Abstract
Human functional MRI studies in acute and various chronic pain conditions have revolutionized how we view pain, and have led to a new theory that complex multi-dimensional pain experience (sensory-discriminative, affective/motivational, and cognitive) is represented by concurrent activity in widely-distributed brain regions (termed a network or pain matrix). Despite these breakthrough discoveries, the specific functions proposed for these regions remain elusive, because detailed electrophysiological characterizations of these regions in the primate brain are lacking. To fill in this knowledge gap, we have studied the cortical areas around the central and lateral sulci of the non-human primate brain with combined submillimeter resolution functional imaging (optical imaging and fMRI) and intracranial electrophysiological recording. In this mini-review, I summarize and present data showing that the cortical circuitry engaged in nociceptive processing is much more complex than previously recognized. Electrophysiological evidence supports the engagement of a distinct nociceptive-processing network within SI (i.e., areas 3a, 3b, 1 and 2), SII, and other areas along the lateral sulcus. Deafferentation caused by spinal cord injury profoundly alters the relationships between fMRI and electrophysiological signals. This finding has significant implications for using fMRI to study chronic pain conditions involving deafferentation in humans.
Keywords: Nociception, Non-human primate, Cortex, Functional MRI, Functional connectivity
Introduction
Pain is a somatic sensation with unique characteristics. Human functional MRI (fMRI) studies in acute and various chronic pain conditions have revolutionized how we view pain, and have led to a new theory that complex multi-dimensional pain experience (sensory-discriminative, affective/motivational, and cognitive) is represented by concurrent activity in widely-distributed brain regions (termed a network or pain matrix). Within this network, the primary somatosensory cortex (SI) has been proposed to subserve a more general sensory discrimination role [1–5], whereas the operculo-insular region, including the secondary somatosensory cortex (SII) and the posterior insula (pIns) of primates, serves as the earliest pain-encoding region. This region is responsible for the subjective recognition of pain, encoding of pain intensity, learning and memory of pain-related events, and possibly the generation and maintenance of chronic pain states in humans. Despite these breakthroughs and discoveries, the specific functions proposed for these regions remain elusive, because detailed electrophysiological characterization of these regions is lacking in the primate brain, and only a limited number of nociceptive neurons have been isolated. The circuits subserving the representation of different aspects of pain information in primates, and the neuronal encoding mechanisms of the different aspects of nociceptive inputs remain to be established.
Modern brain imaging, such as fMRI, has revolutionized our view of pain, allowing the opportunity to investigate the neural mechanisms of pain by simultaneously measuring across multiple brain regions while they are engaged in functions related to the perception and modulation of pain in healthy and disease states. The fundamental tenet in these imaging studies is that the fMRI signal (e.g., Blood Oxygenation Level Dependent, BOLD) changes in parallel with neural activity. The precise relationships between the fMRI signal and neuronal activity (also called neurovascular coupling), however, are not fully understood and remain active topics of research. We do know, however, that neuronal spiking and synaptic activity, whether excitatory or inhibitory, contribute to the blood-flow demand detected by fMRI signals. Much of the existing knowledge stems mainly from studies of the monkey visual system under physiological conditions, which assess the relationships between changes in neuronal electrophysiological signals (spiking and local field potentials, LFPs) and MRI signal amplitudes [6]. Recent evidence indicates that the relationships between fMRI BOLD and electrophysiological signals indeed vary across different brain regions and task conditions, perhaps due to differences in the functional organization of neurons within the specific imaging volume and/or differences in the neuronal engagement during each task [7, 8]. Few studies have directly compared the precise spatiotemporal relationships between these two types of signal, especially in pathological conditions. The widespread application of fMRI has not only identified brain regions that are responsible for the multi-dimensional experience of pain perception, but has also revealed widespread alterations in brain function and structure in various chronic pain conditions. However, accurate interpretations of these MRI findings and a full appreciation of their clinical and behavioral implications require knowledge of the neuronal constituents underlying the MRI signal, given that the BOLD fMRI signal indirectly measures the ensemble properties and activity of relatively large neuron populations [6, 9, 10]. Such information is difficult, often impossible, to obtain in human subjects for both technical and ethical reasons, but it is possible to acquire from animal studies. In contrast, fMRI in animals allows us to directly compare neuronal and MRI activity, and ultimately provides the much-needed information about the physiological basis of the fMRI signals.
In my laboratory, we have taken such an approach by combining high-resolution fMRI (at 9.4 T), multichannel microelectrode recording, and histological investigation. Our studies focus on understanding how different types of nociceptive and innocuous touch inputs under normal and pathological conditions (deafferentation) are processed in early somatosensory cortices (i.e., areas around the central and lateral sulci) in lightly-anesthetized squirrel monkeys. Information provided by each method is independent and complementary, and provides a more comprehensive view of cortical processing. In this paper, I mainly review findings derived from studies in the SI cortex (areas 3a, 3b, and 1/2) in two experimental settings: nociceptive and tactile processes under normal conditions (input-intact) and tactile processes in deafferentation conditions (input-deprived). The deafferentation study was motivated by the inconsistently-detected SI activation in chronic pain conditions, along with numerous reports on plastic changes in the somatotopic map of SI cortex. In our studies, deafferentation is introduced by a unilateral dorsal column lesion (DCL) at a high cervical level, a procedure that selectively disrupts the pathway important for fine discriminative touch. We started our study with this model system, because it has been well studied using other invasive methods (e.g., microelectrode mapping and immunohistological evaluation). Although we have not specifically tested the effects of disruption of the nociceptive pathways on the pain response, we believe that understanding the effects of deafferentation on the fMRI signal and its relationship with neuronal activity has implications for other deafferentation conditions, including some types of chronic pain. In the following sections, I briefly state our motivation, summarize the findings, and discuss the potential implications.
FMRI Study of Nociception in Non-human Primates: Missing Link Between Animal and Human Data
We use the primate as an experimental model to study nociception due to its unique advantages. First, the monkey brain closely resembles the human brain, compared to those of rodents. For example, rather than the single SI and SII areas in rodents, the SI and SII cortices in primates (including humans) are composed of several functionally and anatomically distinct sub-regions. Out of the four well-established sub-region structures in primates, only area 3b is regarded as homologous to SI in rodents [11]. Similar differences in functional organization are known to exist in other high-order pain processing regions, such as those along the lateral sulcus [12–14], including area 7b (part of the inferior parietal cortex in humans), retro-insula, and pIns (which is hard to distinguish from SII in human fMRI images). Thus, information derived from monkey studies provides the important missing link between rodent and human data. Second, the high spatial resolution of fMRI images achievable in monkeys [15–19] allows examination of the pain network at the submillimeter scale [15, 20–24]. This achievement is critical for understanding brain function and pain processing [20, 25], as fine-scale columnar structures are believed to be the building blocks of basic information-processing units for cerebral function. FMRI activation maps not only allow us to examine the nociceptive responses at various network scales (e.g., modular organization within one area and across areas), but they also help tremendously when selecting target regions for the time-consuming electrophysiological mapping and recording studies, particularly in areas of which we have little prior knowledge.
FMRI and Optical Imaging of Intrinsic Signals Reveal Differential Cortical Representations of Mechanical and Thermal Nociception and Innocuous Touch Within SI and Other Cortices Along the Lateral Sulcus
The SI (composed of four sub-regions in primates) and the SII cortices (composed of parietal ventral (PV), S2, and ventral somatosensory sub-regions) are early somatic regions responsible for functions including fine discriminative touch, tactile shape perception, and proprioception, as well as temperature and pain sensing. The roles of these regions in touch processing and perception are much better established than their roles in encoding temperature and pain. We now know that higher primates have four strip-like representations of skin and muscle receptors corresponding to areas 3a, 3b, 1, and 2 of the anterior parietal cortex. Areas 3b and 1 receive cutaneous information from the thalamic ventroposterior nucleus, while the ventroposterior superior nucleus provides areas 3a and 2 with information from muscle receptors. Area 3b provides most of the activating cutaneous inputs to areas 1 and 2. Further processing in S2 and the PV area allows tactile recognition of objects (e.g. shape or surface features). Both S2 and PV receive activating inputs from areas 3a, 3b, 1, and 2, and S2 also projects to PV and to a parietal rostral area where further connections with the amygdala and hippocampus may occur to allow the formation of tactile memories (for a review on this topic see [12]).
The precise role of SI cortex in pain perception has long been debated [1, 26–28]. The doubt comes mainly from three observations: inconsistent detection of fMRI activation in human SI cortex, particularly in chronic pathological pain conditions; the small number of nociceptive neurons identified in corresponding areas 3a, 3b, and 1/2 in monkeys [29–32]; and failure to evoke or diminish pain sensation by stimulating or ablating SI cortex. Based on our own data, we believe that several factors have contributed to these observations, the first being the use of relatively large fMRI signal voxels on sampling signals that originate from functionally heterogeneous neuronal populations. The second factor relates to the compromised fMRI response detection in pathological conditions, such as deafferentation. Findings derived from our high-resolution fMRI studies support our speculation about the first factor. Our SI studies benefit greatly from the fact that the SI cortex of new-world monkeys is a flat cortical region that allows detailed spatial comparison among maps obtained with fMRI, microelectrode mapping and recordings, and histology.
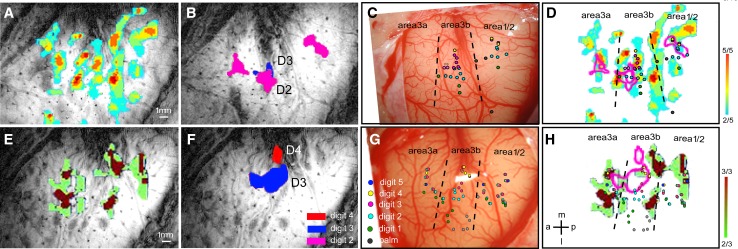
Under light anesthesia (0.5%–1% isoflurane), we found that nociceptive-heat stimulation of digits (47.5 °C) evokes robust (~0.8% BOLD signal change), widely-distributed, and reproducible fMRI activations in topographically appropriate regions of areas 3a, 3b, and 1/2 of SI, and anterior M1 cortex (Fig. 1A, D, E, H) [33]. In contrast, fMRI activations elicited by innocuous tactile stimuli are more constrained spatially; they are located in somatotopically appropriate but different regions of areas 3b and 1 (Fig. 1B and F, see the color-coded microelectrode penetrations in Fig. 1D and H). These differential activation patterns to nociceptive heat versus touch are present across monkeys (for details, see Figure 4 in [33]). Occasionally, we also detected tactile stimulus-related BOLD signal changes in area 3a (Fig. 1B). By characterizing the receptive fields and preferred stimulus properties of neurons at each microelectrode penetration site (colored dots in Fig. 1C and G), we determined the cortical territories of each digit representation in each cortical area and their inter-areal borders (dotted line in Fig. 1C and G). Careful examination of the activation maps and BOLD time-courses in each cortical area led to two main findings in the SI cortex. First, the spatial relationships between heat-nociceptive and tactile fMRI activations vary across areas. In area 3b, heat fMRI activation does not overlap with tactile responses, and is centered at inter-areal borders (areas 3b–1, areas 3a–3b, and areas 1–2). In areas 1/2, heat nociceptive activation foci only partially overlap with tactile activation foci. Different from areas 3b and 1/2, area 3a responds preferentially to nociceptive heat stimuli. Second, BOLD signal changes to heat stimulation in areas 3a and 1 peak earlier than that in area 3b. The areas 3a and 1 to area 3b information flow direction is reversed to a flow from area 3b to area 1 for processing tactile inputs. Mechanical nociceptive stimuli of digits, however, elicit optical signal increases in areas 3a, 3b, and 1/2 regions that co-localize with those to innocuous tactile stimuli [2]. Thus far, targeted microelectrode recordings conducted in our lab have identified nociceptive neurons in each of the fMRI activation clusters we examined ([2, 33] and unpublished observations).
Fig. 1.
Comparison of nociceptive-heat and tactile fMRI activations within SI cortex in two representative monkeys. A, E Color-coded activation probability maps illustrate the frequency of detected activations (in each run) in response to 47.5 °C nociceptive-heat stimulation of digits within each imaging session. Activation in each run was thresholded at P < 0.0001 (uncorrected). B, F Composite digit tactile activation maps in the same animals. Letters indicate the stimulated digits associated with the activation. C, G Digit representation maps as determined electrophysiologically by microelectrode mapping. Receptive field properties of neurons at each penetration (colored dots) are color-coded for different digits. Dotted lines indicate the estimated inter-areal borders. D, H Overlays of nociceptive heat and tactile fMRI activation patterns and electrophysiological maps in each animal. Color-coded scale bars indicate the number of activated runs of the total of scanned runs (far right). a anterior, p posterior, m middle, l lateral. Scale bars 1 mm. Modified from Chen LM, et al., Pain 2011 [23].
In addition to the SI cortex, cortical areas that reside along the lateral sulcus (the Sylvain fissure in human brain), particularly the insula (Ins), have been proposed as the primary pain-specific processing regions [34, 35]. The lack of adequate understanding of how different aspects of nociceptive information are processed and integrated by individual neurons and populations of neurons in these areas has led to heated debates on the functions of cortical areas, and the location(s) or even existence of a primary nociception-specific processing region in the cortex of primates [1, 36, 37] (see most recent discussions on this topic in [37, 38]). To date, only a limited number of scattered nociceptive neurons (primarily wide-dynamic range nociceptive neurons) have been identified in this poorly-defined region [2, 30, 39–42]. To fill in these knowledge gaps, we also mapped the lateral sulcus regions (homologous to the parieto-insula region in human brain) during nociceptive stimulation and discovered complicated fine-scale networks for nociceptive processing [43–46]. For example, robust and reproducible nociceptive stimuli-evoked fMRI activations were identified in posterior SII, pIns, area 7b, and the retro-insula (Fig. 2) [33]. The last two are considered part of the posterior and inferior parietal cortex in humans, an area connecting sensory and limbic regions [47–50]. It is important to note that these regions are not readily distinguishable in human fMRI studies.
Fig. 2.
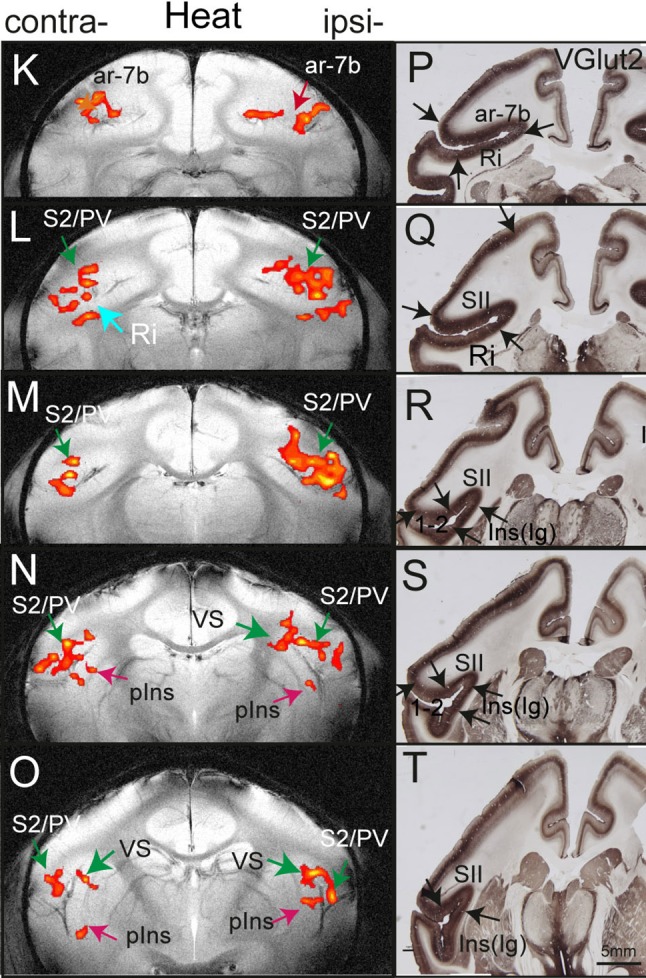
fMRI activation in lateral sulcus areas of SII, posterior insula (pIns), area 7b (ar-7b), and retro-insula (Ri) in response to nociceptive-heat stimulation (left column) and corresponding VGlut2-stained tissue sections. Modified from Chen LM, et al., Pain 2012 [33].
In summary, extending previous neuroimaging findings in the monkey SI cortex [2, 51, 52], our high-resolution fMRI studies have revealed a spatially complex and segregated fine-scale cortical network around the central and lateral sulci for processing nociceptive information. This network includes subregions of SI, SII, pIns, area 7b, and retro-insula. Based on these observations, we hypothesize that there is a distinct nociception-specific network around the central and lateral sulci. These regions likely work together to encode information on the quality, intensity, and location of painful stimuli. We are currently investigating the neuronal basis of these nociceptive stimuli-evoked fMRI responses and their functional connections.
Implications of the Segregated Nociceptive and Innocuous Tactile Responses within SI Cortex of Monkey Brain for Human fMRI Studies
We think that the complexity of spatial and temporal features very likely contributes to the variation in fMRI signals, therefore resulting in inconsistent detection of pain-related fMRI signal changes in SI cortex in human studies, in which the functionally and anatomically distinct four-region structure (areas 3a, 3b, 1, and 2) is treated as a single entity of SI cortex. For instance, from the detection point of view, if a large proportion of fMRI voxels are sampling signals originating from a mixture of different SI subregions or regions containing a mixture of nociceptive and innocuous neurons, then the net detectable fMRI signal changes would likely change drastically depending on the location of those voxels and the composition of neuron clusters with different preferred stimuli across subjects. The editorial by Dr. Davis [9] illustrates very well one aspect of the aforementioned complex: the possible relationships between a single fMRI voxel and the underlying anatomical and functional constituents. As expected, if we reduced the fMRI voxel size to a fine scale (e.g., submillimeter scale in our study), allowing activities that originate from distinct functional cortical columns or regions to be separated, then the detection and reproducibility of fMRI activation evoked by painful stimuli would increase drastically. In fact, these were our findings in both monkey and human high-resolution fMRI studies. In monkeys, fMRI signal changes in response to nociceptive heat, cold (unpublished data), and innocuous touch were consistently detected across imaging runs, sessions, and subjects. In humans, by applying high-resolution fMRI (1 × 1 mm2 in-plane resolution) at 7 T, we consistently detected touch-evoked fMRI signal changes in individual digit regions [53], as well as pain-related signal changes in area 3a and other sub-regions in SI cortex [Stringer EA, Gore JC, and Chen LM unpublished observation].
Differential Intrinsic Inter-areal Functional Connectivity of Areas 3a and 3b of SI Revealed by Resting-State fMRI Signals
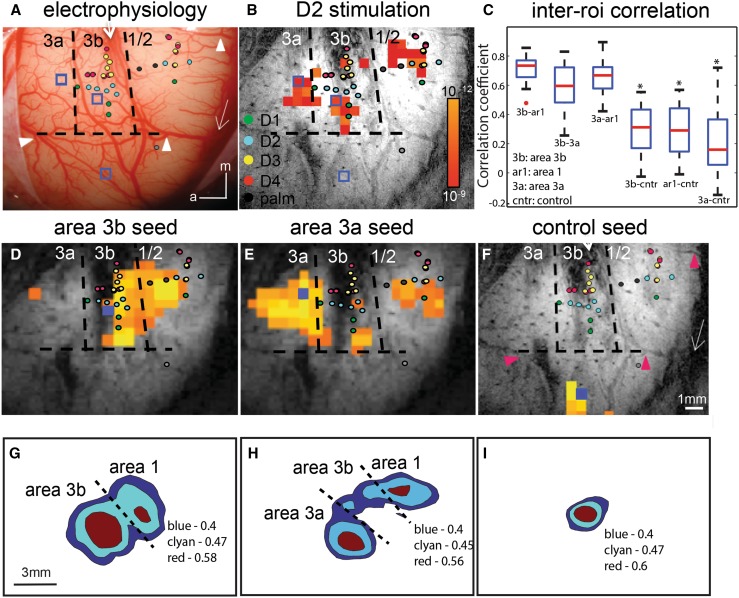
Building upon the evidence for segregated nociceptive versus tactile activation processing circuitries within SI, we further asked the question of whether and how these nociceptive regions are functionally connected. We used the well-documented indicator of functional connectivity, inter-regional correlation of low-frequency fMRI signals in a resting state, to probe the functional connections among areas 3a, 3b, and 1/2. Numerous studies have demonstrated the power and usefulness of resting-state fMRI signals for probing intrinsic resting-state functional connectivity (rsFC) among brain regions in humans and monkeys [54–56]. Using this measure, we found that area 3b and area 3a exhibited differing connectivity patterns (Fig. 3). Area 3b connects strongly to area 1/2, but not area 3a, whereas area 3a connects strongly to area 1/2 (Fig. 3). Importantly, the inter-areal functional connectivity pattern is somatotopically specific (e.g., D2 tip to D2 tip), a feature indicating its functional relevance for spatial localization and the discrimination of sensory inputs. Subsequent microelectrode recording studies indeed revealed synchronized spiking activity among those regions exhibiting strong resting-state fMRI signal correlation [57, 58]. Examination of local intrinsic anatomical connection patterns also identified preferential connections of area 3b and area 1 [57, 59, 60]. For the first time, our study established an anatomical and electrophysiological basis for resting-state fMRI (<0.1 Hz) networks within SI cortex at a local mm-based scale. The robustness of this rsFC measure motivated us to explore the fine-scale whole-brain functional network of each of the nociceptive regions identified. We started by probing the rsFC of SII and the pIns, which resulted in the identification of distinct functional networks of nociceptive regions in these areas. A schematic is shown in Fig. 5.
Fig. 3.
Resting-state fMRI connectivity within SI cortex of squirrel monkeys. One case is shown in A–B and D–F, and population data in C and G–I. A Electrophysiological map of digit region. Colored dots see legend, digits 1–4, palm. Dotted lines estimated borders between areas 3a, 3b, and 1, and between hand/face. White arrows central and lateral sulci. Blue boxes areas 3a, 3b, and 3b face seed regions. White arrowheads vessel markers used for alignment with image in F (pink arrowheads). B BOLD activation in response to vibrotactile stimulation of D2 tip. Activated voxels occur in areas 3a, 3b, and 1. Correlation maps were thresholded at r > 0.7 with a peak correlation value of 0.9. C Box plot of correlation coefficient values between areas 3b and 1 (3b–ar1), 3b and 3a (3b–3a), and 3a and 1 (3a–ar1), with control locations (3b–cntr, 1–cntr, and 3a–cntr). D–F BOLD correlation maps in the resting state. Seed voxels (solid blue boxes in D and E) were placed in the digit regions in areas 3b (C), 3a (D), and the face regions in 3b (F) for voxel-wise correlation analysis. G–I Cross-animal (or population) correlation maps of seeds in areas 3b (G), 3a (H), and face (I) regions. Correlations are a summary of 18 runs (i.e., each map is based on 18 seeds, seeds overlay D2, D3, or D4 digit tip) conducted in 10 animals. To average across animals, seed voxels were used to align all the cross-animal images. Correlation maps are centered on the seed region. Because the seed location is relative to the imaging field of the view across animals, there are some deviations in spatial location between the average correlation map and individual cases. Adapted from Wang Z, Chen LM, et al., Neuron 2013 [57].
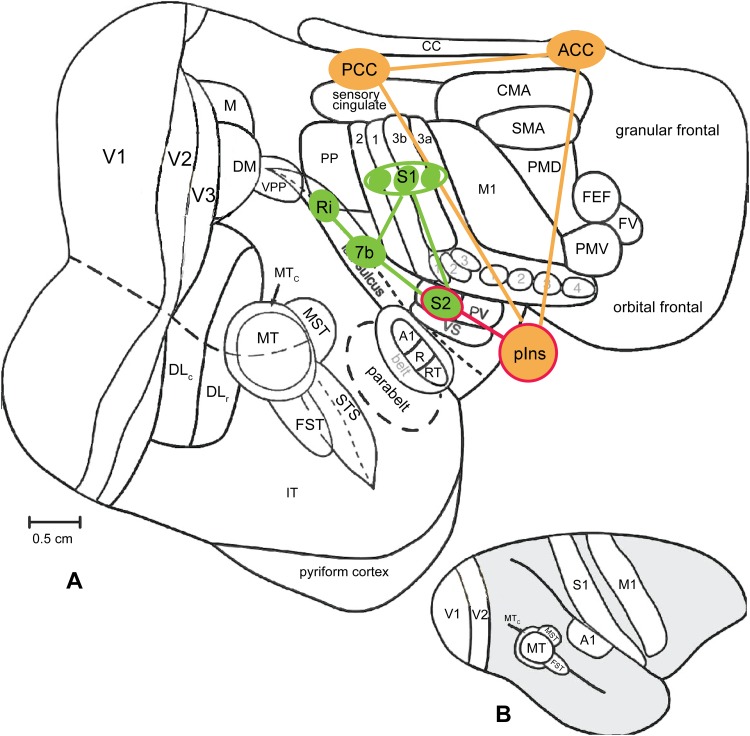
Fig. 5.
Schematic summary of inter-regional relationships among pain networks of squirrel monkeys. A, B Nociceptive processing regions on a flattened view of the entire neocortex (A), and on a lateral view of the intact monkey brain (B). Modified from Kaskan PM, et al., Front Neurosci 2007 with permission [66]. S1 the primary somatosensory cortex, S2 the secondary somatosensory cortex, 7b Brodmann area 7b, pIns posterior insula cortex, Ri retro-insula, PCC posterior cingulate cortex, ACC anterior cingulate cortex.
Proposed Cortical Processing Circuits for Nociception and Touch Within SI Cortex
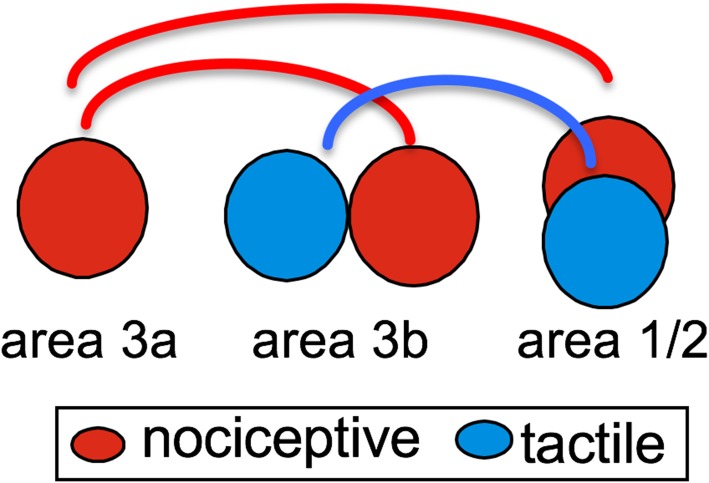
We proposed an inter-areal and inter-regional selective circuitry model for heat and touch input processing within SI cortex based on the findings from stimulus-evoked and resting state fMRI experiments (Fig. 4). This model suggests the following information-processing features within SI: (1) heat-pain processing circuitry within SI is composed of regions in areas 3a, 3b, and 1/2 (red circles), and in a topographic manner. Touch circuitry is independent of the heat-pain circuitry, and consists of areas 3b and 1 (blue circles). (2) There is a general anterior to posterior information-processing hierarchy for both heat-pain and touch. (3) The area 3a to area 1/2 connection is the dominant pathway for pain within SI cortex. Area 3a could be one of the early relay stations for processing heat-nociceptive inputs as proposed by Dr. Craig [36]. We recognize that more in vivo microelectrode recording and mapping studies in monkeys are needed to fully construct the circuitry and to understand the flow for nociceptive information integration within SI cortex. Further studies in humans are also needed to determine the functional relevance of these inter-areal local networks within SI cortex in pain perception.
Fig. 4.
Proposed inter-areal circuitry within SI cortex.
To summarize, evidence obtained from our fMRI and optical imaging experiments supports the involvement of areas 3a, 3b, and 1/2 of SI cortex in processing heat [33, 39, 41, 51], mechanical [2], and cold nociceptive inputs. Local heat and cold nociceptive processing networks within SI appear to be separate from those of innocuous tactile processing. Based on our observations of spatial segregation of heat and tactile fMRI activations and the early-peaked heat fMRI signal changes in area 3a, we propose that nociceptive heat and tactile inputs are processed by different inter-areal circuitries within SI cortex. Specific area 3a to area 1/2 and area 3b to area 1/2 circuitries are engaged in nociceptive heat versus innocuous touch information processes. Based on observations of overlapping mechanical and tactile activations within areas 3b and 1 [2], we also hypothesize that mechanical and heat nociceptive inputs are likely processed by different groups of neurons and local circuitries within and across SI sub-areas. To date, however, clear experimental data for specialized nociceptive processing are only available for the peripheral system (for a recent review, see [61]). Thus, whether the different circuitries identified within and across SI sub-areas are nociception-specific and/or receive functionally-specific thalamic inputs remains to be determined. More neuronal electrophysiological and anatomical connectivity data are necessary to support (or disprove) the proposed networks for the differential representation of heat and mechanical pain in primates [26, 62–64].
The Functional Connectivity Circuits of Nociceptive Regions in SII and Posterior Insula
Beyond the SI cortex, we have also observed separate nociceptive heat and cold processing regions within the SII and the pIns cortices. To date, our fMRI and preliminary electrophysiological evidence support the existence of separate (from core tactile processing regions) nociceptive heat and cold processing circuits within SI and between SI and SII cortices. We hypothesize that nociceptive modality (heat and cold) information is extracted and integrated in early somatosensory areas. Figure 5 shows a schematic of the established functional organization of the whole region along the lateral sulcus based on anatomy, functional organization, neuronal receptive fields, and stimulus response features [65].
Effects of Differentiation on BOLD, LFP, and Spiking Activity in the SI Cortex and the Implications for Detecting SI Nociceptive Responses in Chronic Pain Conditions
Deafferentation is quite common in chronic pain states, such as pain associated with stroke and spinal cord injury. Thus, a better understanding of the effects of deafferentation on fMRI signals, neuronal electrophysiological activity, and the relationships between these two types of signal is critical to accurately interpret fMRI findings involving deafferentation. Toward this goal, we started our study with a well-established deafferentation model in monkeys: unilateral DCL of the spinal cord at the cervical level. This model provides a relatively clear experimental condition, because it selectively disrupts ascending afferents carrying discriminative fine touch information to SI cortex without affecting motor pathways and other ascending pathways, allowing us to quantify the effects of deafferentation on fMRI signal changes in activation size and magnitude, neuronal spiking, and LFP activity, and the spatial and magnitude correlations between changes in these two types of signal in a very well-controlled deafferentation condition. One novel aspect of this model is that the level and percentage of dorsal column afferent disruption can be confirmed histologically and quantified by calculating the number of spared tracer-intake fiber terminals in the dorsal column nuclei in the brainstem (for details, see [67, 68]).
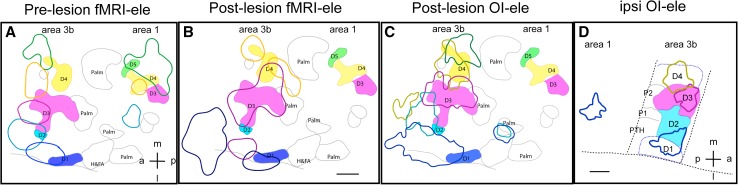
Typical changes in deafferented cortex are illustrated in Fig. 6 [67, 68]. Compared to the fMRI map of pre-lesion digit activation (colored outlines in Fig. 6A), there were apparent spatial shifts in the remaining digit responses toward the palm (open black outlines in Fig. 6A–C) and the inter-area border of areas 3b and 1. D1 (dark blue outline), D3 (dark red outline), and D4 (orange outline) fMRI activations were located at somatotopically appropriate sites, but the activation sizes were larger than normal (Fig. 6B). Unlike the post-lesion fMRI digit maps, the post-lesion optical imaging maps of the digits exhibited close spatial correlations with the post-lesion digit maps defined electrophysiologically (Fig. 6C). The electrophysiology and optical imaging maps of D3 and D4 were in close agreement (Fig. 6C). Portions of the D1 and D2 activations, as well as the second patch of D4 activation, were determined to be in area 3a, as indicated by the border between areas 3b and 3a (dotted lines in Fig. 6A–C). We do not know whether the responsive patches in area 3a are expansions of area 3b digit territory or if they represent newly-acquired responsiveness of area 3a neurons to low-threshold tactile stimuli.
Fig. 6.
Comparisons of fMRI, optical imaging, and electrophysiological maps of digit activation in contralateral and ipsilateral areas 3b and 1 in one monkey after a dorsal column lesion. A Overlay of pre-lesion fMRI and post-lesion electrophysiological maps of digits. Colored outlines location and size of fMRI activations; colored patches location and size of neuronal responses. B Spatial comparison of post-lesion fMRI and post-lesion electrophysiological digit maps. C Spatial comparison of post-lesion optical imaging (OI) and post-lesion electrophysiological digit maps. D Overlay of optical imaging and electrophysiological maps of digits D1–D4 in the ipsilateral areas 3b and 1 of the same animal. CS central sulcus. Dotted black lines estimated inter-areal borders. Scale bars 1 mm. a anterior, p posterior, l lateral, m medial. Modified from Chen LM, et al. J Neurosci 2012 [68].
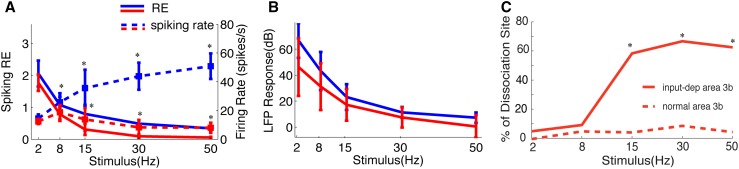
To summarize, after DCLs that result in 78%–99% afferent disruption in monkeys, we found in the input-deprived area 3b that (1) the detection rate of fMRI responses to identical tactile stimulation drops ~30%; (2) the fMRI-responsive region is enlarged; (3) the fMRI activation center shifts spatially, away from its original location, resulting in a reorganization of the digit maps; (4) the response magnitude of fMRI and multi-unit firing activity to 8-Hz stimulation declines significantly [68]; and (5) the fMRI response in the input-deprived region is larger than the digit representation maps determined by neuronal activity. Together, these findings indicate that the previously unresponsive palm region now re-gains responsiveness to spared digit afferent inputs (or ascending via different pathways in the spinal cord [67]) after a spinal cord lesion. In the input-deprived (deafferented) cortex, the responses evoked by tactile stimuli are weaker, spatially shifted, and more diffuse. Most importantly, not only is the spatial extent of fMRI response larger than that defined by neuronal activity, but also simultaneous recordings of the spiking and LFPs from those input-deprived (but reactivated, definition described in [69]) regions reveal dissociated changes in fMRI signal amplitudes, LFPs, and spiking activities. Reduction in multi-unit spiking activity does not occur in parallel with similar changes in LFPs, suggesting a disproportionately enhanced synaptic contribution (reflected by LFP activity) to fMRI signal changes in input-deprived cortex. Specifically, a significant reduction in spiking rates, particularly to high frequency stimuli, but not in LFPs (Fig. 7) suggests that the functional changes occurring in the deafferented cortex are functionally specific, such as the tactile frequency-encoding in our DCL model. The loss of frequency-encoding capacity of spikes in area 3b after DCL may explain contributions to the impaired ability of patients with dorsal column injury to determine the temporal sequence of tactile events.
Fig. 7.
Group quantification of spiking (A) and LFP (B) responses in area 3b as a function of stimulus frequency, and summary of percentage of spike-LFP dissociation in control and input-deprived deafferented cases (C). A The mean response efficacy (RE, solid lines) to different stimulus frequencies declined progressively in deafferented area 3b (red), and was significantly lower than in normal cases (blue) (*P < 0.05, except for 2-Hz stimulus). The firing rates in normal cortex were also significantly higher than in deafferented cortex. B The mean power of evoked LFPs in area 3b also decreased with increasing stimulus frequency. The LFP signal was persistently and robustly modulated by tactile stimulation under all conditions, and there was no difference between signals in normal versus deafferented cortex (*P > 0.05). C Summary of spike-LFP dissociation as a function of stimulus frequency in area 3b of normal and input-deprived subjects. With increasing stimulation frequency, the LFP response was more often dissociated from spiking activity in the input-deprived cases (*P < 0.05). Modified from Wang Z, et al., Exp Neurol 2013 [69].
Altogether, the finding of dissociated spike-LFP in deafferented cortex has important implications for studies using fMRI to infer neuronal activity in pathological conditions. There has been a growing recognition in recent years that LFPs and spiking activity reflect different aspects of neuronal processing at different spatial and temporal scales. The LFP integrates predominantly synaptic input signals from a population of neurons in a relatively larger cortical region, while spiking activity carries the output signal. In general, fMRI signal changes align well with LFP signal changes. To date, the precise relationships of these three different types of signal (fMRI, LFP, and spiking) remain elusive [6, 70–74]. There is evidence for a functional or task-specific relationship between these signals [8, 75, 76]. Our data suggest that, under certain circumstances such as cortical deafferentation, the BOLD signal change, given its dominant contribution from subthreshold synaptic activity, may likely underestimate the changes in spiking activity (for reviews see [6, 75, 77, 78]). To accurately interpret functional imaging findings, one should take into account the specific conditions and states of the cortex and the local circuitry involved in the task. On the other hand, if the integrative subthreshold activity and output spiking activity are dissociated (as in our experimental condition), spiking activity alone very likely would underestimate the degree of ongoing local integration and, by this reasoning, the extent of cortical reorganization. Given what we have shown regarding the dissociation between subthreshold and spiking activity, we believe that systematic investigation of specific pathological brain functions using combined electrophysiological and functional imaging methods is necessary to establish quantitative relationships between fMRI and the underlying activity of populations of neurons.
To summarize, our studies showed that deafferentation alters several aspects of cortical processing. The weakened and diffuse fMRI signal changes to stimuli likely compromise the detection of fMRI activation. This observation calls for more cautious interpretations about failed detection of pain-related activations in SI cortex, particularly in conditions where deafferentation is suspected. The finding of high-frequency-dependent dissociation of LFPs and spiking activity also indicates that the plastic changes that occur in the brain develop in a function-specific manner. Thus, to fully appreciate the role of SI in chronic pain conditions, the uses of appropriate forms of painful stimuli and paradigms are necessary.
Effects of Anesthesia on Nociceptive Stimuli-Evoked and Resting-State fMRI Signals
Although the awake preparation is desirable for studying nociception in animals, anesthesia is necessary for submillimeter resolution fMRI due to technical constrains and for the delivery of prolonged suprathreshold nociceptive stimuli in a controlled manner due to animal welfare concerns. In this context, however, the effects of anesthesia on the systems investigated need to be taken into account in interpreting the findings. In general, anesthesia suppresses neuronal activity, but in a dose-dependent and system-specific manner (for a recent review, see [79]). In our experiments, the effects apply to both stimulus-evoked and resting state fMRI signals. For fMRI activation studies, the use of anesthesia (a low dose of isoflurane in our cases) indeed increases the fMRI signal-to-noise ratio by reducing motion and physiological signal-related noise, which then enhances the detection and magnitude estimation of fMRI signal changes caused by nociceptive stimuli. Anesthesia is expected to alter the neuronal receptive field sizes, stimulus tuning curves, and dynamic features according to observations from somatic and other sensory systems [80, 81]. Since no study has systematically examined the effects of anesthesia on nociceptive neurons, the specifics remain to be determined. From our own experience, nociceptive neurons have been isolated from cortical regions showing nociceptive stimulus-evoked fMRI signal changes under light isoflurane anesthesia. The effect of anesthesia on resting state fMRI signals, particularly within the nociceptive system, is a completely open question.
In a resting state, existing evidence from other sensory systems indicates that anesthesia alters the frequency compositions of the fMRI signal fluctuation and likely neuro-vascular coupling [82–85]. Thus, anesthesia likely affects the dynamics of communication between nociceptive regions. Nevertheless, our observations demonstrate that under light isoflurane anesthesia, robust fMRI signal changes are detectable across the entire brain, including prefrontal cortex (unpublished data). The core and base processes of nociceptive inputs are retained. Moreover, beyond the brain, strong horn-to-horn resting-state functional connectivity has been reported in the spinal cord of monkeys [86], a finding similar to that reported in awake humans [87, 88].
Conclusions and Future Directions
By taking advantage of submillimeter fMRI in a strong MRI field, we provided fMRI evidence supporting the differential engagement of areas 3a, 3b, and 1 of SI cortex in the processing of heat-nociceptive versus tactile inputs. Analysis of resting-state fMRI signals revealed differing inter-areal functional connectivity among the sub-regions. We proposed a model of separate inter-areal processing circuitry for pain and touch within the SI cortex. Our observations of the profound effects of deafferentation on fMRI signals, neuronal electrophysiological activity, and the relationships between the two types of signal highlight the importance of a better general understanding of the neuronal basis of fMRI signals and of nociceptive signals in particular. Our data demonstrate that we now have the tools necessary to start addressing a number of important questions. For example, (1) what specific aspect of nociceptive information is extracted and integrated in each individual cortical region? (2) How do different brain regions communicate and work together to relay nociceptive information to higher-order brain regions? (3) How do cortical nociception-processing regions connect and interact with subcortical pain modulatory regions? And (4) how do sensory pain networks interconnect and interact with affective/emotional and psychological/motivational networks during pain processing and modulation? A combined approach using high-resolution fMRI, electrophysiology, and histology in non-human primates is very well suited to address these remaining key questions in pain research. New knowledge gained will have direct implications for pain studies in humans.
Acknowledgements
I am grateful for the helpful comments of Dr. Robert M Friedman on an early version of this review. I thank all the co-authors for their contributions to the original papers I mentioned here. I also thank Mrs. Chaohui Tang and Mr. Fuxue Xin for their assistance in data collection. Work presented in this chapter was supported by NIH Grant R01 NS069909 and an imaging track Grant from the Dana Foundation.
References
- 1.Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LM, Friedman RM, Roe AW. Area-specific representation of mechanical nociceptive stimuli within SI cortex of squirrel monkeys. Pain. 2009;141:258–268. doi: 10.1016/j.pain.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overduin SA, Servos P. Distributed digit somatotopy in primary somatosensory cortex. Neuroimage. 2004;23:462–472. doi: 10.1016/j.neuroimage.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Ploner M, Schmitz F, Freund HJ, Schnitzler A. Differential organization of touch and pain in human primary somatosensory cortex. J Neurophysiol. 2000;83:1770–1776. doi: 10.1152/jn.2000.83.3.1770. [DOI] [PubMed] [Google Scholar]
- 5.Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 7.Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, et al. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. J Neurosci. 2009;29:3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartolo MJ, Gieselmann MA, Vuksanovic V, Hunter D, Sun L, Chen X, et al. Stimulus-induced dissociation of neuronal firing rates and local field potential gamma power and its relationship to the resonance blood oxygen level-dependent signal in macaque primary visual cortex. Eur J Neurosci. 2011;34:1857–1870. doi: 10.1111/j.1460-9568.2011.07877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis KD. Neurophysiological and anatomical considerations in functional imaging of pain. Pain. 2003;105:1–3. doi: 10.1016/S0304-3959(03)00174-X. [DOI] [PubMed] [Google Scholar]
- 10.Orban GA. Functional MRI in the awake monkey: the missing link. J Cogn Neurosci. 2002;14:965–969. doi: 10.1162/089892902760191171. [DOI] [PubMed] [Google Scholar]
- 11.Kaas JH. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev. 1983;63:206–231. doi: 10.1152/physrev.1983.63.1.206. [DOI] [PubMed] [Google Scholar]
- 12.Kaas JH. The functional organization of somatosensory cortex in primates. Ann Anat. 1993;175:509–518. doi: 10.1016/S0940-9602(11)80212-8. [DOI] [PubMed] [Google Scholar]
- 13.Kaas JH. The evolution of the complex sensory and motor systems of the human brain. Brain Res Bull. 2008;75:384–390. doi: 10.1016/j.brainresbull.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaas JH, Garraghty PE. Hierarchical, parallel, and serial arrangements of sensory cortical areas: connection patterns and functional aspects. Curr Opin Neurobiol. 1991;1:248–251. doi: 10.1016/0959-4388(91)90085-L. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H, Nair G, Walker TA, Kim MK, Pardue MT, Thule PM, et al. Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci U S A. 2006;103:17525–17530. doi: 10.1073/pnas.0605790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duong TQ, Kim DS, Ugurbil K, Kim SG. Spatiotemporal dynamics of the BOLD fMRI signals: toward mapping submillimeter cortical columns using the early negative response. Magn Reson Med. 2000;44:231–242. doi: 10.1002/1522-2594(200008)44:2<231::AID-MRM10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Harel N, Lin J, Moeller S, Ugurbil K, Yacoub E. Combined imaging-histological study of cortical laminar specificity of fMRI signals. Neuroimage. 2006;29:879–887. doi: 10.1016/j.neuroimage.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Harel N, Ugurbil K, Uludag K, Yacoub E. Frontiers of brain mapping using MRI. J Magn Reson Imaging. 2006;23:945–957. doi: 10.1002/jmri.20576. [DOI] [PubMed] [Google Scholar]
- 19.Harel N. Ultra high resolution fMRI at ultra-high field. Neuroimage. 2012;62:1024–1028. doi: 10.1016/j.neuroimage.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SG, Fukuda M. Lessons from fMRI about mapping cortical columns. Neuroscientist. 2008;14:287–299. doi: 10.1177/1073858407309541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SG, Duong TQ. Mapping cortical columnar structures using fMRI. Physiol Behav. 2002;77:641–644. doi: 10.1016/S0031-9384(02)00901-0. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda M, Moon CH, Wang P, Kim SG. Mapping iso-orientation columns by contrast agent-enhanced functional magnetic resonance imaging: reproducibility, specificity, and evaluation by optical imaging of intrinsic signal. J Neurosci. 2006;26:11821–11832. doi: 10.1523/JNEUROSCI.3098-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LM, Dillenburger BC, Wang F, Friedman RM, Avison MJ. High-resolution functional magnetic resonance imaging mapping of noxious heat and tactile activations along the central sulcus in New World monkeys. Pain. 2011;152:522–532. doi: 10.1016/j.pain.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen LM, Friedman RM, Ramsden BM, LaMotte RH, Roe AW. Fine-scale organization of SI (area 3b) in the squirrel monkey revealed with intrinsic optical imaging. J Neurophysiol. 2001;86:3011–3029. doi: 10.1152/jn.2001.86.6.3011. [DOI] [PubMed] [Google Scholar]
- 25.Op de Beeck HP Haushofer J, Kanwisher NG. Interpreting fMRI data: maps, modules and dimensions. Nat Rev Neurosci. 2008;9:123–135. doi: 10.1038/nrn2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vierck CJ, Whitsel BL, Favorov OV, Brown AW, Tommerdahl M. Role of primary somatosensory cortex in the coding of pain. Pain. 2013;154:334–344. doi: 10.1016/j.pain.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vierck CJ, Whitsel BL, Favorov OV, Tommerdahl M. Response to the letter to the editor of pain by S. Canavero. Pain. 2013;154:1158–1159. doi: 10.1016/j.pain.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Kenshalo DR. Pain and the primary somatosensory cortex is its role in pain overrated? Pain Forum. 1996;5:181–183. doi: 10.1016/S1082-3174(96)80027-4. [DOI] [Google Scholar]
- 29.Dong WK, Chudler EH, Sugiyama K, Roberts VJ, Hayashi T. Somatosensory, multisensory, and task-related neurons in cortical area 7b (PF) of unanesthetized monkeys. J Neurophysiol. 1994;72:542–564. doi: 10.1152/jn.1994.72.2.542. [DOI] [PubMed] [Google Scholar]
- 30.Dong WK, Salonen LD, Kawakami Y, Shiwaku T, Kaukoranta EM, Martin RF. Nociceptive responses of trigeminal neurons in SII-7b cortex of awake monkeys. Brain Res. 1989;484:314–324. doi: 10.1016/0006-8993(89)90375-2. [DOI] [PubMed] [Google Scholar]
- 31.Robinson CJ, Burton H. Somatotopographic organization in the second somatosensory area of M. fascicularis. J Comp Neurol. 1980;192:43–67. doi: 10.1002/cne.901920104. [DOI] [PubMed] [Google Scholar]
- 32.Whitsel BL, Petrucelli LM, Sapiro G. Modality representation in the lumbar and cervical fasciculus gracilis of squirrel monkeys. Brain Res. 1969;15:67–78. doi: 10.1016/0006-8993(69)90310-2. [DOI] [PubMed] [Google Scholar]
- 33.Chen LM, Dillenburger BC, Wang F, Tang CH. Differential fMRI activation to noxious heat and tactile stimuli in parasylvian areas of new world monkeys. Pain. 2012;153:158–169. doi: 10.1016/j.pain.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 35.Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 2015;18:499–500. doi: 10.1038/nn.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 37.Davis KD, Bushnell MC, Iannetti GD, St Lawrence K, Coghill R. Evidence against pain specificity in the dorsal posterior insula. F1000Res. 2015;4:362. doi: 10.12688/f1000research.6833.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula is not an island in pain but subserves a fundamental role - Response to: “Evidence against pain specificity in the dorsal posterior insula” by Davis et al. F1000Res 2015, 4: 1207. [DOI] [PMC free article] [PubMed]
- 39.Kenshalo DR, Iwata K, Sholas M, Thomas DA. Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. J Neurophysiol. 2000;84:719–729. doi: 10.1152/jn.2000.84.2.719. [DOI] [PubMed] [Google Scholar]
- 40.Kenshalo DR, Jr, Isensee O. Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol. 1983;50:1479–1496. doi: 10.1152/jn.1983.50.6.1479. [DOI] [PubMed] [Google Scholar]
- 41.Whitsel BL, Favorov OV, Li Y, Quibrera M, Tommerdahl M. Area 3a neuron response to skin nociceptor afferent drive. Cereb Cortex. 2009;19:349–366. doi: 10.1093/cercor/bhn086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang ZH, Dougherty PM, Oppenheimer SM. Monkey insular cortex neurons respond to baroreceptive and somatosensory convergent inputs. Neuroscience. 1999;94:351–360. doi: 10.1016/S0306-4522(99)00339-5. [DOI] [PubMed] [Google Scholar]
- 43.Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- 44.Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- 45.Eickhoff SB, Grefkes C, Zilles K, Fink GR. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex. 2007;17:1800–1811. doi: 10.1093/cercor/bhl090. [DOI] [PubMed] [Google Scholar]
- 46.Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- 47.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- 48.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 49.Robinson CJ, Burton H. Somatic submodality distribution within the second somatosensory (SII), 7b, retroinsular, postauditory, and granular insular cortical areas of M. fascicularis. J Comp Neurol. 1980;192:93–108. doi: 10.1002/cne.901920106. [DOI] [PubMed] [Google Scholar]
- 50.Neal JW, Pearson RC, Powell TP. The ipsilateral cortico-cortical connections of area 7b, PF, in the parietal and temporal lobes of the monkey. Brain Res. 1990;524:119–132. doi: 10.1016/0006-8993(90)90500-B. [DOI] [PubMed] [Google Scholar]
- 51.Tommerdahl M, Delemos KA, Favorov OV, Metz CB, Vierck CJ, Jr, Whitsel BL. Response of anterior parietal cortex to different modes of same-site skin stimulation. J Neurophysiol. 1998;80:3272–3283. doi: 10.1152/jn.1998.80.6.3272. [DOI] [PubMed] [Google Scholar]
- 52.Tommerdahl M, Delemos KA, Vierck CJ, Jr, Favorov OV, Whitsel BL. Anterior parietal cortical response to tactile and skin-heating stimuli applied to the same skin site. J Neurophysiol. 1996;75:2662–2670. doi: 10.1152/jn.1996.75.6.2662. [DOI] [PubMed] [Google Scholar]
- 53.Stringer EA, Chen LM, Friedman RM, Gatenby C, Gore JC. Differentiation of somatosensory cortices by high-resolution fMRI at 7 T. Neuroimage. 2011;54:1012–1020. doi: 10.1016/j.neuroimage.2010.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blatow M, Nennig E, Durst A, Sartor K, Stippich C. fMRI reflects functional connectivity of human somatosensory cortex. Neuroimage. 2007;37:927–936. doi: 10.1016/j.neuroimage.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 55.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 56.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Chen LM, Negyessy L, Friedman RM, Mishra A, Gore JC, et al. The relationship of anatomical and functional connectivity to resting-state connectivity in primate somatosensory cortex. Neuron. 2013;78:1116–1126. doi: 10.1016/j.neuron.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, Mishra A, Newton AT, Morgan VL, Stringer EA, Rogers BP, et al. Fine-scale functional connectivity in somatosensory cortex revealed by high-resolution fMRI. Magn Reson Imaging. 2011;29:1330–1337. doi: 10.1016/j.mri.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashaber M, Palfi E, Friedman RM, Palmer C, Jakli B, Chen LM, et al. Connectivity of somatosensory cortical area 1 form an anatomical substrate for the emergence of multifinger receptive fields and complex feature selectivity in the squirrel monkey (Saimiri sciureus) J Comp Neurol. 2014;522:1769–1785. doi: 10.1002/cne.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Negyessy L, Palfi E, Ashaber M, Palmer C, Jakli B, Friedman RM, et al. Intrinsic horizontal connections process global tactile features in the primary somatosensory cortex: neuroanatomical evidence. J Comp Neurol. 2013;521:2798–2817. doi: 10.1002/cne.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109:5–12. doi: 10.1152/jn.00457.2012. [DOI] [PubMed] [Google Scholar]
- 62.Craig AD. Lamina I, but not lamina V, spinothalamic neurons exhibit responses that correspond with burning pain. J Neurophysiol. 2004;92:2604–2609. doi: 10.1152/jn.00385.2004. [DOI] [PubMed] [Google Scholar]
- 63.Craig AD. Retrograde analyses of spinothalamic projections in the macaque monkey: input to ventral posterior nuclei. J Comp Neurol. 2006;499:965–978. doi: 10.1002/cne.21154. [DOI] [PubMed] [Google Scholar]
- 64.Craig AD. Retrograde analyses of spinothalamic projections in the macaque monkey: input to the ventral lateral nucleus. J Comp Neurol. 2008;508:315–328. doi: 10.1002/cne.21672. [DOI] [PubMed] [Google Scholar]
- 65.Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol. 2000;418:1–21. doi: 10.1002/(SICI)1096-9861(20000228)418:1<1::AID-CNE1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 66.Kaskan PM, Lu HD, Dillenburger BC, Roe AW, Kaas JH. Intrinsic-signal optical imaging reveals cryptic ocular dominance columns in primary visual cortex of New World owl monkeys. Front Neurosci. 2007;1:67–75. doi: 10.3389/neuro.01.1.1.005.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi HX, Chen LM, Kaas JH. Reorganization of somatosensory cortical areas 3b and 1 after unilateral section of dorsal columns of the spinal cord in squirrel monkeys. J Neurosci. 2011;31:13662–13675. doi: 10.1523/JNEUROSCI.2366-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen LM, Qi HX, Kaas JH. Dynamic reorganization of digit representations in somatosensory cortex of non-human primates after spinal cord injury. J Neurosci. 2012;32:14649–14663. doi: 10.1523/JNEUROSCI.1841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z, Qi HX, Kaas JH, Roe AW, Chen LM. Functional signature of recovering cortex: Dissociation of local field potentials and spiking activity in somatosensory cortices of spinal cord injured monkeys. Exp Neurol. 2013;249:132–143. doi: 10.1016/j.expneurol.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berens P, Keliris GA, Ecker AS, Logothetis NK, Tolias AS. Feature selectivity of the gamma-band of the local field potential in primate primary visual cortex. Front Neurosci. 2008;2:199–207. doi: 10.3389/neuro.01.037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berens P, Keliris GA, Ecker AS, Logothetis NK, Tolias AS. Comparing the feature selectivity of the gamma-band of the local field potential and the underlying spiking activity in primate visual cortex. Front Syst Neurosci. 2008;2:2. doi: 10.3389/neuro.06.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boynton GM. Spikes, BOLD, attention, and awareness: a comparison of electrophysiological and fMRI signals in V1. J Vis. 2011;11:12. doi: 10.1167/11.5.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conner CR, Ellmore TM, Pieters TA, DiSano MA, Tandon N. Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans. J Neurosci. 2011;31:12855–12865. doi: 10.1523/JNEUROSCI.1457-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev. 2010;62:233–244. doi: 10.1016/j.brainresrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc Natl Acad Sci U S A. 2008;105:6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci. 2002;25:27–31. doi: 10.1016/S0166-2236(00)01995-0. [DOI] [PubMed] [Google Scholar]
- 78.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/S0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 79.Bonhomme V, Boveroux P, Brichant JF, Laureys S, Boly M. Neural correlates of consciousness during general anesthesia using functional magnetic resonance imaging (fMRI) Arch Ital Biol. 2012;150:155–163. doi: 10.4449/aib.v150i2.1242. [DOI] [PubMed] [Google Scholar]
- 80.Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol. 2008;100:1160–1168. doi: 10.1152/jn.90592.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris KD, Bartho P, Chadderton P, Curto C, de la Rocha J, Hollender L, et al. How do neurons work together? Lessons from auditory cortex. Hear Res. 2011;271:37–53. doi: 10.1016/j.heares.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jonckers E. Delgado y Palacios R, Shah D, Guglielmetti C, Verhoye M, Van der Linden A. Different anesthesia regimes modulate the functional connectivity outcome in mice. Magn Reson Med. 2014;72:1103–1112. doi: 10.1002/mrm.24990. [DOI] [PubMed] [Google Scholar]
- 83.Bonhomme V, Boveroux P, Hans P, Brichant JF, Vanhaudenhuyse A, Boly M, et al. Influence of anesthesia on cerebral blood flow, cerebral metabolic rate, and brain functional connectivity. Curr Opin Anaesthesiol. 2011;24:474–479. doi: 10.1097/ACO.0b013e32834a12a1. [DOI] [PubMed] [Google Scholar]
- 84.Barttfeld P, Petroni A, Baez S, Urquina H, Sigman M, Cetkovich M, et al. Functional connectivity and temporal variability of brain connections in adults with attention deficit/hyperactivity disorder and bipolar disorder. Neuropsychobiology. 2014;69:65–75. doi: 10.1159/000356964. [DOI] [PubMed] [Google Scholar]
- 85.Hutchison RM, Hutchison M, Manning KY, Menon RS, Everling S. Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain’s functional architecture. Hum Brain Mapp. 2014;35:5754–5775. doi: 10.1002/hbm.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen LM, Mishra A, Yang PF, Wang F, Gore JC. Injury alters intrinsic functional connectivity within the primate spinal cord. Proc Natl Acad Sci U S A. 2015;112:5991–5996. doi: 10.1073/pnas.1424106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barry RL, Rogers BP, Conrad BN, Smith SA, Gore JC. Reproducibility of resting state spinal cord networks in healthy volunteers at 7 Tesla. Neuroimage. 2016;133:31–40. doi: 10.1016/j.neuroimage.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barry RL, Smith SA, Dula AN, Gore JC. Resting state functional connectivity in the human spinal cord. Elife. 2014;3:e02812. doi: 10.7554/eLife.02812. [DOI] [PMC free article] [PubMed] [Google Scholar]