Abstract
In this work we evaluated methanolic extracts from different parts (leaves, seeds, fruit peel and pulp) of Chamaerops humilis L. for antioxidant activity and the ability to inhibit enzymes linked with neurodegenerative diseases: acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and tyrosinase (TYR). The total content of phenolics, flavonoids and condensed tannins was also determined. The antioxidant and inhibitory activities of the extracts varied significantly according to the tissue. Seed extracts showed the greatest ability to scavenge DPPH (IC50 = 81.28 µg mL−1) and ABTS (1440.42 µmolTE ) and to reduce iron (1142.46 µmolAAE ). Seed and peel extracts strongly inhibited AChE (IC50 = 660.16 and 653.68 µg mL−1, respectively) and BChE (IC50 = 304.86 and 701.54 µg mL−1, respectively). The strongest inhibition of TYR was achieved by the seed and pulp extracts (268.97 and 279.99 µg mL−1, respectively). The highest levels of phenolics and condensed tannins were found in the seed extract (1564.88 µmolGAE and 170.00 µmolcE , respectively) whereas the leaf extract was the richest in flavonoids (139.88 µmolQE ). HPLC-DAD analysis indicated the presence of flavonoids and phenolic acids (hydroxycinnamic acids) in the leaf and pulp extracts. A high correlation was found between the total condensed tannins content and the antioxidant and enzyme inhibition activities, suggesting these compounds are responsible for the biological activity of the extracts. Overall, our results indicate that C. humilis extracts may provide a new and alternative source of agents for medical and industrial applications.
Keywords: Acetylcholinesterase, Butyrylcholinesterase, Dwarf palm, Tannins, Tyrosinase
Introduction
Recent investigations have shown that many palm species contain high levels of health-promoting bioactive compounds (Kang et al. 2012; Rezaire et al. 2014; Kchaou et al. 2016). Chamaerops humilis L. (Arecaceae) is a dwarf palm that grows on the European Mediterranean coast and in North Africa (Dufaÿ and Anstett 2004). C. humilis is the most northerly palm species in Europe and is also one of the most cold-tolerant (Giovino et al. 2014). The species is cultivated in many Mediterranean countries as an ornamental because it is robust and has decorative characteristics. Moreover, some components of the plant are consumed as food and used in traditional medicine. The husk (higa) is eaten in southern Spain, the fruits in Morocco and the young suckers in Italy (Merlo et al. 1993; Haynes and Mc-Laughlin 2000). In Algeria, the spadices and the heart of the palm are used to treat several disorders of the digestive tract (Hasnaoui et al. 2013), whereas the leaves used in Morocco and Algeria for the treatment of diabetes (Bnouham et al. 2002; Hasnaoui et al. 2013), and the fruits used in several countries as an astringent agent due to their bitterness (Merlo et al. 1993).
The phytochemical properties of C. humilis are not well characterized although several studies have reported the presence of tannins, flavonoids, saponins, sterols, and terpenoids, which may explain its pharmacological effects (Benmehdi et al. 2012; Benahmed-Bouhafsoun et al. 2013; Hasnaoui et al. 2013). A more recent study showed that C. humilis seed oil is rich in bioactive compounds that are resistant to heat and oxidation (Nehdi et al. 2014). Leaf extracts also possess antioxidant activity and the ability to inhibit lipoxygenase (Benahmed-Bouhafsoun et al. 2013; Miguel et al. 2014).
Neurodegenerative diseases, particularly Alzheimer’s disease (AD) and Parkinson’s disease (PD), are major health problems especially in industrialized countries (Metzler-Baddeley 2007; Uc and Rizzo 2008). The pathogenesis of AD includes the depletion of acetylcholine in the brain, and cholinomimetic drugs are therefore used to temporarily improve cognitive function (Francis et al. 1999). Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors are widely used for the treatment of AD because they slow down the rate of acetylcholine depletion. Tyrosinase (TYR), an enzyme that converts l-tyrosine to l-DOPA and oxidizes l-DOPA to form dopachrome, induces the production of melanin (Seo et al. 2003). This pigment helps to prevent UV damage to the skin, hair and eyes, but excess production is associated with hyperpigmentation and neurodegenerative disorders such as PD. TYR is also responsible for browning in fruits and vegetables and therefore TYR inhibitors are frequently applied to plant-based foods. Concerns over the toxicity and side effects of synthetic inhibitors of these enzymes have led to the search for safe and effective inhibitors of natural origin (Zengin et al. 2015).
Oxidative stress is also involved in the pathogenesis of neurodegenerative disorders and other chronic diseases, but progression can be delayed by minimizing redox imbalances that generate reactive oxygen species (ROS). Antioxidants scavenge ROS and other free radicals and therefore plants rich in antioxidants could help to reduce the impact of age-related chronic diseases (Krishnaih et al. 2007). Recent investigations have focused on the identification of plants rich in natural products that scavenge ROS and inhibit enzymes, because these may provide a natural therapeutic approach to prevent or manage diseases such as AD and PD. The aim of this work was to investigate the pharmacological properties of C. humilis by evaluating the antioxidant and enzyme (AChE, BChE and TYR) inhibitory activities of methanolic extracts, and to ascertain if these activities vary with the part of the plant used (leaves, seeds, and fruits pulp and peel).
Materials and methods
Chemicals and reagents
We obtained 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) tablets, ethanol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), methanol, trichloroacetic acid (TCA), K2S2O8, acetylthiocholine iodide (ATCI), butyrylthiocholine chloride (BTCI), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), electric eel AChE (EC 3.1.1.7, type VIS), horse serum BChE (EC 3.1.1.8), mushroom TYR (EC 1.14.18.1), 3,4-dihydroxy-l-phenylalanine (l-DOPA), quercetin, galanthamine hydrobromide and kojic acid from Sigma-Aldrich (Steinheim, Germany). Folin–Ciocalteu’s phenol reagent (F–C reagent), gallic acid, Na2CO3, AlCl3, CH3COONa and FeCl3 were acquired from VWR (Leuven, Belgium). K3[Fe(CN)6], 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and butylated hydroxytoluene (BHT) were purchased from Acros Organics (Geel, Germany). Formic acid and methanol Lichrosolv were obtained from Merck (Darmstadt, Germany). We obtained 5-O-caffeoylquinic acid, catechin, luteolin-8-C-glucoside, apigenin-8-C-glucoside and apigenin-7-O-rutinoside from Extrasynthèse, and 3-O-caffeoylquinic and 4-O-caffeoylquinic from Chengdu Biopurity Phytochemicals Ltd (Sichuan, China).
Plant material and extraction procedure
Chamaerops humilis leaves and ripe fruits were collected in September 2014 from plants growing wild in the Algarve region (South Portugal). The plant was authenticated by JM Rosa Pinto from the herbarium of the University of Algarve (Faro, Portugal). The plant material (leaves, seeds, fruit peel and pulp) was dried at 40 °C until a constant weight was achieved (~ 48 h) and then powdered in a blender (mean particle size < 2 mm). Dried plant material was extracted twice by maceration with methanol for 24 h at room temperature. After filtration, the extracts were concentrated to dryness in a rotary evaporator at 40 °C under reduced pressure, and stored at – 20 °C.
Determination of the total phenolic content
The total phenolic content of the extracts was determined using the F–C colorimetric method (Ainsworth and Gillespie 2007). Briefly, 200 µL of 10% (v/v) F–C reagent was mixed with 100 µL of plant extract in phosphate buffer (75 mM, pH 7.0) before adding 800 µL of 700 mM Na2CO3. The reaction was incubated for 2 h at room temperature. Gallic acid was used as a positive control and phosphate buffer as negative control. The absorbance was measured at 765 nm and the results were expressed as gallic acid equivalents per gram of extract (µmolGAE ).
Determination of the total flavonoid content
The total flavonoid content of each extract was measured using an adapted aluminum chloride colorimetric method (Woisky and Salantino 1998). The extracts and quercetin dilutions (500 µL) were mixed with 80% ethanol (1500 µL), 1% AlCl3 (100 µL), and 1 M CH3COONa (100 µL) and incubated for 30 min at room temperature. The absorbance was measured at 415 nm, and the results were expressed as quercetin equivalents per gram of extract (µmolQE ).
Determination of the total condensed tannins content
The condensed tannins content of each extract was determined using the vanillin-HCl method (Broadhurst and Jones 1978) with modifications. Briefly, 1500 µL of vanillin reagent (4% w/v in methanol) was mixed with 250 µL extract and 750 µL concentrated HCl (37%). The extract samples, catechin dilutions and blanks (with methanol) were incubated for 15 min. The absorbance was measured at 500 nm and the results were expressed in terms of catechin equivalents per gram of extract (µmolCE ).
Antioxidant activity
2,2′-Azinobis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical cation decoloration assay
The ABTS free radical-scavenging activity of the extracts was evaluated as described by Re et al. (1999). A 7 mM stock solution of ABTS·+ was prepared using potassium persulfate as the oxidizing agent. The absorbance was measured at 734 nm and the sample dilution that achieved 20–80% inhibition of the blank absorbance was selected to calculate Trolox equivalent antioxidant capacity (TEAC) values. The results were expressed as trolox equivalents per gram of extract (µmolTE ).
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging assay
The capacity of the extracts to scavenge DPPH radicals was evaluated as described by Soler-Rivas et al. (2000) with some modifications. We mixed 100 µL DPPH (90 µM methanolic solution) with 10 µL extract at different concentrations in a 96-well microplate before diluting the mixture with 190 µL methanol. The extract was replaced with methanol for the negative control and BHT was used as the reference antioxidant. The reduction of DPPH radicals was measured at 515 nm after 30 min incubation at room temperature. Radical-scavenging activity was expressed in terms of the amount of sample necessary to reduce the initial absorbance by 50% (IC50).
Ferric reducing antioxidant power (FRAP)
The reducing properties of the extracts were evaluated as described by Pulido et al. (2000) with slight modifications. The plant extract (100 µL) was mixed with 250 µL sodium phosphate buffer (200 mM, pH 6.6) and 250 µL 1% K3[Fe(CN)6]. The mixture was incubated for 20 min at 50 °C and centrifuged at 650 rpm for 10 min following the addition of 250 µL 10% TCA. In a clear 96-well microplate, 100 µL of the supernatant was diluted with 100 µL water and mixed with 20 µL 0.1% FeCl3. Phosphate buffer was used as the negative control and ascorbic acid as the positive control. The absorbance was measured at 700 nm to determine the reducing activity, and the results were expressed as ascorbic acid equivalents (µmolAAE ).
Enzyme inhibition
Cholinesterase (AChE and BChE) inhibition
The inhibition of AChE and BChE activity was measured according to Ellman et al. (1961). The following components were mixed in the wells of a 96-well microplate: 3 mM DTNB, 15 mM substrate (ATCI or BTCI), 100 mM phosphate buffer (pH 8.0) and extracts at different concentrations. Galanthamine (standard inhibitor) was used as a positive control and buffer without extract as a negative control. AChE or BChE (0.28 U mL−1) was added and the absorbance was measured at 405 nm for 5 min. The inhibitory activity was expressed as IC50 values (the concentration required to inhibit AChE or BChE activity by 50%).
Tyrosinase (TYR) inhibition
The inhibition of TYR was determined using the modified dopachrome method (Masuda et al. 2005). The following components were mixed with the extracts in the wells of a 96-well microplate: 80 µL phosphate buffer (pH 6.8), 40 µL TYR, and 40 µL l-DOPA. The absorbance was measured at 475 nm and the TYR inhibitory activity was expressed as percentage inhibition. Kojic acid was used as the reference.
High-performance liquid chromatography-diode array detection (HPLC-DAD) analysis
The extracts were analyzed on an analytical HPLC unit (Gilson, Villiers le Bel, France), using a Spherisorb ODS2 column (4.6 × 250 mm, 5 µm particle size). The solvent system was a gradient of water:formic acid (19:1) (A) and methanol (B), starting with 5% B and increasing to 15% B at 3 min, 25% B at 13 min, 30% B at 25 min, 35% B at 35 min, 45% B at 39 min, 45% B at 42 min, 55% B at 47 min, 75% B at 56 min, and 100% B at 60 min, at a solvent flow rate of 0.9 mL min−1. Products were detected using a Gilson Diode Array Detector (DAD). Spectral data from all peaks were collected in the range 200–400 nm. The data were processed using Unipoint® system software (Gilson Medical Electronics, Villiers le Bel, France). The compounds in each extract were identified by comparing their retention times and UV–Vis spectra in the 200–400 nm range with authentic standards injected under the same conditions. Tricin-7-O-rutinoside was quantified as apigenin-7-O-rutinoside and the other compounds were quantified as themselves.
Statistical analysis
All the experiments were carried out three times using triplicate samples. The data were presented as mean ± standard error and were processed by one-way analysis of variance (ANOVA). Significant differences between means were identified using Duncan’s New Multiple Range Test (p < 0.05), and correlations were calculated using Pearson’s test. All statistical analysis was carried out using the SPSS statistical package for Windows v18.0 (SPSS Inc., Chicago, IL, USA).
Results and discussion
Biological activities
Antioxidant compounds play a vital role in preventing or delaying the progression of neurodegenerative diseases because they counteract the oxidative stress that produces free radicals (Tabet 2006). Lately there has been worldwide interest in finding antioxidants from natural sources because they can be more potent and less toxic than synthetic antioxidants (Abdel-Hameed et al. 2014). Several plant extracts have been investigated as potential sources of natural antioxidants (Nile et al. 2017; Suluvoy and Grace 2017). Here we tested the antioxidant activity of four C. humilis extracts (leaves, seeds, fruit peel and pulp). The capacity of each extract to scavenge free radicals was assessed using DPPH and ABTS assays, and the results are presented in Table 1. DPPH radical-scavenging activity, in which a hydrogen donor generates the reduced form (DPPH-H·), is used extensively to screen for antioxidants. The seed extract showed the lowest IC50 value, indicating the strongest antioxidant activity (81.28 ± 1.79 µg mL−1). This extract also showed the strongest ability to scavenge ABTS·+ radicals, revealing that both lipophilic and hydrophilic substances with antioxidant activity are present in the same extract (1440.42 ± 49.88 µmolTE ). The reducing capacity of a sample is also regarded as a significant indicator of its potential antioxidant activity. The ability of C. humilis extracts to reduce a ferricyanide/Fe3+ complex was quantified by measuring the formation of a blue color at 700 nm, representing the ferrous ion. The values ranged from 369.59 ± 22.91 to 1142.46 ± 23.24 µmolAAE and indicated that the seed extract was the most potent reductant among the four samples. Although the antioxidant activity of C. humilis has been investigated before, only leaf extracts have been tested. Miguel et al. (2014) reported IC50 values of 0.035 ± 0.061 and 0.035 ± 0.079 mg mL−1 using the DPPH and ABTS methods, respectively, with extracts prepared from leaf material collected in Morocco. Benahmed-Bouhafsoun et al. (2013) reported an IC50 of 180.71 ± 6.6 µg mL−1 in a DPPH assay, using leaf material collected in Algeria. The antioxidant activity of C. humilis fruit extracts is described for the first time in this study, although fruit extracts from other palm species have been tested (Kang et al. 2012; Rezaire et al. 2014; Kchaou et al. 2016). Fruit extracts, particularly those obtained from the peel, displayed strong antioxidant activity in our three assays (Table 1).
Table 1.
Antioxidant activity of Chamaerops humilis extracts determined using the 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2 azino-bis(3-ethylbenzothiazloine-6-sulfonic acid) (ABTS) and ferric reducing antioxidant power (FRAP) assays
| DPPH (IC50, µg mL−1) | ABTS (µmolTE ) | FRAP (µmolTE ) | |
|---|---|---|---|
| Leaves | 346.08 ± 12.63d | 593.23 ± 9.80b | 434.34 ± 13.71c |
| Peel | 180.97 ± 6.49b | 550.08 ± 20.14b | 580.29 8.07b |
| Pulp | 325.03 ± 10.81c | 351.06 ± 11.99c | 369.56 ± 22.91d |
| Seed | 81.28 ± 1.79a | 1440.42 ± 49.88a | 1142.46 ± 23.24a |
Values are expressed as means ± SE (n = 3). Values followed by different letters are significantly different at p < 0.05. Reference in DPPH assay, BHT: IC50 = 462.24 ± 0.03 μg mL−1
The cholinergic neurotransmitter acetylcholine is depleted in AD patients and cholinesterase inhibitors have been shown to augment the activity of surviving cholinergic neurons, improving the memory and cognition in AD patients (Massoud and Gauthier 2010). Furthermore, TYR plays an important role in the formation of neuromelanin in the human brain and catalyzes the transformation of tyrosine into dopaquinone, a neurotoxic substance associated with PD, making TYR inhibitors an attractive approach for the treatment of PD (Khan 2007). Table 2 shows the inhibitory activities of C. humilis extracts against AChE, BChE and TYR. The seed extract was the most potent inhibitor of AChE (IC50 = 660.16 ± 21.94 µg mL−1), BChE (IC50 = 304.86 ± 26.41 µg mL−1) and TYR (IC50 = 268.97 ± 17.75 µg mL−1). Peel and pulp extracts were also able to inhibit cholinesterase and TYR activities, respectively (Table 2). In contrast, the leaf extract showed the lowest ability to inhibit all three enzymes (IC50 = 7005.82 ± 287.85, 8358.87 ± 343.57 and 1633.62 ± 62.33 µg mL−1 for AChE, BChE and TYR, respectively). To the best of our knowledge, the anti-cholinesterase and anti-TYR activities of the extracts studied herein have not been reported previously.
Table 2.
Inhibitory activity of Chamaerops humilis extracts against acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and tyrosinase (TYR) (IC50, µg mL−1)
| AChE | BChE | TYR | |
|---|---|---|---|
| Leaves | 7005.82 ± 287.85c | 8358.87 ± 343. 57c | 1633.62 ± 62.33c |
| Peel | 653.68 ± 18.20a | 701.54 ± 65.84a | 747.46 ± 66.23b |
| Pulp | 1486.37 ± 178.69b | 1582.11 ± 185.26b | 279.99 ± 26.87a |
| Seed | 660.16 ± 21.94a | 304.86 ± 26.41a | 268.97 ± 17.75a |
| Standard | 2.20 ± 0.04 | 11.70 ± 1.09 | 49.23 ± 0.70 |
Values are expressed as means ± SE (n = 3). Values followed by different letters are significantly different at p < 0.05. The standard inhibitor was galanthamine for AChE and BChE, and kojic acid for TYR
Bioactive compounds
Phenolic compounds are a group of plant compounds with biotechnological and industrial significance and are the major contributors to the diverse biological activities of plant extracts (Robards et al. 1999). The total phenolic content of the C. humilis extracts was determined, revealing values of 344.33 ± 11.82 to 1564.88 ± 29.91 µmolGAE (Table 3). The highest phenolic content was found in the seed extract, and the lowest in the pulp extract. Flavonoids and tannins are widely distributed in the plant kingdom and they are also have diverse bioactive properties. We therefore measured the flavonoid and condensed tannin contents of the C. humilis extracts. The total flavonoid content ranged from 19.48 ± 0.64 to 139.88 ± 2.38 µmolQE (Table 3), with the highest flavonoid content found in the leaf extract, and the lowest in the pulp extract. In contrast, the highest level of total condensed tannins (170.00 ± 12.42 µmolCE ) was found in the seed extract (Table 3).
Table 3.
Total phenolic, flavonoid and condensed tannin contents of Chamaerops humilis extracts determined using the Folin–Ciocalteu (F–C), AlCl3 and vanillin-HCl assays, respectively
| Total phenolics (µmolGAE ) | Total flavonoids (µmolGAE ) | Total condensed tannins (µmolGAE ) | |
|---|---|---|---|
| Leaves | 591.88 ± 23.60b | 139.88 ± 2.38a | 38.75 ± 6.06c |
| Peel | 567.59 ± 12.97b | 26.91 ± 2.20c | 111.46 ± 9.78b |
| Pulp | 344.33 ± 11.82c | 19.48 ± 0.64d | 61.27 ± 5.12c |
| Seed | 1564.88 ± 29.91a | 42.92 ± 2.99b | 170.00 ± 12.42a |
Values are expressed as means ± SE (n = 3). Values followed by different letters are significantly different at p < 0.05
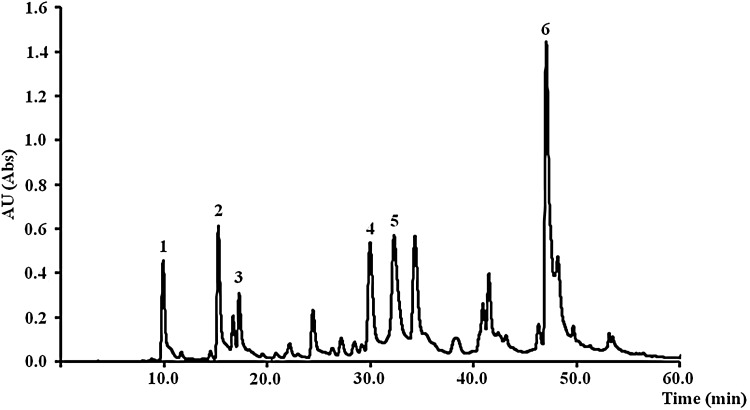
HPLC-DAD analysis indicated the presence of flavonoids and phenolic acids (hydroxycinnamic acids) in the leaf and pulp extracts. The leaf extract contained three hydroxycinnamic acids: 3-O-caffeoylquinic acid (compound 1, 3.27 ± 0.24 mg g−1 dry extract), 4-O-caffeoylquinic acid (compound 2, 4.81 ± 0.47 mg g−1 dry extract) and 5-O-caffeoylquinic acid (compound 3, 2.97 ± 0.15 mg g−1 dry extract). It also contained the flavonoids luteolin-8-C-glucoside (compound 4, 3.88 ± 0.59 mg g−1 dry extract) and apigenin-8-C-glucoside (compound 5, 7.16 ± 1.06 mg g−1 dry extract) (Fig. 1; Table 4). Furthermore, compound 6 was the major compound in the leaf extract (7.72 ± 0.99 mg g−1 dry extract) and a comparison with previously reported UV spectra allowed a tentative annotation as tricin-7-O-rutinoside (Hirai et al. 1986; Harborne et al. 1974). In the pulp extract, we identified 3-O-caffeoylquinic acid (0.18 ± 0.03 mg g−1 dry extract) and (+)-catechin (0.54 ± 0.13 mg g−1 dry extract). The preliminary chemical characterization therefore agrees with the data obtained using colorimetric methods (Table 3). Although phytochemical studies in C. humilis are scarce, the presence of tannins and flavonoids has been reported in methanolic extracts of leaves and fruits, as well as saponins and quinones in leaves (Benmehdi et al. 2012; Benahmed-Bouhafsoun et al. 2013). Furthermore, the analysis of a methanolic leaf extract by GC/MS and FT-IR spectroscopy identified lucenin 2 (luteolin-6,8-di-C-glucoside), dasycarpidan-1-methanol, acetate (ester), 1,3-d-5-hexan-2-one-2, 4-dinitrophenylhydrazone and 9-hexadecenoic acid as the most abundant compounds (Left et al. 2013).
Fig. 1.
HPLC-DAD chromatogram of C. humilis leaf extract. Detection at 350 nm: (1) 3-O-caffeoylquinic acid; (2) 4-O-caffeoylquinic acid; (3) 5-O-caffeoylquinic acid; (4) luteolin-8-C-glucoside; (5) apigenin-8-C-glucoside; (6) tricin-7-O-rutinoside. AU arbitrary units
Table 4.
Phenolic compounds identified in C. humilis leaf extract
| Peak | Compound | RT (min) | mg g−1 dry extract |
|---|---|---|---|
| 1 | 3-O-Caffeoylquinic acid | 9.81 | 3.27 ± 0.24 |
| 2 | 4-O-Caffeoylquinic acid | 15.53 | 4.81 ± 0.47 |
| 3 | 5-O-Caffeoylquinic acid | 17.53 | 2.97 ± 0.15 |
| 4 | Luteolin-8-C-glucoside | 30.19 | 3.88 ± 0.59 |
| 5 | Apigenin-8-C-glucoside | 32.47 | 7.16 ± 1.06 |
| 6 | Tricin-7-O-rutinoside | 46.31 | 7.72 ± 0.99 |
Values are expressed as mean ± SD (n = 3)
We also evaluated the correlation between the total content of each class of bioactive metabolite, the antioxidant activity and the capacity to inhibit enzymes. We observed a strong correlation between the total phenolic content and antioxidant activity in all three assays (Table 5). Furthermore, a significant correlation was observed between the total content of condensed tannins and both forms of biological activity (antioxidant and enzyme inhibition) suggesting that this group of compounds may play an important role in the bioactivity of the extracts (Table 5). There was a strong correlation between the abundance of phenols and tannins (r = 0.824, p < 0.05), suggesting that the tannins are an important group of phenols in C. humilis extracts. Tannins are structurally diverse compounds that vary greatly in abundance within and among plant species (Zhang et al. 2010) and demonstrate a wide range of biological effects including antioxidant and antimicrobial activities (Serrano et al. 2009; Zhang et al. 2010). In addition to its important medical applications, the demand for tannins has increased due to their applications in leather tanning and glue production (Sousa and Brito 2015).
Table 5.
Pearson’s correlation coefficients between assays
| Total bioactive compounds | Antioxidant activity | Enzyme inhibitory activity | ||||
|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | AChE | BChE | TYR | |
| TPC | − 0.825** | 0.990** | 0.976** | − 0.289 | − 0.327 | − 0.325 |
| TFC | 0.457 | − 0.018 | − 0.208 | 0.956** | 0.949** | 0.899** |
| TCTC | − 0.956** | 0.806** | 0.919** | − 0.707* | − 0.717** | − 0.601* |
Data represents Pearson’s correlation coefficient R
TPC total phenolic content, TFC total flavonoid content, TCTC total condensed tannin content, DPPH 1,1-diphenyl-2-picrylhydrazyl, ABTS 2,2 azino-bis(3-ethylbenzothiazloine-6-sulfonic acid), FRAP ferric reducing antioxidant power, AChE acetylcholinesterase, BChE butyrylcholinesterase, TYR tyrosinase
* p < 0.05
** p < 0.01
Conclusion
Our investigation has shown that extracts from C. humilis, particularly seed extracts, have potent antioxidant activity and also the ability to inhibit AChE, BChE and TYR. There was a strong correlation between the total content of condensed tannins and both of these biological activities, suggesting that the presence of tannins may explain the biological effects of the extracts. Although more detailed phytochemical investigations are required to identify the specific bioactive compounds in each extract, our results suggest that C. humilis could be developed as an important source of compounds for the treatment of global health problems such as neurodegenerative diseases.
Acknowledgements
We would like to acknowledge financial support from the EU (FEDER funds through COMPETE) and from National Funds (FCT/MEC, Fundação para a Ciência e Tecnologia/Ministério da Educação e Ciência) through project UID/QUI/50006/2013, co-financed by the EU (FEDER under the Partnership Agreement PT2020). S. Gonçalves acknowledges a Grant from FCT (SFRH/BPD/84112/2012) and C. Grosso thanks FCT for the FCT Investigator award (IF/01332/2014).
Abbreviations
- AAE
Ascorbic acid equivalents
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
- AChE
Acetylcholinesterase
- AD
Alzheimer’s disease
- ATCI
Acetylthiocholine iodide
- BChE
Butyrylcholinesterase
- BTCI
Butyrylthiocholine chloride
- CE
Catechin equivalents
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- l-DOPA
3,4-dihydroxy-l-phenylalanine
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- F–C reagent
Folin–Ciocalteu reagent
- FRAP
Ferric reducing antioxidant power
- GAE
Gallic acid equivalents
- HPLC-DAD
High-performance liquid chromatography-diode array detection
- PD
Parkinson’s disease
- QE
Quercetin equivalents
- TCA
Trichloroacetic acid
- TE
Trolox equivalents
- Trolox
6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
- TYR
Tyrosinase
Authors’ contribution
The work presented here was accomplished with the collaboration of all authors. The research topic and framework were defined by S. Gonçalves and A. Romano. E. Moreira and C. Grosso preformed the HPLC analysis under the supervision of P.B. Andrade and P. Valentão. S. Gonçalves and J. Medronho prepared the plant material and conducted the biological activity assays. S. Gonçalves analyzed the data and wrote the paper. All authors revised and approved the manuscript.
Compliance with ethical standards
Conflict of interest
Authors declared no conflict of interest.
References
- Abdel-Hameed E-SS, Nagaty MA, Salman MS, Bazaidm SA. Phytochemicals, nutritionals and antioxidant properties of two prickly pear cactus cultivars (Opuntia ficus indica Mill.) growing in Taif, KSA. Food Chem. 2014;160:31–38. doi: 10.1016/j.foodchem.2014.03.060. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Benahmed-Bouhafsoun A, Djied S, Mouzaz F, Kaid-Harche M. Phytochemical composition and in vitro antioxidant activity of Chamaerops humilis L. extracts. Int J Pharm Pharm Sci. 2013;5:741–744. [Google Scholar]
- Benmehdi H, Hasnaoui O, Benali O, Salhi F. Phytochemical investigation of leaves and fruits extracts of Chamaerops humilis L. J Mater Environ Sci. 2012;3:320–337. [Google Scholar]
- Bnouham M, Mekhfi H, Legssyer A, Ziyyat A. Medicinal plants used in the treatment of diabetes in Morocco. Int J Diabetes Metab. 2002;10:33–50. [Google Scholar]
- Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. J Sci Food Agric. 1978;29:788–794. doi: 10.1002/jsfa.2740290908. [DOI] [Google Scholar]
- Dufaÿ M, Anstett M-C. Cheating is not always punished: killer female plants and pollination by deceit in the dwarf palm Chamaerops humilis. J Evol Biol. 2004;17:862–868. doi: 10.1111/j.1420-9101.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino A, Scibetta S, Saia S, Guarino C. Genetic and morphologic diversity of European fan palm (Chamaerops humilis L.) populations from different environments from Sicily. Bot J Linn Soc. 2014;176:66–81. doi: 10.1111/boj.12195. [DOI] [Google Scholar]
- Harborne JB, Williams CA, Greenham J. Distribution of charged favones and caffeylshikimic acid in Palmae. Phytochemistry. 1974;13:1557–1559. doi: 10.1016/0031-9422(74)80327-4. [DOI] [Google Scholar]
- Hasnaoui O, Benali O, Bouazza M, Benmehdi H. Ethnobotanical approaches and phytochemical analysis of Chamaerops humilis L. (Arecaceae) in the area of Tlemcen (western Algeria) Res J Pharm Biol Chem Sci. 2013;4:910–918. [Google Scholar]
- Haynes J, Mc-Laughlin J (2000) Edible palms and their uses. Fact sheet MDCE-00-50-1. Homestead, Fla. http://www.plantapalm.com/vpe/ethnobotany/EdiblePalms.PDF. Accessed 23 Feb 2016
- Hirai Y, Sanada S, Ida Y, Shoji J. Studies on the constituents of Palmae plants. III. The constituents of Chamaerops humilis L. and Trachycarpus wagnerianus Becc. Chem Pharm Bull. 1986;34:82–87. doi: 10.1248/cpb.34.82. [DOI] [Google Scholar]
- Kang J, Thakali KM, Xiem C, Kondo M, Tong Y, Ou B, Jensen G, Medina MB, Schauss AG, Wu X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012;133:671–677. doi: 10.1016/j.foodchem.2012.01.048. [DOI] [Google Scholar]
- Kchaou W, Abbès F, Mansour RB, Blecker C, Attia H, Besbes S. Phenolic profile, antibacterial and cytotoxic properties of second grade date extract from Tunisian cultivars (Phoenix dactylifera L.) Food Chem. 2016;194:1048–1055. doi: 10.1016/j.foodchem.2015.08.120. [DOI] [PubMed] [Google Scholar]
- Khan MTH. Heterocyclic compounds against the enzyme tyrosinase essential for melanin production: biochemical features of inhibition. Top Heterocycl Chem. 2007;9:119–138. [Google Scholar]
- Krishnaih D, Sarbatly R, Bono A. Phytochemical antioxidants for health and medicine—a move towards nature. Biotechnol Mol Biol Rev. 2007;1:97–104. [Google Scholar]
- Left DB, Zertoubi M, Khoudali S, Benaissa M, Irhzo A, Azzi M. Effect of methanol extract of Chamaerops humilis L. leaves (MECHLL) on the protection performance of oxide film formed on reinforcement steel surface in concrete simulated pore solution. Int J Electrochem Sci. 2013;8:11768–11781. [Google Scholar]
- Massoud F, Gauthier S. Update on the pharmacological treatment of Alzheimer’s disease. Curr Neuropharmacol. 2010;8:69–80. doi: 10.2174/157015910790909520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Yamashita D, Takeda Y, Yonemori S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci Biotechnol Biochem. 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- Merlo ME, Aleman MM, Cabello J, Penasm J. On the Mediterranean fan palm (Chamaerops humilis) Principes. 1993;37:151–158. [Google Scholar]
- Metzler-Baddeley C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer’s disease and Parkinson’s disease with dementia. Cortex. 2007;43:583–600. doi: 10.1016/S0010-9452(08)70489-1. [DOI] [PubMed] [Google Scholar]
- Miguel M, Bouchmaaa N, Aazza S, Gaamoussi F, Lyoussi B. Antioxidant, anti-inflammatory and anti-acetylcholinesterase activities of Moroccan plants. Fresenius Environ Bull. 2014;23:1–14. [Google Scholar]
- Nehdi IA, Mokbli S, Sbihi H, Tan CP, Al-Resayesm SI. Chamaerops humilis L. var. argentea André date palm seed oil: a potential dietetic plant product. J Food Sci. 2014;79:C534–C539. doi: 10.1111/1750-3841.12420. [DOI] [PubMed] [Google Scholar]
- Nile SH, Nile AS, Keum Y-S. Total phenolics, antioxidant, antitumor, and enzyme inhibitory activity of Indian medicinal and aromatic plants extracted with different extraction methods. 3 Biotech. 2017;7:76. doi: 10.1007/s13205-017-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rezaire A, Robinson J-C, Bereaum D, Verbaere A, Sommerer N, Khan MK, Durand P, Prost E, Fils-Lycaon B. Amazonian palm Oenocarpus bataua (‘‘patawa’’): chemical and biological antioxidant activity—phytochemical composition. Food Chem. 2014;149:62–70. doi: 10.1016/j.foodchem.2013.10.077. [DOI] [PubMed] [Google Scholar]
- Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999;66:401–436. doi: 10.1016/S0308-8146(99)00093-X. [DOI] [Google Scholar]
- Seo SY, Sharma VK, Sharma N. Mushroom tyrosinase: recent propects. J Agric Food Chem. 2003;51:2837–2853. doi: 10.1021/jf020826f. [DOI] [PubMed] [Google Scholar]
- Serrano J, Puupponen-Pimi R, Dauer A, Aura A-M, Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53:S310–S329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- Soler-Rivas C, Espín JC, Wichers HJ. An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem Anal. 2000;11:330–338. doi: 10.1002/1099-1565(200009/10)11:5<330::AID-PCA534>3.0.CO;2-G. [DOI] [Google Scholar]
- Sousa AD, Brito ESD. Optimization of condensed tannin aqueous extraction from cashew tree pruning residue using response surface methodology and its drying. Waste Biomass Valoriz. 2015;6:569–577. doi: 10.1007/s12649-015-9381-4. [DOI] [Google Scholar]
- Suluvoy JK, Grace VMB. Phytochemical profile and free radical nitric oxide (NO) scavenging activity of Averrhoa bilimbi L. fruit extract. 3 Biotech. 2017;7:85. doi: 10.1007/s13205-017-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabet N. Acetylcholinesterase inhibitors for Alzheimer’s disease: anti-inflammatories in acetylcholine clothing. Age Ageing. 2006;35:336–338. doi: 10.1093/ageing/afl027. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M. Driving and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2008;8:377–383. doi: 10.1007/s11910-008-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisky RG, Salantino A. Analysis of propolis: some parameters and procedures for chemical quality control. J Apicult Res. 1998;37:99–105. doi: 10.1080/00218839.1998.11100961. [DOI] [Google Scholar]
- Zengin G, Uysal S, Ceylan R, Aktumsek A. Phenolic constituent, antioxidative and tyrosinase inhibitory activity of Ornithogalum narbonense L. from Turkey: a phytochemical study. Ind Crop Prod. 2015;70:1–6. doi: 10.1016/j.indcrop.2015.03.012. [DOI] [Google Scholar]
- Zhang S-J, Lin Y-M, Zhou HC, Wei S-D, Lin G-H, Ye G-F. Antioxidant tannins from stem bark and fine root of Casuarina equisetifolia. Molecules. 2010;15:5658–5670. doi: 10.3390/molecules15085658. [DOI] [PMC free article] [PubMed] [Google Scholar]