Abstract
Recent studies have shown that the chemokine receptor CXCR3 and its ligand CXCL10 in the dorsal root ganglion mediate itch in experimental allergic contact dermatitis (ACD). CXCR3 in the spinal cord also contributes to the maintenance of neuropathic pain. However, whether spinal CXCR3 is involved in acute or chronic itch remains unclear. Here, we report that Cxcr3 −/− mice showed normal scratching in acute itch models but reduced scratching in chronic itch models of dry skin and ACD. In contrast, both formalin-induced acute pain and complete Freund’s adjuvant-induced chronic inflammatory pain were reduced in Cxcr3 −/− mice. In addition, the expression of CXCR3 and CXCL10 was increased in the spinal cord in the dry skin model induced by acetone and diethyl ether followed by water (AEW). Intrathecal injection of a CXCR3 antagonist alleviated AEW-induced itch. Furthermore, touch-elicited itch (alloknesis) after compound 48/80 or AEW treatment was suppressed in Cxcr3 −/− mice. Finally, AEW-induced astrocyte activation was inhibited in Cxcr3 −/− mice. Taken together, these data suggest that spinal CXCR3 mediates chronic itch and alloknesis, and targeting CXCR3 may provide effective treatment for chronic pruritus.
Keywords: Chronic itch, Alloknesis, Dry skin, CXCR3, CXCL10, Spinal cord
Introduction
Chemokines are a family of small secreted proteins, which are well-known regulators of peripheral immune cell trafficking [1]. Chemokines are also expressed in the central nervous system, where they regulate its function under both physiological and pathological conditions, including neuronal development, synaptic transmission, and disease-associated neuroinflammation [1–3]. Several chemokines have been shown to mediate neuroinflammation in the spinal cord and contribute to chronic pain [4, 5]. Chronic itch is a debilitating symptom of inflammatory skin conditions, such as contact dermatitis and atopic dermatitis, as well as systemic diseases, for which existing treatment is largely ineffective [6]. Recent studies have revealed that chronic itch and chronic pain share some common mechanisms [7, 8]. For example, toll-like receptor 4 (TLR4) in the spinal cord contributes to neuropathic pain, arthritis pain, and chemotherapy-induced pain [9–11]. TLR4 also plays an important role in mediating the chronic itch induced by dry skin and allergic contact dermatitis (ACD) [12]. Whether chemokines in the spinal cord are involved in chronic itch remains to be investigated.
Chemokines consist of >50 members and 4 subfamilies. CXCL10 belongs to the CXC subfamily and exerts its biological function via the G-protein coupled receptor CXCR3 [13]. CXCL10/CXCR3 signaling has been implicated in a variety of human diseases including chronic inflammation, immune dysfunction, cancer, and metastasis [14, 15]. Our recent study showed that CXCL10 and CXCR3 are upregulated in the spinal cord after peripheral nerve injury and contribute to the maintenance of neuropathic pain [16]. Spinal CXCL10/CXCR3 is also involved in experimental autoimmune encephalomyelitis-evoked hyperalgesia [17] and bone cancer-induced pain hypersensitivity [18]. Recently, Qu et al. reported that CXCL10 and CXCR3 are increased in the dorsal root ganglion (DRG) in an ACD model, and CXCL10 directly activates a subset of cutaneous DRG neurons through neuronal CXCR3 [19]. In peripheral tissues, CXCL10 is produced by epidermal cells and is upregulated in the challenged skin of contact hypersensitivity [20, 21]. These studies suggest a role of peripheral CXCL10/CXCR3 in mediating ACD-induced itch. Whether spinal CXCR3 contributes to chronic itch has not been explored.
In this study, using acute itch models induced by compound 48/80 or chloroquine, and chronic itch models induced by dry skin or ACD, we investigated whether Cxcr3-deficiency affects acute or chronic itch. Given that astrocyte activation in the spinal dorsal horn is associated with chronic itch [22], we also checked the expression of the astrocytic marker GFAP in the spinal cord in the dry skin model.
Materials and Methods
Animals
Adult C57BL/6 mice (male, 8 weeks old) were purchased from the Experimental Animal Center of Nantong University. Cxcr3 −/− mice (B6.129P2-Cxcr3 tm1Dgen/J, stock number 005796) were purchased from the Jackson Laboratory. The animals were maintained in a specific-pathogen-free facility under a 12:12 h light-dark cycle at a room temperature of 22 ± 1 °C. All animal procedures were reviewed and approved by the Animal Care and Use Committee of Nantong University.
Drugs and Administration
The potent and selective CXCR3 antagonist (±)-NBI-74330 was from Tocris (Bristol, UK); compound 48/80, chloroquine (CQ), and complete Freund’s adjuvant (CFA) were from Sigma-Aldrich (St. Louis, MO); diphenylcyclopropenone (DCP) was from Shanghai Aladdin Biochem Technology Co., Ltd (Shanghai, China); and 2,4-dinitro-1-fluorobenzene (DNFB) was from TCI (Shanghai) Development Co., Ltd (Shanghai, China). Intrathecal injection was done with a 30 G needle into the intervertebral space between L5 and L6 to deliver reagents to the cerebrospinal fluid.
Behavioral Analysis
Neck Models of Acute Itch
Mice were habituated to the testing environment for 2 days. The back of the neck was shaved 2 days before experiments. On the day of behavioral testing, mice were individually placed in small plastic chambers (15 × 15 × 15 cm3) on an elevated metal mesh floor and allowed at least 30 min for habituation. Under brief anesthesia with isoflurane, mice were given an intradermal injection of 50 μL of compound 48/80 (100 μg) or CQ (200 μg) via a 30 G needle into the nape of the neck [12]. Immediately after the injection, mice were returned to the chambers and recorded for 30 min. The video was subsequently played back offline and the scratching behavior was quantified in a blinded manner. A scratch was counted when a mouse lifted the hindpaw to scratch the shaved skin and returned the paw to the floor or mouth [23].
Cheek Model of Acute Itch
The animal’s cheek was shaved 2 days before experiments. On the day of experiment, after brief anesthesia with isoflurane, 10 μL of compound 48/80 (100 μg) or CQ (200 μg) was injected into the cheek, and the animal’s behavior was recorded for 30 min [12]. The numbers of wipes and scratches were counted offline in a blinded manner. Wipes were defined as a unilateral wipe with the forelimb. Scratches were defined as a lifting of the hindpaw toward the injection site on the cheek and then returning the paw to the floor or to the mouth [24].
Dry Skin-Induced Mouse Model of Chronic Itch
The hair of the nape was shaved 2 days prior to treatment. Dry skin was induced by application of a 1.5 mL 1:1 mixture of acetone and diethyl ether for 10 min, followed by clean water for 30 s (AEW) twice a day (morning and evening) for 7–8 days [12]. Control animals were treated with water only. The spontaneous scratching behavior was recorded for 1 h. Bouts of scratching were then counted in a blinded manner.
Diphenylcyclopropenone (DCP)-Induced ACD in Neck Skin
The hair of the nape was shaved and then DCP (1% in acetone, 0.2 mL) was painted onto it. Seven days later, the mice were challenged by painting the nape skin with 0.2 mL 0.5% DCP [12], which was applied daily for 10 days. The spontaneous scratching behavior was video-recorded for 1 h after each DCP application. Bouts of scratching were then counted in a blinded manner.
2,4-Dinitro-1-fluorobenzene (DNFB)-Induced ACD in Neck Skin
DNFB was dissolved in a mixture of acetone and olive oil (4:1). The surface of the abdomen and the nape of the neck were shaved 2 days before sensitization. Mice were sensitized with 50 μL of 0.5% DNFB by topical application to the shaved abdominal skin. Five days later, the mice were challenged with 30 μL of 0.25% DNFB by painting the nape of neck, and then on days 3, 5, and 7 [12]. The scratching behavior was video-recorded for 1 h on days 4, 6, and 8. Bouts of scratching were then counted in a blinded manner.
Formalin-Induced Spontaneous Pain
Mice were habituated in an individual observation cage for at least 30 min prior to an injection of 5% formalin (20 μL) into the ventral surface of the right hindpaw using a 30 G needle. After the injection, they were immediately placed into the observation cage and video-recorded for 45 min. The time spent licking, biting, and flinching of the injected paw was recorded in 5-min intervals in a blinded manner.
Von Frey Test
The animals were put in boxes on an elevated metal mesh floor and allowed 30 min for habituation before examination. The plantar surface of the hindpaw was stimulated with a series of von Frey hairs with logarithmically incremental stiffness (0.02–2.56 g; Stoelting, Wood Dale, IL) presented perpendicular to the plantar surface (2–3 s for each hair). The 50% paw withdrawal threshold was determined using Dixon’s up-down method [25].
Hargreaves Test
The animals were put in a plastic box placed on a glass plate, and the plantar surface was exposed to a beam of radiant heat through a transparent glass surface (IITC model 390 Analgesia Meter; Life Science, Woodland Hills, CA). The baseline latencies were adjusted to 10–14 s with a maximum of 20 s as a cutoff to prevent potential injury. The latencies were averaged over 3 trials, separated by 5-min intervals [26].
Rota-Rod Test
Mice were trained on the rota-rod for 3 min at a speed of 10 rpm, until they no longer fell off. For testing, the speed was set at 10 rpm for 60 s and subsequently accelerated to 80 rpm in 5 min. The latency to fall after the beginning of the acceleration was recorded [27].
Real-Time Quantitative PCR (qPCR)
Total RNA from the spinal cord or DRG was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). One microgram of total RNA was reverse-transcribed using an oligo(dT) primer according to the manufacturer’s protocol (Takara, Shiga, Japan). The qPCR analysis was performed in the Real-time Detection System (Rotor-Gene 6000, Hamburg, Germany) by SYBR green I dye detection (Takara). The cDNA was amplified using the following primers: Cxcr3 forward (5′-TAC CTT GAG GTT AGT GAA CGT CA-3′) and reverse (5′-CGC TCT CGT TTT CCC CAT AAT C-3′); Cxcl10 forward (5′-TGA ATC CGG AAT CTA AGA CCA TCA A-3′) and reverse (5′-AGG ACT AGC CAT CCA CTG GGT AAA G-3′); and Gapdh forward (5′-AAA TGG TGA AGG TCG GTG TGA AC-3′) and reverse (5′-CAA CAA TCT CCA CTT TGC CAC TG-3′). The PCR amplifications were performed at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 45 s. Gapdh was used as an endogenous control to normalize differences. Melting curves were constructed on completion of the cycles to ensure the absence of nonspecific products. Quantification was performed by normalizing Ct (cycle threshold) values with the Gapdh Ct and analyzed with the 2−ΔΔCT method.
Immunohistochemistry
Animals were deeply anesthetized with isoflurane and perfused through the ascending aorta with PBS followed by 4% paraformaldehyde in 0.16 mol/L phosphate buffer. After perfusion, the C1–2 spinal segments were removed and postfixed in the same fixative overnight. Spinal cord sections (30 μm, free-floating) were cut on a cryostat and processed for immunofluorescence as we described previously [28]. The sections were first blocked with 5% donkey serum for 2 h at room temperature, then incubated overnight at 4 °C with the following primary antibodies: CXCR3 (rabbit, 1:200, Boster Biological Technology), CXCL10 (goat, 1:100, R&D Systems), and glial fibrillary acidic protein (GFAP, mouse, 1:5000, Millipore). The sections were then incubated for 1 h at room temperature with Cy3- or FITC-conjugated secondary antibodies (1:400, Jackson ImmunoResearch, West Grove, PA). The stained sections were examined under a Leica fluorescence microscope, and images were captured with a CCD Spot camera.
Quantification and Statistics
All data are expressed as mean ± SEM. For the analysis of CXCR3, CXCL10, and GFAP immunoreactivity, images of the cervical dorsal horn were captured and numerical values of the intensity were calculated with a computer-assisted imaging analysis system (ImageJ) [29]. Three nonadjacent sections were randomly selected from each mouse, and 3–4 mice were included in each group. The behavioral data were analyzed by two-way repeated measures ANOVA followed by the Bonferroni test as the post-hoc multiple comparison analysis. Student’s t-test was applied if only 2 groups were to be compared. The criterion for statistical significance was P < 0.05.
Results
Cxcr3−/− Mice Show Normal Acute Itch Induced by Compound 48/80 or CQ
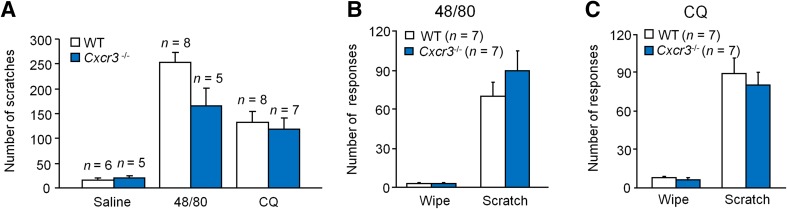
It is known that acute itch can be characterized as histaminergic itch induced by compound 48/80, and non-histaminergic itch induced by CQ. We found that compound 48/80- and CQ-induced itch were comparable between WT (C57BL/6) and Cxcr3 −/− mice (Fig. 1A). To further define the possible role of CXCR3 in pain and itch, a cheek model was used to distinguish pain versus itch, as indicated by distinct pain-like wiping by forelimbs and itch-like scratching by the hindlimbs [24]. Cheek injection of compound 48/80 or CQ induced marked scratching behavior but very mild wiping behavior. The number of wipes and scratches did not differ between WT and Cxcr3 −/− mice after compound 48/80 (Fig. 1B) or CQ (Fig. 1C) injection. These data suggest that CXCR3 is dispensable for both histaminergic and non-histaminergic acute itch.
Fig. 1.
CXCR3 is not required in models of acute itch in the neck and cheek. A Acute itch induced by intradermal injection of compound 48/80 (48/80, 100 μg) or chloroquine (CQ, 200 μg) into the neck skin was comparable in WT and Cxcr3 −/− mice. B, C Acute itch and pain, which were induced by intradermal injection of 48/80 (B) or CQ (C) into the cheek, induced similar wipes and scratches in WT and Cxcr3 −/− mice.
Chronic Itch Induced by Dry Skin or ACD is Reduced in Cxcr3−/− Mice
We then checked the effect of CXCR3 on chronic itch. AEW treatment causes skin dehydration and induces chronic itch [30], and we found that the number of scratches gradually increased from day 3 to day 7 after daily treatment with AEW in WT mice (Fig. 2A). AEW also induced scratching in Cxcr3 −/− mice, but the number of scratches was significantly less than that in WT mice. The area under the curve also showed decreased scratch responses in Cxcr3 −/− mice (Fig. 2B).
Fig. 2.
CXCR3 is required for chronic itch in the dry skin and allergic contact dermatitis models. A AEW treatment induced chronic itch in WT mice, which was reduced in Cxcr3 −/− mice (*P < 0.05, two-way repeated measures ANOVA). B Area under the curve of (A) (*P < 0.05, Student’s t test). C Chronic itch induced by DCP was substantially reduced in Cxcr3 −/− mice (*P < 0.05 two-way repeated measures ANOVA). D Area under the curve of (C) (*P < 0.05, Student’s t test). E Chronic itch induced by DNFB was markedly reduced in Cxcr3 −/− mice (*P < 0.05, two-way repeated measures ANOVA). F Area under the curve of (E) (*P < 0.05, Student’s t test).
To further confirm the role of CXCR3 in chronic itch, we treated the animals with either DCP or DNFB to induce ACD [31, 32]. Treatment with DCP induced robust and persistent itch in WT mice, which was substantially reduced in Cxcr3 −/− mice (Fig. 2C, D). In addition, treatment with DNFB also induced spontaneous itch on days 4, 6, and 8 in WT mice (Fig. 2E), and the number of scratches was dramatically reduced in Cxcr3 −/− mice (Fig. 2E, F). These data suggest that CXCR3 is involved in dry skin- and ACD-induced chronic itch.
Formalin-Induced Spontaneous Pain and CFA-Induced Pain Hypersensitivity are Reduced in Cxcr3−/− Mice
Our recent study showed that CXCR3 plays an important role in nerve injury-induced chronic neuropathic pain [16]. We then asked if CXCR3 is involved in acute or chronic inflammatory pain. Formalin (5%) injection into the paw induced typical two-phase spontaneous pain in WT mice, which was reduced in Cxcr3 −/− mice (Fig. 3A). Further analysis showed that the nociceptive response in the first phase was similar in WT and Cxcr3 −/− mice, while the response in the second phase in Cxcr3 −/− mice was significantly less than that in WT mice (Fig. 3B).
Fig. 3.
CXCR3 is required for both formalin-induced and CFA-induced inflammatory pain. A Intraplantar injection of formalin-induced spontaneous pain was reduced in Cxcr3 −/− mice (*P < 0.05, two-way repeated measures ANOVA). B Spontaneous pain in the first phase (0–10 min) was comparable between WT and Cxcr3 −/− mice, but that in the second phase (10–45 min) was reduced in Cxcr3 −/− mice (*P < 0.05, Student’s t test). C Mechanical allodynia induced by intraplantar injection of CFA declined from day 3 to day 10 in Cxcr3 −/− mice (*P < 0.05, two-way repeated measures ANOVA followed by Bonferroni test). D CFA-induced heat hyperalgesia was greatly attenuated from day 1 to day 10 in Cxcr3 −/− mice (*P < 0.05, two-way repeated measures ANOVA followed by Bonferroni test).
Intraplantar injection of CFA is a widely-used model of chronic inflammatory pain. As shown in Fig. 3C and D, CFA injection induced rapid and persistent mechanical allodynia and heat hyperalgesia in WT mice, starting from day 1 and continuing for >10 days. However, in Cxcr3 −/− mice, the CFA-induced mechanical allodynia was alleviated from day 3, and CFA induced mild heat hyperalgesia on day 1, which recovered after day 3. These data suggest that CXCR3 is involved in both acute and chronic inflammatory pain.
AEW Increases the Expression of CXCR3 and CXCL10 in the Spinal Cord
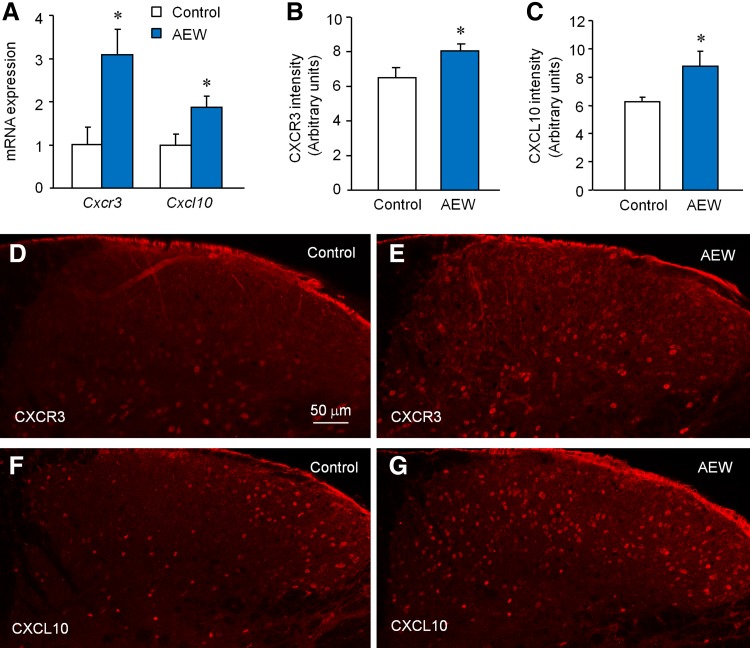
To check if spinal CXCR3 is involved in chronic itch, we examined the expression of CXCR3 and CXCL10 in the spinal cord 7 days after AEW treatment. Compared to the water-treated control animals, AEW treatment increased both Cxcr3 mRNA and Cxcl10 mRNA in the spinal cord (Fig. 4A). Immunostaining further showed increased CXCR3-immunoreactivity (Fig. 4B–D) and CXCL10-immunoreactivity (Fig. 4B, E, F) in the cervical dorsal horn, indicating that spinal CXCL10 and CXCR3 are involved in chronic itch.
Fig. 4.
The expression of CXCR3 and CXCL10 is upregulated in the spinal cord after AEW treatment. A qPCR showed increased Cxcr3 and Cxcl10 mRNA 7 days after AEW (*P < 0.05, Student’s t test, n = 6–8 mice/group). B, C The intensity of CXCR3-IR (B) and CXCL10-IR (C) was also increased 7 days after AEW (*P < 0.05, student’s t test, n = 3–4 mice/group). D, E Representative images of CXCR3 immunostaining from control (D) and AEW-treated (E) mice. F, G Representative images of CXCL10 immunostaining from control (F) and AEW-treated (G) mice.
CXCR3 Antagonist Attenuates AEW-Induced Chronic Itch
To confirm the effect of spinal CXCR3 on chronic itch, we intrathecally injected a CXCR3 antagonist, NBI-74330 (20 μg) [16], on day 7 of AEW treatment. One hour after injection, scratching behaviors were counted for 1 h. NBI-74330 significantly reduced scratching compared to the vehicle group (Fig. 5A). The same treatment did not affect motor function, as assessed by the rota-rod test. These results suggest that (1) the reduction in the number of scratches after NBI-74330 injection was not due to a defect in locomotor activity, and (2) spinal CXCR3 may contribute to AEW-induced chronic itch.
Fig. 5.
Spinal CXCR3 is required for AEW-induced chronic itch. A Intrathecal injection of the CXCR3 antagonist NBI-74330 (20 μg) on day 7 of AEW treatment reduced scratching behavior (*P < 0.05, two-way repeated measures ANOVA followed by Bonferroni test). B The same dose of NBI-74330 did not affect motor function as assessed by the rota-rod test.
Compound 48/80- or AEW-Induced Alloknesis is Reduced in Cxcr3−/− Mice
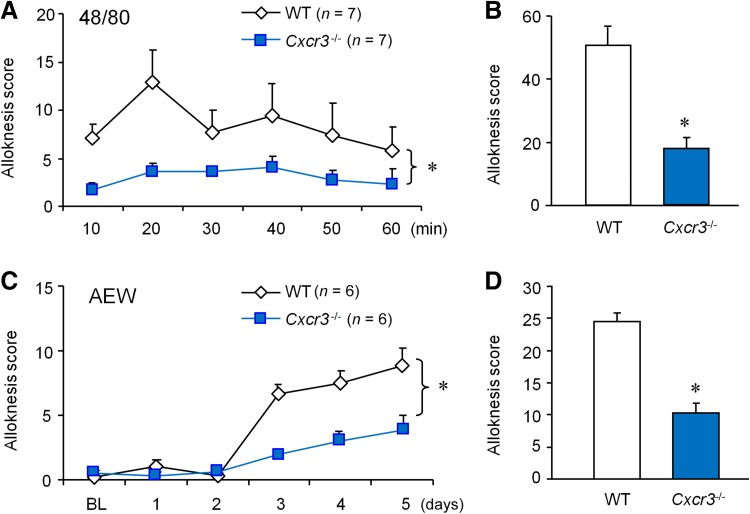
Touch-evoked itch (alloknesis) is mediated by central sensitization [33]. We scored alloknesis by counting the bouts of scratching after application of 0.7 mN von Frey stimuli 30 min after compound 48/80 injection [12]. Cxcr3 −/− mice displayed a significant reduction in alloknesis score during a 60-min period (Fig. 6A, B). Alloknesis was also induced after AEW treatment in WT mice, starting from day 3 and reaching a high level on day 5 (Fig. 6C). However, the AEW-induced development of alloknesis was reduced in Cxcr3 −/− mice (Fig. 6D). These data suggest that CXCR3 plays an important role in alloknesis after acute itch and during chronic itch.
Fig. 6.
CXCR3 is required for touch-evoked scratching (alloknesis) after compound 48/80 or AEW treatment. A Alloknesis, induced 30 min after compound 48/80 treatment, was reduced in Cxcr3 −/− mice (*P < 0.05, two-way repeated measures ANOVA). B The total alloknesis score was reduced in Cxcr3 −/− mice (*P < 0.05, Student’s t test). C Alloknesis, induced after AEW-induced dry skin, was reduced in Cxcr3 −/− mice (*P < 0.05, two-way repeated measures ANOVA). D The total alloknesis score was reduced in Cxcr3 −/− mice (*P < 0.05, Student’s t test).
AEW-Induced Astrocyte Activation is Reduced in Cxcr3−/− Mice
Given that astrogliosis is important in the maintenance of chronic itch [12, 34], we checked the expression of the astrocytic marker GFAP in the spinal cord of WT and Cxcr3 −/− mice 7 days after AEW treatment, and found that AEW increased GFAP expression in WT mice, but not in Cxcr3 −/− mice (Fig. 7A). Quantitative analysis of the intensity of GFAP+ immunofluorescence further showed that the GFAP expression was significantly increased in WT-AEW mice, compared to either WT-control or Cxcr3 −/−-AEW mice (Fig. 7B), suggesting an association of CXCR3 with astrogliosis in the chronic itch state.
Fig. 7.
AEW-induced astrocyte activation is reduced in Cxcr3-deficient mice. A Immunostaining of GFAP shows activated astrocytes 7 days after AEW treatment in WT mice, but not in Cxcr3 −/− mice. B Quantitative analysis of GFAP-IR in WT and Cxcr3 −/− mice (*P < 0.05, Student’s t test, n = 3–4 mice/group).
Discussion
In the present study, we provide the first evidence that CXCR3 is involved in chronic itch and chronic pain. Particularly, CXCR3 in the spinal cord plays an important role in mediating chronic itch, and this process may be associated with spinal astrogliosis.
Compound 48/80 and CQ are commonly used to induce acute itch. Compound 48/80-evoked itch is mainly mediated through mast-cell degranulation, a process causing histamine release. This kind of itch can be blocked by histamine receptor H1 and H4 antagonists [35]. However, CQ-induced itch cannot be treated effectively by antihistamine drugs, and may act through MrgprA3 (Mas-related G-protein coupled receptor member A3) and PAR2 (protease activated receptor 2) [36, 37]. Cxcr3-deficient mice exhibited normal scratch responses to compound 48/80 and CQ. These mice also show normal responses to noxious heat and mechanical stimuli [16], implying that CXCR3 does not appear to be essential for the spinal transmission of itch and pain signaling.
Chronic itch is usually associated with inflammatory skin diseases, such as dry skin, atopic dermatitis, and ACD [38]. In Cxcr3-deficient mice, chronic itch was substantially reduced in the dry skin and ACD models, confirming the involvement of CXCR3 in chronic itch. Our recent study showed that spinal nerve injury-induced neuropathic pain is persistently reduced in Cxcr3-deficient mice [16]. These mice also showed mild mechanical allodynia and heat hyperalgesia after CFA injection (Fig. 3B), indicating that CXCR3 is also necessary for the pathogenesis of chronic pain. However, different from acute itch, formalin-induced acute pain was decreased in Cxcr3-deficient mice, with the reduction in the second phase. It is known that the first-phase of formalin-induced spontaneous pain is a result of direct activation of nociceptors and peripheral sensitization, while the second-phase pain may be a result of central sensitization [39–41]. The reduction of spontaneous pain behaviors in the second phase in Cxcr3-deficient mice suggests that CXCR3 is involved in pain via regulating central sensitization. Indeed, intrathecal injection of CXCL10 induces CXCR3-dependent pain hypersensitivity and extracellular signal-regulated kinase activation in dorsal horn neurons [16]. Perfusion of spinal cord slices with CXCL10 increases spontaneous excitatory postsynaptic currents and NMDA- and AMPA-induced currents of lamina II neurons [16], supporting a role of CXCR3 in central sensitization.
Peripheral and central sensitization contribute to both chronic pain and chronic itch [7, 38, 42, 43]. Intradermal injection of CXCL10 cannot induce either pain or itch under normal conditions [19]. However, CXCR3 and CXCL10 are increased in DRG neurons after ACD-induced chronic itch [19]. CXCL10 also directly activates DRG neurons via neuronal CXCR3. In addition, administration of a CXCR3 antagonist to the site of ACD attenuates spontaneous itch [19]. These data suggest that CXCR3 in the DRG is involved in chronic itch via peripheral sensitization. We showed that AEW treatment increased the expression of CXCR3 and CXCL10 in the spinal cord. Intrathecal injection of the CXCR3 antagonist NBI-74330, targeting spinal CXCR3, reduced the number of AEW-induced scratches, supporting the role of spinal CXCR3 in the persistence of dry skin-induced itch. Furthermore, alloknesis was reduced in Cxcr3-deficient mice during compound 48/80-induced acute itch or AEW-induced chronic itch. Alloknesis is a result of central sensitization in the spinal cord, as activation of low-threshold mechanoreceptors excites sensitized itch-signaling neurons in the dorsal horn [12, 33]. These data suggest that CXCR3 is involved in chronic itch and alloknesis via central sensitization. As we only checked the spinal effect of a CXCR3 antagonist in dry skin-induced itch, future studies are needed to determine the role of such antagonists in acute itch, in chronic itch other than the dry skin model, and in alloknesis.
Glial cells such as astrocytes and microglia are important in the development and maintenance of chronic pain. Tissue damage or nerve injury induces spinal gliosis, and inhibition of glial activation attenuates chronic pain [44, 45]. Recent studies have shown that chronic itch is associated with astrogliosis [12, 34], but not with microgliosis in the spinal cord [12]. In addition, the transcription factor signal transducer and activator of transcription 3 (STAT3) and TLR4 are expressed in astrocytes and mediate astrocytic activation under chronic itch conditions [12, 34]. Consistent with previous reports, AEW treatment increased expression of the astrocytic marker GFAP in WT mice (Fig. 7). Also, using an Elizabethan collar to protect mice from scratching, AEW-induced astrogliosis is prevented in the cervical spinal cord [12], indicating that scratching-induced sensory input is essential for astrogliosis in the dry skin model. In this study, Cxcr3-deficient mice did not show astrogliosis after AEW treatment. This may be due to the decreased scratching in Cxcr3 −/− mice during AEW treatment. On the other hand, as CXCL10 can increase excitatory synaptic transmission via CXCR3 [16], the blocked astrogliosis in Cxcr3 −/− mice may also be attributed to the decreased central sensitization. Further studies, including the effects of intrathecal injection of CXCR3 antagonists on astrogliosis and the cellular distribution of CXCR3 and CXCL10 in the cervical spinal cord after AEW treatment are required to clarify the correlation between CXCR3 and astrogliosis in chronic itch.
In conclusion, our findings demonstrate that CXCR3 is involved in chronic itch and alloknesis, but not acute itch. Particularly, CXCR3 in the spinal cord plays a pivotal role in the pathogenesis of chronic itch induced by dry skin and ACD. Taken together with our previous data [16], these results suggest that targeting spinal CXCR3 may offer effective treatment for both chronic itch and chronic pain.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (31371121, 81300954, and 31671091), and the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Footnotes
Peng-Bo Jing and De-Li Cao contributed equally to this work.
References
- 1.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 2.Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/S0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 3.Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics. 2007;4:590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel T, Yosipovitch G. Therapy of pruritus. Expert Opin Pharmacother. 2010;11:1673–1682. doi: 10.1517/14656566.2010.484420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji RR. Neuroimmune interactions in itch: Do chronic itch, chronic pain, and chronic cough share similar mechanisms? Pulm Pharmacol Ther. 2015;35:81–86. doi: 10.1016/j.pupt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, et al. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152:2881–2891. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain. 2014;15:712–725. doi: 10.1016/j.jpain.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T, Han Q, Chen G, Huang Y, Zhao LX, Berta T, et al. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain. 2016;157:806–817. doi: 10.1097/j.pain.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ransohoff RM, Liu L, Cardona AE. Chemokines and chemokine receptors: multipurpose players in neuroinflammation. Int Rev Neurobiol. 2007;82:187–204. doi: 10.1016/S0074-7742(07)82010-1. [DOI] [PubMed] [Google Scholar]
- 14.Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26:311–327. doi: 10.1016/j.cytogfr.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann N Y Acad Sci. 2009;1173:310–317. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 16.Jiang BC, He LN, Wu XB, Shi H, Zhang WW, Zhang ZJ, et al. Promoted interaction of C/EBPalpha with demethylated Cxcr3 gene promoter contributes to neuropathic pain in mice. J Neurosci. 2017;37:685–700. doi: 10.1523/JNEUROSCI.2262-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz K, Pickert G, Wijnvoord N, Haussler A, Tegeder I. Dichotomy of CCL21 and CXCR3 in nerve injury-evoked and autoimmunity-evoked hyperalgesia. Brain Behav Immun. 2013;32:186–200. doi: 10.1016/j.bbi.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Guan XH, Fu QC, Shi D, Bu HL, Song ZP, Xiong BR, et al. Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Exp Neurol. 2015;263:39–49. doi: 10.1016/j.expneurol.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Qu L, Fu K, Yang J, Shimada SG, LaMotte RH. CXCR3 chemokine receptor signaling mediates itch in experimental allergic contact dermatitis. Pain. 2015;156:1737–1746. doi: 10.1097/j.pain.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992;89:1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flier J, Boorsma DM, Bruynzeel DP, Van Beek PJ, Stoof TJ, Scheper RJ, et al. The CXCR3 activating chemokines IP-10, Mig, and IP-9 are expressed in allergic but not in irritant patch test reactions. J Invest Dermatol. 1999;113:574–578. doi: 10.1046/j.1523-1747.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- 22.Tsuda M. Spinal dorsal horn astrocytes: New players in chronic itch. Allergol Int. 2017;66:31–35. doi: 10.1016/j.alit.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Zhou FM, Cheng RX, Wang S, Huang Y, Gao YJ, Zhou Y, et al. Antioxidants attenuate acute and chronic itch: peripheral and central mechanisms of oxidative stress in pruritus. Neurosci Bull. 2016 doi: 10.1007/s12264-016-0076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 26.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 27.Jiang BC, Cao DL, Zhang X, Zhang ZJ, He LN, Li CH, et al. CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J Clin Invest. 2016;126:745–761. doi: 10.1172/JCI81950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang ZJ, Cao DL, Zhang X, Ji RR, Gao YJ. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain. 2013;154:2185–2197. doi: 10.1016/j.pain.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88:285–292. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- 31.Inagaki N, Shiraishi N, Igeta K, Itoh T, Chikumoto T, Nagao M, et al. Inhibition of scratching behavior associated with allergic dermatitis in mice by tacrolimus, but not by dexamethasone. Eur J Pharmacol. 2006;546:189–196. doi: 10.1016/j.ejphar.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Vennegaard MT, Bonefeld CM, Hagedorn PH, Bangsgaard N, Lovendorf MB, Odum N, et al. Allergic contact dermatitis induces upregulation of identical microRNAs in humans and mice. Contact Dermatitis. 2012;67:298–305. doi: 10.1111/j.1600-0536.2012.02083.x. [DOI] [PubMed] [Google Scholar]
- 33.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) J Invest Dermatol. 2012;132:1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiratori-Hayashi M, Koga K, Tozaki-Saitoh H, Kohro Y, Toyonaga H, Yamaguchi C, et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med. 2015;21:927–931. doi: 10.1038/nm.3912. [DOI] [PubMed] [Google Scholar]
- 35.Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol. 2004;142:374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Berta T, Xu ZZ, Liu T, Park JY, Ji RR. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain. 2011;152:419–427. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carstens E, Akiyama T. Central mechanisms of itch. Curr Probl Dermatol. 2016;50:11–17. doi: 10.1159/000446011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–S28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]