Abstract
To investigate the behavioral and biomolecular similarity between neuralgia and depression, a trigeminal neuralgia (TN) mouse model was established by constriction of the infraorbital nerve (CION) to mimic clinical trigeminal neuropathic pain. A mouse learned helplessness (LH) model was developed to investigate inescapable foot-shock-induced psychiatric disorders like depression in humans. Mass spectrometry was used to assess changes in the biomolecules and signaling pathways in the hippocampus from TN or LH mice. TN mice developed not only significant mechanical allodynia but also depressive-like behaviors (mainly behavioral despair) at 2 weeks after CION, similar to LH mice. MS analysis demonstrated common and distinctive protein changes in the hippocampus between groups. Many protein function families (such as cell-to-cell signaling and interaction, and cell assembly and organization,) and signaling pathways (e.g., the Huntington’s disease pathway) were involved in chronic neuralgia and depression. Together, these results demonstrated that the LH and TN models both develop depressive-like behaviors, and revealed the involvement of many psychiatric disorder-related biomolecules/pathways in the pathogenesis of TN and LH.
Keywords: Trigeminal neuralgia, Learned helplessness, Depression, Allodynia, Mass spectrometry
Introduction
Clinically, pain and mental diseases frequently coexist. Chronic pain plays an important role in psychiatric disorders. Patients with many forms of pain are more susceptible to depression and anxiety [1, 2]. Most studies of the relationship between chronic pain and mood disorders have focused on depression [3], and accumulating supporting evidence has been reported over the last two decades [4, 5]. Studies have reported that ~50% of patients with chronic pain suffer from major depression [6], but the mechanisms underlying this comorbidity remain unclear.
Injury of the infraorbital nerve (a branch of the trigeminal nerve) has been used to study facial pain, especially trigeminal neuralgia (TN). TN attacks can be excruciating for patients even with a light touch [7]. Rodent TN models are generally established by constriction of the infraorbital nerve (CION). In light of the current lack of research on the relationship between neuralgia and depression, CION could be a valuable tool and warrants particular attention in neuropathic pain and depression trials. The learned helplessness (LH) model is well established and has been widely used to induce behavioral disorders, despair in particular [8, 9]. In this paradigm, animals are exposed to inescapable and unpredictable foot-shock stress and finally develop depressive-like behaviors [10–12]. The behavioral characteristics of susceptible LH animals are very similar to clinical depression [13].
It is fully recognized that hippocampal changes are involved in the pathogenesis of depression [14, 15]. Compared with other brain regions, the hippopotamus is the most widely-investigated and directly-influenced region in the field of depression [16, 17]. However, most investigations were based on classical depression models (like LH and chronic mild stress). With regard to the depressive-like expression under chronic neuropathic pain conditions, the corresponding molecular mechanisms remain largely elusive. In the face of the complex links between neuropathic pain and depression, the following questions interested us: Is the CION neuropathic pain model sufficient to develop depressive-like behaviors? If so, what proteins and their related signaling pathways as well as functional families are commonly involved in the hippocampal changes induced by CION and LH? In order to answer these questions, mass spectrometry (MS) was used to analyze protein samples from the hippocampus of mice exposed to CION and LH.
Materials and Methods
Animals
C57BL/6 male mice aged 7–9 weeks were provided by the Experimental Animal Center, Chinese Academy of Sciences (Shanghai, China). The mice were group-housed at 5 per cage with a 12 h/12 h light-dark cycle (lights on from 08:00 to 20:00) in an air-conditioned room (23 ± 2 °C), with free access to food and water. Animals were habituated for 1 week before experiments. All animal experiments were approved by the Committee on the Use of Animal Experiments of Fudan University (Permit Number: SYXK 2009-0082) and followed the policies on the use of laboratory animals issued by the International Association for the Study of Pain.
Procedures
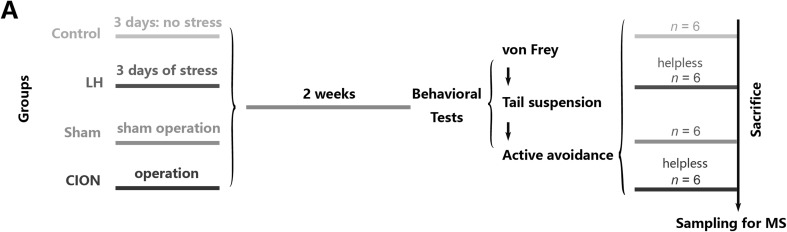
The mice were divided into four groups: control, LH, sham-CION, and CION groups (n = 9/group). The LH and control groups underwent 3 days of inescapable shock stress or control treatment, respectively; the sham-CION and CION groups received sham or CION surgery, respectively. Afterwards, the four groups were kept in home cages for 2 weeks before the behavioral tests. The animals in the LH and CION groups were verified to be depressed (helpless) individuals based on the loss of active avoidance behaviors. Six helpless mice per group were selected for mass spectrometry (MS) sampling. The timeline of the study is shown in Fig. 1.
Fig. 1.
Schematic of the groups and experimental procedures. Four groups of animals were subjected to treatments, 2 weeks in the home-cage, behavioral tests, and then sampling.
Learned Helplessness
Each mouse was placed in a Plexiglas chamber (30 × 30 × 16 cm3) with a stainless-steel grid floor (0.3 × 0.5 cm2) attached to an electric shock generator (Shanghai Mobile Datum Information Technology Co., Shanghai, China) and exposed to 60 cycles of randomly-assigned inescapable electric foot-shocks (0.8 mA, 10 s shock duration, 7.5–22.5 s intervals) [18–20]. The LH protocol was performed between 09:00 and 12:00 for 3 consecutive days. The control animals were placed in the same apparatus but did not receive any foot-shocks.
Trigeminal Neuralgia Model
The TN model was produced by chronic constriction injury to the unilateral infraorbital nerve via an intraoral approach as described previously [21, 22]. The animal was anesthetized with sodium pentobarbital (4%, i.p., 50 mg/kg) and the head was fixed and the mouth kept open during the operation. A 1-cm incision was made along the left gingivobuccal margin in the buccal mucosa, beginning immediately next to the first molar. The left infraorbital nerve was freed and loosely tied with 2 to 4 chromic gut (4-0) ligatures 1.5–2 mm apart, after which the incision was closed. Sham-operated animals received only nerve exposure but no ligation. All surgical procedures were performed aseptically.
von Frey Test
Before tests, each mouse was shaved around the mystacial vibrissae and handled for 3–5 days (once a day for 30 min). The mouse was gently held by an experimenter wearing a regular leather work-glove. The habituation required more than normal petting of the mouse, and this was achieved within half an hour [23, 24]. A series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) ranging from 0.07 to 4 g were lightly applied to the skin within the infraorbital territory, near the center of the vibrissal pad on hairy skin surrounding the mystacial vibrissae. A brisk or active withdrawal of the head from the probing filament was defined as a response. Each filament was tested 5 times at 5-s intervals. The withdrawal threshold was defined as the lowest force in grams that produced at least 3 withdrawal responses in 5 consecutive applications [23, 24]. All behavioral tests, including those below, were conducted under blind conditions.
Active-Avoidance Shuttle-Box Test
The shuttle-box used in the active-avoidance test had white Plexiglas walls and a stainless-steel floor consisting of rods spaced 0.5 cm apart. Two equal-sized chambers (30 × 30 × 16 cm3) were separated by a gate (10 × 10 cm2) which was located in the middle of the box. The conditioned stimuli (a tone signal: 85 dB, 3 s) were given before the escapable foot shock at the beginning of each trial. If the mouse had no response to the tone, it would receive a 0.4 mA escapable foot shock for 30 s. The test contained 30 trials, the escape latency of each trial was recorded, and the mean latency was calculated. Those which exhibited a mean latency >15 s were considered to have developed “helplessness”, and only those animals were sampled for MS analysis.
Tail-Suspension Test
The tail-suspension test was performed to assess the depressive-like behavior. In the test, mice were subjected to the short-term inescapable stress of being suspended (50 cm above the floor) by fixing the tail using adhesive tape. The duration of an immobile posture was recorded and analyzed.
Mass Spectrometry
For each hippocampal sample, proteins were precipitated with ice-cold acetone, and then re-dissolved in dissolution buffer (consisting of 0.5 mol/L triethylammonium bicarbonate and 0.1% SDS). Then the proteins were quantified by the BCA protein assay, 100 μg protein was typically digested, and the resultant peptide mixture was labeled using chemicals from the iTRAQ reagent kit (Applied Biosystems, Pleasanton, CA). Disulfide bonds were reduced in 5 mmol/L Tris-(2-carboxyethy) phosphine for 1 h at 60 °C, followed by blocking cysteine residues in 10 mmol/L methyl methane thiosulfonate for 30 min at room temperature, before digestion with sequence-grade modified trypsin (Promega, Madison, WI). For labeling, each iTRAQ reagent was dissolved in 50 μL isopropanol and added to the respective peptide mixture. Proteins were labeled with the iTRAQ tags.
High-pH reverse-phase separation was then performed. The peptide mixture was re-dissolved in buffer A (20 mmol/L ammonium formate in water, pH 10.0, adjusted with ammonium hydroxide), and then fractionated by high-pH separation using an Acquity UPLC system (Waters Corp., Milford, MA) connected to a reverse-phase column (Acquity UPLC Peptide C18 column, 2.1 × 150 mm2, 1.7 μm, 130 Å; Waters Corp.). High-pH separation was performed using a linear gradient. Starting from 5% buffer (20 mmol/L ammonium formate in 90% ACN, pH 10.0, adjusted with ammonium hydroxide) to 35% in 25 min. The column was re-equilibrated at initial conditions for 15 min. The flow-rate was maintained at 600 μL/min and the column was maintained at room temperature. Forty fractions were collected and each was dried in a vacuum concentrator for the next step.
Afterwards, low-pH nano-HPLC-MS/MS analysis was carried out. Briefly, the mixed peptides were separated by nano-HPLC (Eksigent Technologies, Silicon Valley, CA) on the secondary RP analytical column (Eksigent, C18, 3 µm, 150 × 75 µm2). Peptides were subsequently eluted using the following gradient conditions with phase B (98% ACN with 0.1% formic acid) from 5% to 45% (5–70 min) and total flow rate was maintained at 300 nL/min. Electrospray voltage of 2.3 kV versus the inlet of the mass spectrometer was used.
Finally, a triple TOF 4600 mass spectrometer was operated in data-dependent mode to switch automatically between MS and MS/MS acquisition. MS spectra were acquired across the mass range 350–1250 m/z in high-resolution mode with a 250 ms accumulation time per spectrum. Tandem mass spectra were scanned from 100–1250 m/z in high-sensitivity mode with rolling collision energy. The 20 most intense precursors were selected for fragmentation per cycle with a dynamic exclusion time of 9 s.
Each MS sample that represented a group consisted of equivalent total protein from six independent individuals; and six groups (six samples) in three comparisons were assessed as follows: the ipsilateral hippocampus of the CION-treated group vs sham (Ipsi. CION vs sham), the contralateral hippocampus of the CION-treated group vs sham (Con. CION vs sham), and LH vs control.
Western Blot
Mice were sacrificed and the hippocampus was rapidly removed. Hippocampal tissues were homogenized in lysis buffer containing a mixture of protease inhibitors and phenylmethylsulfonyl fluoride (Roche Diagnostics, Indianapolis, IN). Samples (a total of 15 μg protein) were separated on 10% SDS-PAGE gel and transferred to PVDF membranes. The membranes were blocked in blocking buffer containing 5% milk in Tris-buffered saline (TBST) for 2 h at room temperature and incubated overnight at 4°C with rabbit anti-BDNF (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were washed 3 times with TBST and incubated with HRP-conjugated secondary antibodies (1:20000; Pierce) for 2 h at 4 °C. Signals were visualized using enhanced chemiluminescence (Pierce Manufacturing, Appleton, WI), and captured by a ChemiDoc XRS system (Bio-Rad, Hercules, CA). The immunoreactivity of Western blots was quantified by densitometry and normalized to GAPDH. The antibody for GAPDH was HRP-labeled anti-GAPDH (1:50000; Yasunari Biological Engineering Co., Ltd., Shanghai, China).
Data Analysis and Statistics
All behavioral data are presented as mean ± SEM and were analyzed using Student’s t test or one-way ANOVA followed by post hoc Student-Newman-Keuls analysis. A P value <0.05 was considered statistically significant. Analysis of MS data was performed using the Ingenuity Pathway Analysis (IPA) system (Qiagen, Berlin, Germany). The P value was calculated using the right-tailed Fisher’s exact test. The function-related changes were predicted by downstream regulator analysis applying the Ingenuity® Knowledge Base (Ingenuity® Systems, http://www.ingenuity.com). Signaling pathway changes were obtained using the IPA Canonical Pathway database tool.
Results
Inescapable Shock Induces Depression-Like Behaviors
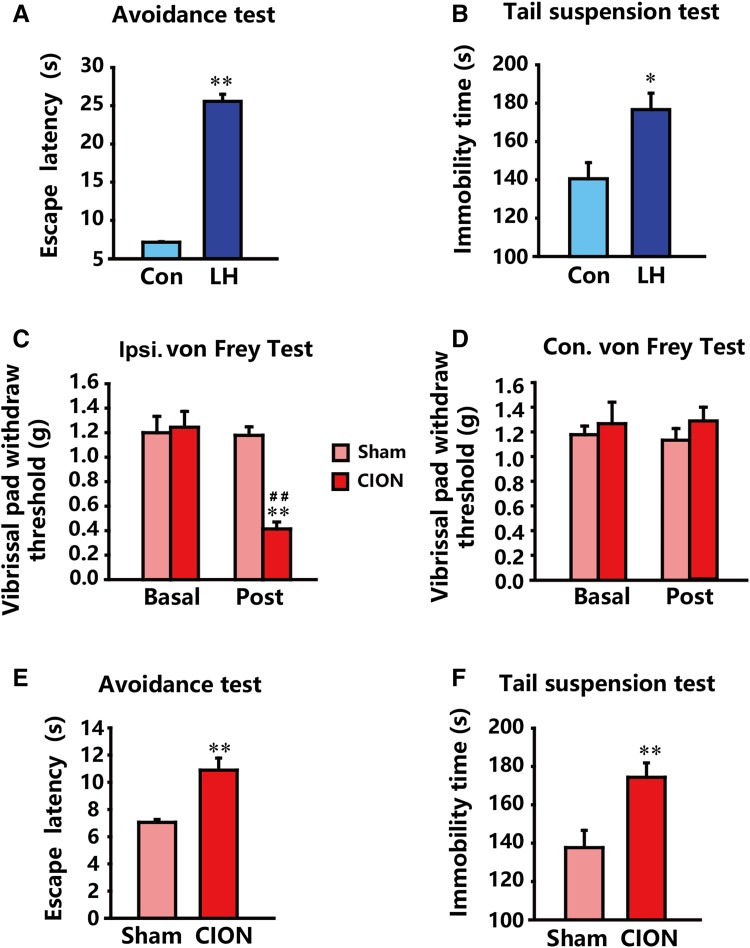
The active-avoidance and tail-suspension tests were performed 2 weeks after inescapable shock in order to validate the development of a typical depression-like phenotype. The results showed that the LH animals exhibited a significantly prolonged escape latency (P < 0.01 vs control) in the avoidance test (Fig. 2A) and immobility time (P < 0.05 vs control) in the suspension test (Fig. 2B). These results, in line with our expectations, demonstrated that the 3-day LH protocol was efficient to establish the depression model with typical behavioral despair.
Fig. 2.
Nociceptive behavior of CION-treated animals and depression-like behaviors of LH and CION-treated animals (n = 9/group). A, B LH animals exhibited prolonged escape latency in the active-avoidance test (A) and immobility time in the tail-suspension test (B). C, D CION induced significant mechanical allodynia in the ipsilateral face (C) but not contralaterally (D). E, F CION induced depression-like behaviors similar to LH stress, as indicated by prolonged escape latency (E) and immobility time (F). *P < 0.05, **P < 0.01 compared with control (Con) or sham; ## P < 0.01 compared with basal level.
CION Induces not Only Allodynia but Also Depression-Like Behaviors
Compared with the sham group, CION-treated animals exhibited a significantly decreased mechanical threshold in the ipsilateral vibrissa pad (Fig. 2C, P < 0.01 vs sham or basal level), while no difference was observed in the pad contralateral to CION (Fig. 2D). These data suggested that the CION-treated mice developed stable mechanical allodynia, which persisted for at least 2 weeks. Notably, the escape latency in the avoidance test (Fig. 2E) and immobility time (Fig. 2F) in the tail-suspension test were also markedly increased (P < 0.01 vs sham), highlighting that chronic TN can result in the development of depression.
Function-Related Biomolecular Changes in TN and LH Models
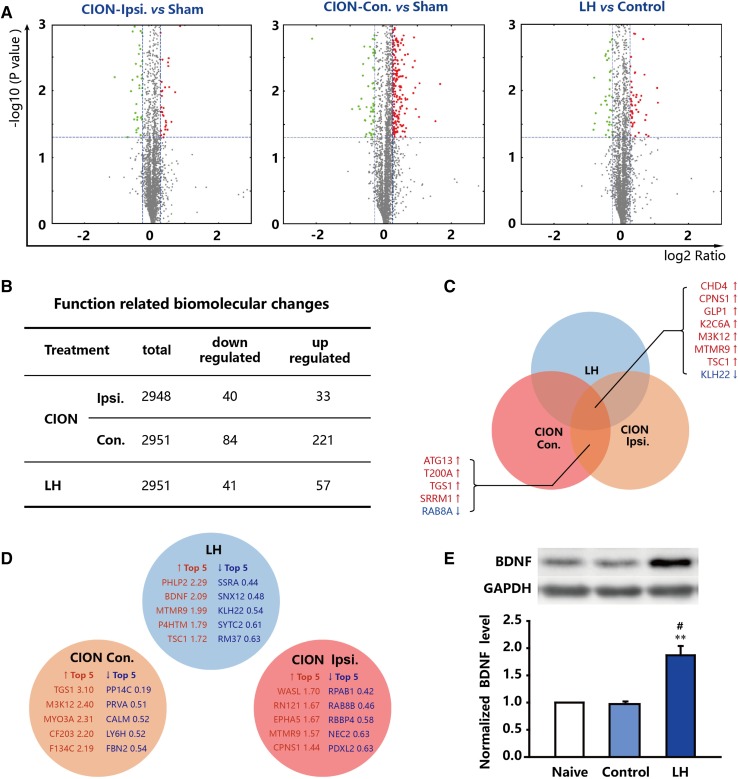
MS was used to analyze the biomolecular and signaling pathway changes in the TN and LH models. Based on the MS data and comprehensive bio-informatics analysis, the function-related biomolecular changes were significant on both sides of the hippocampus in CION-treated and LH animals. In the CION-treated animals, 40 proteins were down-regulated and 30 were up-regulated on the ipsilateral side among 2949 proteins with clear biological backgrounds (Fig. 3A, B). On the contralateral side 84 proteins were down-regulated and 221 were up-regulated among 2952 proteins analyzed (Fig. 3A, B). Some proteins were commonly regulated in all three comparisons (Ipsi. CION vs sham, Con. CION vs sham, and LH vs control): CHD4, CPNS1, GLP1, K2C6A, M3K12, MTMR9, TSC1, and KLH22 (Fig. 3C). Some were changed on both sides of the hippocampus in CION-treated animals: ATG13, T200A, TGS1, SRRM1, and RAB8A (Fig. 3C). The top 5 up-regulated and down-regulated proteins are shown in Fig. 3D. Of these, brain-derived neurotrophic factor (BDNF) is an interesting, important and significant target as it participates in many brain functions and nuclei under conditions of stress. We therefore used Western blots to further assess BDNF in the LH model. A similar result was obtained, which showed that BDNF was significantly increased in LH animals (P < 0.01 vs control; Fig. 3E).
Fig. 3.
Biomolecular changes in the hippocampus of CION- and LH-treated animals (n = 6/group). A Green, downregulated proteins; red, upregulated proteins; grey, unchanged proteins; x-axis, log2 of change ratio; y-axis, −log of P-value calculated by Fisher’s exact test, right-tailed. B Summary of the above biomolecular changes in three comparisons. C Proteins commonly regulated in the three comparisons (two models and both sides of the hippocampus with CION). D The top 5 up/down-regulated biomolecules in the three comparisons. E Verification of the BDNF changes in the LH model using Western blotting. **P < 0.01 compared with naive; # P < 0.05 compared with control.
Function-Related Families and Signaling Pathways in the TN and LH Models
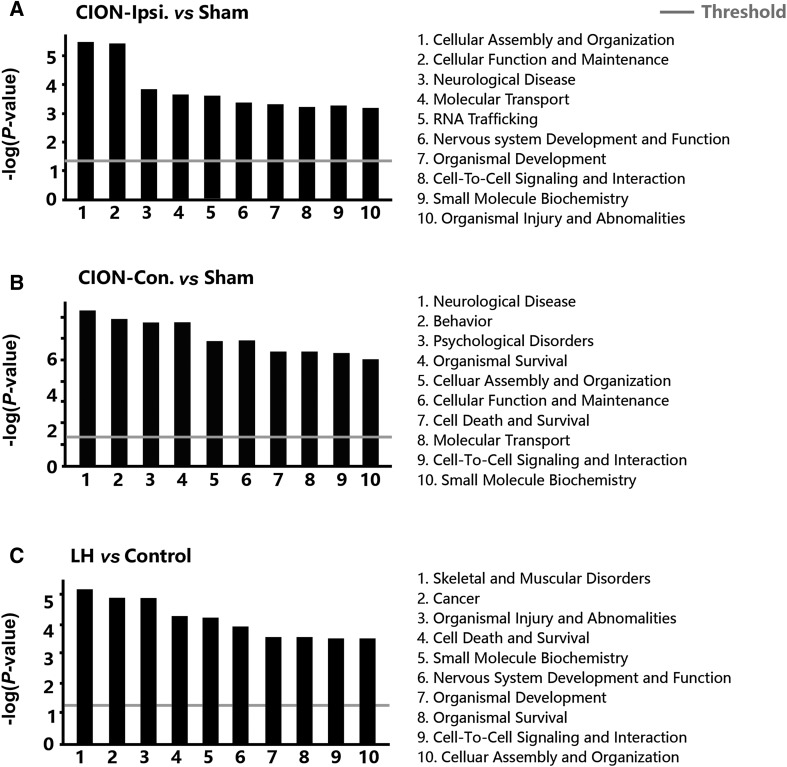
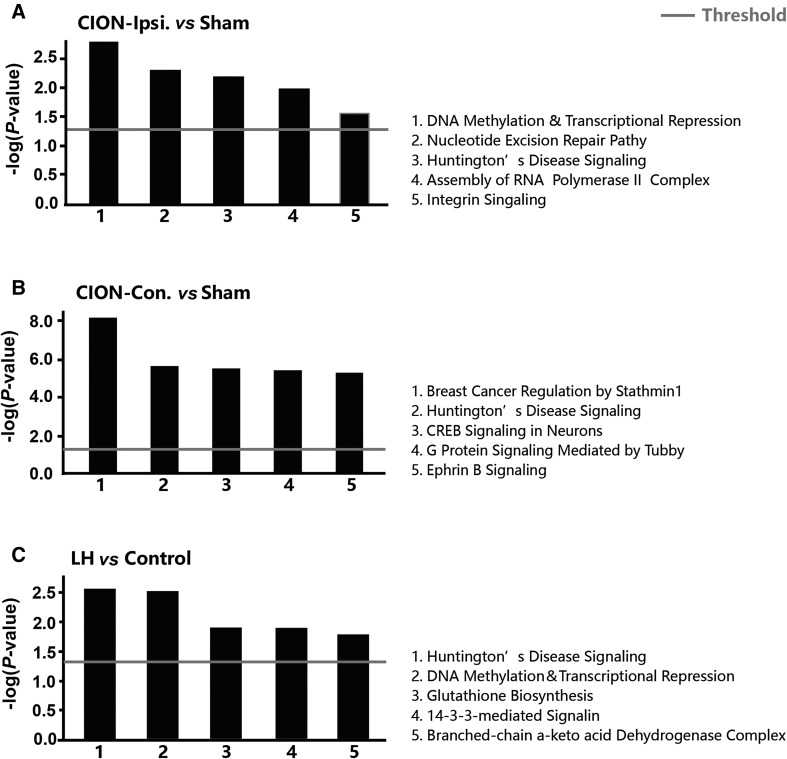
Next, we investigated the function-related biomolecular families participating in the pathogenesis under CION and LH stresses (Fig. 4A–C). Although significant differences in functional families were found between the ipsilateral and contralateral hippocampi, changes in the following were observed on both sides: (1) cell assembly and organization; (2) cell function and maintenance; (3) cell-to-cell signaling and interaction; (4) neurological diseases; (5) molecular transport; and (6) small molecule biochemistry. On the other hand, the following changes were found in both CION and LH models: (1) small molecule biochemistry; (2) nervous system development and function; (3) organismal development; (4) organismal survival; (5) cell-to-cell signaling and interaction; and (6) cell assembly and organization. Furthermore, we examined the alteration of signaling pathways in two models. The top 5 pathways changed in different treatments were listed in Fig 5A–C. Interestingly, there was a marked difference in the signaling changes between the ipsilateral and contralateral hippocampus in CION-treated animals. In the ipsilateral hippocampus, the DNA methylation and transcriptional repression signaling pathways scored highest, and the Huntington’s disease signaling pathway also showed a significant increase. In the contralateral hippocampus, there were not only a greater number of up/down-regulated proteins (Fig. 3A, B), but also a large number of signaling pathways with significantly larger change probability, such as breast cancer regulation by stathmin 1 and Huntington’s disease signaling pathway. In the LH animals, Huntington’s disease, DNA methylation, and transcriptional repression signaling pathways had a high probability. The two models shared a pathway that may regulate the depressive-like behaviors, the Huntington’s disease signaling pathway.
Fig. 4.
Change probabilities of functional families predicted by proteins in three comparisons.
Fig. 5.
Change probabilities of signaling pathways predicted by proteins in three comparisons.
Discussion
Chronic neuropathic pain caused by peripheral nerve injury, such as CION, sciatic nerve ligation, and spinal nerve injury, affects millions of people. Trigeminal nerve compression results in TN [25], which is characterized by brief, stabbing, shock-like pain [26]. So far, the etiopathogenesis and mental-disorder-related consequence of TN remain to be elucidated. Rodent studies have demonstrated that CION induces allodynia in response to mechanical and cold stimulation of the vibrissa pad [27, 28]. In our work, CION resulted in not only mechanical allodynia but also depression-like behaviors. The causes of mood or psychiatric disorders are various and confused. Hereditary, genetic studies have revealed significant overlaps of risk genes across major psychiatric disorders including bipolar disorder, major depressive disorder, schizophrenia, and autism [29, 30]. Numbers of studies have suggested that chronic injury of peripheral nerve results in behavioral abnormalities and then induces mental disorders [31, 32]. It is also widely known that stresses such as chronic unpredictable stress, chronic mild stress, social stress, and LH result in mental disorders [11, 12, 33–37]. LH studies have repeatedly reported that inescapable electrical stimulation drives the initiation of depression in rodent models [11, 38, 39]. Here, we used LH as a positive model control and found behavioral despair in common with the TN model. Our study is among the very few to directly show that TN can be a depression-leading stressor as well. As far as we know, this is the first animal evidence that only 2 weeks of TN can induce behavioral despair. In line with this, some clinical studies have revealed that treatment of TN can immediately attenuate depressive disorders [40], and conversely, when a comorbid patient was treated with the antidepressant duloxetine, the TN was unexpectedly and rapidly relieved [41]. Together, these findings point out that neuralgia and depression might be modulated similarly. Previously, we reported that, in the inflammatory pain state induced by complete Freund’s adjuvant, C57BL/6J and FVB/NJNju mice exhibit significant anxiety-like behaviors as assessed by the open field and elevated plus maze tests [42]. It is generally agreed that anxiety and despair frequently co-occur during the development of depression, while behavioral despair is a more malignant indication than anxiety [43–45]. Unlike the widely-recognized finding that depression-like behaviors (especially despair and anhedonia) are induced after a long period of stress exposure, we noted that despair was a side-effect of just 2 weeks of TN. The present work further deepens our understanding of the close and complex connections between neuropathic pain and depression.
Psychiatric disorders may share similarities in physiological functions and cellular mechanisms. In our MS results, most striking was the Huntington’s disease signaling pathway, which was significantly up-regulated on both sides of the hippocampus in the TN and LH models. This similarity has seldom been noted, but some references shed light in support. It has been reported that the incidence of depression in Huntington’s disease patients is more than twice that in the general population [46]. Besides, serious impairments in negative emotion recognition and empathy for pain have been found in Huntington’s disease families [47]. These reports consistently indicate a potential regulatory role of the hippocampus in pain and depression through Huntington’s disease signaling. We strongly suggest that Huntington’s disease signaling plays a role in depression/pain-related cognitive deficits.
Several molecules were commonly up-regulated in the three comparisons. These molecules may play key roles in the comorbidity of neuralgia and depression. CHD4 (chromodomain-helicase DNA-binding protein 4) has been reported to promote the self-renewal and multi-lineage differentiation of hematopoietic stem cells [48]. CHD4, CHD5, and CHD7 play pivotal roles in the function and differentiation of neural stem cell niches in the dentate gyrus of the hippocampus through cooperation with major epigenetic modifiers, transcription factors, and signaling pathways [49]. During neurogenesis, CHD4 interacts with PRC2 (polycomb repressive complex 2), specifically with the H3K27 methyltransferase enzyme, and the CHD4/PRC2 complex directly binds to the promoter of the GFAP gene (Gfap), represses its expression, and prevents glial differentiation [50]. In view of its role in promoting newborn neurons, we speculate that the increased CHD4 in the TN and LH models may be a homeostatic counter-stress response. GLP1 (glucagon-like peptide 1) is a gut hormone as well as a centrally-active neuropeptide, whose neuroprotective and neurotrophic actions have been investigated [51]. It has been hypothesized that GLP-1 plays a key role in the activation of stress responses, which may be connected with its role in the central regulation of energy homeostasis [52]. In our MS data, GLP-1 up-regulation in both models may be similar to the role of CHD4, as an adaptation to stress. MTMR9 (myotubularin-related protein 9), a new member of the myotubularin family, plays a role in autophagy and apoptosis [53]. Its relationship with depression- or pain-driven hippocampal changes has never been reported. Given that a mechanism underlying depression involves the loss of neural stem cells and neurons by apoptosis [43, 54–56], the accumulation of MTMR9 in our two models is likely to be a crucial pathological link. To be brief, and due to the lack of direct reports, the other interesting but novel proteins shown in Fig. 3C are not discussed in detail. However, their potential effects on neuralgia and depression remain to be determined.
Intriguingly, differences in proteins and pathways between the two sides of the hippocampus in CION-treated animals were evident. In the light of a greater number of up/down-regulated proteins (Fig. 3B), a larger amplitude of variation (Fig. 3B), a higher probability of signaling changes (Fig. 5B), and a multitude of signaling pathways (not shown), the contralateral hippocampus seemed to play a more important role in the pathogenesis of TN or TN-induced depression.
Besides the hippocampus, studies have also used proteomic analysis to describe protein changes in other brain regions. Gellén et al. induced depression in adult rats by neonatal clomipramine treatment and then conducted a proteomic study. They identified 32 significantly altered proteins (e.g., macrophage migration inhibitory factor) in the prefrontal cortex [57]. A clinical study by Martins-de-Souza used shotgun proteome analyses to reveal protein changes in the dorsolateral prefrontal cortex of patients with major depressive disorder (MDD). They validated some proteins associated with energy metabolism and synaptic function, and especially noted a change in the histidine triad nucleotide-binding protein 1 [58]. Cox et al. evaluated molecular changes associated with environmental stress in both rodent models and MDD patients, and seven important functional domains (e.g., carbohydrate metabolism and cellular respiration) were reported [59]. In combination with these published data, our findings provide a wide range of targets for comparison and therapeutic applications.
Collectively, we found that both the LH and TN models developed depressive-like behaviors, and explored many of the biomolecular/pathway targets associated with neuralgia and psychiatric disorders that are involved in the pathogenesis of TN and LH.
Acknowledgements
We thank Dr. Sheng-Yuan Yu at the Department of Neurology, Chinese PLA General Hospital for help with CION surgery. This work was supported by the National Natural Science Foundation of China (31421091, 81471130, 31371123 and 31420103903) and a Development Project of Shanghai Peak Disciplines Integrated Chinese and Western Medicine, China.
Footnotes
Qing-Huan Guo and Qing-He Tong have contributed equally to this work.
Contributor Information
Hong Cao, Email: hongcao@fudan.edu.cn.
Yu-Qiu Zhang, Email: yuqiuzhang@fudan.edu.cn.
References
- 1.Yap AU, Chua EK, Dworkin SF, Tan HH, Tan KB. Multiple pains and psychosocial functioning/psychologic distress in TMD patients. Int J Prosthodont. 2002;15:461–466. [PubMed] [Google Scholar]
- 2.Dworkin SF, Von Korff M, LeResche L. Multiple pains and psychiatric disturbance. An epidemiologic investigation. Arch Gen Psychiatry. 1990;47:239–244. doi: 10.1001/archpsyc.1990.01810150039007. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- 4.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/S0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- 5.Varija D, Kumar KP, Reddy KP, Reddy VK. Prolonged constriction of sciatic nerve affecting oxidative stressors & antioxidant enzymes in rat. Indian J Med Res. 2009;129:587–592. [PubMed] [Google Scholar]
- 6.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 7.Ma F, Zhang L, Lyons D, Westlund KN. Orofacial neuropathic pain mouse model induced by Trigeminal Inflammatory Compression (TIC) of the infraorbital nerve. Mol Brain. 2012;5:44. doi: 10.1186/1756-6606-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwivedi Y, Mondal AC, Rizavi HS, Shukla PK, Pandey GN. Single and repeated stress-induced modulation of phospholipase C catalytic activity and expression: role in LH behavior. Neuropsychopharmacology. 2005;30:473–483. doi: 10.1038/sj.npp.1300605. [DOI] [PubMed] [Google Scholar]
- 9.Henn V. The treatment of chronic headache. Schweiz Rundsch Med Prax. 1993;82:540–543. [PubMed] [Google Scholar]
- 10.Borsini F, Cesana R. Further characterisation of potential antidepressant action of flibanserin. Psychopharmacology (Berl) 2001;159:64–69. doi: 10.1007/s002130100876. [DOI] [PubMed] [Google Scholar]
- 11.Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res Brain Res Protoc. 2005;16:70–78. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Singh GK, Garabadu D, Muruganandam AV, Joshi VK, Krishnamurthy S. Antidepressant activity of Asparagus racemosus in rodent models. Pharmacol Biochem Behav. 2009;91:283–290. doi: 10.1016/j.pbb.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Willner P. Animal models of depression: validity and applications. Adv Biochem Psychopharmacol. 1995;49:19–41. [PubMed] [Google Scholar]
- 14.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 15.Iwata M, Shirayama Y, Ishida H, Hazama GI, Nakagome K. Hippocampal astrocytes are necessary for antidepressant treatment of learned helplessness rats. Hippocampus. 2011;21:877–884. doi: 10.1002/hipo.20803. [DOI] [PubMed] [Google Scholar]
- 16.Khan SA, Ryali V, Bhat PS, Prakash J, Srivastava K, Khanam S. The hippocampus and executive functions in depression. Ind Psychiatry J. 2015;24:18–22. doi: 10.4103/0972-6748.160920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC. Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry. 2014;22:1504–1512. doi: 10.1016/j.jagp.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, Yang CJ, Yue N, Liu Y, Yu J, Wang YQ, et al. Clomipramine reverses hypoalgesia/hypoesthesia and improved depressive-like behaviors induced by inescapable shock in rats. Neurosci Lett. 2013;541:227–232. doi: 10.1016/j.neulet.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 19.Dwivedi Y, Mondal AC, Payappagoudar GV, Rizavi HS. Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology. 2005;48:204–214. doi: 10.1016/j.neuropharm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Papolos DF, Edwards E, Marmur R, Lachman HM, Henn FA. Effects of the antiglucocorticoid RU 38486 on the induction of learned helpless behavior in Sprague-Dawley rats. Brain Res. 1993;615:304–309. doi: 10.1016/0006-8993(93)90042-L. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima A, Tsuboi Y, Suzuki I, Honda K, Shinoda M, Kondo M, et al. PKCgamma in Vc and C1/C2 is involved in trigeminal neuropathic pain. J Dent Res. 2011;90:777–781. doi: 10.1177/0022034511401406. [DOI] [PubMed] [Google Scholar]
- 22.Constandil L, Goich M, Hernandez A, Bourgeais L, Cazorla M, Hamon M, et al. Cyclotraxin-B, a new TrkB antagonist, and glial blockade by propentofylline, equally prevent and reverse cold allodynia induced by BDNF or partial infraorbital nerve constriction in mice. J Pain. 2012;13:579–589. doi: 10.1016/j.jpain.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Liang YC, Huang CC, Hsu KS. The synthetic cannabinoids attenuate allodynia and hyperalgesia in a rat model of trigeminal neuropathic pain. Neuropharmacology. 2007;53:169–177. doi: 10.1016/j.neuropharm.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 25.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Tallawy HN, Farghaly WM, Rageh TA, Shehata GA, Abdel Hakeem MN, Badry R, et al. Prevalence of trigeminal neuralgia in Al-Quseir city (Red sea Governorate) Egypt. Clin Neurol Neurosurg. 2013;115:1792–1794. doi: 10.1016/j.clineuro.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Luiz AP, Schroeder SD, Chichorro JG, Calixto JB, Zampronio AR, Rae GA. Kinin B(1) and B(2) receptors contribute to orofacial heat hyperalgesia induced by infraorbital nerve constriction injury in mice and rats. Neuropeptides. 2010;44:87–92. doi: 10.1016/j.npep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Chichorro JG, Zampronio AR, Souza GE, Rae GA. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain. 2006;123:64–74. doi: 10.1016/j.pain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Kaiser T, Monteiro P, Zhang X, Van der Goes MS, Wang D, et al. Mice with Shank3 mutations associated with ASD and schizophrenia display both shared and distinct defects. Neuron 2016, 89: 147–162. [DOI] [PMC free article] [PubMed]
- 30.Lee MM, Reif A, Schmitt AG. Major depression: a role for hippocampal neurogenesis? Curr Top Behav Neurosci. 2013;14:153–179. doi: 10.1007/7854_2012_226. [DOI] [PubMed] [Google Scholar]
- 31.Attal N, Perrot S, Fermanian J, Bouhassira D. The neuropathic components of chronic low back pain: a prospective multicenter study using the DN4 Questionnaire. J Pain. 2011;12:1080–1087. doi: 10.1016/j.jpain.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier MJ, et al. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry. 2011;70:946–953. doi: 10.1016/j.biopsych.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Yu M, Zhang Y, Chen X, Zhang T. Antidepressant-like effects and possible mechanisms of amantadine on cognitive and synaptic deficits in a rat model of chronic stress. Stress 2016, 19: 104–113. [DOI] [PubMed]
- 34.Zhang Z, Wang W, Zhong P, Liu SJ, Long JZ, Zhao L, et al. Blockade of 2-arachidonoylglycerol hydrolysis produces antidepressant-like effects and enhances adult hippocampal neurogenesis and synaptic plasticity. Hippocampus 2015, 25: 16–26. [DOI] [PMC free article] [PubMed]
- 35.Liu W, Zhou C. Corticosterone reduces brain mitochondrial function and expression of mitofusin, BDNF in depression-like rodents regardless of exercise preconditioning. Psychoneuroendocrinology 2012, 37: 1057–1070. [DOI] [PubMed]
- 36.Yang L, Shi LJ, Tang B, Han QQ, Yu J, Wu GC, et al. Opposite sex contact and isolation: a novel depression/anxiety model. Neurosci Bull. 2016;32:92–98. doi: 10.1007/s12264-015-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan HC, Cao X, Das M, Zhu XH, Gao TM. Behavioral animal models of depression. Neurosci Bull. 2010;26:327–337. doi: 10.1007/s12264-010-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anisman H, Merali Z. Rodent models of depression: learned helplessness induced in mice. Curr Protoc Neurosci 2001, Chapter 8: Unit 8 10C. [DOI] [PubMed]
- 39.Vollmayr B, Gass P. Learned helplessness: unique features and translational value of a cognitive depression model. Cell Tissue Res. 2013;354:171–178. doi: 10.1007/s00441-013-1654-2. [DOI] [PubMed] [Google Scholar]
- 40.Tang Y, Ma L, Li N, Guo Y, Yang L, Wu B, et al. Percutaneous trigeminal ganglion radiofrequency thermocoagulation alleviates anxiety and depression disorders in patients with classic trigeminal neuralgia: A cohort study. Medicine (Baltimore) 2016;95:e5379. doi: 10.1097/MD.0000000000005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu CC, Chang CW, Peng CH, Liang CS. Rapid management of trigeminal neuralgia and comorbid major depressive disorder with duloxetine. Ann Pharmacother. 2014;48:1090–1092. doi: 10.1177/1060028014532789. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Yang L, Yu J, Zhang YQ. Persistent, comorbid pain and anxiety can be uncoupled in a mouse model. Physiol Behav. 2015;151:55–63. doi: 10.1016/j.physbeh.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Yang L, Yue N, Zhu X, Han Q, Li B, Liu Q, et al. Electroacupuncture promotes proliferation of amplifying neural progenitors and preserves quiescent neural progenitors from apoptosis to alleviate depressive-like and anxiety-like behaviours. Evid Based Complement Alternat Med. 2014;2014:872568. doi: 10.1155/2014/872568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malyszczak K, Szechinski M. Comorbidity of different forms of anxiety disorders and depression. Psychiatr Pol. 2004;38:603–609. [PubMed] [Google Scholar]
- 45.Boyer P. Do anxiety and depression have a common pathophysiological mechanism? Acta Psychiatr Scand Suppl 2000: 24–29. [PubMed]
- 46.Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, et al. Depression and stages of Huntington’s disease. J Neuropsychiatry Clin Neurosci. 2005;17:496–502. doi: 10.1176/jnp.17.4.496. [DOI] [PubMed] [Google Scholar]
- 47.Baez S, Herrera E, Gershanik O, Garcia AM, Bocanegra Y, Kargieman L, et al. Impairments in negative emotion recognition and empathy for pain in Huntington’s disease families. Neuropsychologia. 2015;68:158–167. doi: 10.1016/j.neuropsychologia.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida T, Hazan I, Zhang J, Ng SY, Naito T, Snippert HJ, et al. The role of the chromatin remodeler Mi-2beta in hematopoietic stem cell self-renewal and multilineage differentiation. Genes Dev. 2008;22:1174–1189. doi: 10.1101/gad.1642808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Micucci JA, Sperry ED, Martin DM. Chromodomain helicase DNA-binding proteins in stem cells and human developmental diseases. Stem Cells Dev. 2015;24:917–926. doi: 10.1089/scd.2014.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sparmann A, Xie Y, Verhoeven E, Vermeulen M, Lancini C, Gargiulo G, et al. The chromodomain helicase Chd4 is required for Polycomb-mediated inhibition of astroglial differentiation. EMBO J. 2013;32:1598–1612. doi: 10.1038/emboj.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166:1586–1599. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav. 2013;122:201–207. doi: 10.1016/j.physbeh.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou J, Chang SC, Marjanovic J, Majerus PW. MTMR9 increases MTMR6 enzyme activity, stability, and role in apoptosis. J Biol Chem. 2009;284:2064–2071. doi: 10.1074/jbc.M804292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L, Yue N, Zhu X, Han Q, Liu Q, Yu J, et al. Electroacupuncture upregulates ERK signaling pathways and promotes adult hippocampal neural progenitors proliferation in a rat model of depression. BMC Complement Altern Med. 2013;13:288. doi: 10.1186/1472-6882-13-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Liu W, Zhou Y, Ma C, Li S, Cong B. Endoplasmic reticulum stress is involved in restraint stress-induced hippocampal apoptosis and cognitive impairments in rats. Physiol Behav. 2014;131:41–48. doi: 10.1016/j.physbeh.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 56.Zhang JQ, Wu XH, Feng Y, Xie XF, Fan YH, Yan S, et al. Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: involvement of the neuroinflammatory pathway. Acta Pharmacol Sin. 2016;37:1141–1153. doi: 10.1038/aps.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gellen B, Volgyi K, Gyorffy BA, Balogh B, Darula Z, Hunyadi-Gulyas E, et al. Proteomic investigation of the prefrontal cortex in the rat clomipramine model of depression. J Proteomics. 2016;153:53–64. doi: 10.1016/j.jprot.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 58.Martins-de-Souza D, Guest PC, Harris LW, Vanattou-Saifoudine N, Webster MJ, Rahmoune H, et al. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl Psychiatry. 2012;2:e87. doi: 10.1038/tp.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox DA, Gottschalk MG, Stelzhammer V, Wesseling H, Cooper JD, Bahn S. Evaluation of molecular brain changes associated with environmental stress in rodent models compared to human major depressive disorder: A proteomic systems approach. World J Biol Psychiatry 2016: 1–12. [DOI] [PubMed]