 days after intraperitoneal injection of 250 mg/kg bw to mice50).
5. Shape and Size of Administered MWCNT Fibers
In general, particle shapes have an important role in toxicity, and this holds true for nanomaterial toxicity and in particular for the toxicity to the lung of inhaled nanomaterials. Based on their shapes, MWCNTs are roughly classified into two types, straight and tangled. The straight fiber type has a needle-like shape and is firm like asbestos, and the tangled type is thin enough to bend and self-assemble into tangled aggregates. The fiber paradigm, formulated from asbestos toxicity studies, theorizes that longer fibers of straight needle-like shape exert stronger toxic and carcinogenic effects than shorter ones36).
The long straight MWCNT, MWNT-7, were used in studies that administered MWNT-7 to p53 heterozygous mice by intraperitoneal injection4,8), and studies that administered MWNT-7 to rats by intrascrotal injection7). In these studies, MWNT-7 was carcinogenic. In contrast, Muller et al.38) examined the carcinogenicity of a MWCNT that was about 0.7 μm in length in a 2-year study using intraperitoneal injection to rats. This MWCNT did not induce peritoneal mesothelioma or an inflammatory response. TEM images of this MWCNT showed tangled self-aggregates. In a pilot study, Poland et al.9) found that MWCNTs injected into the peritoneal cavity of mice showed asbestos-like pathogenicity. They reported that exposing the mesothelial lining of peritoneal cavity of mice, as surrogate for mesothelial lining of the pleural cavity, to long MWCNTs resulted in asbestos-like, length-dependent, pathogenic behavior. Murphy et al.51) examined pleural toxicity by injection of MWCNTs into the pleural cavity of mice. Long straight MWCNTs fibers induced acute inflammation, leading to progressive fibrosis on the parietal pleura, where stomata of strictly defined size limits the egress of long, but not short fibers. This was confirmed by demonstrating clearance of short fibers through stomata, but not long fibers. They concluded that pathogenicity of long MWCNT fibers arises as a result of length-dependent retention at the stomata on the parietal pleura. Thus, it is clear that long straight fibers have carcinogenic potential in the peritoneal and pleural cavities. Donaldson and his co-workers36) hypothesize that the retention of long MWCNT fibers at stomatal openings on the parietal pleura, coupled with frustrated phagocytosis of pleural leukocytes that attempt to ingest them, produce chronic inflammation that is known to be a driver for proliferation, genotoxicity, growth factor synthesis and release. It is likely to culminate in pathology such as fibrosis, pleural effusion, and mesothelioma.
The situation in the lung, of course, is different from that in the peritoneal and pleural cavities. Removal of fibers from the lung is not limited by size restricting stomata. However, like the peritoneal and pleural cavities, longer fibers are more carcinogenic in the lung than shorter ones. We exposed rats to airborne MWNT-7, a long straight MWCNT, for two years, showing clear lung carcinogenicity27). MWNT-7 was also used in initiation/promotion carcinogenesis study that exposed mice to airborne MWNT-7 for short duration16). While MWNT-7 was not a complete carcinogen in this study, it did promote MCA-initiated lung carcinogenesis. In contrast, we found that MWNT-7, which was cut to a shorter length (about 3.3 μm in length), when administered by intratracheal instillation resulted in no histological inflammatory changes in the rat lung (unpublished data), while ordinary length MWNT-7 did induce inflammatory and granulomatous lesions of the lung42). Moreover, Donaldson and Poland52) mentioned that there is no evidence that particles show below 100 nm show any step-change in hazard meaning that there is no evidence of nano-specific hazard. As discussed below, phagocytosis of MWCNT fibers by macrophages may relate the size and shape of MWCNT fibers to their carcinogenicity.
Another aspect to consider is the direct interaction of MWCNT fibers with cells, such as mesothelial cells. Toyokuni53) reported that rigid MWCNT fibers 50 nm in diameter are able to pierce mesothelial cells and cause inflammation and mesothelial tumor development, while 15 nm diameter MWCNT fibers formed a tangled globule-like structure that did not pierce mesothelial cells, had little mesothelial toxicity, and did not induce mesothelial carcinogenesis5,37). In our in vitro studies of chromosome aberrations using CHL cells, long MWCNTs including MWNT-7 induced polyploidy, but short MWCNTs did not (unpublished data). However, in contrast to the results reported by Toyokuni53), we also found that one tangled type of MWCNT was a weak inducer of polyploidy (unpublished data).
In summary, shape and size are important factors in MWCNT fiber carcinogenicity. For the straight, needle fiber type of MWCNTs, it is certain that the threshold length for carcinogenicity seems to be shorter based on the results of inhaled MWNT-7 carcinogenicity and initiation/promotion carcinogenesis studies in rats and mice, and subacute and subchronic toxicity studies and studies in vitro. Further studies are required for estimation of carcinogenic threshold length.
6. Impurity of MWCNTs
Impurities might influence the toxicity of MWCNTs54). Metals are known to catalyze free radical reactions. Therefore, Fe impurities of MWCNTs have been considered to possibly play a role in MWCNT toxicity. Fe-rich MWCNT was cytotoxic and genotoxic and induced potent cellular oxidative stress, while Fe-free MWCNT did not exert any adverse effects in vitro55). Iron might also contribute to MWCNT carcinogenicity. Wang et al.56) reported that adsorption of hemoglobin and transferrin onto the surface of MWCNT (diameter, 50 nm) played a role in causing mesothelial iron overload, contributing to oxidative damage and possibly subsequent carcinogenesis in mesothelial cells. In our 2-year inhalation carcinogenicity study of MWNT-727), the iron content of the MWNT-7 was 4,400 ppm, while in a 2-year intratracheal instillation carcinogenicity study, the iron content of the MWCNT-N was only 460 ppm32), suggesting that if iron played a role in the carcinogenicity of MWCNT-N, it could have been iron adsorbed from the tissues. Porter et al.15) reported that iron present in MWNT-7 was not capable of generating measurable reactive oxygen species (ROS), presumably because the iron is encapsulated by carbon. Further studies on the role of iron in MWCNT carcinogenicity in vivo are warranted.
7. Surface Properties of MWCNTs
Oberdorester et al.10) discusses the concept that surface properties of nanomaterials may be important determinants of biological and toxicological activities. For example, surface charge can affect cellular uptake and surface modifications, such as carboxylation, can affect biopersistence. Because it is the surface of the MWCNT fiber that interacts with the tissue, an important surface property is surface area (cm2/g material), and this dose metric has been widely applied in nanoparticle toxicology. However, this metric is often misunderstood to assume that all nanomaterials fit a common dose-response relationship, when the specific surface area of each material is used as a metric. Instead, the surface area concept argues that for nano-sized particles of the same chemistry expressing an effect as a function of surface area provides a more meaningful toxicological dose metric for hazard ranking. This is considerably more effective than using mass or number of the tested nanomaterial, while dose-response relationships using surface area can be different for materials of different chemistries57). It is possible that the carcinogenicity of MWNT-7 might correlate to surface area, however, further study is required to resolve this issue.
8. Genotoxicity of MWCNTs
The determination of whether a genotoxic or non-genotoxic mechanism operates in MWNT-7 induced carcinogenicity is qualitatively important for carcinogen risk assessment. Genotoxicity data on MWNT-7 is briefly introduced in this section.
Ames tests of MWNT-7 were negative using Salmonella typhimurium or Escherichia coli58,59). However, bacterial mutagenicity testing systems (Ames tests) using prokaryotic cells do not appear to be suitable for the assessment of MWCNTs60). Mutagenicity in these systems is presumably related to the degree of MWCNT uptake by bacteria, which is likely to be less than in eukaryote cells for two reasons. First, prokaryotic cells cannot perform endocytosis and, second, their cell wall forms a barrier against simple diffusion of MWCNTs (particularly those in agglomerated form) into the interior of the bacteria. This lack of uptake could lead to false negative results. In addition, given that there are potential issues with the use of bacterial mutagenicity testing systems, it is prudent to utilize a mammalian cell alternative for assessing mutagenicity60). Asakura et al.61) reported that MWNT-7 was negative for mutagenicity at the hgprt locus of a mammalian cell line, a CHL/IU derived sub-clone, indicating that MWNT-7 is not a direct DNA mutagen.
However, while MWNT-7 is not a direct DNA mutagen, genotoxic effects have been reported. Ema et al.58) and Asakura et al.61) reported that MWNT-7 genotoxicity was characterized by the formation of polyploidy without structural chromosomal aberration. Asakura et al.61) also reported increased numbers of bi- and multi-nucleated cells without micronucleus induction. Yasui et al.62) observed that comparatively long MWNT-7 (approximately ≥ 20 μm) inhibited cytokinesis during cell division and induced the formation of bi-nucleated cells, whereas short MWNT-7 did not. In addition, Kato et al.63) reported that MWNT-7 induced micronucleus formation and sister chromatid exchange. In addition, Siegrist et al.64) described significant disruption of the mitotic spindle in cultured cells by MWCNT (Nanolab Inc. PD15 L5-20) at occupationally relevant exposure levels. Sasaki et al.65) reported that straight MWCNTs were the strongest inducer of polyploidy and curved MWCNTs were moderate inducers of polyploidy, and tangled MWCNTs were the weakest inducers of polyploidy. None of MWCNTs induced structural chromosomal aberrations. Taken together, these results indicate that MWCNTs including MWNT-7 do not induce mutations through direct interaction with the DNA (negative results in hgprt gene mutation assay) but rather induce polyploidy through direct interference with biological processes during cytokinesis.
Importantly, Kato et al.63) report that intratracheal instillation of MWNT-7 in mice increased gpt mutant frequencies, DNA damage by comet assay, and DNA oxidative damage. This type of damage is consistent with damage due to oxidative stress and inflammatory responses, but gpt mutations and oxidative damage are not consistent with aberrant cytokinesis. Therefore, these results indicate that MWNT-7 genotoxicity, namely the mutagenicity of MWNT-7 in vivo, is primarily due to secondary mechanisms such as oxidative stress and inflammatory responses.
9. Mode of Action of the Carcinogenicity of MWNT-7
Available data cannot fully explain the mode of action (MOA) of MWNT-7 induced carcinogenicity; however, the data on the MOA of non-neoplastic pulmonary toxicity, which is characterized by inflammation and fibrosis induced by MWCNT fibers, are suggestive. Biopersistence of MWCNTs in the lung, phagocytosis of MWCNTs by macrophages, generation of ROS and the resulting oxidative stress, and MWCNT-induced inflammation are involved in the subchronic toxicity of MWCNTs54,66).
Physicobiological properties of MWCNT fibers, such as shape and size (length and diameter), surface area, and contaminants such as iron, are considered to be important contributing factors to MWCNT-induced inflammation and fibrosis, and shape and size are regarded as being particularly significant in the carcinogenicity of MWCNTs. Longer, rigid MWCNT fibers, similar to asbestos, seem to possess biopersistence and increased induction of ROS formation, oxidative stress, inflammation, and genotoxicity including secondary mutagenicity in the lung. In our MWNT-7 inhalation carcinogenicity study, MWNT-7 lung burden increased linearly in concentration manner and exposure-period manner, exhibiting biopersistence27). We also observed numerous macrophages with phagocytosed MWNT-7 in the alveolar space or wall. SEM findings showed macrophages that had accumulated in the lung containing dense aggregates of MWNT-7 fibers. The persistence of these macrophages indicate phagocytosis that would lead to the generation of ROS and oxidative stress; this scenario was expanded into a hypothesis for the carcinogenicity of fibers several decades ago, a scenario that holds true today67). In our separate 4-week inhalation study, we found an increase in 8-hydroxy-2'-deoxyguanosine (8-OHdG) formation in the lungs of rats exposed to MWNT-7 (unpublished data), demonstrating a genotoxic mechanism that could contribute to the development of rat lung carcinogenesis. Overall, the data support the paradigm that biopersistence of MWNT-7 fibers in the lung leads to persistent generation of oxidants and cytokines, which in turn lead to inflammation, resulting in cycles of cell damage and repair coupled with oxidant-mediated DNA damage, this milieu can lead to the development of initiated cells and preneoplastic lesions that have the potential to develop into tumors.
The same MOA could contribute to the induction of mesothelioma (mesothelioma genesis). It is known that MWCNTs can be translocated into the pleural cavity from the alveoli33,34,49,68). Fibrous materials such as asbestos cannot be cleared from the pleural cavity, resulting in biopersistence in the pleural cavity36). These biopersistent fibers could then lead to phagocytosis, persistent generation of oxidants and cytokines, and chronic inflammation, as discussed above, producing a microenvironment that supports generation of DNA mutations, including the survival and proliferation of cells harboring these mutations, and ultimately leading to development of mesothelioma.
10. International Evaluation of MWCNTs
The carcinogenicity of MWCNTs were assessed by the International Agency for Research on Cancer (IARC) Monograph Working Group. The working group convened 21 experts from ten countries69). MWNT-7 (referred to as MWCNT-7 in Ref. 69) caused peritoneal mesothelioma in male and female rats in one intraperitoneal injection study5) and one intrascrotal injection study7), and in male p53 heterozygous mice in two intraperitoneal injection studies4,8). Inhalation of MWNT-7 promoted MCA-initiated lung carcinogenesis in male mice16). In one intraperitoneal injection study, two other types of MWCNTs with physical dimensions similar to those of MWNT-7 caused mesotheliomas in male and female rats5). Therefore, the working group concluded that there was sufficient evidence for MWNT-7 carcinogenicity in experimental animals and limited evidence for two other types of MWCNTs. No human cancer data were available, therefore, there was inadequate evidence for the carcinogenicity of MWCNTs in humans. Mechanistic studies indicated that MWCNT induced genetic alterations64) by mechanisms relevant to humans. However, there were no other data pertaining to the mechanism of carcinogenicity. In conclusion, MWNT-7 was classified as possibly carcinogenic to humans (Group 2B).
11. Quantitative Assessment of Genotoxic Carcinogens
Because cancer is a fatal disease, the carcinogenicity of chemicals is a major concern for human health. Carcinogens in general are classified into genotoxic or non-genotoxic types by qualitative assessment, which includes assays such as the Ames test, chromosome aberration test and micronucleus test, and the potency of the carcinogens is assessed quantitatively. Typically, the potency of a carcinogen is initially assessed using a linear model, i.e., a model that assumes a linear relationship between carcinogenic response and dose at low doses. For assessing risk to humans of genotoxic carcinogens, a linear non-threshold approach is used. The non-threshold approach assumes that there is no threshold for risk and generates S-shaped curves that are linear at low doses and reach the point (0 dose, 0 risk). The threshold approach, which is used for non-genotoxic carcinogens, assumes that at some measurable dose there is zero risk of cancer development, and consequently does not reach the (0 dose, 0 risk) point. The non-threshold concept of genotoxic carcinogenicity reflects the idea that a single event caused by the genotoxic carcinogen influences cancer development. However, it is well known that cancer development is a multistep process, and that active biological mechanisms exist that guard against cell transformation and aberrant cell proliferation, which suggests that thresholds should exist for genotoxic carcinogens as well as for non-genotoxic carcinogens.
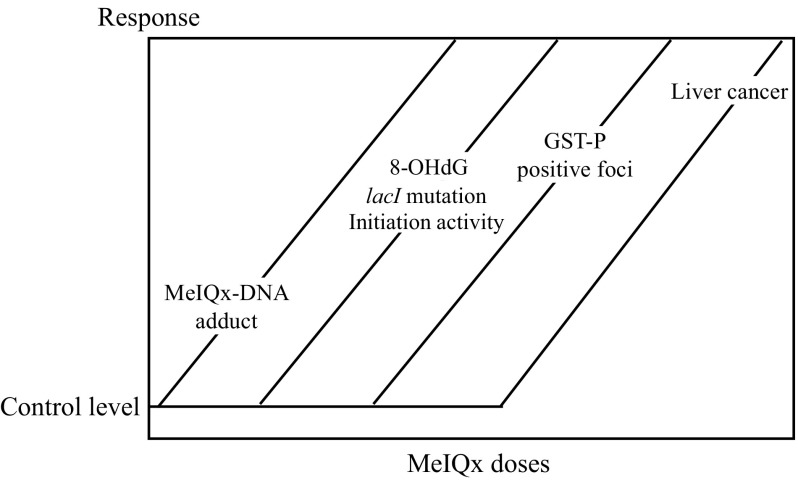
The chemical carcinogen, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) is a heterocyclic amine contained in baked meat and fish. MeIQx is carcinogenic for the rat liver and is a direct mutagen. To investigate its carcinogenic effect, male rats were administered MeIQx in the diet at doses of 0.001 ppm (speculated human exposure level) to 100 ppm (the hepatocarcinogenic level in rats)70-72). The result is summarized in Fig. 7. Starting at very low doses, levels of MeIQx-DNA adducts increased, then formation of 8-OHdG, which is a maker of oxidative stress, lacI gene mutations and initiation activity began to increase, and this was followed by the development of glutathione S-transferase placental form (GST-P) positive foci, which is a preneoplastic lesion and a marker of rat hepatocarcinogenicity. These results demonstrated that different no-effect levels exist for different parameters relevant to carcinogenesis in rats exposed to low doses of the genotoxic carcinogen, MeIQx. Moreover, rats exposed to low doses of two other genotoxic hepatocarcinogens contained in food, N-nitrosodiethylamine and 2-amino-3-methylimidazo[4,5-f]quinoline, also showed the existence of different no-effect levels for different parameters relevant to carcinogenesis70,73). In addition, Williams et al.74,75) found NOAEL for DNA adduct formation in the livers of rats treated with low doses of the genotoxic hepatocarcinogen, 2-acetylaminofluorene. These studies concluded that a threshold, at least a practical threshold, exists for the carcinogenicity of genotoxic carcinogens, including direct mutagens.
Fig. 7.
Relationship between qualitative events and quantitative doses in MeIQx hepatocarcinogenesis in rats.
Recently, it was recognized that genotoxic carcinogens, including carcinogens that induce DNA mutations via secondary mechanisms, should be considered to have a practical threshold76,77). This directly impacted cancer risk assessment in humans. As mentioned before, MWNT-7 is classified as a mutagen due to secondary mechanisms. Therefore, the threshold approach is accepted for MWNT-7 carcinogenicity.
12. Occupational exposure assessment to MWCNTs
In 2013, NIOSH reported that the best data to use for quantitative risk assessment and as the basis for a recommended exposure limit (REL, an occupational exposure limit [OEL] that has been recommended by NIOSH) were the non-neoplastic pulmonary data from MWCNT studies using experiment animals78). NIOSH considered the pulmonary responses of inflammation and fibrosis observed in short-term and subchronic studies in animals to be relevant to humans, as inflammatory and fibrotic effects were also observed in occupational lung diseases associated with workplace exposures to other inhaled particles and fibers. NIOSH concluded that all types of MWCNTs should be considered a respiratory hazard and that occupational exposures be kept below an 8-hour time-weighted average of 1 μg/m3 of respirable elemental carbon. Nakanishi et al.35) assessed the health risks via inhalation of six types of CNTs and derived an OEL for the CNT group rather than individual CNTs. Based on the results of their 4 week inhalation study and intratracheal instillation study, they proposed an OEL period of 15 years for CNTs of 0.03 mg/m3, a value considerably higher than the NIOSH recommendation. Thus, both the OEL recommended by NIOSH and the OEL by Nakanishi et al. pertain to CNTs as a group and the two OELs were considerably different.
It is an important issue to assess OEL of MWCNTs in the workplace for protection of occupational cancer. Therefore, we calculated the OEL of MWNT-7 from our inhalation carcinogenicity data. As discussed above, the genotoxicity data on MWNT-7 indicates that it is a genotoxic (mutagenic) agent that acts via a secondary mechanism (not directly DNA reactive). At present it is accepted that there should be a practical threshold for this kind of carcinogen76,77), and our carcinogenic threshold studies support this concept71,72). Based on the evidence of the existence of a practical threshold for MWNT-7 carcinogenicity, the NOAEL for carcinogenicity of MWNT-7 is 0.02 mg/m3 in rats since exposure to this level did not result in the development of lung carcinomas in our study. Using the NOAEL-based approach, we calculated that the OEL is 0.15 μg/m3 for a human worker, based on human equivalent working time concentration and uncertainty factors (species differences and genotoxic cancer factor). This value is lower than the OEL recommended by NIOSH and markedly lower than the OEL calculated by Nakanishi et al35), however, it should be noted that the OEL we calculated using the NOAEL-based approach is for a carcinogenic MWCNT. In addition, to calculate the OEL, minute ventilation and alveolar deposition fraction could be considered.
For risk analysis, a point of departure (PoD) from the observed carcinogenesis data should be estimated to mark the beginning of extrapolation to lower doses. The lower confidence limits of the benchmark dose yielding a response with a 10% extra risk (BMCL10) were calculated with US.EPA's benchmark dose software Version 2.679). In our carcinogenicity study, since induction of lung carcinoma in male rats was observed at 0.2 mg/m3 MWNT-7, the BMCL10 in exposed male rats was 0.01 mg/m3. Using this value and the method of Nakanishi35), the calculated 15 year period limited OEL is 0.15 μg/m3. Using the BMCL10 of 0.01 mg/m3 and the NIOSH method78), which gave an OEL that NIOSH believes would be protective of worker safety and health over a working lifetime of 45 years, the calculated OEL is 0.05 μg/m3.
13. Epilogue
Further studies are required to evaluate the risk of MWCNTs to human health, particularly occupational health. Special emphasis should be placed on the carcinogenicity of different types of MWCNTs, including tangled types. It is very important that the effect of shape and size on MWCNT carcinogenicity be experimentally defined. In addition, other factors that potentially impact on MWCNT carcinogenicity, such as impurities, surface area, and surface reactivity, need to be investigated. It is impossible to examine the carcinogenicity of all the different MWCNTs in rodent 2-year inhalation carcinogenicity tests. Therefore, development of alternative methods is required for hazard identification of MWCNTs. In addition, the MOA of MWCNT carcinogenicity in animals should be investigated by both in vivo and in vitro methods. Once the MOA is described and can be extrapolated to humans, short term qualitative and quantitative approaches are accepted for risk assessment of that MWCNTs carcinogenicity. Exposure measurement data in the workplace must be collected, and it will be necessary that simple and appropriate methods be developed for airborne measurement of MWCNTs. When managed properly, MWCNTs will be of great benefit to human society.
Acknowledgments: The authors would like to thank all members of JBRC for their collaboration of MWNT-7 inhalation studies in rats. The authors also are very grateful for Dr. David B. Alexander for his valuable editorial comments.
Funding: MWNT-7 inhalation studies in JBRC were contracted and supported by Ministry of Health, Labour and Welfare of Japan.
Conflicts of interest: The authors declare that have no conflicts of interest.
References
- 1). Organisation for Economic Co-operation and Development. OECD: Testing Programme of Manufactured Nanomaterials - Dossiers and Endpoints. Chemical safety and biosafety. Safety of manufactured nanomaterials. [Online]. 2016; Available from: URL: http://www.oecd.org/chemicalsafety/nanosafety/dossiers-and-endpoints-testing-programme-manufactured-nanomaterials.html
- 2). Pott F. Animal experiments on biological effects of mineral fibers. In: Wagner JC. Biological effects of mineral fibers. IARC Scientific Publication No. 30. Lyon: International Agency for Research on Cancer; 1980. p. 261-272. [PubMed] [Google Scholar]
- 3). Stanton MF, Layard M, Tegeris A, et al. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst 1981; 67: 965-975. [PubMed] [Google Scholar]
- 4). Takagi A, Hirose A, Nishimura T, et al. Induction of mesothelioma in p53+/- mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci 2008; 33: 105-116. [DOI] [PubMed] [Google Scholar]
- 5). Nagai H, Okazaki Y, Chew SH, et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. PNAS 2011; 108: 1330-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Rittinghausen S, Hackbarth A, Creutzenberg O, et al. The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Part Fiber Toxicol 2014; 11: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Sakamoto Y, Nakae D, Fukumori N, et al. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci 2009; 34: 65-76. [DOI] [PubMed] [Google Scholar]
- 8). Takagi A, Hirose A, Futakuchi M, Tsukada H, Kanno J. Dose-dependent mesothelioma induction by intraperitoneal administration of multi-wall carbon nanotubes in p53 heterozygous mice. Cancer Sci 2012; 103: 1440-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nature Nanotechnol 2008; 3: 423-428. [DOI] [PubMed] [Google Scholar]
- 10). Oberdörster G, Castranova V, Asgharian B, et al. Inhalation exposure to carbon nanotubes (CNT) and carbon nanofibers (CNF): Methodology and dosimetry. J Toxicol Environ Health, Part B 2015; 18: 121-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Kasai T, Gotoh K, Nishizawa T, et al. Development of a new multi-walled carbon nanotube (MWCNT) aerosol generation and exposure system and confirmation of suitability for conducting a single-exposure inhalation study of MWCNT in rats. Nanotoxicol 2014; 8: 169-178. [DOI] [PubMed] [Google Scholar]
- 12). Kasai T, Umeda Y, Ohnishi M, et al. Thirteen-week study of toxicity of fiber-like multi-walled carbon nanotubes with whole-body inhalation exposure in rats. Nanotoxicol 2015; 9: 413-422. [DOI] [PubMed] [Google Scholar]
- 13). Umeda Y, Kasai T, Saito M, et al. Two-Week Toxicity of multi-walled carbon nanotubes by whole-body inhalation exposure in rats. J Toxicol Pathol 2013; 26: 131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). McKinney W, Chen B, Frazer D. Computer controlled multi-walled carbon nanotube inhalation exposure system. Inhal Toxicol 2009; 21: 1053-1061. [DOI] [PubMed] [Google Scholar]
- 15). Porter DW, Hubbs AF, Chen BT, et al. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicol 2012; 7: 1179-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Sargent LM, Porter DW, Staska LM, et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol 2014; 11: 3. (doi: 10.1186/1743-8977-11-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Fujitani Y, Furuyama A, Hirano S. Generation of airborne multi-walled carbon nanotubes for inhalation studies. Aerosol Sci Technol 2009; 43: 881-890. [Google Scholar]
- 18). Kim JS, Sung JH, Song KS, et al. Persistent DNA damage measured by comet assay of Sprague Dawley rat lung cells after five days of inhalation exposure and 1 month post-exposure to dispersed multi-wall carbon nanotubes (MWCNTs) generated by new MWCNT aerosol generation system. Toxicol Sci 2012; 128: 439-448. [DOI] [PubMed] [Google Scholar]
- 19). Ma-Hock L, Gamer AO, Landsiedel R, et al. Generation and characterization of test atmospheres with nanomaterials. Inhal Toxicol 2007; 19: 833-848. [DOI] [PubMed] [Google Scholar]
- 20). Mitchell LA, Gao J, Wal RV, et al. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol Sci 2007; 100: 203-214. [DOI] [PubMed] [Google Scholar]
- 21). Myojo T, Oyabu K, Nishi C, et al. Aerosol generation and measurement of multi-wall carbon nanotubes. J Nanoparticle Research 2009; 11: 91-99. [Google Scholar]
- 22). Pauluhn J, Thiel A. A simple approach to validation of directed-flow noseonly inhalation chambers. J Appl Toxicol 2007; 27: 160-167. [DOI] [PubMed] [Google Scholar]
- 23). Ryman-Rasmussen JP, Cesta MF, Brody AR, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nature Nanotechnol 2009; 4: 747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Taquahashi Y, Ogawa Y, Takagi A, et al. An improved dispersion method of multi-wall carbon nanotube for inhalation toxicity studies of experimental animals. J Toxicol Sci 2013; 38: 619-628. [DOI] [PubMed] [Google Scholar]
- 25). Ma-Hock L, Treumann S, Strauss V, et al. Inhalation toxicity of multi-wall carbon nanotubes in rats exposed for 3 months. Toxicol Sci 2009; 112: 468-481. [DOI] [PubMed] [Google Scholar]
- 26). Pauluhn J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol Sci 2010; 113: 226-242. [DOI] [PubMed] [Google Scholar]
- 27). Kasai T, Umeda Y, Ohnishi M, et al. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part Fibre Toxicol 2016; 13: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Takaya M, Serita F, Yamazaki K, et al. Characteristics of multiwall carbon nanotubes for an intratracheal instillation study with rats. Ind Health 2010; 48: 452-459. [DOI] [PubMed] [Google Scholar]
- 29). Mauderly JL. Relevance of particle-induced rat lung tumors for assessing lung carcinogenic hazard and human lung cancer risk. Environ Health Perspect 1997; 105: 1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Reeves AL, Puro HE, Smith RG, Vorwald AJ. Experimental asbestos carcinogenesis. Environ Res 1971; 4: 496-511. [Google Scholar]
- 31). Donaldson K, Poland CA. Nanotoxicology: New insights into nanotubes. Nat Nanotechnol 2009; 4: 708-710. [DOI] [PubMed] [Google Scholar]
- 32). Suzui M, Futakuchi M, Fukamachi K, et al. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Sci 2016; 107: 924-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Xu J, Futakuchi M, Shimizu H, et al. Multi-walled carbon nanotubes translocate into the pleural cavity and induce visceral mesothelial proliferation in rats. Cancer Science 2012; 103: 2045-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Xu J, Alexander DB, Futakuchi M, et al. Size- and shape-dependent pleural translocation, deposition, fibrogenesis, and mesothelial proliferation by multiwalled carbon nanotubes. Cancer Sci 2014; 105: 763-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Nakanishi J, Morimoto Y, Ogura I, et al. Risk Assessment of the Carbon Nanotube Group. Risk Analysis 2015; 35: 1940-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Donaldson K, Murphy FA, Duffin R, Poland DC. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol 2010; 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Nagai H, Okazaki Y, Chew SH, et al. Intraperitoneal administration of tangled multiwalled carbon nanotubes of 15 nm in diameter does not induce mesothelial carcinogenesis in rats. Pathol Int 2013; 63: 457-462. [DOI] [PubMed] [Google Scholar]
- 38). Muller J, Delos M, Panin N, Rabolli V, Huaux F, Lison D. Absence of carcinogenic response to multi-wall carbon nanotubes in a 104-week bioassay in the peritoneal cavity of the rat. Toxicol Sci 2009; 110: 442-448. [DOI] [PubMed] [Google Scholar]
- 39). Muller J, Decordier I, Hoet PH, et al. Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis 2008; 29: 427-433. [DOI] [PubMed] [Google Scholar]
- 40). Muller J, Huaux F, Fonseca A, et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: toxicological aspects. Chem Res Toxicol 2008; 21: 1698-1705. [DOI] [PubMed] [Google Scholar]
- 41). Takanashi S, Hara K, Aoki K, et al. Carcinogenicity evaluation for the application of carbon nanotubes as biomaterials in rasH2 mice. Sci Rep 2012; 2: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Aiso S, Kubota H, Umeda Y, et al. Translocation of intratracheally instilled multiwall carbon nanotubes to lung-associated lymph nodes in rats. Ind Health 2011; 49: 215-220. [DOI] [PubMed] [Google Scholar]
- 43). Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal toxicol 2004; 16: 437-445. [DOI] [PubMed] [Google Scholar]
- 44). Tamura M, Inada M, Nakazato T, et al. A determination method of pristine multiwall carbon nanotubes in rat lungs after intratracheal instillation exposure by combustive oxidationnondispersive infrared analysis. Talanta 2011; 84: 802-808. [DOI] [PubMed] [Google Scholar]
- 45). Doudrick K, Corson N, Oberdörster G, et al. Extraction and quantification of carbon nanotubes in biological matrices with application to rat lung tissue. ACS Nano 2013; 7: 8849-8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Ohnishi M, Yajima H, Kasai T, et al. Novel method using hybrid markers: development of an approach for pulmonary measurement of multi-walled carbon nanotubes. J Occup Med Toxicol 2013; 8: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Ohnishi M, Suzuki M, Yamamoto M, et al. Improved method for measurement of multi-walled carbon nanotubes in rat lung. J Occup Med Toxicol 2016; 11: 44. (doi: 10.1186/s12995-016-0132-7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Mercer R, Scabilloni J, Hubbs A, et al. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol 2013; 10: 33. (doi: 10.1186/1743-8977-10-33). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Mercer RR, Scabillon JF, Hubb AF, et al. Extrapulmonary transport of MWCNT following inhalation exposure. Part fibre toxicol 2013; 10: 38. (doi: 10.1186/1743-8977-10-38). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Liang G, Yin L, Zhang J, et al. Effects of subchronic exposure to multi-walled carbon nanotubes on mice. J Toxicol Environmen Part A 2010; 73: 463-470. [DOI] [PubMed] [Google Scholar]
- 51). Murphy FA, Poland CA, Duffin R, et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol 2011; 178: 2587-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Donaldson K, Poland CA. Nanotoxicity: challenging the myth of nano-specific toxicity. Curr Opin Biotechnol 2013; 24: 724-734. [DOI] [PubMed] [Google Scholar]
- 53). Toyokuni S. The origin and future of oxidative stress pathology: From the recognition of carcinogenesis as an iron addiction with ferroptosis-resistance to non-thermal plasma therapy. Pathol Int 2016; 66: 245-259. [DOI] [PubMed] [Google Scholar]
- 54). Donaldson K, Aitken R, Tran L, et al. Carbon Nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci 2006; 92: 5-22. [DOI] [PubMed] [Google Scholar]
- 55). Aldieri E, Fenoglio I, Cesano F, et al. The role of iron impurities in the toxic effects exerted by short multiwalled carbon nanotubes (MWCNT) in murine alveolar macrophages. J Toxicol Environ Health A 2013; 76: 1056-1071. [DOI] [PubMed] [Google Scholar]
- 56). Wang Y, Okazaki Y, Shi L, et al. Role of hemoglobin and transferrin in multi-wall carbon nanotube-induced mesothelial injury and carcinogenesis. Cancer Sci 2016; 107: 250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ health perspect 2005; 113: 823-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Ema M, Imamura T, Suzuki H, Kobayashi N, Naya M, Nakanishi J. Evaluation of genotoxicity of multi-walled carbon nanotubes in a battery of in vitro and in vivo assays. Regul Toxicol Pharmacol 2012; 63: 188-195. [DOI] [PubMed] [Google Scholar]
- 59). Umbuzeiro GA, Coluci VR, Honorio JG, et al. Understanding the interaction of multi-walled carbon nanotubes with mutagenic organic pollutants using computational modeling and biological experiments. Trends Analyt Chem 2011; 30: 437-446. [Google Scholar]
- 60). Doak SH, Manshian B, Jenkins GJS, Singh N. In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat Res 2012; 745: 104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Asakura M, Sasaki T, Sugiyama T, et al. Genotoxicity and cytotoxicity of multi-wall carbon nanotubes in cultured Chinese hamster lung cells in comparison with chrysotile A fibers. J Occup Health 2010; 52: 155-166. [DOI] [PubMed] [Google Scholar]
- 62). Yasui M, Kamoshita N, Nishimura T, Honma M. Mechanism of induction of binucleated cells by multiwalled carbon nanotubes as revealed by live-cell imaging analysis. Genes Environ 2015; 37: 6. (doi: 10.1186/s41021-015-0003-y). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Kato T, Totsuka Y, Ishino K, et al. Genotoxicity of multi-walled carbon nanotubes in both in vitro and in vivo assay systems. Nanotoxicology 2013; 7: 452-461. [DOI] [PubMed] [Google Scholar]
- 64). Siegrist KJ, Reynolds SH, Kashon ML, et al. Genotoxicity of multi-walled carbon nanotubes at occupationally relevant doses. Part Fibre Toxicol 2014; 11: 6. (doi: 10.1186/1743-8977-11-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Sasaki T, Asakura M, Ishioka C, et al. In vitro chromosomal aberrations induced by various shapes of multi-walled carbon nanotubes (MWCNTs). J Occup Health 2016; 58: 622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Driessen MD, Mues S, Vennemann A, et al. Proteomic analysis of protein carbonylation: a useful tool to unravel nanoparticle toxicity mechanisms. Part Fibre Toxicol 2015; 12: 36. (doi: 10.1186/s12989-015-0108-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Archer VE, Dixon WC. Carcinogenicity of fibers and films: a theory. Med Hypotheses 1979; 5: 1257-1262. [DOI] [PubMed] [Google Scholar]
- 68). Mercer RR, Hubbs AF, Scabilloni JF, et al. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol 2010; 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69). Grosse Y, Loomis D, Guyton KZ, et al. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol 2014; 15: 1427-1428. [DOI] [PubMed] [Google Scholar]
- 70). Fukushima S, Wanibuchi H, Morimura K, et al. Lack of a dose-response relationship for carcinogenicity in the rat liver with low doses of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline or N-nitrosodiethylamine. Jpn J Cancer Res 2002; 93: 1076-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Fukushima S, Wei M, Kakehashi A, Wanibuchi H. Threshold for genotoxic carcinogen: evidence from mechanism-based carcinogenicity studies. Cancer risk assessment 2010. (doi: 10.1002/9780470622728.ch8.). [DOI] [Google Scholar]
- 72). Fukushima S, Gi M, Kakehashi A, Wanibuchi H. Qualitative and quantitative assessment on loe-dose carcinogenicity of genotoxic hepatocarcinogens: Dose-response for key events in rat hepatocarcinogenesis. In: Nohmi T, Fukushima S, editors. Thresholuds of genotoxic carcinogens of DNA-reactive compound. Amsterdam: Elesevier Publisher; 2016. p. 1-18. [Google Scholar]
- 73). Wei M, Wanibuchi H, Nakae D, et al. Low-dose carcinogenicity of 2-amino-3-methylimidazo[4,5-f ]quinoline in rats: Evidence for the existence of no-effect levels and a mechanism involving p21 (Cip / WAF1). Cancer Sci 2011; 102: 88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Williams GM, Duan JD, Iatropulos MJ, Koberts T. A no observed adverse effect level for DNA adduct formation in rat liver with repeat prolonged dosing of the hepatocarcinogen 2-acetylaminofluorene. Toxicol Res 2015; 4: 233-240. [Google Scholar]
- 75). Kobets T, Williams GM. Thresholuds for hepatocarcinogenicity of DNA-reactive compounds. In: Nohmi T, Fukushima S, editors. Thresholuds of genotoxic carcinogens of DNA-reactive compounds. Amsterdam: Elesevier Publisher; 2016. p. 19-36. [Google Scholar]
- 76). Bolt HM, Hermann M. The concept of "practical thresholds" in the derivation of occupational exposure limits for carcinogens by the scientific committee on occupational exposure limits (SCOEL) of the European Union. Genes and Environ 2008; 30: 114-119. [Google Scholar]
- 77). Bolt HM. Practical thresholds in the derivation of occupational exposure limits (OELs) for carcinogens. In: Nohmi T, Fukushima S, editors. Thresholuds of genotoxic carcinogens of DNA-reactive compounds. Amsterdam: Elesevier Publisher; 2016. p. 117-126. [Google Scholar]
- 78). National Institute for Occupational Safety and Health (NIOSH). Current Intelligence Bulletin 65, Occupational Exposure to Carbon nanotubes and nanofibers, DHHS (NIOSH) Publication No. 2013-145, April 2013. [Online]. Available from: URL: https://www.cdc.gov/niosh/docs/2013-145/pdfs/2013-145.pdf
- 79). United States Environmental Protection Agency (US.EPA). Benchmark dose software version 2.6 user's manual. EPA/100/R-12/001. Washington, DC: US EPA; 2015. [Google Scholar]