Abstract
Recently, the advances in the synthesis of new types of nanomaterials have created several opportunities in drug delivery and targeted therapy applications. Among the various nanostructures, gold nanostructures with controllable physical and chemical properties have received attention for various biomedical uses, including sensing of biomolecules, in vitro and in vivo bioimaging (as advanced contrast agents for photothermal and bioimaging techniques), photothermolysis of cancer cells, and targeted drug delivery. The attractive properties of gold nanomaterials, particularly, anti-angiogenic properties, are highly useful in a variety of cancers studies. In addition, they can bind many proteins and drugs and can be actively targeted to cancer cells over-expressing cell surface receptors and they are biocompatible in nature with a high atomic number, which directs to greater absorption of kilovoltage X-rays and provides greater contrast than standard agents. In this review, we have summarized the synthesis, structure and functionalization of gold nanostructures, and their biomedical applications with special reference to cancer studies.
Keywords: Gold nanomaterial, Synthesis, Characterization, Biomedical applications
Introduction
Targeted drug delivery has received attention from researchers for development of pharmaceuticals. Particularly, the researchers have focused on developing drug delivery systems for treatment of advanced cancers. Drug localization, the absence of side effects, and the dosage are some advantages of targeted drug delivery (Kumar et al. 2013). Photothermal therapy (PTT) is a unique strategy for the treatment of cancers (Cheng et al. 2008). Necroptosis plays an important role in the patterned cell death in PTT (Parida et al. 2017). Gold nanostructures are capable of photothermal energy conversion, i.e., the conversion of light energy into heat energy. This special characteristic can be used to control the release of drugs. Gold nanostructures can also be used for cell ablation and killing of cancer cells in plasmonic PTT. Based on these properties, gold nanostructures can serve as a multifunctional platform in the biomedical field (Yang et al. 2015).
Gold has a significant history in terms of biomedical and biological applications. In 1857, “fine particles” were discovered by Faraday by the reaction of HAuCl4 with phosphorus dissolved in CS2. In this reaction, the “fine particles” formed had a ruby red color; and these have been stable for more than 100 years (Faraday 1857). In 1908, Gustav Mie established solutions of the Maxwell equation; he suggested that the intense red color of gold nanoparticles (AuNPs) was caused by their absorption and light scattering (Mie 1908). The first report of gold being used in the treatment of arthritis was in 1934 (Forestier 1932); it reduced inflammation in the patients. In 1950s, AuNPs were used as radiotracers for lymph node biopsies in humans (Sherman and Ter-Pogossian 1953). In 1957, John Turkevich prepared colloidal AuNPs that were spherical, polydisperse, and 1–160 nm in size (Turkevich 1985). A publication by British biological researchers titled “An immunocolloid method for the electron microscope” in 1971 brought a revolution in immunochemistry. They described the conjugation of antibodies with AuNPs, which served as immunochemical markers. Thereafter, colloidal gold conjugates were used in various medical and biological fields (Dykman and Khlebtsov 2012).
Figure 1 shows that AuNPs are used in a very wide range of medical and biological fields, especially in biosensors, immunoassays, immunoanalysis, clinical chemistry, cancer studies, and genomics (Dykman and Khlebtsov 2011). Moreover, AuNPs have been receiving more attention in all biomedical applications, particularly in therapy, prophylaxis, and hygiene. The most important uses of colloidal gold are based on its physical and chemical properties. When the AuNPs are smaller in size than the free path bulk material, the conduction electrons are enhanced by the surface of the nanomaterials. Gold nanostructures can easily modify the surface chemistry, size, and shape of bio-inert surfaces. This favorable property makes gold nanostructures a good platform for biomedical applications (Mulvaney 1996; Cobley et al. 2011). Our review aims to provide a concise summary of gold nanostructures of different structures for drug delivery and therapy in the biomedical field.
Fig. 1.
Schematic diagram for various applications for gold nanomaterials
Synthesis of gold nanomaterials
Numerous physico-chemical and biological routes are used to synthesize AuNPs that are highly stable against the oxidation. The surface area, size, and shape of the AuNPs can be tuned according to their applications by modifying synthesis procedures (Dhas et al. 2014; Priyadarshini and Pradhan 2017). The synthesis and characterization of noble metal-containing compounds should be carried out carefully due to their biomedical applications. There are many methods for the synthesis of AuNPs including chemical or green synthesis methods. According to the synthesis method, different shapes of AuNPs can be prepared (Fig. 2). Moreover, their applications vary depending on the synthesis method (Pal et al. 2005).
Fig. 2.
Different structure of gold nanomaterials
Chemical syntheses
Gold spheres
To synthesize AuNPs, a variety of methods, such as sodium citrate-stabilized microemulsion, seeding growth, photochemistry, and radiolysis have been used. A previous study showed that the physiochemical properties differed depending on the synthesis procedure adopted (Zhou et al. 2009). Nanospheres have resonance wavelengths in the visible region. Adjusting the size and resonance wavelengths of nanospheres is useful in biomedical applications. The most common method for controlling the size of AuNPs is the use of capping agents, which include surfactants, ligands, or polymers. Synthesis of AuNPs larger than 100 nm without sharp edges and corners is difficult (Jana et al. 2001). Huang et al. (2005) prepared chitosan–gold composite, spherical nanoparticles and films, which had a wine-red color and showed plasmon absorption bands at 522 and 532 nm. They reported that during film formation, a nanocomposite was formed with a branch-like structure, but when the film was cast from 30 μL, no branch-like structures were formed. Later, Bhumkar et al. (2007) synthesized chitosan-reduced AuNPs as novel drug carriers; the main advantages of chitosan-reduced nanomaterials are biocompatibility, improved surface properties, and low cost. Similarly, using chitosan as a stabilizing agent, Jin et al. synthesized plasma AuNPs under atmospheric conditions (Jin et al. 2013).
Citrate is a commonly used chemical for stabilizing the AuNPs. Using the citrate method, Zhou et al. (2012) synthesized 20–30 nm AuNPs; these nanoparticles were highly stable and well dispersed. Ascorbic acid is an excellent reducing agent as it is stable against hydrolysis, is biodegradable, and has low toxicity. Zhang et al. (2005) synthesized AuNPs with different sizes using ascorbic acid and by varying the surfactant to water ratio. Link et al. (1999) proposed that different chemical synthesis procedures could be used for synthesizing different size of AuNPs. For example, a mixture of chloroauric acid and gold solution under reflux could be used for synthesizing 20 nm AuNPs, whereas 10 nm AuNPs could be synthesized by the sodium citrate reduction method. Sulfur-containing ligands such as trithiols, disulfides, and xanthates can also be used in the synthesis of AuNPs. Although disulfides, as well as thiols, cannot be stabilized, they have catalytic activity (Daniel and Astruc 2004).
Gold clusters
Gold nanoclusters (AuNCs) are tiny particles that contain few to ten atoms. They show different fluorescence properties in visible region and NIR region depending on their size. Moreover, their quantum yield is up to 0.001 million times stronger than the bulk metal ions (Huang et al. 2007). Lin et al. (2009) synthesized water-soluble AuNCs by forming the AuNPs in an organic phase and then extracting them into aqueous solution, where they exhibited red photoluminescence (PL) at 650 nm. Similarly, Wu et al. (2010) prepared ultra-small NIR AuNCs with a size of about 2.7 nm when synthesized by the same procedure (i.e., HAuCl4 with BSA). The red fluorescence of the prepared AuNCs showed an emission peak at 710 nm. Glutathione is an excellent reducing and stabilizing agent for the synthesis of AuNCs with sodium borohydride; it has a quantum yield of 10−3 (Link et al. 2002). In another study, Santiago et al. (2010) synthesized AuNCs containing only 2–3 atoms by a simple electrochemical method using polyvinylpyrrolidone; the prepared AuNCs also had magnetic properties. Singh et al. (2017) developed bovine serum albumin (BSA) and glucose-conjugated AuNCs (Glu-AuNCs) for targeting cancer cells. BSA-AuNCs showed better response in the A431 cell line than in the HaCaT cell line. The glucose-conjugated AuNCs were not internalized in the HaCaT cells but were internalized in the A431 cells, which was suggested to have happened through the Glut-1 receptors.
Experimental methods of synthesis can be classified into “bottom–up” and “top–down” approaches. Atomic species form AuNCs in the “bottom–up” approach. Many researchers synthesized AuNCs with phosphine acting as a reducing and stabilizing agent (Bellon et al. 1972; Weare et al. 2000). Thiol is used as a capping ligand for the synthesis of AuNCs in the “bottom–up” approach; it also acts as a reducing agent to change the oxidation state of gold from +3 to +1 (Zhu et al. 2008). The “top–down” method is also a very effective method for the synthesis of AuNCs. For “top–down” synthesis of AuNCs, two methods, namely the thiol conventional and citrate-stabilized methods (Jin et al. 2004).
Rods
In 1989, Wiesner and Wokaun reported a seed-mediated growth method by reducing HAuCl4 in the presence of colloidal gold nuclei by adding white phosphorus, and then starting growth by addition of H2O2 (Xia et al. 2015). Jana et al. proposed the use of a cationic surfactant, cetyltrimethylammonium bromide (CTAB), for the synthesis of gold nanorod (GNR) in 2001 (Jana 2005; Nikoobakht et al. 2003). According to their report, there are three main seed growth methods:
Three-step seeded growth.
One-step, silver-assisted seeded growth.
A seedless approach to silver growth.
In the first method, three solutions are used: (1) citrate-capped AuNPs that act as seeds, (2) HAuCl4 and CTAB, the latter of which acts as a surfactant, and (3) ascorbic acid that acts as a reducing agent. After a specified time, the first growth solution is mixed with the second growth solution, and then finally transferred into the third solution. Nikoobakht et al. synthesized a CTAB-capped seed to replace the citrate-capped growth seed and added silver nitrate to the growth solution. This modification overcame many disadvantages and limitations of the previous methods (Nikoobakht et al. 2003). Using this modified second method, high yields of GNR were obtained in the ratio of 1.5–4.5 without centrifugation. Many researchers now utilize various modifications of the one-step, silver-assisted seeded growth method (Xia et al. 2015). Finally, third method is one-pot synthesis of GNR by adding sodium borohydride to the growth solution without using seed solution; it is also called “seedless” GNR synthesis method.
Busbee et al. developed a GNR synthesis method requiring minimal purification; they synthesized GNR with 90% yield using this method (Busbee et al. 2003). Photochemical synthesis is a good way to get uniformly sized GNR. The addition of silver ions in the synthesis dictates the fine shape of GNR (Kim et al. 2002). Monodispersed GNR with high yield (50%) were synthesized by control of the surfactant concentration, temperature, and stability of growth (Perez-Juste et al. 2005). By a variety of different seeds, having different surfactants, GNR with size ranging from 4 to 18 nm were synthesized. The large size seeds resulted in lower sized GNR (Gole and Murphy 2004).
Plates
Gold nanoplates are plate-like structures that are roofed by two relatively basal (111) planes. Chen et al. prepared triangular gold nanoplates by one-pot seedless growth method with high yield. Compared to the previously used methods, the iodide ions (used in their method) act as dual functional reagents, removing other shape impurities and forming gold nanoplates at the binding sites (Chen et al. 2014). Zhu et al. developed a novel chemical method at high and low temperatures for the synthesis of hexagonal gold nanoplates. By PVP and CTAB as surfactants, they also synthesized different shapes, such as star-like, shield-like, and trigonal (Zhu et al. 2011). Alloyeau et al. (2015) proposed a novel method that involved radiolysis within a scanning electron microscope, which allowed for both the control and measurement of the growth of the gold nanoplates. Luo et al. (2015) synthesized a single crystalline structure of nanoplates using radiation microscopy. This method was more advanced than those used to make previous, traditional polycrystalline metal films, because the flat surface was formed from atoms (James et al. 2015). James et al. optimized a new technique, “Daisynth,” to synthesize gold nanoplates. This technique produced near-infrared absorbance with the required localized surface plasmon resonance (LSPR) at a higher yield than other conventional methods (James et al. 2015).
Cubes
Due to their square cross section and sharp edges, nanocubes are receiving more attention than other metallic nanomaterials for plasmonic optics and biomedical applications. Cube-shaped, bimetallic nanomaterials with a gold core range from 13.4 to 50 nm. Zhu et al. (2015) found that the size, shape, and yield of these nanocubes were dependent on temperature; they determined that the absorbance peak blue-shifted with a variation in temperature. Ding et al. (2015) synthesized gold nanocubes by adjusting the volume of gold seed and silver nitrate, which altered the edge lengths of the gold cubes. They developed a single-core shell nanostructure for surface enhanced Raman scattering (SERS) activity. In another study, Chu et al. (2015) reported a new method with higher sensitivity for glucose on the thiol graphene surface of uniformly sized gold cubes. A well-distributed crystallization of gold on the graphene layer was made possible by bridging of the sulfhydryl groups with the gold. By the Langmuir–Blodgett technique, Mahmoud and group examined well-organized 2D arrays of gold nanocubes. Gold nanocubes were organized by changing the length of the polyethylene glycol (PEG) polymer (Mahmoud 2015). Using the discrete dipole approximation (DDA) method, Bordley and group investigated the field coupling of gold nanocubes in face-to-face dimer configurations, due to the loss of dipole distance or electromagnetic field. They found clear evidence of a new region of near field coupling, marked by a new large plasmonic mode (Bordley et al. 2015).
Green synthesis of gold nanoparticles
Recently, researchers have been interested in developing eco-friendly green chemistry methods to synthesize metal nanoparticles. The green synthesis method has several advantages over conventional methods, such as being simple, nontoxic, inexpensive, efficient, requiring ambient temperatures and pressures, and needing no additional reducing or capping agents. Using this method, AuNPs were synthesized with Butea monosperma leaf extract acting as a capping and reducing agent (Patra et al. 2015). Using bovine serum albumin (BSA), Xie and group synthesized red fluorescent AuNCs containing 25 gold atoms and having an emission of 640 nm by using a simple, one-pot, “green” method and this material was stable in a pH range of 3–12 (Xie et al. 2009). Ateeq et al. (2015) used patuletin, which was isolated from Tagetes patula, as a reducing agent for the one-pot synthesis of AuNPs; they obtained a higher yield of 63.2%. Suarasan et al. (2015) synthesized AuNPs at different concentrations and temperatures with gelatin as a reducing agent. Gelatin has a high level of biocompatibility toward osteoblasts and does not alter cell proliferation and viability. Chlorogenic acid can be used as a reducing and capping agent for the green synthesis of AuNPs. Chlorogenic acid inhibits pro-inflammatory cytokines and other inflammation-related genes. Functionalization of chlorogenic acid in AuNPs produces stronger anti-inflammatory activity than chlorogenic acid alone (Hwang et al. 2015). Li and group synthesized water-soluble L-carnosine-assisted AuNCs via an easy, one-step method, which had a strong red fluorescence with a quantum yield of 3.4% (Li et al. 2016).
Bagci et al. (2015) used a simple and rapid method to synthesize AuNPs for cysteine detection, using apple juice at room temperature. The particle color, stability, and color change during the reaction with cysteine were suitable for cysteine detection. In other study, Annadhasan et al. (2015) described a natural sunlight irradiation method for the synthesis of AuNPs for colorimetric detection. The reaction started within 2 min, and AuNPs were synthesized within 20 min of sunlight irradiation. N-cholyl-l-valine was used to avoid aggregation and control the size and shape of the nanoparticles. Dong et al. (2004) proposed a photochemical method for synthesis of AuNPs by UV solar radiation. By adjusting the concentration of Au(III) ions, they were able to synthesize different sized AuNPs with the solar radiation (Dong et al. 2004). Dhas et al. (2012) synthesized novel AuNPs by using Sargassum myriocystum extract. These synthesized materials were well dispersed and small in size ranging from 10 to 23 nm.
Functionalization of AuNPs
Considering the profitable chemical, physical, and biological properties of AuNPs, they have been used in many applications, fabricated with metal ions, polymers, carbohydrates, and organic compounds. Polyethylene glycol (PEG) is commonly used to functionalize AuNPs alone or in combination with biotin, peptides or oligonucleotides; it can help to interact with cells (Tiwari et al. 2011). The internalization of functionalized AuNPs in cells depends on the attached ligands, size of the nanoparticles, and molecular weight of the PEG. The efficiency and specificity of nanoparticle-based drug delivery systems can be enhanced by functionalization with the amino acids and peptides (Ghosh et al. 2008). Functionalization of AuNPs with the peptides like CALNN and its derivative CALNNR8 is used for targeting the intracellular components and as an effective cell-targeting agent (Sun et al. 2008).
Glucosamine functionalization of AuNPs enhanced their antibacterial activity. Naturally, glucosamine has an electrostatic nature; it prevents aggregation of AuNPs and also increases bacterial oxidative stress (Govindaraju et al. 2015). Xiaoning et al. functionalized AuNPs with different ligands to impart antimicrobial activity against multi-drug-resistance bacteria. Cationic- and hydrophobic-functionalized AuNPs successfully inhibited the growth of 11 clinical, multi-drug-resistance bacteria (Li et al. 2014). Mitra et al. developed drug-conjugated (i.e., 9AA-HCl, AY, AO, and Pro) AuNPs for antibacterial activity. These drugs were conjugated through a simple, stirring method; the addition of the drugs decreased the plasmon band. These composites provided better antibacterial activity than the bare drugs (Mitra et al. 2014).
AuNPs were coated with lysostaphin to produce a novel material with antibacterial activity against methicillin-resistant S. aureus. This product had slightly greater antibacterial activity than lysostaphin-functionalized silver nanoparticles (Jun et al. 2015). Isoluminol (6-amino-2,3-dihydrophthalazine-1,4-dione), a highly luminescent molecule that can function as a reducing agent, was used to functionalize AuNPs to produce uniquely shaped particles (Li et al. 2013). Recently, AuNPs were doped with graphene oxide, which increased their antibacterial activity (Govindaraju et al. 2016). GNR functionalized with PSS, CTAB, and PEG was prepared to reduce their cytotoxicity to the cells. PSS-AuNRs showed low toxicity to the cells, whereas PEG-AuNRs showed no toxicity (Alex et al. 2017).
Applications of gold nanomaterials
Drug delivery
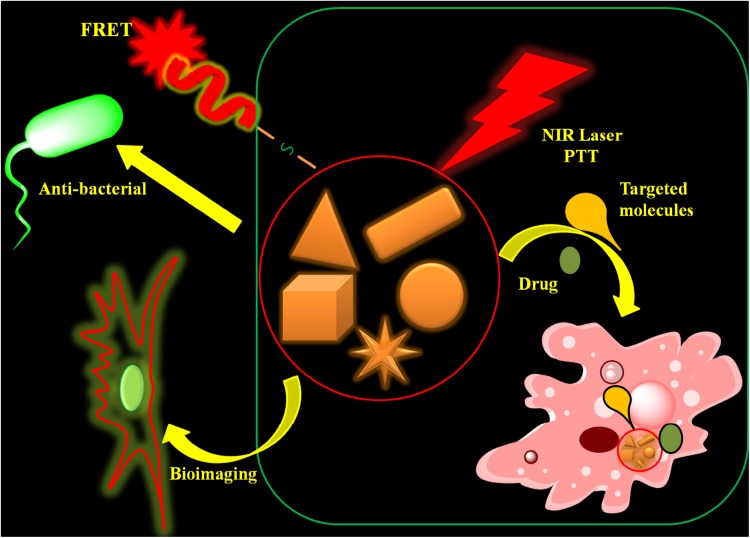
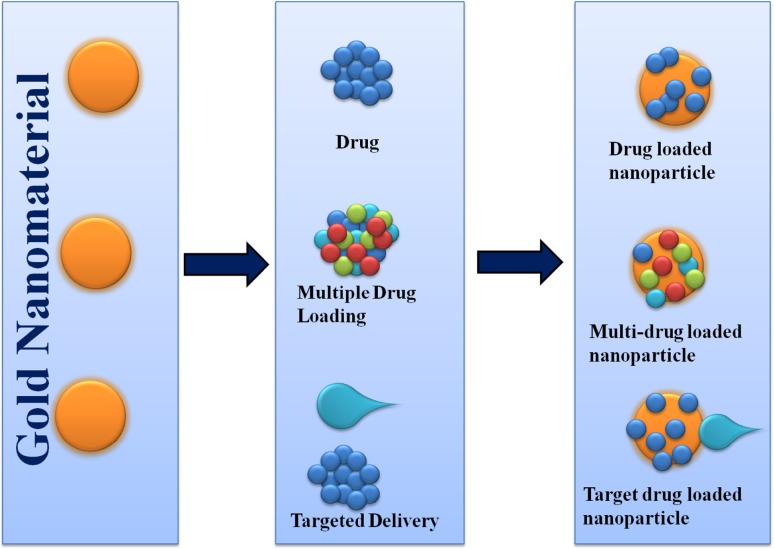
Gold nanomaterials are promising nanomaterials in medicine, especially for the targeted drug delivery. Due to their varying structure, small size, easy synthesis method, and surface modifications, they are emerging as a new class of targeted drug delivery systems. Many methods have been developed for drug loading in the AuNPs, including direct conjugation involving Au–S or –N linkages, capping ligands, electrostatic interactions, van der Waals forces, and hydrogen bonding. Examples of these drug delivery systems are illustrated in Fig. 3.
Fig. 3.
Schemes for different versions of drug delivery system
Tian et al. (2016) developed a new technique for monitoring drug release using gold nanostar. Using this technique, it is possible to track and monitor real-time drug release from gold nanostars in living cells and mice. Highly hydrophobic GW627368X was conjugated with GNR embedded block copolymer micelles to play a dual role in both drug delivery and photothermal therapy. High concentrations of glutathione help to release the drug from the material (Parida et al. 2017). Chemical conjugation of Taxol to AuNPs was reported to show greater toxicity to the T47D cell line than that shown by non-conjugated and Taxol-free material (Alhalili et al. 2017). This study demonstrated that AuNPs conjugated with Taxol are an efficient drug delivery system. Negatively charged, 39.9 nm, PEG-AuNPs had a 9.7% loading capacity for doxorubicin (DOX). Glioma-bearing mice treated with PEG-DOX-AuNPs had a median survival time that was 189% longer than that of the saline control group. This demonstrated that the DOX release produced a better anti-glioma effect than in other studies (Ruan et al. 2015). Liposomal drug carriers with encapsulated AuNPs have achieved target- and time-controlled drug release. When exposed to laser light, light energy is converted to heat energy; liposomes release the AuNPs that release the drug to the target site. This technology is an attractive option for targeted and controlled drug delivery (Lajunen et al. 2015). Bishop et al. developed polymer-coated AuNPs for co-delivery of DNA and siRNA. These hybrid nanoparticles are a new platform for gene therapy of human cells (Bishop et al. 2015). Different materials are functionalized with gold nanomaterials used in drug delivery system (Table 1).
Table 1.
List of functionalized gold nanomaterials in drug delivery system
| Nanomaterial | Drug | Target | Mechanism | Reference |
|---|---|---|---|---|
| PEG-DOX-AuNPs | Doxorubicin | Nuclei | Induce the cell apoptosis | (Ruan et al. 2015) |
| Magnetic AuNPs | Doxorubicin | Liver, heart, lung and spleen | EPR effect | (Elbialy et al. 2015) |
| EPI–FA–AuNPs | Epirubicin | Cell wall | Induce the cell apoptosis | (Kumar et al. 2016) |
| cRGD-uPIC-AuNP | – | Cellular internalization | Enhanced gene silencing | (Yi et al. 2016) |
| MSNs- AuNPs | Doxorubicin | αvβ3 integrin | Enhanced apoptosis | (Chen et al. 2016) |
| Au NCs | – | Cytoplasm | Penetrate in cells | (Yahia-Ammar et al. 2016) |
| AuNPs-NKCT1 | Hepatic enzymes and cancer marker | Mitochondrial membrane | Cell cycle inhibition | (Bhowmik et al. 2017) |
| PTX-COS AuNPs | Paclitaxel | DNA and mitochondrial membrane | Apoptosis | (Manivasagan et al. 2016) |
Photothermal therapy
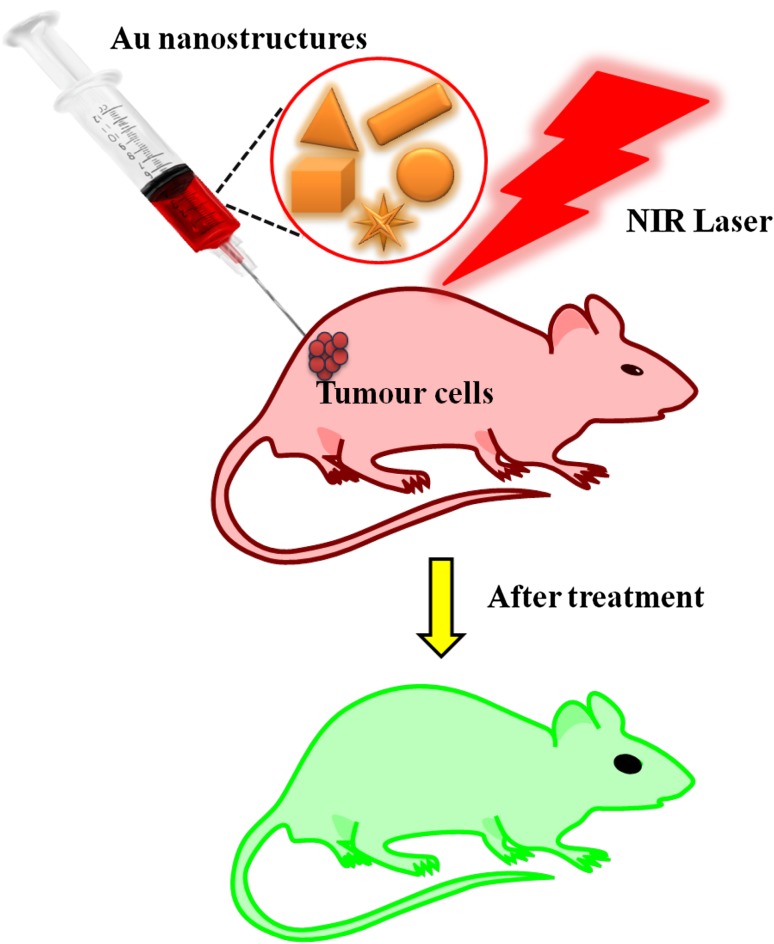
Photothermal killing of a cancerous cell is a promising technique in cancer treatment. Figure 4 illustrates the basic concept of PTT. During PTT, the AuNPs become hot when they reach their maximum absorption in the visible or near infrared region, leading to cell death (Kennedy et al. 2011). Thus, laser-irradiated AuNPs could act as a therapeutic agent without the presence of a drug. Selectively inducing cell death is possible by adjusting the size, shape, particle number, and laser energy. This property makes AuNPs potentially useful in PTT and treating other diseases. Figure 5 shows the basic principle of PTT, which is the conversion of light into heat energy. Many methods of heating are available in the PTT, such as using lasers, microwaves, or ultrasound (Svaasand et al. 1990; Philipp et al. 1995; Seki et al. 1999; Jolesz and Hynynen 2001). Gamal-Eldeen et al. developed gum Arabic-AuNPs as efficient PTT agents against the liver cell line PNLS. These nanoparticles interacted with hepatocytes and activated caspase-3, leading to apoptosis (Gamal-Eldeen et al. 2016). Pitisillides et al. developed a new technique to target cancer cells using 20 ns laser pulses to generate heat around the particles of 20 and 30 nm (Pitsillides et al. 2003). In the K562 cancer line, 2–3 J/cm3 was reported to be sufficient to damage the cells with 10–15 nanoparticles of 20 nm size (Zharov et al. 2003).
Fig. 4.
Schematic diagram for photothermal treatment for cancer cell
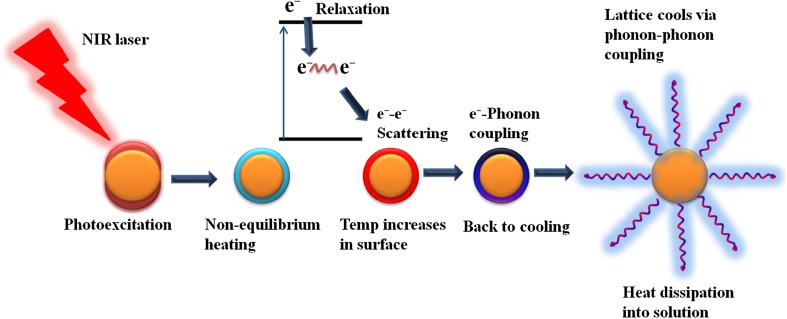
Fig. 5.
Schematic explanation for the principle of photothermal therapy, light energy is converting into heat energy
Similar to AuNPs, other gold structures and conjugates also are useful candidates for PTT. Some prostate and metastatic liver cancer cells give good responses to immune nanoshells in PTT (Choi et al. 2007). PEGylated gold SiO2 nanoshells with a thickness of 10 nm were used for photothermal, anticancer activity by Halas and West (Hirsch et al. 2003). Von Maltzahn et al. reported that PEG coated with GNR showed increased blood circulation time in vivo and increased accumulation at the cancer site (Von Maltzahn et al. 2009). After irradiation with a continuous-wave (CW) laser for 10 min, gold nanocages increased the temperature of cancer cells to 50 °C, demonstrating their potential for PTT cancer treatment (Chen et al. 2010). To enhance their activity during PTT, AuNPs can be functionalized with different nanomaterials or active targeting moieties on their surface (Loo et al. 2005; El-Sayed et al. 2006; Chen et al. 2007; Huff et al. 2007; Lu et al. 2009; Melancon et al. 2014). In CW laser PPT, two mechanisms can kill the cells. First, heat increases upon irradiation, leading to hyperthermia. Second, during laser irradiation, the high photon density and repetitive absorption of photons by gold materials lead to cavitations (i.e., vapor bubble formation) (Tong et al. 2009; Qin and Bischof 2012; Nanocavitation 2013).
Gold nanostructures are promising candidates for PTT of cancer and other diseases. However, there are still some drawbacks and unanswered questions regarding PTT, such as material stability, biocompatibility, physiochemical interactions, and blood circulation time. The killing mechanisms of PTT with gold nanomaterials need to be investigated in more detail. The high cost of gold may also play a major role in limiting its use in PTT (Dykman and Khlebtsov 2012; Yang et al. 2015). Gold nanomaterials and their efficiency against cancer cell lines are listed in Table 2.
Table 2.
Efficiency of gold nanomaterials against cancer cell lines
| Nanomaterial | Cancer line | Efficiency | Reference |
|---|---|---|---|
| Lipos Au NPs | HT1080-fluc2-turboFP | Temperature increment up to 7 °C with 4 min of laser irradiation | (Rengan et al. 2015) |
| AuNRs | Cervical cancer cells | Enhances therapeutic efficacy | (Parida et al. 2017) |
| AuNPs | 4T1 cells | Small nanoparticles shifted to NRI region | (Cheng et al. 2016) |
| AuNPs | HSC-3 | PTT and real-time monitoring the molecular changes | (Aioub and El-Sayed 2016) |
| AuNPs | HeLa and AMN3 | Quickly expand and burst, ripping apart of cancer cells | (Hussein et al. 2016) |
| Gum Arabic-AuNPs | HepG2 | Efficacy in inhibiting liver PNLs and inflammation | (Gamal-Eldeen et al. 2016) |
| AuNPs | SUM-159 and U87-MG | Photothermal ablation and could favor tumor accumulation | (Iodice et al. 2016) |
Photodynamic therapy
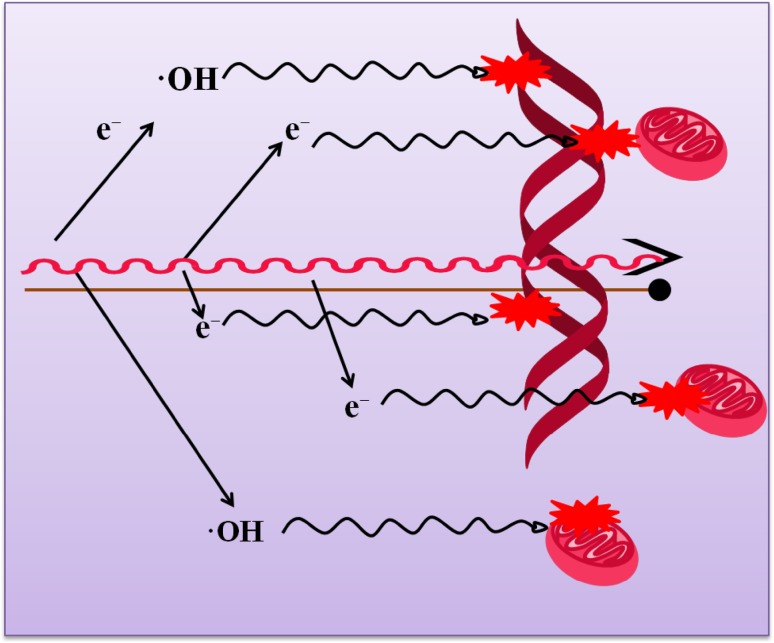
The use of photosensitizer drugs that are activated by visible light of a particular wavelength for the treatment of diseases is called photodynamic therapy (PDT). Affected cancer cells are irradiated with a wavelength of laser light, corresponding to the peak of drug absorption (Dykman and Khlebtsov 2012). In addition to the aforementioned heat therapy, another mechanism plays a role, namely the photochemical generation of oxygen free radicals, which induces necrosis and apoptosis in cancer cells (Wilson 2008). Gold nanomaterials have received considerable attention in the PDT field. A phosphatidylcholine-conjugated AuNPs showed improved solubility in polar solvents, increased yield of singlet oxygen (up to 50%), and improved selectivity for tumor targeting compared to free Pc molecules. When the AuNPs absorb light, electrons transfer to the excited state and lead to overproduction of reactive oxygen species (Negishi et al. 2004). Figure 6 illustrates the mechanism of radiation damage to the tumor cell.
Fig. 6.
Mechanism of radiation damage
Two important properties of gold-based photosensitizers are that they can penetrate to the depth needed for effective cancer treatment, and they are more stable than the organic dyes (Vankayala et al. 2014). Recently, Hwang et al. confirmed that GNR have a high potential to destroy cancer cells by irradiation of laser light at 915 nm. Along with gold, nanoconjugates composed of, for example, peptides, proteins, photoactive substances, nanoshells, nanoechinus, and nanocages are also useful for PDT (Dykman and Khlebtsov 2012; Yang et al. 2015). However, the photosensitizer should persist in the targeted area for a long time, and the heating should be low for the dye alone. Functionalization of gold nanomaterials and their efficiency in the photodynamic therapy is listed in Table 3.
Table 3.
Efficiency of functionalized gold nanomaterials in photodynamic therapy
| Nanomaterial | Cell line | Efficiency | Reference |
|---|---|---|---|
| Liposomes with AuNPs | ARPE-19 (A–F) and HUVEC (G–O) | pH-sensitivity and light sensitivity | (Lajunen et al. 2015) |
| EGFpep-AuNPs | 9L.E29 cells | Enhanced drug delivery and potentially efficacy | (Meyers et al. 2015) |
| GNR@MSNP | KB | Control the release behaviors | (Liu et al. 2015) |
| AuNPs | BRAIN TUMOR | Selective drug delivery | (Dixit et al. 2016) |
| PEG-AuNPs | Female C57/BL6 mice | Efficacy of the in vivo drug delivery | (Camerin et al. 2016) |
| MB-conjugated AuNPs | Fibroblast cells, MRSA (UTMC 1442) | Fast and efficient destruction of immature and mature biofilms | (Darabpour et al. 2017) |
| PLGA- AuNPs | PANC-1, HEK-293 | Target with tumour associated molecules | (Deng et al. 2016) |
| PR-AuNPs-PEG-Ab | SK-BR-3 breast cancer | Water soluble | (Penon et al. 2017) |
Radio-labeled AuNPs as multifunctional target therapy
Multifunctional systems have been synthesized with different targeting agents to the nanoparticles. AuNPs can be utilized in multifunctional target therapy when using multifunctional probes that have properties such as optical quenching, and SERS and X-ray absorption properties. These specific properties may be useful in multi-modal imaging and in radiotherapy in the pharmaceuticals fields.
For example, Luna-Gutierrez et al. (2012) developed peptide-conjugated AuNPs for targeted radionuclide therapy of tumors expressing α(ν)β(3) integrins. The 177Lu-labeled AuNPs were conjugated with the c (RGDfK)C (cyclo (Arg-Gly-Asp-Phe-Lys) Cys) and their dosage was compared with the monomeric and dimeric RGD peptides for efficiency against tumors in mice. Overall, the conjugated AuNPs showed higher efficiency against the cancer. 177Lu-AuNP-RGD were used to treat cancer and their effect was compared with the AuNPs and their RGD conjugates with the 177Lu (Vilchis-Juarez et al. 2014). This multivalent system using 117Lu can also be used in combination with thermoablative therapy using laser heating or an RF field given its high cell internalization (Luna-Gutierrez et al. 2013).
Rambanapasi et al. (2015) studied the biodistribution of dual-radiolabeled citrate-coated AuNPs for the oral and intravenous administrations. They found that the AuNPs and citrate-coated NPs showed different biodistribution, which was dependent on the dose level. AuNPs were labeled with the 125l and 111ln and functionalized with the MMP9 cleaved peptide, which makes the multifunctional targeted nanomaterial for the SPECT imagining. These accumulate around the edges of the tumors. This kind of multispectral SPECT imaging was used to differentiate the tumors from each other (Black et al. 2015). AuNPs coated with the 64Cu–DOTA and PEG2K-DOTA were used to check the radio labeling efficiency for the cancer imaging therapy. It showed 81.3% efficiency and more stable with the serum samples. This material was reported to be suitable for the radio labeling and cancer imaging and therapy (Xing et al. 2014).
Other biomedical applications
The AuNPs have various applications including in Surface Enhance Raman spectroscopy, glucose sensors, detection of cancer cells, and biological activities such as anti-diabetic and anti-inflammatory. Karthick et al. developed novel Gymnema sylvestre containing AuNPs for anti-inflammatory activity on diabetic rats. The synthesized nanomaterials effectively reduced the blood glucose levels and provided good anti-inflammatory activity, assessed by inflammatory markers TNF-α, IL-6, and hsCRP (Karthick et al. 2014). Properties of AuNPs, such as high-scattering cross section and superior photo-stability, make them a good candidate for imaging based applications. The AuNPs were suggested to be the part of next generation bioimaging platforms for cancer imaging (Huang et al. 2007). Wu et al. (2007) developed a nine-layer multiwall carbon nanotube conjugate with AuNPs and glucose oxidase enzyme. It provided the best result among the glucose sensors available with the limit of detection being 6.7 µM only in 7 s.
Sokolov et al. (2003) developed molecularly targeted AuNPs against cancer cells by conjugating anti-epidermal growth factor receptor to the AuNPs. By scattering the nanoparticles through monochromatic or normal laser pens, these AuNPs could be used for optical labeling of the cancer biomarkers. Owing to their availability and simple design, white-light illuminations have more advantages than the single-wave length illuminations. Another study used 40 nm AuNPs for cancer detection in vitro in the dark field mode (El-Sayed et al. 2005). The greenish SPR light scattering at 530 nm is used for the optical imaging of AuNPs; anti-EGFR anti-bodies are conjugated for specific binding to the cancer cell surface. Two-photon excitation using femtosecond NIR laser was successfully used for imaging mouse ear cancer cells in vitro (Wang et al. 2005).
Conclusion
Stability, adaptable structure, functionalization, and ability to control drug release are some of the desirable properties of targeted drug delivery systems. Great achievement has been made in gold nanostructures in recent years. In this review, we have discussed the different shapes, synthesis procedures, functionalization, and the use of AuNPs as drug delivery agents and therapeutics. Gold nanomaterials are synthesized in aqueous media by the use of ligands, polymers and dendrimers. But removal of ligands often causes instability and agglomeration in the gold nanomaterials, leading to ineffective responses in vitro and in vivo. Another challenge in the use of the gold nanomaterials is aggregation in the biological buffer. Gold-aided targeted delivery can be used for drugs, antigens, and DNA. Finally, there is a need to the study the gold nanomaterials in detail to develop biocompatible AuNPs. Although researchers have extensively studied the gold nanostructures and their applications in the past few year, several important aspects remain to be studied. It should be noted that biodistribution and toxicity can be affected the by factor including morphology and synthesis method. The introduction of adjuvant properties in the gold nanomaterials has added potential for the development of next generation drug delivery systems. Finally, its advantages make gold a promising material in many biomedical fields.
Acknowledgement
This research was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP) (No.2017R1A2B4004700).
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
References
- Aioub M, El-Sayed MA. A real-time surface enhanced raman spectroscopy study of plasmonic photothermal cell death using targeted gold nanoparticles. J Am Chem Soc. 2016;138:1258–1264. doi: 10.1021/jacs.5b10997. [DOI] [PubMed] [Google Scholar]
- Alex SA, Rajiv S, Chakravarty S, Chandrasekaran N, Mukherjee A. Significance of surface functionalization of gold nanorods for reduced effect on igg stability and minimization of cytotoxicity. Mater Sci Eng C. 2017;71:744–754. doi: 10.1016/j.msec.2016.10.061. [DOI] [PubMed] [Google Scholar]
- Alhalili Z, Figueroa D, Johnston MR, Shapter J, Sanderson B. Effect of modification protocols on the effectiveness of gold nanoparticles as drug delivery vehicles for killing of breast cancer cells. Aust J Chem. 2017;69:1402–1412. doi: 10.1071/CH16430. [DOI] [Google Scholar]
- Alloyeau D, Dachraoui W, Javed Y, Belkahla H, Wang G, Lecoq H, Ammar S, Ersen O, Wisnet A, Gazeau F, Ricolleau C. Unravelling kinetic and thermodynamic effects on the growth of gold nanoplates by liquid transmission electron microscopy. Nano Lett. 2015;15:2574–2581. doi: 10.1021/acs.nanolett.5b00140. [DOI] [PubMed] [Google Scholar]
- Annadhasan M, Kasthuri J, Rajendiran N. Green synthesis of gold nanoparticles under sunlight irradiation and their colorimetric detection of Ni2+ and Co2+ ions. RSC Adv. 2015;5:11458–11468. doi: 10.1039/C4RA14034F. [DOI] [Google Scholar]
- Ateeq M, Shah MR, ul Ain N, Bano S, Anis I, Faizi S, Bertino MF, Naz SS. Green synthesis and molecular recognition ability of patuletin coated gold nanoparticles. Biosens Bioelectron. 2015;63:499–505. doi: 10.1016/j.bios.2014.07.076. [DOI] [PubMed] [Google Scholar]
- Bagci PO, Wang YC, Gunasekaran S. A simple and green route for room-temperature synthesis of gold nanoparticles and selective colorimetric detection of cysteine. J Food Sci. 2015;80:2071–2078. doi: 10.1111/1750-3841.12974. [DOI] [PubMed] [Google Scholar]
- Bellon P, Manassero M, Sansoni M. Crystal and molecular structure of tri-iodoheptakis (tri-p-fluorophenylphosphine) undecagold. Dalton Trans. 1972;14:1481–1487. doi: 10.1039/dt9720001481. [DOI] [Google Scholar]
- Bhowmik T, Saha PP, Sarkar A, Gomes A. Evaluation of cytotoxicity of a purified venom protein from Naja kaouthia (NKCT1) using gold nanoparticles for targeted delivery to cancer cell. Chem Biol Interact. 2017;261:35–49. doi: 10.1016/j.cbi.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24:1415–1426. doi: 10.1007/s11095-007-9257-9. [DOI] [PubMed] [Google Scholar]
- Bishop CJ, Tzeng SY, Green JJ. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater. 2015;11:393–403. doi: 10.1016/j.actbio.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KC, Akers WJ, Sudlow G, Xu B, Laforest R, Achilefu S. Dual-radiolabeled nanoparticle SPECT probes for bioimaging. Nanoscale. 2015;7:440–444. doi: 10.1039/C4NR05269B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordley JA, Hooshmand N, El-Sayed MA. The coupling between gold or silver nanocubes in their homo-dimers: a new coupling mechanism at short separation distances. Nano Lett. 2015;15:3391–3397. doi: 10.1021/acs.nanolett.5b00734. [DOI] [PubMed] [Google Scholar]
- Busbee BD, Obare SO, Murphy CJ. An improved synthesis of high-aspect-ratio gold nanorods. Adv Mater. 2003;15:414–416. doi: 10.1002/adma.200390095. [DOI] [Google Scholar]
- Camerin M, Moreno M, Marin MJ, Schofield CL, Chambrier I, Cook MJ, Coppellotti O, Jori G, Russell DA. Delivery of a hydrophobic phthalocyanine photosensitizer using PEGylated gold nanoparticle conjugates for the in vivo photodynamic therapy of amelanotic melanoma. Photochem Photobio Sci. 2016;15:618–625. doi: 10.1039/C5PP00463B. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang D, Xi J, Au L, Siekkinen A, Warsen A, Li ZY, Zhang H, Xia Y, Li X. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007;7:1318–1322. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Glaus C, Laforest R, Zhang Q, Yang M, Gidding M, Welch MJ, Xia Y. Gold nanocages as photothermal transducers for cancer treatment. Small. 2010;6:811–817. doi: 10.1002/smll.200902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ji F, Xu Y, He L, Mi Y, Bao F, Sun B, Zhang X, Zhang Q. High-yield seedless synthesis of triangular gold nanoplates through oxidative etching. Nano Lett. 2014;14:7201–7206. doi: 10.1021/nl504126u. [DOI] [PubMed] [Google Scholar]
- Chen G, Xie Y, Peltier R, Lei H, Wang P, Chen J, Hu Y, Wang F, Yao X, Sun H. Peptide-decorated gold nanoparticles as functional nano-capping agent of mesoporous silica container for targeting drug delivery. ACS Appl Mater Interfaces. 2016;8:11204–11209. doi: 10.1021/acsami.6b02594. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei B, Burda C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J Am Chem Soc. 2008;130:10643–10647. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Sun R, Yin L, Chai Z, Shi H, Gao M. Light-triggered assembly of gold nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo. Adv Mater. 2016 doi: 10.1002/adma.201604894. [DOI] [PubMed] [Google Scholar]
- Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ. A cellular trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- Chu Z, Liu Y, Xu Y, Shi L, Peng J, Jin W. In-situ fabrication of well-distributed gold nanocubes on thiol graphene as a third-generation biosensor for ultrasensitive glucose detection. Electrochim Acta. 2015;176:162–171. doi: 10.1016/j.electacta.2015.06.123. [DOI] [Google Scholar]
- Cobley CM, Chen J, Cho EC, Wang LV, Xia Y. Gold nanostructures: a class of multifunctional materials for biomedical applications. Chem Soc Rev. 2011;40:44–56. doi: 10.1039/B821763G. [DOI] [PubMed] [Google Scholar]
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- Darabpour E, Kashef N, Amini SM, Kharrazi S, Djavid GE. Fast and effective photodynamic inactivation of 4-day-old biofilm of methicillin-resistant Staphylococcus aureus using methylene blue-conjugated gold nanoparticles. J Drug Deliv Sci Technol. 2017;37:134–140. doi: 10.1016/j.jddst.2016.12.007. [DOI] [Google Scholar]
- Deng W, Kautzka Z, Chen W, Goldys EM. PLGA nanocomposites loaded with verteporfin and gold nanoparticles for enhanced photodynamic therapy of cancer cells. RSC Adv. 2016;6:112393–112402. doi: 10.1039/C6RA21997G. [DOI] [Google Scholar]
- Dhas TS, Kumar VG, Abraham LS, Karthick V, Govindaraju K. Sargassum myriocystum mediated biosynthesis of gold nanoparticles. Spectrochim Acta A. 2012;99:97–101. doi: 10.1016/j.saa.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Dhas TS, Kumar VG, Karthick V, Angel KJ, Govindaraju K. Facile synthesis of silver chloride nanoparticles using marine alga and its antibacterial efficacy. Spectrochim Acta A. 2014;120:416–420. doi: 10.1016/j.saa.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Ding SJ, Zhu J. Tuning the surface enhanced raman scattering activity of gold nanocubes by silver coating. App Surf Sci. 2015;357:487–492. doi: 10.1016/j.apsusc.2015.09.077. [DOI] [Google Scholar]
- Dixit S, Zhu Y, Moore A, Broome AM. Hg-118multi-functional gold nanoparticle photosensitizer drug delivery for brain tumors. Neuro Oncol. 2016;18:iii75. doi: 10.1093/neuonc/now067.05. [DOI] [Google Scholar]
- Dong S, Tang C, Zhou H, Zhao H. Photochemical synthesis of gold nanoparticles by the sunlight radiation using a seeding approach. Gold Bull. 2004;37:187–195. doi: 10.1007/BF03215212. [DOI] [Google Scholar]
- Dykman L, Khlebtsov N. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3:9. [PMC free article] [PubMed] [Google Scholar]
- Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012;41:2256–2282. doi: 10.1039/C1CS15166E. [DOI] [PubMed] [Google Scholar]
- Elbialy NS, Fathy MM, Khalil WM. Doxorubicin loaded magnetic gold nanoparticles for in vivo targeted drug delivery. Int J Pharm. 2015;490:190–199. doi: 10.1016/j.ijpharm.2015.05.032. [DOI] [PubMed] [Google Scholar]
- El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239:129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Faraday M. Experimental relations of gold (and other metals) to light. Philos Trans R Soc. 1857;147:145–181. doi: 10.1098/rstl.1857.0011. [DOI] [Google Scholar]
- Forestier J. The treatment of rheumatoid arthritis with gold salts injections. Lancet. 1932;219:441–444. doi: 10.1016/S0140-6736(01)24417-1. [DOI] [Google Scholar]
- Gamal-Eldeen AM, Moustafa D, El-Daly SM, El-Hussieny EA, Saleh S, Khoobchandani M, Bacon KL, Gupta S, Katti K, Shukla R, Katti KV. Photothermal therapy mediated by gum Arabic-conjugated gold nanoparticles suppresses liver preneoplastic lesions in mice. J Photochem Photobiol. 2016;163:47–56. doi: 10.1016/j.jphotobiol.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Ghosh PS, Kim CK, Han G, Forbes NS, Rotello VM. Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. ACS Nano. 2008;2:2213–2218. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gole A, Murphy CJ. Seed-mediated synthesis of gold nanorods: role of the size and nature of the seed. Chem Mater. 2004;16:3633–3640. doi: 10.1021/cm0492336. [DOI] [Google Scholar]
- Govindaraju S, Ramasamy M, Baskaran R, Ahn SJ, Yun K. Ultraviolet light and laser irradiation enhances the antibacterial activity of glucosamine-functionalized gold nanoparticles. Int J Nanomed. 2015;10:67. doi: 10.2147/IJN.S88318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju S, Samal M, Yun K. Superior antibacterial activity of GlcN-AuNP-GO by ultraviolet irradiation. Mater Sci Eng C. 2016;69:366–372. doi: 10.1016/j.msec.2016.06.052. [DOI] [PubMed] [Google Scholar]
- Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Yuan Q, Yang X. Morphology study of gold–chitosan nanocomposites. J Colloid Interface Sci. 2005;282:26–31. doi: 10.1016/j.jcis.2004.08.063. [DOI] [PubMed] [Google Scholar]
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine. 2007;2(5):681–693. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- Huff TB, Tong L, Zhao Y, Hansen MN, Cheng J-X, Wei A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine. 2007;2(1):125–132. doi: 10.2217/17435889.2.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein SI, Sultan AS, Yaseen NY. Gold nanoparticles for photothermal therapy of cancerous cells in vitro. Int J Curr Microbiol App Sci. 2016;5:261–266. doi: 10.20546/ijcmas.2016.510.029. [DOI] [Google Scholar]
- Hwang SJ, Jun SH, Park Y, Cha SH, Yoon M, Cho S, Lee HJ, Park Y. Green synthesis of gold nanoparticles using chlorogenic acid and their enhanced performance for inflammation. Nanomedicine. 2015;11:1677–1688. doi: 10.1016/j.nano.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Iodice C, Cervadoro A, Palange A, Key J, Aryal S, Ramirez MR, Mattu C, Ciardelli G, O’Neill BE, Decuzzi P. Enhancing photothermal cancer therapy by clustering gold nanoparticles into spherical polymeric nanoconstructs. Opt Lasers Engg. 2016;76:74–81. doi: 10.1016/j.optlaseng.2015.04.017. [DOI] [Google Scholar]
- James KT, O’Toole MG, Patel DN, Zhang G, Gobin AM, Keynton RS. A high yield, controllable process for producing tunable near infrared-absorbing gold nanoplates. RSC Adv. 2015;57:12498–12505. doi: 10.1039/C4RA14889D. [DOI] [Google Scholar]
- Jana NR. Gram-scale synthesis of soluble, near-monodisperse gold nanorods and other anisotropic nanoparticles. Small. 2005;1:875–882. doi: 10.1002/smll.200500014. [DOI] [PubMed] [Google Scholar]
- Jana NR, Gearheart L, Murphy CJ. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater. 2001;13:1389. doi: 10.1002/1521-4095(200109)13:18<1389::AID-ADMA1389>3.0.CO;2-F. [DOI] [Google Scholar]
- Jin R, Egusa S, Scherer NF. Thermally-induced formation of atomic Au clusters and conversion into nanocubes. J Am Chem Soc. 2004;126:9900–9901. doi: 10.1021/ja0482482. [DOI] [PubMed] [Google Scholar]
- Jin Y, Li Z, Hu L, Shi X, Guan W, Du Y. Synthesis of chitosan-stabilized gold nanoparticles by atmospheric plasma. Carbohydr Polym. 2013;91:152–156. doi: 10.1016/j.carbpol.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Jolesz FA, Hynynen K. Magnetic resonance image-guided focused ultrasound surgery. Cancer J. 2001;8:100–112. [PubMed] [Google Scholar]
- Jun SH, Cho S, Park Y. Functionalization of lysostaphin on gold and silver nanoparticles and their in vitro antibacterial activities against methicillin-resistant staphylococcus aureus. Nanosci Nanotechnol Lett. 2015;7:433–440. doi: 10.1166/nnl.2015.1954. [DOI] [Google Scholar]
- Karthick V, Kumar VG, Dhas TS, Singaravelu G, Sadiq AM, Govindaraju K. Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats-An in vivo approach. Colloids Surf B. 2014;122:505–511. doi: 10.1016/j.colsurfb.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, West JL, Drezek RA. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–183. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- Kim F, Song JH, Yang P. Photochemical synthesis of gold nanorods. J Am Chem Soc. 2002;124:14316–14317. doi: 10.1021/ja028110o. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zhang X, Liang XJ. Gold nanoparticles: emerging paradigm for targeted drug delivery system. Biotech Adv. 2013;31:593–606. doi: 10.1016/j.biotechadv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Kumar CS, Mahesh A, Antoniraj MG, Vaidevi S, Ruckmani K. Ultrafast synthesis of stabilized gold nanoparticles using aqueous fruit extract of limonia acidissima and conjugated epirubicin: targeted drug delivery for treatment of breast cancer. RSC Adv. 2016;6:26874–26882. doi: 10.1039/C6RA01482H. [DOI] [Google Scholar]
- Lajunen T, Viitala L, Kontturi LS, Laaksonen T, Liang H, Vuorimaa-Laukkanen E, Viitala T, Le Guével X, Yliperttula M, Murtomäki L, Urtti A. Light induced cytosolic drug delivery from liposomes with gold nanoparticles. J Control Release. 2015;203:85–98. doi: 10.1016/j.jconrel.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Li F, Tian D, Cui H. Synthesis and characterizations of iso-luminol-functionalized, tadpole-shaped, gold nanomaterials. Luminescence. 2013;28:7–15. doi: 10.1002/bio.1380. [DOI] [PubMed] [Google Scholar]
- Li X, Robinson SM, Gupta A, Saha K, Jiang Z, Moyano DF, Sahar A, Riley MA, Rotello VM. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 2014;8:10682–10686. doi: 10.1021/nn5042625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen J, Huang H, Feng JJ, Wang AJ, Shao LX. Green and facile synthesis of l-carnosine protected fluorescent gold nanoclusters for cellular imaging. Sens Actuators B. 2016;223:40–44. doi: 10.1016/j.snb.2015.09.052. [DOI] [Google Scholar]
- Lin CA, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M, Li JK, Shen JL, Wang HH, Yeh HI, Parak WJ. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano. 2009;3:395–401. doi: 10.1021/nn800632j. [DOI] [PubMed] [Google Scholar]
- Link S, El-Sayed MA. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J Phys Chem B. 1999;103:4212–4217. doi: 10.1021/jp984796o. [DOI] [Google Scholar]
- Link S, Beeby A, FitzGerald S, El-Sayed MA, Schaaff TG, Whetten RL. Visible to infrared luminescence from a 28-atom gold cluster. J Phys Chem B. 2002;106:3410–3415. doi: 10.1021/jp014259v. [DOI] [Google Scholar]
- Liu J, Detrembleur C, Pauw-Gillet D, Mornet S, Jerome C, Duguet E. Gold nanorods coated with mesoporous silica shell as drug delivery system for remote near infrared light-activated release and potential phototherapy. Small. 2015;11:2323–2332. doi: 10.1002/smll.201402145. [DOI] [PubMed] [Google Scholar]
- Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5:709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog–conjugated hollow gold nanospheres. Clin Cancer Res. 2009;15:876–886. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Gutierrez M, Ferro-Flores G, Ocampo-Garcia B, Jimenez-Mancilla N, Morales-Avila E, Leon-Rodriguez D, Isaac-Olive K. 177Lu-labeled monomeric, dimeric and multimeric RGD peptides for the therapy of tumors expressing α (ν) β (3) integrins. J. Label. Compd. Radiopharm. 2012;55:140–148. doi: 10.1002/jlcr.2910. [DOI] [Google Scholar]
- Luna-Gutierrez M, Ferro-Flores G, Ocampo-Garcia BE, Santos-Cuevas CL, Jimenez-Mancilla N, Leon-Rodriguez D, Azorin-Vega E, Isaac-Olive K. A therapeutic system of 177 Lu-labeled gold nanoparticles-RGD internalized in breast cancer cells. Rev Soc Quim Mex. 2013;57:212–219. [Google Scholar]
- Luo S, Yang H, Yang Y, Zhao D, Chen X, Qiu M, Li Q. Controlling wave-vector of propagating surface plasmon polaritons on single-crystalline gold nanoplates. Sci Rep. 2015 doi: 10.1038/srep13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud MA. Super-radiant plasmon mode is more efficient for SERS than the sub-radiant mode in highly packed 2D gold nanocube arrays. J Chem Phys. 2015 doi: 10.1063/1.4928734. [DOI] [PubMed] [Google Scholar]
- Manivasagan P, Bharathiraja S, Bui NQ, Lim IG, Oh J. Paclitaxel-loaded chitosan oligosaccharide-stabilized gold nanoparticles as novel agents for drug delivery and photoacoustic imaging of cancer cells. Int J Pharm. 2016;511:367–379. doi: 10.1016/j.ijpharm.2016.07.025. [DOI] [PubMed] [Google Scholar]
- Melancon MP, Zhou M, Zhang R, Xiong C, Allen P, Wen X, Huang Q, Wallace M, Myers JN, Stafford RJ, Liang D. Selective uptake and imaging of aptamer-and antibody-conjugated hollow nanospheres targeted to epidermal growth factor receptors overexpressed in head and neck cancer. ACS Nano. 2014;8:4530–4538. doi: 10.1021/nn406632u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JD, Cheng Y, Broome AM, Agnes RS, Schluchter MD, Margevicius S, Wang X, Kenney ME, Burda C, Basilion JP. Peptide-targeted gold nanoparticles for photodynamic therapy of brain cancer. Part Part Syst Char. 2015;32:448–457. doi: 10.1002/ppsc.201400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mie G. Beitrage zur Optik trüber Medien, speziell kolloidaler Metallosungen. Ann Phys (Berl.) 1908;330:377–445. doi: 10.1002/andp.19083300302. [DOI] [Google Scholar]
- Mitra P, Chakraborty PK, Saha P, Ray P, Basu S. Antibacterial efficacy of acridine derivatives conjugated with gold nanoparticles. Int J Pharm. 2014;473:636–643. doi: 10.1016/j.ijpharm.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 1996;12(3):788–800. doi: 10.1021/la9502711. [DOI] [Google Scholar]
- Nanocavitation PM. Photothermal effects in ultrafast laser irradiation of gold nanorods in water Boulais, Etienne; Lachaine, Remi; Meunier. J Phys Chem C. 2013;117:9386–9396. [Google Scholar]
- Negishi Y, Takasugi Y, Sato S, Yao H, Kimura K, Tsukuda T. IX-s structures, stabilities and physicochemical properties of organometallic hybrid clusters. J Am Chem Soc. 2004;126:6518–6519. doi: 10.1021/ja0483589. [DOI] [PubMed] [Google Scholar]
- Nikoobakht B, El-Sayed MA. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater. 2003;15:1957–1962. doi: 10.1021/cm020732l. [DOI] [Google Scholar]
- Pal A, Esumi K, Pal T. Preparation of nanosized gold particles in a biopolymer using UV photoactivation. J Colloid Interface Sci. 2005;288:396–401. doi: 10.1016/j.jcis.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Parida S, Maiti C, Rajesh Y, Dey KK, Pal I, Parekh A, Patra R, Dhara D, Dutta PK, Mandal M. Gold nanorod embedded reduction responsive block copolymer micelle-triggered drug delivery combined with photothermal ablation for targeted cancer therapy. Biochim Biophys Acta. 2017;1861:3039–3052. doi: 10.1016/j.bbagen.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Patra S, Mukherjee S, Barui AK, Ganguly A, Sreedhar B, Patra CR. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater Sci Eng C. 2015;53:298–309. doi: 10.1016/j.msec.2015.04.048. [DOI] [PubMed] [Google Scholar]
- Penon O, Marin MJ, Russell DA, Perez-Garcia L. Water soluble, multifunctional antibody-porphyrin gold nanoparticles for targeted photodynamic therapy. J Colloid Interface Sci. 2017;496:100–110. doi: 10.1016/j.jcis.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Perez-Juste J, Pastoriza-Santos I, Liz-Marzan LM, Mulvaney P. Gold nanorods: synthesis, characterization and applications. Coord Chem Rev. 2005;249:1870–1901. doi: 10.1016/j.ccr.2005.01.030. [DOI] [Google Scholar]
- Philipp CM, Rohde E, Berlien H. Nd: YAG laser procedures in tumor treatment. Semin Surg Oncol. 1995;11:290–298. doi: 10.1002/ssu.2980110404. [DOI] [PubMed] [Google Scholar]
- Pitsillides CM, Joe EK, Wei X, Anderson RR, Lin CP. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys J. 2003;84:4023–4032. doi: 10.1016/S0006-3495(03)75128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshini E, Pradhan N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: a review. Sens Actuator B Chem. 2017;238:888–902. doi: 10.1016/j.snb.2016.06.081. [DOI] [Google Scholar]
- Qin Z, Bischof JC. Thermophysical and biological responses of gold nanoparticle laser heating. Chem Soc Rev. 2012;41:1191–1217. doi: 10.1039/C1CS15184C. [DOI] [PubMed] [Google Scholar]
- Rambanapasi C, Barnard N, Grobler A, Buntting H, Sonopo M, Jansen D, Jordaan A, Steyn H, Zeevaart JR. Dual radiolabeling as a technique to track nanocarriers: the case of gold nanoparticles. Molecules. 2015;20:12863–12879. doi: 10.3390/molecules200712863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengan AK, Bukhari AB, Pradhan A, Malhotra R, Banerjee R, Srivastava R, De A. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015;15:842–848. doi: 10.1021/nl5045378. [DOI] [PubMed] [Google Scholar]
- Ruan S, Yuan M, Zhang L, Hu G, Chen J, Cun X, Zhang Q, Yang Y, He Q, Gao H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials. 2015;37:425–435. doi: 10.1016/j.biomaterials.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Santiago GB, Rodríguez MJ, Blanco C, Rivas J, López-Quintela MA, Martinho JM. One step synthesis of the smallest photoluminescent and paramagnetic PVP-protected gold atomic clusters. Nano Lett. 2010;10:4217–4221. doi: 10.1021/nl1026716. [DOI] [PubMed] [Google Scholar]
- Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, Yamashiki N, Okamura A, Inoue K. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma. Cancer. 1999;85:1694–1702. doi: 10.1002/(SICI)1097-0142(19990415)85:8<1694::AID-CNCR8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sherman AI, Ter-Pogossian M. Lymph-node concentration of radioactive colloidal gold following interstitial injection. Cancer. 1953;6:1238–1240. doi: 10.1002/1097-0142(195311)6:6<1238::AID-CNCR2820060618>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Singh S. Glucose decorated gold nanoclusters: a membrane potential independent fluorescence probe for rapid identification of cancer cells expressing glut receptors. Colloids Surf B. 2017;155:25–34. doi: 10.1016/j.colsurfb.2017.03.052. [DOI] [PubMed] [Google Scholar]
- Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, Richards-Kortum R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999–2004. [PubMed] [Google Scholar]
- Suarasan S, Focsan M, Soritau O, Maniu D, Astilean S. One-pot, green synthesis of gold nanoparticles by gelatin and investigation of their biological effects on osteoblast cells. Colloids Surf B. 2015;132:122–131. doi: 10.1016/j.colsurfb.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Sun L, Liu D, Wang Z. Functional gold nanoparticle–peptide complexes as cell-targeting agents. Langmuir. 2008;24:10293–10297. doi: 10.1021/la8015063. [DOI] [PubMed] [Google Scholar]
- Svaasand LO, Gomer CJ, Morinelli E. On the physical rationale of laser induced hyperthermia. Lasers Med Sci. 1990;5:121–128. doi: 10.1007/BF02031373. [DOI] [Google Scholar]
- Tian F, Conde J, Bao C, Chen Y, Curtin J, Cui D. Gold nanostars for efficient in vitro and in vivo real-time SERS detection and drug delivery via plasmonic-tunable Raman/FTIR imaging. Biomaterials. 2016;106:87–97. doi: 10.1016/j.biomaterials.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Tiwari PM, Vig K, Dennis VA, Singh SR. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 2011;1:31–63. doi: 10.3390/nano1010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Wei Q, Wei A, Cheng JX. Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects. J. Photochem Photobiol. 2009;85:21–32. doi: 10.1111/j.1751-1097.2008.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkevich J. Colloidal gold. part i historical and preparative aspects, morphology and structure. Gold Bull. 1985;18:125–131. doi: 10.1007/BF03214694. [DOI] [Google Scholar]
- Vankayala R, Huang YK, Kalluru P, Chiang CS, Hwang KC. First demonstration of gold nanorods-mediated photodynamic therapeutic destruction of tumors via near infra-red light activation. Small. 2014;10:1612–1622. doi: 10.1002/smll.201302719. [DOI] [PubMed] [Google Scholar]
- Vilchis-Juarez A, Ferro-Flores G, Santos-Cuevas C, Morales-Avila E, Ocampo-Garcia B, Diaz-Nieto L, Luna-Gutierrez M, Jimenez-Mancilla N, Pedraza-Lopez M, Gomez-Olivan L. Molecular targeting radiotherapy with cyclo-RGDFK (C) peptides conjugated to 177Lu-labeled gold nanoparticles in tumor-bearing mice. J Biomed Nanotechnol. 2014;10:393–404. doi: 10.1166/jbn.2014.1721. [DOI] [PubMed] [Google Scholar]
- Von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Huff TB, Zweifel DA, He W, Low PS, Wei A, Cheng JX. In vitro and in vivo two-photon luminescence imaging of single gold nanorods. Proc Natl Acad Sci USA. 2005;102:15752–15756. doi: 10.1073/pnas.0504892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weare WW, Reed SM, Warner MG, Hutchison JE. Improved synthesis of small (d core ≈ 1.5 nm) phosphine-stabilized gold nanoparticles. J Am Chem Soc. 2000;122:12890–12891. doi: 10.1021/ja002673n. [DOI] [Google Scholar]
- Wilson R. The use of gold nanoparticles in diagnostics and detection. Chem Soc Rev. 2008;37:2028–2045. doi: 10.1039/b712179m. [DOI] [PubMed] [Google Scholar]
- Wu BY, Hou SH, Yin F, Zhao ZX, Wang YY, Wang XS, Chen Q. Amperometric glucose biosensor based on multilayer films via layer-by-layer self-assembly of multi-wall carbon nanotubes, gold nanoparticles and glucose oxidase on the Pt electrode. Biosens Bioelectron. 2007;22:2854–2860. doi: 10.1016/j.bios.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Wu X, He X, Wang K, Xie C, Zhou B, Qing Z. Ultrasmall near-infrared gold nanoclusters for tumor fluorescence imaging in vivo. Nanoscale. 2010;2:2244–2249. doi: 10.1039/c0nr00359j. [DOI] [PubMed] [Google Scholar]
- Xia K, Zhang L, Huang Y, Lu Z. Preparation of gold nanorods and their applications in photothermal therapy. J Nanosci Nanotechnol. 2015;15:63–73. doi: 10.1166/jnn.2015.9586. [DOI] [PubMed] [Google Scholar]
- Xie J, Zheng Y, Ying JY. Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc. 2009;131:888–889. doi: 10.1021/ja806804u. [DOI] [PubMed] [Google Scholar]
- Xing Y, Zhao J, Shi X, Conti PS, Chen K. Recent development of radiolabeled nanoparticles for PET imaging. Austin J Nanomed Nanotech. 2014;2:1016. [Google Scholar]
- Yahia-Ammar A, Sierra D, Merola F, Hildebrandt N, Le Guevel X. Self-assembled gold nanoclusters for bright fluorescence imaging and enhanced drug delivery. ACS Nano. 2016;10:2591–2599. doi: 10.1021/acsnano.5b07596. [DOI] [PubMed] [Google Scholar]
- Yang X, Yang M, Pang B, Vara M, Xia Y. Gold nanomaterials at work in biomedicine. Chem Rev. 2015;115:10410–10488. doi: 10.1021/acs.chemrev.5b00193. [DOI] [PubMed] [Google Scholar]
- Yi Y, Kim HJ, Mi P, Zheng M, Takemoto H, Toh K, Kim BS, Hayashi K, Naito M, Matsumoto Y, Miyata K. Targeted systemic delivery of siRNA to cervical cancer model using cyclic RGD-installed unimer polyion complex-assembled gold nanoparticles. J Control Release. 2016;244:247–256. doi: 10.1016/j.jconrel.2016.08.041. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jiang X, Wang E, Dong S. Attachment of gold nanoparticles to glassy carbon electrode and its application for the voltammetric resolution of ascorbic acid and dopamine. J Electroanal Chem. 2005;583:292–299. doi: 10.1016/j.jelechem.2005.06.014. [DOI] [Google Scholar]
- Zharov VP, Galitovsky V, Viegas M. Photothermal detection of local thermal effects during selective nanophotothermolysis. Appl Phys Lett. 2003;83:4897–4899. doi: 10.1063/1.1632546. [DOI] [Google Scholar]
- Zhou J, Ralston J, Sedev R, Beattie DA. Functionalized gold nanoparticles: synthesis, structure and colloid stability. J Colloid Interface Sci. 2009;331:251–262. doi: 10.1016/j.jcis.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Kong Y, Kundu S, Cirillo JD, Liang H. Antibacterial activities of gold and silver nanoparticles against escherichia coli and bacillus calmette-guerin. J Nanobiotechnol. 2012;10:1–9. doi: 10.1186/1477-3155-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Lanni E, Garg N, Bier ME, Jin R. Kinetically controlled, high-yield synthesis of Au25 clusters. J Am Chem Soc. 2008;130:1138–1139. doi: 10.1021/ja0782448. [DOI] [PubMed] [Google Scholar]
- Zhu J, Kan C, Li H, Cao Y, Ding X, Wan J. Synthesis and growth mechanism of gold nanoplates with novel shapes. J Cryst Growth. 2011;321:124–130. doi: 10.1016/j.jcrysgro.2011.02.015. [DOI] [Google Scholar]
- Zhu J, Zhang F, Chen BB, Li JJ, Zhao JW. Tuning the shell thickness-dependent plasmonic absorption of ag coated au nanocubes: the effect of synthesis temperature. Mater Sci Eng B. 2015;199:113–120. doi: 10.1016/j.mseb.2015.06.001. [DOI] [Google Scholar]