Abstract
Empathy is traditionally thought to be a unique ability of humans to feel, understand, and share the emotional state of others. However, the notion has been greatly challenged by the emerging discoveries of empathy for pain or distress in rodents. Because empathy is believed to be fundamental to the formation of prosocial, altruistic, and even moral behaviors in social animals and humans, studies associated with decoding the neural circuits and unraveling the underlying molecular and neural mechanisms of empathy for pain or distress in rodents would be very important and encouraging. In this review, the author set out to outline and update the concept of empathy from the evolutionary point of view, and introduce up-to-date advances in the study of empathy and its neural correlates in both humans and rodents. Finally, the author highlights the perspectives and challenges for the further use of rodent models in the study of empathy for pain or distress.
Keywords: Empathy, Pain, Distress, Neural correlates
Introduction
Although known for a long time, it has not been documented until recently that pain can be modulated by social factors that may affect both the development and maintenance of chronic pain and the outcomes for patients (pain sufferers) in clinical settings [1, 2]. As a consequence, it has been suggested that the term pain should be redefined as “a distressing experience associated with actual or potential tissue damage with sensory, emotional, cognitive, and social components”, which extends the official definition of pain by the International Association for the Study of Pain (IASP) [3]. The IASP’s official definition of pain stated that “pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”[4]. A sentence was added later to the IASP’s definition, emphasizing that “the inability to communicate in no way negates the possibility that an individual is experiencing pain and is in need of appropriate pain relieving treatment” (IASP Newsletter, 2001 (2), p2), but this clearly neglected the social and cognitive components of pain per se. However, emerging evidence has demonstrated that positive social support from a healthy spouse, kinship, colleagues, and friends or the establishment of a realistic physician-patient rapport is beneficial to the relief of pain due to social buffering, whereas social transfer of negative emotions from an unhealthy spouse/kinship, bad social relationships, and physician-patient conflicts exacerbate the severity of pain due to social stress [1, 2].
These facts from clinical practice are very important cues for both basic and clinical pain researchers to reconsider the results from the past several decades and reshape experimental design and clinical practice into a ‘bio-psychosocial-behavioral’ paradigm. A social communication model of pain proposed recently by Craig attempted to highlight the chronological process of interactions among the pain event, suffering person, and caregiver (physician/nurse/other) in terms of historical and current biological and social determinants imprinted in the brain of a patient [5] (for details see [6]). In that model, at least three major social players who hold different points of view are involved in the process of the pain problem: the patient, patient’s kinship, and physicians (and nurses/hospital staff). The English word patient originally meant ‘one who suffers’ and comes from the Latin word ‘patiens’. The modern meaning of a physician is a medical professional practitioner (also called medical doctor or doctor) who is tightly associated with promoting, maintaining, or restoring the health of patients through research-based diagnosis and treatment of disease, injury, and other physical and mental impairments. From the social and psychological points of view, each player has his/or her own empathic ‘bubble’ that exists separately. However, from the evolutionary point of view, the empathic bubbles of patient and patient’s kinship can soon fuse to form a larger empathic bubble to provide shared feeling and understanding, and motivations of caring and helping for the sufferer due to in-group empathy for pain and distress. Because empathy is thought to be produced only among familiars, the physician’s ability to empathize during patient/patient’s kinship-physician interactions would be very important and necessary for establishing a physician-patient rapport, a fusion of the empathic bubbles of the patient/patient’s kinship and the physician (and nurses/hospital staff). This proposition is strongly supported by a well-designed experimental report showing that a physician’s high perspective-taking scores (skills) are highly correlated with increased activity of the rostral anterior cingulate cortex (rACC) during patient-physician interaction [7], an area that has been demonstrated to be a key brain region for the mediation of both vicariously felt pain (empathy for pain) and emotional response to directly experienced pain in both humans [8–10] and animals [11–15](for review see [16]).
Empathy was traditionally thought to be a characteristic unique to human beings. However, this notion has been strongly challenged in recent years, because empathy for pain and fear and prosocial altruistic behaviors have also been found in both non-human primates and rodents (for commentaries and reviews see [1, 2, 17–25]). The discovery of empathy for pain or distress in rodents is of particular importance and critical for understanding the brain mechanisms underlying empathy, prosocial behaviors and altruism, the root of morality in humans at both the molecular/cellular and neural circuit levels [1, 2, 11, 20, 21, 23–27]. In this review, I outline the concept of empathy and advances in the findings of neural correlates of empathy in humans and animal models in experimental studies of empathy for distress or pain in rodents. Some of the ideas about empathy in animals have been published in Chinese [28].
Definition of Empathy: History and Update
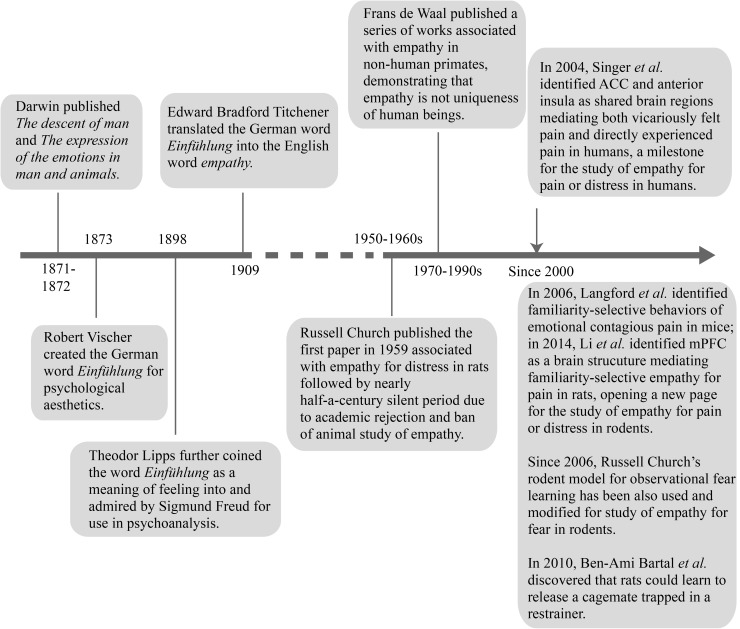
The English word “empathy” was used in translation from the German word Einfühlung with the meaning of ‘feeling into’ by Edward Bradford Titchener (1867–1927), a British psychologist, in 1909. Theodor Lipps (1851–1914), an influential German philosopher, is believed to have coined the word Einfühlung which was initially created by Robert Vischer (1847–1933), a German philosopher, in 1873. The etymology of the word “empathy” is rooted in the ancient Greek word empatheia, meaning ‘physical affection or passion’ which is derived from en (‘in’ or‘at’) and pathos (‘passion’ or ‘suffering’). In the last century, the study of empathy developed very slowly and the term was mainly adopted in the fields of social science, psychology, the practice of psychoanalysis, and non-human primate ethology [17, 29, 30]. However, in the past decade, empathy has suddenly become a topic of interest in the field of neuroscience due to advancing progress in neuroimaging studies of brain responses associated with one person’s empathy for another’s pain or distress (for reviews see [31–35]). The timeline of historical and current ideas about empathy is shown in Fig. 1 and Box 1.
Fig. 1.
Timeline of historical and current ideas about empathy.
Box 1.
Definitions of empathy
| The definition of empathy has long been under debate since it appeared with an academic meaning more than one hundred years ago, probably due to different interpretations of its meaning by scholars from different fields, ranging from philosophy (human-natural esthetics) to social science, psychoanalysis, psychology, and ethology by means of empirical and phenomenal observations, and finally has been narrowed down experimentally to brain science and affective (emotional) neuroscience in terms of biopsychosocial or biobehavioral brain functions due to neuroimaging discoveries of some brain areas involved in it [17, 30–35]. However, the current definitions of empathy are confounding and incomplete due to neglect of its evolutionary root by academic fields, with a long-existing traditional notion that empathy is only possessed by humans and non-human primates in terms of affection and emotion. This traditional notion has been greatly challenged by new advances in discoveries of empathy for pain or distress in rodents [1, 2, 11, 20, 21, 23–28]. Historically, in the domain of social science and psychology, empathy was simply defined as a unique ability or capacity of human beings to feel and understand other’s emotional state for which theoretical models of theory-theory, theory of mind, mentalizing/mindreading, and perspective-taking have been proposed to underlie the development of empathy in humans [32]. However, from the neuroevolutionary point of view, empathy has been defined as ‘an integrated affective response stemming from the perception of another’s emotional state or condition similar to what the other person is feeling or would be expected to feel in the given situation’ in terms of ontogeny, phylogeny, neuroevolution, brain mechanisms, context, and psychopathology [22, 34]. Empathy is also believed to consist of the capacity to (1) be affected by and share the emotional state of another; (2) assess the reasons for the other’s state; and (3) identify with the other, adopting his or her perspective; and finally these empathic responses motivate one’s empathy-based altruism, leading to sharing, caring, and helping in facing to another’s distress or pain [17]. However, neither of the above ideas and definitions about empathy can be fully accepted at the moment due to neglect of its evolutionary root. Although rodents have been shown to have behaviors associated with empathy for pain or distress [11, 26] and prosocial altruistic behaviors such as motivation for helping to release a trapped cage-mate from a restrainer [36, 37] and cooperative behaviors for food-seeking [38, 39], further well-designed laboratory work is still required to obtain support for the evolution of empathy from different species (lower animals to human beings) through natural selection. Because empathy has multiple facets associated with prosocial and social constructs that are hierarchical and expressed variously in terms of emotional contagion, social attachment, social bonding, affiliation, empathic concern, perspective-taking, sympathy, altruism, and even morality [17, 30, 32, 35], it is not possible to precisely define it from the social and psychological points of view only (for an updated definition of empathy, see the text). |
In a seminal paper, Singer and colleagues have demonstrated that empathy for pain (pain observed or felt vicariously by a lover) selectively activates her bilateral anterior insula, rACC, and other areas that overlap with areas involved in mediation of the affective component of pain directly experienced by her lover, whereas the sensory component of pain such as the somatosensory cortex is not activated, implying for the first time that empathy is a function of the brain [8]. From a meta-analysis of many functional magnetic resonance imaging (fMRI) studies, it has been concluded that a core network that includes the bilateral anterior insular cortex and medial/anterior cingulate cortex is associated with empathy for pain, although different results might occur under different contextual or cue-based conditions [10]. These neuroimaging studies no doubt shed new light on the old concept of empathy and opened a new venue for understanding the neural mechanisms of empathy [31, 33, 35]. As a strong line of evidence supporting an evolutionary view of empathy, we demonstrated for the first time that the medial prefrontal cortex (mPFC), which includes prelimbic cortex (PrLC), infralimbic cortex (ILC), and the anterior cingulate cortex (ACC), is involved in the mediation of empathy for pain or distress in rats based on lesion studies [11] (details below). Moreover, prosocial altruistic behaviors have also been found in rodents [36–39] (details in Box 1).
Based upon the emerging and cumulative experimental evidence from both humans and lower animals, empathy can be redefined as an evolutionary behavior of social animals and humans associated with prosocial reciprocity, altruism, and morality by the ability and capacity to feel, to recognize, and/or to understand the emotional states of others. This new definition may bridge the gap revealed by Zaki and Ochsner [40] who pointed out that the existing neuroimaging studies only focus on the imaged brain activity in response to the other’s emotional state without behavioral observations. Through validation of the rodent models of empathy, the gap caused by the complexity of empathy which has been produced by historical, theoretic, and methodological disparities in the two major fields of empathy study, psychology and neuroscience, could be bridged through studies of the underpinnings of empathy in terms of bio-psychosocial-behavioral paradigms across species [21]. The updated definition of empathy proposed here has an attempt to: (1) acknowledge the evolutionary issue of empathy in the frame of biology but not pure psychology; (2) endow empathy as a behavior of all social animals and humans; (3) focus on the roles of brain functions in the development of empathy associated with multidimensional components including sensory, emotional, cognitive, and executive (motor action and decision-making) issues; and finally (4) put the empathy back into biology as a root of the prosocial reciprocity, altruism, and morality in human beings.
Affective (Emotional) and Cognitive Empathy in Humans
Empathy is believed to be the fundament or root of prosociality, altruism, and morality in humans [17–19, 35]. It has multidimensional facets in terms of emotional contagion, social attachment, social bonding, affiliation, empathic concern, sympathy, perspective-taking, theory of mind, empathic altruism, and altruistic and moral behaviors. Thus it is difficult to classify empathy in terms of philosophy, social science, and even psychology without recruiting the power of brain science.
To see whether different brain regions are involved in the mediation of different levels of empathy, namely (1) emotional empathy (‘I feel what you feel’) that can be taken into account as primitive empathy (e.g., emotional contagion) and (2) cognitive empathy (‘I understand what you feel’) that can be considered as advanced empathy, a clinical data-based analysis was carried out on patients with CT- and MRI-identified brain lesions [41]. After structural identification of the areas of the lesions, the patients were assessed for the two levels of empathy using tools for both a basic emotional contagion system and a more advanced cognitive perspective-taking system. The basic emotional contagion system was used to assess patients’ skills in an emotion recognition task consisting of 52 photographs of eyes reflecting 14 basic and complex emotions (happy, sad, afraid, surprised, distressed, disgusted, angry, interested, worried, confident, fantasizing, preoccupied, friendly, and suspicious). The cognitive perspective-taking system was used to assess patients’ skills in a second-order false belief task that evaluates ‘Theory of Mind’, namely, one’s ability to understand what someone else (A) thinks about what someone else (B) thinks. Based on this well-designed clinical analysis, it has been found that emotional empathy is mainly mediated by the inferior frontal gyrus (Brodmann area 44, equivalent to Broca’s area) and cognitive empathy is mainly mediated by the ventromedial prefrontal cortex (vmPFC, Brodmann areas 10 and 11) [41]. It is well known that the emotional state of humans and animals can be evolutionary and well expressed by facial expressions, vocalization, laughter, crying, groaning, moaning, and even spoken language [42–44], thus the involvement of Broca’s area (motor language center or Brodmann area 44) in emotional empathy is scientifically acceptable. As for the involvement of the vmPFC (Brodmann areas 10 and 11) in cognitive empathy, it is also scientifically explainable because the mPFC, which includes the rACC and limbic cortex (referred to as PrLC and ILC in rodents), has wide-spread afferent and efferent connections in the brain and is involved in the mediation of many higher cognitive and emotional functions including attention, self-awareness, spontaneous thought, consciousness, mindfulness, recognition, affection, decision-making, learning, and memory [45–53]. The mPFC is also involved in the modulation of directly experienced distress (pain, fear, and catastrophe) [16, 54–58] and vicariously felt or observational pain or distress in both human beings and rodents [8, 11], implicating the existence of a core brain network underlying empathy for distress or pain [10]. The discovery of the two separate neural systems responsible for the two levels of empathy—emotional and cognitive—is very important for further understanding the ontogeny, phylogeny, and evolution of empathy in bio-psychosocial-behavioral terms. Under this framework, emotional (affective) empathy is believed: (1) to be mediated anatomically by the inferior frontal gyrus (Brodmann area 44) which contains unimodal dysgranular cortex in cytoarchitectural appearance; (2) to occur in human infants ontogenetically, and in rodents, birds, and probably other lower social animals phylogenetically; (3) to be behaviorally expressed as emotional contagion, personal distress, empathic concern, and emotional recognition; and (4) to be explained by a theory of simulation such as the perception-action model proposed by Preston and de Waal [30]. Meanwhile, cognitive empathy is believed: (1) to be mediated anatomically by the vmPFC (Brodmann areas 10, 11) which contains heteromodal granular cortex in cytoarchitectural appearance; (2) to occur in children and adolescents ontogenetically, and in non-human primates (chimpanzees, bonobos, and probably other higher social animals) phylogenetically; (3) to be expressed as perspective-taking, imagination of emotional future outcomes, and theory of mind (ToM) behaviorally; and (4) to be explained by theories of mentalizing/mindreading and ToM models [32]. The discovery of the two separate neural systems responsible for the two levels of empathy is also very important for further understanding of the ontogeny, phylogeny, and evolution of empathy across different species, especially in laboratory animals such as rodents, by means of genetic, epigenetic, and optogenetic approaches.
Core Neural Networks Associated with Empathy for Pain or Distress in Humans
In The Descent of Man [42], one of his most important works, Darwin stated that ‘many animals, however, certainly sympathise with each other’s distress or danger’ (p 126) and ‘as man is a social animal, it is almost certain that he would inherit a tendency to be faithful to his comrades, and obedient to the leader of his tribe; for these qualities are common to most social animals’ (pp 132–133). This was clearly the first description of the common phenomenon of empathy for pain or distress in social animals in the history of biological science (Fig. 1). The term ‘sympathy’ is derived from the Greek words syn (‘together’) and pathos (‘feeling’) which means ‘fellow-feeling’ and coined by Adam Smith in his book The Theory of Moral Sentiments (1759). Notably sympathy and empathy are often used interchangeably, but sometimes have different meanings in modern time. As noted above, “to empathize” (the verb of empathy) has the meaning “to feel, to understand, and to share” the other’s emotions of both positive (happiness and joyfulness) and negative states (angriness, sadness, sorrowfulness, fear, and panic), however, “to sympathize” (the verb of sympathy) only means “to feel and to understand” but not “to share” the other’s negative emotional state caused by trouble, misfortune, pain, distress, and catastrophe. Namely, empathy can motivate altruistic help behaviors regardless of whether it is dangerous or not, while sympathy cannot (I am sorry but…). This is because empathy that is associated with emotional responses to both the positive and negative emotions of other people is most likely to occur among familiars but not strangers as Darwin stated: “…, with all animals, sympathy is directed solely towards the members of the same community, and therefore towards known, and more or less beloved members, but not to all the individuals of the same species.” (p 130). This familiarity-based empathy has also been confirmed by many observations carried out in communities of non-human primates, such as chimpanzees and bonobos [17–19, 30] and rodents [1, 2, 11, 26]. In contrast, sympathy that is specifically associated with emotional response to other people’s negative emotional states is likely to occur among both familiars and strangers. Because the Descent was first published in 1871, the English word ‘empathy’ had not appeared in the literature before it was translated from the German word Einfühlung into English in 1909. What Darwin stated clearly intended the meaning of empathy, but not the real meaning of sympathy, because he also continued that [42]:
with mankind, selfishness, experience, and imitation, probably add, …, to the power of sympathy; for we are led by the hope of receiving good in return to perform acts of sympathetic kindness to others; and sympathy is much strengthened by habit. (p 130).
The social animals which stand at the bottom of the scale are guided almost exclusively, and those which stand higher in the scale are largely guided, by special instincts in the aid which they give to the members of the same community; but they are likewise in part impelled by mutual love and sympathy, assisted apparently by some amount of reason. (p 133).
As indicated by James Moore and Adrian Desmond in the Introduction to the 2nd edition of the Descent, Darwin was trying to explain the origin of morality and compassion in humans and believed that ‘sympathy’ (empathy in real meaning) and selflessness had more value than competition for ‘group selection’— the tribe as a whole competes, not the individual. Darwin’s evolutionary view of the roles of empathy, sympathy, prosocial, and altruistic behaviors in the emergence of human morality has been followed and backed up by many lines of updated evidence [17–19, 30, 35]. However, where does empathy come from?
In the first neuroimaging study [8], 16 couples were recruited to serve as paired subjects for the investigation of pain-related empathy. Using fMRI, brain activity was recorded and imaged in the female partner who could observe through a mirror which reflected the process of pain produced by painful electrical stimulation of her own and the male partner’s right hand. When she was experiencing pain herself, activity increased in the contralateral primary somatosensory cortex (S1)/primary motor cortex (M1), bilateral SII, bilateral anterior insula and ACC. However, when she was observing pain of her lover, activity only increased in the ACC, bilateral anterior insula, and inferior prefrontal cortex, cerebellum, and brainstem but without activation of S1 and SII. This result is interesting and inspiring because it indicates that the same brain areas (ACC and anterior insular cortex) are activated by both vicariously felt pain (empathy for pain or distress) and directly experienced pain [8]. Moreover, a comparison was made between the brain activities and the values assessed by the Empathic Concern Scale of Davis and the Balanced Emotional Empathy Scale of Mehrabian and the results showed that the activation level in the ACC and the anterior insula was highly correlated with the empathy-related scores measured immediately after the fMRI scan [8]. After repeated fMRI studies, a core network that included the anterior insular cortex and ACC was confirmed to be selectively associated with empathy for pain or distress, although different results might occur under different contextual or cue-based conditions [10]. For example, when viewing pictures of body parts in painful situations (picture-based studies), brain areas including the anterior insula, dmPFC, dlPFC, inferior frontal gyrus, premotor cortex, inferior parietal cortex, as well as S1/SII were strongly activated [10]. On the other hand, in cue-based studies in which one person witnessed another’s painful state, brain areas including the vmPFC, precuneus, superior temporal gyrus, and temporo-parietal junction were strongly activated [10]. The distinct activation of brain areas by picture-based and cue-based paradigms is interesting but remains to be further studied. Collectively, based on the results of meta-analysis of fMRI studies, it has become clear that familiarity-based empathy for pain or distress is likely to be mediated by the ACC/MCC and insular cortex, while cue-based and picture-based sympathy for painful or distressful events activates different areas associated with either mentalizing/ToM or action understanding, probably reflecting the complexity of empathy and/or sympathy across different levels. The anterior insular cortex is an intriguing area which has also been shown to be involved in the observational response to another’s disgust [31–33, 59]. Interestingly, the empathy-related activity in the ACC and anterior insular cortex has also been demonstrated to be modulatable by cultural context [60]. When observing painful stimulation applied to racial in-group faces, activity in the ACC, inferior frontal, and insular cortex was significantly increased in both Caucasians and Chinese participants; however, the empathic neural response in the ACC was significantly decreased when participants viewed the faces of other races [60]. This racial in-group/out-group bias in empathy for pain can be enhanced by the intranasal administration of oxytocin [61], a neuropeptide essential for social behaviors such as empathy, mother-child bonding, social cognition/recognition, affiliation, attachment, and sociability in humans [62–68].
Rodent Models of Empathy for Pain or Distress
Can rodents or other lower animals have empathy, sympathy, or altruistic behaviors? Can they feel, understand, and even share others’ pain or distress? These questions have not been touched for more than a century due to silence in the field of scientific research. As noted above, Darwin provided many examples for the existence of empathy/sympathy for pain or distress in social animals [42, 43], however, unfortunately the study of empathy from the evolutionary point of view was neglected or ignored for more than a century, probably due to the influence of theism, racism, and sexism. To the best of our knowledge, the scientific taboo was also largely caused by the traditional notion that endowing animals with human emotions was undesirable and taboo. Nonetheless, in the last half of the last century and especially in more recent years of the new century, emerging evidence from both non-human primates [17–19] and lower animals including rodents [11, 26, 69, 70], birds [71–73], and even ants [74] has provided support for establishing the idea (notion) that human empathy, sympathy, altruism, and even morality may result from biological evolution [1, 2, 17–25, 34, 35]. Here, we provide an overview or outline of the advances in the study of empathy for pain or distress in rodents. Distress can be caused by both physical and spiritual pain or suffering. The available rodent models of empathy for distress are divided into two types –empathy for pain and empathy for fear—based on different experimental paradigms (data on familiarity-based empathy for distress are summarized in Table 1).
Table 1.
Familiarity-based empathy for pain or distress in rodents.
| Species (strain, sex) | Dyads | Experimental design | Nociceptive stimulus | Time for testing | Observer’s empathic responses | Mechanisms | Sources | |
|---|---|---|---|---|---|---|---|---|
| Empathy for pain | Rat (SD, M) | Cagemate/Cagemate Non-cagemate/Non-cagemate | Social interaction for 30 min and only partner in pain | s.c. bee venom (0.2 mg in 50 μL) | 30 min after social interaction | Familiarity-selective: PWMT: ↓ PWTL: - Increase in paw flinching |
Increased c-Fos expression in spinal cord Blocked by chemical lesion of bilateral mPFC |
[11] |
| Mouse (CD-1, M/F) | Cagemate/Cagemate Non-cagemate/Non-cagemate | Social interaction for 30 min and both animals in pain | i.p. acetic acid (0.9%) or s.c. formalin (1% or 5%) | During social interaction | Familiarity-selective: PWMT: n.a. PWTL: ↓ Increase in writhing or paw flinching |
Blocked only by visual blockade Inducible by suppressing glucocorticoid synthesis in strangers |
[26] [95] |
|
| Empathy for fear | Rat (Wistar, M) | Cagemate/cagemate Cagemate/cagemate Non-cagemate/non-cagemate |
Social interaction for 10 min with recent fear conditioning cagemate in home cage | FC by foot-shocks (9×1 s, 55 s ITI, 1.3 mA, 50 Hz) | During social interaction | Increased acoustic startle response to 20×110 dB white-noise (20 ms pulse with 2 ms rise-time) and increased exploration; Increased sniffing and allogrooming; Enhanced acquisition of avoidance response and conditioned freezing (memory) in familiar and unfamiliar dyads; Unchanged thermal pain sensitivity. |
Increased c-Fos expression in lateral and central amygdala | [77] [78] |
| Rat (Long-Evans, M/F) | Cagemate/cagemate M/M OF/OF EF/EF |
Social interaction for 10 min with recent fear conditioning cagemate in the home cage | As above | During social interaction | Similar social exploratory behaviors among M, OF and EF; Enhanced acquisition of avoidance response in M and OF but not in EF. |
Increased c-Fos expression in lateral and central amygdala and mPFC (PrLC and ILC) only in male and not female of either OF or EF. | [82] | |
| Rat (SD, M) Rat (SD, F) Rat (SD, M/F) |
Cagemate/cagemate Co-housed sisters for 180 days/co-housed non-sisters for 7 days Cagemate/cagemate Dominance/subordinate |
On day 1, a demonstrator was fear conditioned (FC), on day 2, an observer was paired with FC+CS followed by social interaction with each other in home cage for 24 h | FC produced by 3×20 s 80 dB tones co-terminating with foot-shock (3×0.5 s, 180 s ITI, 0.7 mA, 5 Hz) | On day 3, long-term memory test performed with CS | Increased freezing in response to mild CS in 50% in observer Long-term co-housed sisters showed more social contact and freezing than briefly co-housed non-sisters Subordinate showed more freezing when socially interacting with FC dominant conspecific |
n.a. n.a. Increased c-Fos activity in ACC, lateral amygdala, and ventral hippocampus |
[83] [84] [85] |
|
| Rat (SD, M) | Cagemate/cagemate | Social interaction between naïve-receiver and FC-sender or between fear-experienced receiver (3 unsignaled foot-shocks: 1 s, 60 s ITI, 1 mA) and FC-sender or between fear-experienced receiver and naïve-sender | FC produced by 10×20 s tones (82 dB, 2.9 kHz) co-terminating with foot-shock (10×1 s, 120 s ITI, 2 mA) | 24 h after social interaction in the home cage | Increased freezing in fear-experienced receiver (observer) but not naïve-receiver in response to FC-sender’s 22kHz ultrasonic vocalization induced by 8 min tone CS | Blocked by electrical lesion or lidocaine infusion at bilateral MGN of fear-experienced receiver | [86] | |
| Rat (Long-Evans, F) | Cagemate/cagemate | Social interaction for 40 min between naïve-witness and a distressed demonstrator or between fear experienced witness (foot-shocks: 4×1 s, 240–360 s ITI, 0.8 mA and 1 s, 0,8 mA, every 24 h) and a distressed demonstrator | Produced by foot-shocks (5×5-s, 120–180-s ITI, 0.8 mA) | During witnessing a distressed demonstrator 24 h after consolidation of fear memory following 2-day foot-shock training | Increased freezing and decreased locomotor activity in fear experienced witness but not naïve observer | n.a. | [87] | |
| Rat (SD, M) | Cagemate/cagemate Post-weaning social isolated/ post-weaning social isolated |
Social interaction with an FC distressed demonstrator | FC produced by 8 tones (2000 Hz, 20 s) co-terminating with foot-shocks (8×1 s, 80 s ITI, 0.5 mA or 0.8 mA,) | During social interaction with an FC demonstrator and 24 h after acquisition of observational fear learning | Cagemates showed more freezing time than post-weaning socially-isolated conspecific | Impaired observational fear learning in post-weaning socially-isolated observer | [88] | |
| Rat (Wistar, M) | Cagemate/cagemate | Two naïve subjects co-housed with two chronic stress-experienced cagemates for 15 days | Stress produced by exposure to 8×4 h white noise (100 dB) during 15 days | 15 days after social interaction with chronic stress-experienced cagemates | Increased level of plasma cortisol and anxiety | n.a. | [89] | |
| Rat (Wistar, M) | Cagemate/cagemate Non-cagemate/non-cagemate |
FC subject was allowed to communicate with familiar or unfamiliar odorized neighboring chamber for 1 h on day 1 and 30 min on day 2 | FC produced by 7×3 s tone (65 dB, 8 kHz) co-terminating with foot-shocks (7×0.5 s, 50–270 s ITI, 0.65 mA) | Fear expression test performed on fear-experienced subject by 5×3s CS after odorization | Social buffering of conditioned fear learning obtained in subject odorized by familiar conspecific, resulting in less freezing and more sniffing and walking | Suppression of FC-induced c-Fos expression in PVN and amygdala | [90] | |
| Mouse (C57BL/6 vs BALB/cJ, M/F) | Non-cagemates Demonstrator was F1 offspring of C57BL/6×BALB/cJ |
On day 1, two observers from both C57BL/6 and BALB/cJ were exposed to two distressed demonstrators with CS-UCS contingency; on day 2, the same FC procedure was repeated | FC produced by 10×120 s trials randomly sequenced by (1) 30 s CS (85 dB, 1 kHz) co-terminating with UCS foot-shock (2 s, 120 s ITI, 0.5 mA), (2) 30 s tone followed by 2 s UCS, (3) only 2 s UCS, and (4) only 30 s CS. | Immediately after day 2 FC procedure, observer received 5×120s CS followed by 5×120s CS-UCS contingency | C57BL/6 strain showed more freezing and social approach than BALB/cJ strain | n.a. | [79] | |
| Mouse (C57BL/6, M/F) Mouse (11 inbred strains: C57BL/6J, C57BL/6NTac, 129S1/SvImJ, 129S4/SvJae, BTBR T(+) Itpr3(tf) /J, AKR/J, BALB/cByJ, C3H/HeJ, DBA/2J, FVB/NJ and NOD/ShiLtJ) |
Siblings/nonsiblings Male/female mating partner |
On day 1, social interaction between a naïve observer and a distressed demonstrator with foot-shock; 24 h after FC, the observer was placed back to the same chamber to acquire observational fear learning | FC produced by 240 s foot-shock (24×2 s, 10 s ITI, 1 mA) | (1) During social interaction with distressing conspecific; (2) 24 h after being placed back to same chamber (contextual cue) |
When sibling and female mating partner (co-housed >10 weeks) served as distressed demonstrator, observer showed more freezing in both testing sessions | (1) Partially blocked by visual blockade; (2) Significantly blocked by unilateral lesion of medial pain system (PF/MD-ACC) and lateral amygdala, but not lateral pain system (VPL/VPM-S1) (3) Cav1.2 VGCC subunit involved Variability in empathy for fear and C57BL/6J, C57BL/6NTac, 129S1/SvImJ, 129S4/SvJae and BTBR T(+) Itpr3(tf) /J showed more observational fear responses, however, AKR/J, BALB/cByJ, C3H/HeJ, DBA/2J, FVB/NJ and NOD/ShiLtJ mice showed low empathic fear response |
[80] [81] |
|
| Mouse (C57BL/6, M) | Cagemate/cagemate | On day 1, subjects acquired FC with 5×CS-UCS in context A (cleaned by 1% acid) and extinguished with 6×10 CS in context B (cleaned by 1% ammonium hydroxide); on day 2, tested with 10×CS | FC produced by 5×20 s CS tones (85 dB, 5 kHz) co-terminating with foot-shocks (5×1 s, 0.6 mA) | During extinction test on day 1 and during renewal CS test on day 2 in the presence or absence of a distressed cagemate | In the presence of a distressed cagemate, extinguished fear rekindled by 10×20s CS tones | n.a. | [91] | |
| Mouse (C57BL/6, M) | Cagemate/cagemate Co-housed for >8 weeks |
Three-chambered test: (1) Social approach choice: restrained mate vs empty or free-moving mate vs empty or restrained vs free-moving mate; (2) Social approach choice after alternate social interaction with restrained or free-moving mate for 7 days (3) Social approach choice after alternate social interaction with mate in pain or mate without pain for 7 days |
Restrained Restrained s.c. injection of 4% formalin |
30 min observation after training | Subject mouse spent more time in restrained or free-moving cagemate relative to empty chamber, however, it spent almost the same time with restrained and free-moving mates More cagemates (60%) spent more time with restrained relative to free-moving mate Subject mouse spent more time with mate in pain relative to mate without pain |
n.a. | [92] | |
| Mouse (C57BL/6J, M) | Cagemate/cagemate Non-cagemate/non-cagemate |
Social interaction between an observer and a distressed demonstrator during FC | FC produced by 240 s foot-shock (1 s, 10 s ITI, 0.5 mA) | Baseline (0–5 min), during foot-shock test (6–9 min) and recovery (10 min) recorded. | Familiar observer showed more freezing than unfamiliar | n.a. | [93] |
↓, decrease; -, no change; cagemate, co-housed for ≥2 weeks; non-cagemate, housed in different cages; isolated, housed singly
ACC, anterior cingulate cortex; CS, conditioning stimulus; EF, estral female; F, female; i.p., intraperitoneal; ILC, infralimbic cortex; ITI, intertrial interval, M, male; MD, mediodorsal nucleus of the thalamus; MGN, medial geniculate nucleus of the thalamus; mPFC, medial prefrontal cortex; n.a., not available; OF, ovariectomized female; FC, Pavlovian contextual fear condition; PF, parafasciculus nucleus of the thalamus; PrLC, prelimbic cortex; PVN, paraventricular nucleus of hypothalamus; PWMT, paw withdrawal mechanical threshold; PWTL, paw withdrawal thermal latency; s.c., subcutaneous; SD, Sprague-Dawley; UCS, unconditioned stimulus; VPL, ventral posterolateral nucleus of the thalamus; VPM, ventral posteromedial nucleus of the thalamus
Historically, experimental investigations of empathy for pain or distress in laboratory rodents appeared transiently in the middle of the last century [75]. In the first experimental report by Russell Church [69], rats were trained to obtain food by pressing a lever. Then, while a rat (perceiver or observer) was trying to press the lever to get food, another rat (stimulator or demonstrator), which could be seen by the observer rat, was given an electrical shock from the electrified cage floor. It was found that the ‘observer’ rat stopped pressing the lever while witnessing its conspecific suffering from pain or distress. This behavior was explained as empathic feeling and caring of rats in response to pain or distress of a conspecific. Another pioneering work also showed that a rat spent more time trying to rescue a conspecific by releasing it from a suspended state and this was explained as altruistic behavior [70]. It is said that the topic of empathy for pain or distress and altruism in animals existed briefly in the 1960s and then disappeared due to academic rejection by the prevailing behaviorists. After nearly 50 years, a report entitled ‘Social modulation of pain as evidence for empathy in mice’ authored by Langford et al. [26] was published in the journal Science; this is thought to mark the opening of a new page for the study of empathy in animals [20, 75, 76]. The work did not result in immediate attention from the field of neuroscience but was greatly appreciated by world-renowned ethologists and primatologists [20, 75]. Eight years later, the empathy for pain or distress was examined and confirmed in rats by our group [11]. On the other hand, Russell Church’s rat model of empathy for distress was modified and used in rats and mice referred to as an empathy for fear or ‘observational fear learning’ model [77–93]. The literature associated with empathy for fear is summarized in Table 1 in which only familiarity-related studies are included, although most of the reports had no stranger controls. Because rats and mice are laboratory animals and their behavior can be modified or modulated by genetic, epigenetic, optogenetic, and pharmacological approaches, models of empathy in rodents have become increasingly attractive and appreciated as valuable for the study of empathy and its underpinnings, the ‘neuroscience of empathy’ suddenly emerged as a ‘hot’ topic last year [1, 2, 20, 21, 23–25].
Emotional Contagious Pain Transferred through On-Site Social Interaction
In the mouse model from Jeffery Mogil’s lab at McGill University, Canada [26] (see Table 1), emotional contagion of pain can be studied by real-time (on-site) social reciprocal interactions between a pair of mice in pain. Now it is generally accepted as a model for the study of empathy for pain because social transfer of pain was only identified between cagemates (familiars, co-housed for more than two weeks), but not between non-cagemates (strangers). The discovery of familiarity-based social transfer of pain in mice is consistent with the current conceptual account of emotional empathy [17–21, 30–35, 41] (also see [42, 43]). In that model, a pair of sex-matched CD-1 mice was placed into a transparent Plexiglas cylinder (15 cm diameter; 22.5 cm high) in which the dyadic mice could move freely and socially interact by perception and reciprocal physical contacts on a ¼-inch-thick glass floor. The pain behaviors were recorded by a video-camera system and analyzed and quantified using off-line software. The experiment was designed as: (1) group 1, both of the paired mice received an intraperitoneal (i.p.) injection of 0.9% glacial acetic acid in a volume of 10 mL/kg and the writhing behavior occurred concurrently. Under this condition, both mice served as Observer and Demonstrator; (2) group 2, only one mouse received an injection of acetic acid and served as Observer, while the other remained untreated. It was found that the number of episodes of writhing—a sign of pain or distress—was significantly greater in ‘double-writhing’ mice than in the ‘single-writhing’ mouse. Because the social transfer of pain-related behaviors was only identified in cagemates and siblings (co-housed for >2–3 weeks) but not strangers, it was concluded that emotional contagion of pain exists in mice. The emotional contagious pain transferred through on-site reciprocal interactions was also confirmed in the formalin test in which 1% or 5% formalin (20 μL) was injected into the plantar surface of the right hind paw of paired mice using a 50 μL Hamilton microsyringe with a 30-gauge needle. The results showed that the number of paw flinches measured in the mouse with more pain (5% formalin) declined when socially interacting with a cagemate with less pain (1% formalin), while that measured in the mouse with less pain (1% formalin) increased when socially interacting with a cagemate with more pain (5% formalin), implying that the familiarity-based social transfer of pain-related behaviors might be bidirectional.
Moreover, the paw-withdrawal thermal latency (PWTL) measured using a radiant heat stimulator in ‘double-writhing’ cagemate mice was significantly shorter than that in a ‘single-writhing’ cagemate mouse, while thermal pain hypersensitivity did not occur in stranger mice, suggesting the existence of familiarity-based social transfer of thermal pain hypersensitivity through on-site interaction. However, changes in paw withdrawal mechanical threshold (PWMT) were not measured in that study and whether empathy-related mechanical pain hypersensitivity occurs is not yet known. It was also shown that facilitation of the spinally-organized nociceptive reflex by social reciprocal interaction only occurred in familiars, but not strangers. In sharp contrast, the PWTL measured in the stranger ‘double-writhing’ mice was significantly prolonged, suggesting occurrence of the stress-induced analgesia. However, the stress-induced analgesia in strangers only occurred in male dyadic mice and was testosterone-dependent [94].
To answer the question of why emotional contagion of pain does not occur in strangers, Mogil and colleagues examined the roles of social stress mediated by the hypothalamic-pituitary-adrenal (HPA) axis and found that pharmacological inhibition of glucocorticoid synthesis by metyrapone (50 mg/kg) evoked empathy for pain in both stranger (non-cagemate) dyadic mice and stranger people [95]. Combined administration of mifepristone (RU 486, 10 mg/kg), an antagonist of glucocorticoid receptors, and RU 26752 (5 mg/kg), an antagonist of mineralocorticoid receptors, was also effective in eliciting emotional contagion of pain in stranger mice. Moreover, shared gaming experience resulted in a decrease in the level of serum cortisol and elicited emotional contagion of pain in human strangers. These results provided a line of new evidence strongly supporting the idea that mice share the underpinnings of emotional contagion of pain with human beings.
Collectively, it is generally accepted that the mouse model of emotional contagious pain can be used to study the lower type of empathy, especially emotional contagion. However, further investigations of empathic behaviors influenced by emotional contagion of pain are encouraged, although some results have already been published. In a study of social approach to pain [96], CD-1 and oxytocin receptor-null mutant mice were used. In that study, only CD-1 female mice showed more approach behavior towards a cagemate in pain, while either CD-1 male or oxytocin receptor-null mutant mice did not. The explanation of that result of a failure in male social approach towards a cagemate in pain should be cautious because variability in empathic response has been found across 11 inbred strains of mice measured using an observational fear learning model (empathy for fear) [81]. Among the 11 inbred mice, five strains – C57BL/6J, C57BL/6NTac, 129S1/SvImJ, 129S4/SvJae, and BTBR T(+) Itpr3(tf)/J – showed greater observational fear responses; however, AKR/J, BALB/cByJ, C3H/HeJ, DBA/2J, FVB/NJ, and NOD/ShiLtJ mice showed lower empathic fear responses [81]. Although the CD-1 strain was not included in that study, it has been shown to be much more aggressive than C57BL/6J mice [97]. In a social defeat stress model, Zhang and colleagues used the CD-1 strain as an aggressive invader to cause social defeat stress in the paired C57BL/6J strain through 10 days of repeated aggression. The repeated aggression against a partner clearly showed that CD-1 mice may have a genetic background of more apathy but with less empathy. The difference in empathic response has also been reported in CD-1 mice in dominant/subordinate relationships [98]. In that report, two unfamiliar CD-1 mice were housed in a cage separated by a wire-mesh partition for 24 h. The partition was removed daily to allow the two mice socially interact for 10 min for a total of six days until a clear and stable dominant/submissive relationship was established by the criterion that the dominant status meant it attacked its partner without ever being attacked, while the subordinate status meant it was continuously attacked and defeated by its partner, showing fully defensive and submissive behavior. Then the dominant and subordinate mice were paired for testing under two conditions: (1) the dominant mouse received a 1% formalin injection while the subordinate did not receive any treatment; (2) the subordinate mouse received a 1% formalin injection while the dominant did not receive any treatment. The result showed that the dominant mouse spent less time staring at the subordinate in pain, whereas the subordinate mouse spent more time staring at the dominant mouse in pain, suggesting that the dominant/submissive status plays an important role in determining the level of empathic response in CD-1 mice. However, in a three-chamber test, a C57/BL6 mouse was shown to prefer to spend more time with its cagemate in pain (social approach) relative to a cagemate free of pain for the first choice, but repeated exposure of one mouse to a cagemate in pain resulted in aversive or avoidance behavior [92]. The difference in empathic response has also been reported in the model of empathy for fear, namely the C57BL/6J strain is more empathic than the BALB/cJ strain [79]. These results from different labs strongly support the idea that the level of empathic response in mice might vary according to different genetic backgrounds.
To answer the question of how pain is transferred from one mouse to another through social interaction, chemical lesions were used to destroy auditory and olfactory afferents, while an opaque wall was used to block visual communications between dyadic mice. The results showed that only visual blockade eliminated emotional contagion of pain [26] although it has been argued in a new report showing the involvement of olfactory cues in mediating the transfer of both mechanical and thermal pain hypersensitivity (hyperalgesia) from inflammatory or morphine/alcohol withdrawal conspecifics in pain to the bystander mice regardless of familiarity and unfamiliarity [99]. In that study, mice were not allowed to interact in a socially physical contact environment, but instead, they were housed and tested in the same bedding room where inflammatory or morphine/alcohol withdrawal conspecifics in pain had been housed. Moreover, a period of at least 24 h exposure to the bedding from hyperalgesic mice was shown to be essential for social transfer of pain hypersensitivity to other bystander mice, suggesting the involvement of an olfactory mechanism as well [99]. However, whether this type of social transfer of pain involves empathy is not known and requires further study.
Finally, what would happen if a mouse were co-housed with a cagemate in chronic pain or distress or with a brain disorder? Several studies have shown that mice co-housed with a cagemate with chronic neuropathic pain for two weeks [100] or with a cagemate suffering from epilepsy/or repeated electrical shock-induced distress for 6–8 weeks [101] resulted in both empathy impairment and emotional disorders such as anxiety, depression, and even cognitive impairment. Those results suggested that the brain functions of empathy for pain or distress might be changed by time, and social interaction with a patient suffering from chronic pain or distress or other chronic diseases might cause aversion or avoidance. This is also a common phenomenon experienced by humans, as stated in the Chinese proverb “There would be no filial piety at the bedside of a chronic patient”.
Empathy for Pain Produced through Priming Social Interaction
As noted above, empathy includes both emotional and cognitive forms. From the evolutionary point of view, both forms should be possessed across mammals. To exclude pure mimicry of behaviors transferred through on-site real-time social interaction, we re-designed the experiment in which dyadic Sprague-Dawley (SD) albino rats were placed in a transparent plastic observing box (30×30×30 cm3) for free social interaction for 30 min (referred to as priming social interaction, see [2]) before pain sensitivity and pain-related behaviors were evaluated [11] (also see Table 1). Briefly, after environmental acclimation and measurement of basal pain sensitivity, rats were randomly divided into four groups: (1) control, a pair of cagemate rats interacted freely within the testing box for 30 min but neither had any irritant treatment; (2) cagemate observer, a rat was allowed to interact freely with a cagemate demonstrator in pain produced by subcutaneous (s.c.) injection of bee venom (0.2 mg BV dissolved in 50 μL saline) (for detailed methods see [102, 103]; (3) non-cagemate observer, a rat was allowed to interact freely with a non-cagemate demonstrator in pain produced by s.c. BV injection; and (4) naïve rats housed in isolation and allowed to stay in the same testing box for 30 min alone. We found that after priming social interaction, the observer rat that had socially interacted with a cagemate in pain or distress showed a significant decrease in PWMT while PWTL remained unchanged. However, no significant changes in either mechanical or thermal pain sensitivity were found in non-cagemate observer rats which had also socially interacted with a stranger demonstrator in pain or distress. These results cannot be simply explained by emotional contagion of pain because when pain sensitivity was measured the demonstrator rat was absent and the observer rat was changed into a new testing box free of context and cues. The changes in mechanical pain sensitivity were not likely to be caused by social stress because there was no distinct change in the level of serum corticosterone among control, cagemate observer, and non-cagemate observer rats. Moreover, there was no significant difference in the anxiety-like behaviors between cagemate observer and non-cagemate observer although the two groups of rats showed more anxiety than control rats. Collectively, it is suggested that priming social transfer of mechanical pain hypersensitivity is familiarity-based empathy for pain in rats.
To determine whether the familiarity-based empathy for pain affects pain responses, s.c. BV injection was made into the plantar surface of one hind paw of rats from naïve, control, cagemate observer and non-cagemate observer groups. The results showed that the cagemate observer rats displayed more signs of pain such as paw flinches, licking, and lifting of the injected hind paw than the other three groups. Because it is well known that paw flinches induced by s.c. BV are mediated by spinally-organized nociceptive reflex circuitry and paw licking and lifting behaviors are mediated by supraspinal components such as the ACC [12] (for reviews see [102, 103]), studies of the underlying neural mechanisms at both the spinal and supraspinal levels became of particular importance. The enhanced pain-related behavioral response observed in the familiar (cagemate) observer but not the unfamiliar (non-cagemate) observer indicates that there must be changes in the brain and the spinal cord dorsal horn responsible for the process of empathy for pain. To test this assumption, we first evaluated c-Fos expression, a biomarker representing nociceptive neuronal activity, in the dorsal horn of the spinal cord after 30 min priming social interaction in rats from naïve, cagemate observer, and non-cagemate observer groups. Surprisingly, the c-Fos-labeled neurons in the spinal dorsal horn induced by s.c. BV injection were significantly increased within both the superficial (laminae I–II) and deep layers (IV–VI) in cagemate observer rats relative to non-cagemate observer rats. This for the first time indicated that empathy for pain can facilitate spinally-organized nocifensive or nociceptive reflex responses.
However, what brain regions are responsible for this social enhancement of pain? As indicated by our previous studies and by many neuroimaging studies in humans and animals [16, 54–58], the mPFC, amygdala, and entorhinal cortex – major cortical areas sending axonal projections to the dentate gyrus of the hippocampal formation through the perforant path – are candidates for examination. Using chemical lesions, it was shown that bilateral disruption of the mPFC eliminated both empathy-related pain enhancement and pain hypersensitivity; however, bilateral disruptions of the amygdala and entorhinal cortices did not influence empathy-related pain, strongly implying an essential role of the mPFC in mediating empathy for pain. The importance of this discovery lies in that it was the first evidence showing involvement of the central nervous system in the biological process of empathy for pain or distress in rodents since Darwin proposed it in 1871–1872 [42, 43]. Although the topic of whether there is empathy for pain or distress in rodents is still under debate across different research fields [27, 76], the study of empathy in laboratory animals such as rats and mice is very important because it provides at least a new experimental paradigm in terms of bio-psychosocial-behavioral models for the study of the sensory, emotional, cognitive, and social components of pain [1–3, 5, 6, 27]. As promoted by Frans de Waal, that “To endow animals with human emotions has long been a scientific taboo. But if we do not, we risk missing something fundamental about both animals and us.”[17], the study of empathy for pain or distress in laboratory animals has developed very rapidly and is expected to become a major branch of neuroscience in the near future [17–21, 23–25].
In addition to what has been discovered and published before [11], several results from our on-going unpublished experiments also support the evolutionary view of empathy. The results are as follows: (1) Familiar (cagemate) observer rats have higher levels of both circulating oxytocin in serum and oxytocin expression in the mPFC after 30 min priming social interaction with a familiar cagemate in pain but not in unfamiliar (non-cagemate) observer rats who witness a stranger in pain. As introduced above, oxytocin is a very important neuropeptide essential for empathy, the root of social behaviors such as mother-child bonding, social cognition/recognition, affiliation, attachment, and sociability in humans [62–68], the finding of increased levels of oxytocin associated with empathy for pain in familiar observer rats is very important for further establishing an evolutionary construct of empathy. (2) Intranasal administration of oxytocin elicits empathy for pain in stranger rats which had experienced 30 min priming social interaction with an unfamiliar (non-cagemate) demonstrator rat in pain. (3) Microinjections of an oxytocin receptor antagonist into the bilateral mPFC results in the complete elimination of empathy for pain in both cagemate observer rats and non-cagemate observer rats given intranasal oxytocin. (4) Both SD rats and C57/BL6 mice show social approach towards the cagemate in pain, but bilateral disruption of mPFC eliminates empathy-related social approach behaviors. Collectively, these unpublished data further add to the accumulating lines of evidence supporting the evolutionary root of empathy in humans.
Emotional Contagious Fear and Observational Fear Learning
As shown in Table 1, Russell Church’s rat model of empathy for fearful distress has been modified and used in rats and mice and is referred to as empathy for fear that includes emotional contagious fear and observational fear learning induced by Pavlovian contextual fear conditioning with different parameters for combinations of conditional stimulus (CS) and unconditional stimulus (UCS) [77–93]. Regardless of the various parameters used in different reports, a common pattern of conditioned fear was produced by a repetitive CS tone co-terminating with a distressful electrical foot-shock (UCS) (details in Table 1). The naïve observer animal displayed a freezing response while it was witnessing another distressed conspecific demonstrator receiving an electrical foot-shock. The time spent freezing was rated through off-line video recording analysis or recorded by experimenters. As for the observational fear learning test, the time spent freezing by the observer animal was recorded after it was recalled or retrieved by the CS tone only or after it was placed back in the same test chamber or box where a demonstrator had been fearfully conditioned by a coupled CS-UCS pattern. For example, Shin and colleagues from the Center for Neural Science, Korea Institute of Science and Technology, established a simple mouse model of empathy for fear that was used to study both emotional contagious fear and observational fear learning [23, 80]. In that conditioning setting, two male C57BL/6J mice, one in each component of a double-chambered apparatus, were separated by a transparent Plexiglas partition and one observer mouse was allowed to witness the other demonstrator mouse in pain or distress caused by repetitive electrical foot shocks (Table 1). The demonstrator mouse was allowed to habituate in one side of the two-chambered apparatus for 5 min and then received a period of 4 min training with 240 s foot shocks (24×2 s, 1 mA, 10 s intertrial interval). Then the time spent freezing was rated and it was assumed that the longer the time spent freezing, the stronger the empathic response for the observer mouse. The observation was composed of two parts: (1) freezing time spent by the observer mouse while witnessing a partner in distress on the training day, and (2) freezing time when the observer mouse was placed back into the same observing chamber 24 h after the training. The results showed that the observer mouse showed distinct freezing behavior while witnessing its partner in distress and this behavior was partially blocked by visual blockade with an opaque partition. Moreover, the observer mouse also showed distinct freezing behavior when being placed back into the same observing chamber 24 h after the training, but this behavior was not observed when it was placed in a new box, suggesting the existence of context-specific observational fear learning. Although this social transfer of fear was not specific to familiars, siblings and female mating partners caused longer freezing behavior in observer mice when they served as demonstrator in distress, suggesting partial involvement of empathy for fear and expressed as both emotional contagious fear and observational fear learning transferred through social stress [89, 93]. Furthermore, Shin and colleagues demonstrated that the emotional contagious fear and observational fear learning were mediated by the medial pain system, namely the parafascicular/mediodorsal thalamic nuclei-ACC pathway, but not by the lateral pain system (the ventral posterolateral/posteromedial thalamic nuclei-somatosensory cortical pathway), suggesting an overlap of neural circuitry between vicariously felt fear (observational fear learning) and directly experienced pain or distress. Moreover, the neural activity in the ACC has been shown to be synchronized with the theta rhythm frequency in the lateral amygdala during observational fear learning and the Cav1.2 subunit of the voltage-gated Ca2+ channel in the ACC is critically involved in the process, providing a molecular basis for the emotional contagious fear and observational fear learning.
It is interesting to note that the conditioned fear response, also expressed as freezing behavior, has been shown to be modulated by social interaction or communication and the phenomenon has been referred to as social buffering. In a series of studies by Kiyokawa and colleagues from the Laboratory of Veterinary Ethology, The University of Tokyo, the social buffering effect has been well studied using a well-designed experimental paradigm [90, 104–106]. In that model setting, the subject Wistar rat was first trained by 20 min conditioned fear stimulation through receiving 7 repetitions of a 3-s tone (8 Hz, 65 dB) that terminated concurrently with a foot shock (0.5 s, 0.65 mA). The rat was then placed into one side of two compartments (14×44×15 cm3 each) with a partition penetrated by 175 holes that allowed the fear conditioned rats to communicate with another conspecific of either a male or female cagemate or non-cagemate through olfactory signals so as to be socially buffered [107]. It was found that social buffering significantly ameliorated these conditioned fear responses of the subject rat through inhibition of the HPA axis [108]. Moreover, a familiar conspecific was shown to be more effective than an unfamiliar conspecific for social buffering of conditioned fear responses [90]. The social buffering is likely to be mediated by suppression of neural activity of the paraventricular nucleus of the hypothalamus and lateral amygdala which are involved in conditioned fear responses [108, 109].
Perspectives and Challenges
As Sir Karl Raimund Popper (1902 –1994), one of the greatest philosophers of science of the 20th century, and Sir John Carew Eccles (1903–1997), a great neurophysiologist and Nobel laureate in Physiology or Medicine (1963) proposed in The Self and Its Brain, cosmic evolution can be divided into three stages: (1) world 1 is the world of physical objects, composed of living organisms and non-living inorganic chemical elements; (2) world 2 is the world of subjective experiences performed by consciousness of humans and sentience (animal consciousness); and (3) world 3 is the products of the human mind such as human languages, works of art, science and technology (see Table 1 in [110]). Now it is generally accepted that both world 2 and world 3 of Popper are mediated by the brain. With regard to the functions of the brain, it may be divided into three hierarchical levels: level 1, the lowest level, is associated with sensation, perception, learning and memory, cognition, and motor response through decision-making and execution which is the basis of a ‘view of the world’ (view of Popper’s world 1); level 2, the middle level, is associated with recognition of self and others including empathy, personality, dreams, consciousness, spontaneous thought, rationale, compassion, morality, and ethnicity which are the basis of a ‘view of life’ (equivalent to Popper’s world 2); and level 3, the highest level, is associated with languages, abstract thinking, conceptualization, computation, creative works of art, science and technology, belief, and strategy which are the basis of a ‘view of value’ (equivalent to Popper’s world 3). Thus, one of the most challenging areas in the field of brain science in the 21st century is to understand how the human brain can distinguish or recognize the self and others. Can animals recognize the self and the others? What is consciousness? How is the consciousness produced in the brain? Popper believed that there are lower and higher stages of consciousness. He argued that “If the fact that animals cannot speak is a sufficient reason to deny consciousness to them, it would also be a sufficient reason to deny it to babies at an age before they learn to speak.” [110]. Therefore, I would like to borrow this sentence and say if the fact that animals cannot feel, understand, and share the emotional state of others is a sufficient reason to deny empathy to them, it would also be a sufficient reason to deny it to babies at an age before they can do so. In a paper entitled “Can we share a pain we never felt?” published in Neuron [111], the authors examined the neural correlates of empathy in patients with congenital insensitivity to pain (CIP). The results are surprisingly interesting and showed that the empathic skills assessed by the Balanced Emotional Empathy Score and the Interpersonal Reactivity Index in patients with CIP were almost the same as healthy controls in terms of empathic concern score, perspective-taking score, fantasy score, and personal distress score. There was no significant difference in anxiety score (Zung) and depression score (QD2A) between patients with CIP and healthy controls. Through testing the pain score for pictures of painful situations and pain scores for facial expressions of pain, only pain scores for pictures of painful situations were significantly lower in patients with CIP than in healthy controls. Exposure to the picture-based cues of another’s body in pain and of facial expressions of pain resulted in a similar brain activity pattern in the CIP patients and controls, although significantly lower activation of bilateral visual occipito-temporal cortex was seen in CIP patients relative to healthy controls. This result suggests that the patient with CIP has normal skills of both emotional empathy and cognitive empathy and can feel, understand, and share the pain of others with the same neural network in the brain. It also highlights the roles of both instinct sentience and learned ability in development of human empathy for pain or distress through integrated perceptions at the cortical level from visual, olfactory, and auditory rather than somatosensory inputs, that may occur as well in animals. Thus, it would be interesting and a great challenge to decode the underlying neural circuits of empathy for pain or distress in rodents using genetic, epigenetic, optogenetic, and even magnetogenetic approaches.
Because empathy/sympathy for distress and altruistic behaviors are largely dependent upon familiarity in both humans and rodents, it is also very important to study the neuroscience of familiarity and unfamiliarity that so far is almost unknown. For human beings, because empathy can be influenced by many factors from ‘world 3’ as defined by Popper such as religion, culture, belief, education, economic status, politics, and social formation, it is very difficult to exclude the complex roles of various factors in human studies of empathy using the current assessment tools and models and even theoretical hypotheses. However, at the world 2’ stage, if consciousness is a common brain function for both humans and animals to recognize the self and the other, it would be practical to study empathy in lower animals excluding the influencing factors from Popper’s ‘world 3’. Thus, from the evolutionary point of view, the discoveries of some forms of empathy in rodents shed new light on studies of the underpinnings of empathy in the future. The rodent models of empathy for pain or distress introduced above are very useful tools for the study of the biological evolutionary aspect of empathy using the same bio-psychosocial-behavioral paradigm —an umbrella for the study of empathy in both humans and animals.
Mirror cells, discovered in the late 1990s, are a distinct class of neurons that discharge both when individuals are performing a goal-directed action and when they observe another person performing a motor act with a similar goal [59]. Thus, mirror neurons are believed to be neuronal correlates involved in the mediation of empathy in both humans and animals through mirroring and understanding emotions of others [59]. Mirror neurons were first recorded in monkey premotor cortex [112, 113] and later were also found in humans [114]. Now it is generally known that mirror neurons exist in many brain areas and work as a basic underlying mechanism involved in the transformation of sensory representations of others’ behavior into one’s own motor or visceromotor representations concerning that behavior [115–120]. For example, in both humans and monkeys the anterior insular cortex is involved in empathy for disgust. The human insula is composed of four distinct functional areas: (1) sensorimotor; (2) olfactory-gustatory; (3) socio-emotional; and (4) cognitive. Electrical stimulation of the ventral part of the insula induces disgust in monkeys who express typical disgust grimaces, followed occasionally by retching and spitting, and sometimes refusal to eat. Electrical stimulation of the insula before surgical procedures for patients with epilepsy also induces disgust and vomiting. fMRI studies have also shown selective activation of the anterior insula in a human subject who witnessed disgusted facial expressions. These results strongly support a role of the anterior insula in mediating both vicariously felt disgust and directly experienced disgust. During both forms of disgust, mirror neurons in the anterior insula discharged concurrently, strongly supporting the role of a mirror mechanism in the mediation of empathy for disgust in both humans and monkeys. So far, whether mirror neurons are involved in empathy for pain in rodents has not been reported and we suggest that the multi-electrode array recording technique be used to map out the neuronal patterns in the mPFC and anterior insula in awake rats or mice that are socially interacting with a cagemate or non-cagemate in pain or distress. Mirror mechanism may exist.
As noted above, oxytocin plays very important roles in establishing social behaviors such as empathy, mother-child bonding, social cognition/recognition, affiliation, attachment, and sociability in humans [62–68]. Intranasal administration of oxytocin enhances in-group empathy, trust, cooperation, conformity, and defense against out-group invaders [121]. It has also been proposed that oxytocin plays important roles in the establishment of human-animal relationships that are beneficial to the pet-owner’s social attention, social behaviors, interpersonal interactions, mood, and social buffering by decreasing stress-related parameters such as cortisol, heart rate, blood pressure, self-reported fear, anxiety, and depression [122]. More recently, it has been reported separately that oxytocin is essential for maternal behaviors by balancing auditory cortical processing [123], performing consolation behavior [124], and attenuating fear responses by evoking axonal oxytocin release in the central amygdala in rodents [125]. Alcohol (ethanol) has long been known to influence the processes of emotion, social cognition, and somatic sensation through social drinking and experimental administration. It has been proposed that acute alcohol consumption and intranasal oxytocin have a common effect on emotion, social cognition, and behavior through acting on limbic and prefrontal cortical structures in humans, resulting in an enhancement of empathy-based altruism such as helping motivation, trust, generosity, and morality [126]. Microinjections of both ethanol (20 mmol/L or 90 mg/dL, equivalent to a peak blood concentration of 1.0 g/kg, defined as moderate consumption) and muscimol (5 mmol/L), a γ-aminobutyric acid A (GABAA) receptor agonist, in a volume of 0.5 μL into the bilateral mPFC of rats resulted in mechanical pain hypersensitivity and enhancement of BV-induced pain-related behavioral responses but without affecting thermal pain sensitivity [15]. This result is similar to that of empathy for pain or distress in rats [11]. Moreover, the same treatment with bicuculline (20 μmol/L), a GABAA receptor antagonist, in the bilateral mPFC blocked the development of mechanical pain hypersensitivity and enhancement of BV-induced pain-related behavioral response produced by bilateral microinjections of ethanol and muscimol, supporting a role of the GABAA receptor in mediating both oxytocin and ethanol at the mPFC in the development of empathy for pain or distress in rodents [15].
Impairment of empathy development may be associated with psychopathology and psychiatric disorders such as autism, narcissism, alexithymia, personality disorders, schizophrenia, and depression. It has been proposed that empathy impairment disorders or syndromes are caused by an imbalance between cognitive empathy (CE) and emotional empathy (EE) and classified into four categories: (1) CE deficit disorder (e.g., autism spectrum disorders, ASDs) that is an EE-dominant empathic imbalance with low CE ability but high EE sensitivity; (2) EE deficit disorder (e.g., psychopathy, conduct disorder, and antisocial personality disorder) that is a CE-dominant empathic imbalance with low EE sensitivity but high CE ability; (3) general empathy deficit disorder (e.g., schizophrenia) that is alternatively dominated by CE or EE with low CE ability and low EE sensitivity; and (4) general empathy surfeit disorder (e.g., empathic overdevelopment) with high CE ability and high EE sensitivity expressed as exceptionally good social skills but with some mental deficits [127, 128]. Low levels of oxytocin in the peripheral blood and polymorphisms in the oxytocin receptor gene are associated with both antisocial behaviors (e.g., callous-unemotional traits such as a history of aggression, over-aggression, and self-reported delinquency in children and adolescents), ASDs, and other psychiatric disorders [65, 66, 68, 129]. Intranasal administration of oxytocin also increases the ability to ‘read the mind’ of others as assessed by the “Reading the Mind in the Eyes” test [130, 131] and to be effective for the treatment of ASDs by improving performance in the mind-reading task [132]. ASDs are a family of neurodevelopmental disorders characterized by (1) qualitative deficits in social interactions (e.g., lack of social approach or play behavior); (2) qualitative impairments in communication (e.g., no or delayed language acquisition); and (3) restricted repetitive and stereotyped patterns of behavior, interests, and activities [133]. Because the critical criteria for both the diagnosis and treatment of ASDs are associated with cognitive and emotional empathy, the use of rodent models of empathy is also important for understanding the mechanisms of ASDs and other empathy impairment disorders or syndromes [25, 134]. Because impairment of empathy is the root of many psychiatric disorders, and oxytocin is one of the major neuropeptides involved in development of social ability and social communication [135, 136], efforts made to target the neural circuitry involving oxytocin and its receptors may be promising for the development of novel therapeutic approaches to ASD treatment if the underlying mechanisms of empathy is unraveled [25, 137, 138].
Acknowledgements
This work was supported by grants from the National Basic Research Development Program of China (2013CB835100) and the Natural Science Foundation of China (81571072) to JC.
Footnotes
Many animals, however, certainly sympathise with each other’s distress or danger.
Charles Darwin
References
- 1.Martin LJ, Tuttle AH, Mogil JS. The interaction between pain and social behavior in humans and rodents. Curr Top Behav Neurosci. 2014;20:233–250. doi: 10.1007/7854_2014_287. [DOI] [PubMed] [Google Scholar]
- 2.Mogil JS. Social modulation of and by pain in humans and rodents. Pain. 2015;156:S35–S41. doi: 10.1097/01.j.pain.0000460341.62094.77. [DOI] [PubMed] [Google Scholar]
- 3.Williams AC, Craig KD. Updating the definition of pain. Pain. 2016;157:2420–2423. doi: 10.1097/j.pain.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 4.Merskey H, Bogduk N. Classification of chronic pain. Seattle: IASP Press; 1994. [Google Scholar]
- 5.Craig KD. Social communication model of pain. Pain. 2015;156:1198–1199. doi: 10.1097/j.pain.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 6.Hadjistavropoulos T, Craig KD, Duck S, Cano A, Goubert L, Jackson PL, Mogil JS, et al. Abiopsychosocial formulation of pain communication. Psychol Bull. 2011;137:910–939. doi: 10.1037/a0023876. [DOI] [PubMed] [Google Scholar]
- 7.Jensen KB, Petrovic P, Kerr CE, Kirsch I, Raicek J, Cheetham A, Spaeth R, et al. Sharing pain and relief: neural correlates of physicians during treatment of patients. Mol Psychiatry. 2014;19:392–398. doi: 10.1038/mp.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 9.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 10.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Lu YF, Li CL, Wang Y, Sun W, He T, et al. Social interaction with a cagemate in pain facilitates subsequent spinal nociception via activation of the medial prefrontal cortex in rats. Pain. 2014;155:1253–1261. doi: 10.1016/j.pain.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Ren LY, Lu ZM, Liu MG, Yu YQ, Li Z, Shang GW, Chen J. Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors. Neuroscience. 2008;153:268–278. doi: 10.1016/j.neuroscience.2008.01.067. [DOI] [PubMed] [Google Scholar]
- 13.Gong KR, Cao FL, He Y, Gao CY, Wang DD, Li H, et al. Enhanced excitatory and reduced inhibitory synaptic transmission contribute to persistent pain-induced neuronal hyper-responsiveness in anterior cingulate cortex. Neuroscience. 2010;171:1314–1325. doi: 10.1016/j.neuroscience.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Lu YF, He Y, Wang Y, Zhang FK, He T, Wang RR, et al. Spatial and temporal plasticity of synaptic organization in anterior cingulate cortex following peripheral inflammatory pain: multi-electrode array recordings in rats. Neurosci Bull. 2014;30:1–20. doi: 10.1007/s12264-013-1344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng KW, He T, Wang RR, Li CL, Luo WJ, Wu FF, et al. Ethanol increases mechanical pain sensitivity in rats via activation of GABAA receptors in medial prefrontal cortex. Neurosci Bull. 2016;32:433–444. doi: 10.1007/s12264-016-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu MG, Chen J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: challenges and perspectives. Prog Neurobiol. 2014;116:13–32. doi: 10.1016/j.pneurobio.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 17.de Waal FBM. Putting the altruism back into altruism: The evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 18.de Waal FBM. The antiquity of empathy. Science. 2012;336:874–876. doi: 10.1126/science.1220999. [DOI] [PubMed] [Google Scholar]
- 19.de Waal FBM. The Bonobo and the Atheist. In Search for Humanism among the Primates. New York: W.W. Norton & Company, 2013.
- 20.Panksepp JB, Lahvis GP. Rodent empathy and affective neuroscience. Neurosci Biobehav Rev. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panksepp J, Panksepp JB. Toward a cross-species understanding of empathy. Trend Neurosci. 2013;36:489–496. doi: 10.1016/j.tins.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Liencres C, Shamay-Tsoory SG, Brüne M. Towards a neuroscience of empathy: ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neurosci Biobehav Rev. 2013;37:1537–1548. doi: 10.1016/j.neubiorev.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Keum S, Shin HS. Rodent models for studying empathy. Neurobiol Learn Mem. 2016;135:22–26. doi: 10.1016/j.nlm.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Sivaselvachandran S, Acland EL, Abdallah S, Martin LJ. Behavioral and mechanistic insight into rodent empathy. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Meyza KZ, Bartal IB, Monfils MH, Panksepp JB, Knapska E. The roots of empathy: Through the lens of rodent models. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 27.Langford DJ, Williams AC. The caring, sharing rat? Pain. 2014;155:1183–1184. doi: 10.1016/j.pain.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Li Z, Lv YF, Li CL, Wang Y, Wang RR, et al. Empathy for pain: A novel bio-psychosocial-behavioral laboratory animal model. Acta Physiol Sin. 2015;67:561–570. [PubMed] [Google Scholar]
- 29.Pigman GW. Freud and the history of empathy. Int J Psychoanal. 1995;76:237–256. [PubMed] [Google Scholar]
- 30.Preston SD, de Waal FBM. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–72. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- 31.de Vignemont F, Singer T. The empathic brain: how, when and why? Trend Cogn Sci. 2006;10:435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Leiberg S, Anders S. The multiple facets of empathy: a survey of theory and evidence. Prog Brain Res. 2006;156:419–440. doi: 10.1016/S0079-6123(06)56023-6. [DOI] [PubMed] [Google Scholar]
- 33.Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- 34.Decety J, Norman GJ, Berntson GG, Cacioppo JT. A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Prog Neurobiol. 2012;98:38–48. doi: 10.1016/j.pneurobio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Decety J, Bartal IB, Uzefovsky F, Knafo-Noam A. Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philos Trans R Soc Lond B Biol Sci. 2016 doi: 10.1098/rstb.2015.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-Ami Bartal I, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, Mason P. Pro-social behavior in rats is modulated by social experience. Elife 2014, 3: e01385. [DOI] [PMC free article] [PubMed]
- 38.Márquez C, Rennie SM, Costa DF, Moita MA. Prosocial choice in rats depends on food-seeking behavior displayed by recipients. Curr Biol. 2015;25:1736–1745. doi: 10.1016/j.cub.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Lallement J, van Wingerden M, Marx C, Srejic M, Kalenscher T. Rats prefer mutual rewards in a prosocial choice task. Front in Neurosci. 2015;8:443. doi: 10.3389/fnins.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaki J, Ochsner KN. The neuroscience of empathy: progress, pitfalls and promise. Nat Neurosci. 2012;15:675–680. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
- 41.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;32:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 42.Darwin C. The descent of man. 2. London: Penguin Group; 1879. [Google Scholar]
- 43.Darwin C. The expression of the emotions in man and animals. 2. London: Penguin Group; 1890. [Google Scholar]
- 44.Langford DL, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 45.Condé F, Audinat E, Maire-Lepoivre E, Crépel F. Afferent connections of the medial frontal cortex of the rat. A study using retrograde transport of fluorescent dyes. I. Thalamic afferents. Brain Res Bull. 1990;24:341–354. doi: 10.1016/0361-9230(90)90088-h. [DOI] [PubMed] [Google Scholar]
- 46.Condé F, Maire-Lepoivre E, Audinat E, Crépel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- 47.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 48.Christoff K, Irving ZC, Fox KC, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: a dynamic framework. Nat Rev Neurosci. 2016;17:718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- 49.Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci. 2007;11:413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 51.Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16:213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 52.Liu D, Gu X, Zhu J, Zhang X, Han Z, Yan W, et al. Medial prefrontal activity during delay period contributes to learning of a working memory task. Science. 2014;346:458–463. doi: 10.1126/science.1256573. [DOI] [PubMed] [Google Scholar]
- 53.Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends Neurosci. 2014;37:455–464. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015, 16: 317–331. (Erratum in: Nat Rev Neurosci 2015, 16:439). [DOI] [PubMed]
- 57.Bennett MR. The prefrontal-limbic network in depression: A core pathology of synapse regression. Prog Neurobiol. 2011;93:457–467. doi: 10.1016/j.pneurobio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Bennett MR. The prefrontal-limbic network in depression: Modulation by hypothalamus, basal ganglia and midbrain. Prog Neurobiol. 2011;93:468–487. doi: 10.1016/j.pneurobio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Rizzolatti G, Sinigaglia C. The mirror mechanism: a basic principle of brain function. Nat Rev Neurosci. 2016;17:757–765. doi: 10.1038/nrn.2016.135. [DOI] [PubMed] [Google Scholar]
- 60.Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. J Neurosci. 2009;29:8525–8529. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheng F, Liu Y, Zhou B, Zhou W, Han S. Oxytocin modulates the racial bias in neural responses to others’ suffering. Biol Psychol. 2013;92:380–386. doi: 10.1016/j.biopsycho.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 62.Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Cur Opin Neurobiol. 2000;10:784–789. doi: 10.1016/s0959-4388(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 63.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 64.Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Cur Opin Neurobiol. 2004;14:248–253. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 65.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 66.Heinrichs M, Domes G. Neuropeptides and social behavior: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- 67.Lee HJ, Macbeth AH, Pagani JH, Young3rd WS. Oxytocin: The great facilitator of life. Prog Neurobiol 2009, 88: 127–151. [DOI] [PMC free article] [PubMed]
- 68.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 69.Church RM. Emotional reactions of rats to the pain of others. J Comp Physiol Psychol. 1959;52:132–134. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- 70.Rice GE, Gainer P. “Altruism” in the albino rat. J Comp Physiol Psychol. 1962;55:123–125. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe S, Ono K. An experimental analysis of “empathic” response: Effects of pain reactions of pigeon upon other pigeon’s operant behavior. Behav Processes. 1986;13:269–277. doi: 10.1016/0376-6357(86)90089-6. [DOI] [PubMed] [Google Scholar]
- 72.Edgar JL, Lowe JC, Paul ES, Nicol CJ. Avian maternal response to chick distress. Proc Biol Sci. 2011;278:3129–3134. doi: 10.1098/rspb.2010.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edgar JL, Paul ES, Harris L, Penturn S, Nicol CJ. No evidence for emotional empathy in chickens observing familiar adult conspecifics. PLoS One. 2012;7:e31542. doi: 10.1371/journal.pone.0031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nowbahari E, Scohier A, Durand JL, Hollis KL. Ants, Cataglyphis cursor, use precisely directed rescue behavior to free entrapped relatives. PLoS One. 2009;4:e6573. doi: 10.1371/journal.pone.0006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Waal FBM. Do animals feel empathy? Scientific American Mind. 2007;18(6):28–35. [Google Scholar]
- 76.Miller G. Animal behavior. Signs of empathy seen in mice. Science. 2006;312:1860–1861. doi: 10.1126/science.312.5782.1860b. [DOI] [PubMed] [Google Scholar]
- 77.Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, Kaczmarek L. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc Natl Acad Sci USA. 2006;103:3858–3862. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knapska E, Mikosz M, Werka T, Maren S. Social modulation of learning in rats. Learn Mem. 2010;17:35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keum S, Park J, Kim A, Park J, Kim KK, Jeong J, Shin HS. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav. 2016;15:231–242. doi: 10.1111/gbb.12278. [DOI] [PubMed] [Google Scholar]
- 82.Mikosz M, Nowak A, Werka T, Knapska E. Sex differences in social modulation of learning in rats. Sci Rep. 2015;5:18114. doi: 10.1038/srep18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruchey AK, Jones CE, Monfils MH. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav Brain Res. 2010;214:80–84. doi: 10.1016/j.bbr.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones CE, Riha PD, Gore AC, Monfils MH. Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Anim Cogn. 2014;17:827–834. doi: 10.1007/s10071-013-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones CE, Monfils MH. Dominance status predicts social fear transmission in laboratory rats. Anim Cogn. 2016;19:1051–1069. doi: 10.1007/s10071-016-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5:e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V. Experience modulates vicarious freezing in rats: a model for empathy. PLoS One. 2011;6:e21855. doi: 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yusufishaq S, Rosenkranz JA. Post-weaning social isolation impairs observational fear conditioning. Behav Brain Res. 2013;242:142–149. doi: 10.1016/j.bbr.2012.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akyazi I, Eraslan E. Transmission of stress between cagemates: a study in rats. Physiol Behav. 2014;123:114–118. doi: 10.1016/j.physbeh.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 90.Kiyokawa Y, Honda A, Takeuchi Y, Mori Y. A familiar conspecific is more effective than an unfamiliar conspecific for social buffering of conditioned fear responses in male rats. Behav Brain Res. 2014;267:189–193. doi: 10.1016/j.bbr.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 91.Nowak A, Werka T, Knapska E. Social modulation in extinction of aversive memories. Behav Brain Res. 2013;238:200–205. doi: 10.1016/j.bbr.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe S. Distress of mice induces approach behavior but has an aversive property for conspecifics. Behav Processes. 2012;90:167–173. doi: 10.1016/j.beproc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, Brüne M. Emotional contagion in mice: the role of familiarity. Behav Brain Res. 2014;263:16–21. doi: 10.1016/j.bbr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 94.Langford DJ, Tuttle AH, Briscoe C, Harvey-Lewis C, Baran I, Gleeson P, et al. Varying perceived social threat modulates pain behavior in male mice. J Pain. 2011;12:125–132. doi: 10.1016/j.jpain.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 95.Martin LJ, Hathaway G, Isbester K, Mirali S, Acland EL, Niederstrasser N, et al. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr Biol. 2015;25:326–332. doi: 10.1016/j.cub.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 96.Langford DJ, Tuttle AH, Brown K, Deschenes S, Fischer DB, Mutso A, et al. Social approach to pain in laboratory mice. Soc Neurosci. 2010;5:163–170. doi: 10.1080/17470910903216609. [DOI] [PubMed] [Google Scholar]
- 97.Yang L, Shi LJ, Yu J, Zhang YQ. Activation of protein kinase A in the amygdala modulates anxiety-like behaviors in social defeat exposed mice. Mol Brain. 2016;9:3. doi: 10.1186/s13041-015-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gioiosa L, Chiarotti F, Alleva E, Laviola G. A trouble shared is a trouble halved: social context and status affect pain in mouse dyads. PLoS One. 2009;4:e4143. doi: 10.1371/journal.pone.0004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith ML, Hostetler CM, Heinricher MM, Ryabinin AE. Social transfer of pain in mice. Sci Adv. 2016;2:e1600855. doi: 10.1126/sciadv.1600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baptista-de-Souza D, Nunciato AC, Pereira BC, Fachinni G, Zaniboni CR, Canto-de-Souza A. Mice undergoing neuropathic pain induce anxiogenic-like effects and hypernociception in cagemates. Behav Pharmacol. 2015;26:664–672. doi: 10.1097/FBP.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 101.Yang H, Jung S, Seo J, Khalid A, Yoo JS, Park J, et al. Altered behavior and neural activity in conspecific cagemates co-housed with mouse models of brain disorders. Physiol Behav. 2016;163:167–176. doi: 10.1016/j.physbeh.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 102.Chen J, Lariviere WR. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword. Prog Neurobiol. 2010;92:151–183. doi: 10.1016/j.pneurobio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen J, Guan SM. Bee venom and Pain. In: Gopalakrishnakone P. (Ed.). Toxinology: Toxins and Drug Discovery. Springer Netherlands, 2015: 1–34. DOI 10.1007/978-94-007-6726-3_1-1.
- 104.Kiyokawa Y, Hiroshima S, Takeuchi Y, Mori Y. Social buffering reduces male rats’ behavioral and corticosterone responses to a conditioned stimulus. Horm Behav. 2014;65:114–118. doi: 10.1016/j.yhbeh.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 105.Ishii A, Kiyokawa Y, Takeuchi Y, Mori Y. Social buffering ameliorates conditioned fear responses in female rats. Horm Behav. 2016;81:53–58. doi: 10.1016/j.yhbeh.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 106.Mikami K, Kiyokawa Y, Takeuchi Y, Mori Y. Social buffering enhances extinction of conditioned fear responses in male rats. Physiol Behav. 2016;163:123–128. doi: 10.1016/j.physbeh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 107.Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, Mori Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav Brain Res. 2013;240:46–51. doi: 10.1016/j.bbr.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 108.Kiyokawa Y, Wakabayashi Y, Takeuchi Y, Mori Y. The neural pathway underlying social buffering of conditioned fear responses in male rats. Eur J Neurosci. 2012;36:3429–3437. doi: 10.1111/j.1460-9568.2012.08257.x. [DOI] [PubMed] [Google Scholar]
- 109.Fuzzo F, Matsumoto J, Kiyokawa Y, Takeuchi Y, Ono T, Nishijo H. Social buffering suppresses fear-associated activation of the lateral amygdala in male rats: behavioral and neurophysiological evidence. Front Neurosci. 2015;9:99. doi: 10.3389/fnins.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Popper KR, Eccles JC. The Self and Its Brain. An Argument for Interactionism. London: Taylor & Francis Group, 1977.
- 111.Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61:203–212. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 112.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 113.Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 114.Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 115.Rizzolatti G. Confounding the origin and function of mirror neurons. Behav Brain Sci. 2014;37:218–219. doi: 10.1017/S0140525X13002471. [DOI] [PubMed] [Google Scholar]
- 116.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 117.Rizzolatti G, Cattaneo L, Fabbri-Destro M, Rozzi S. Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol Rev. 2014;94:655–706. doi: 10.1152/physrev.00009.2013. [DOI] [PubMed] [Google Scholar]
- 118.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 119.Rizzolatti G, Fabbri-Destro M. The mirror system and its role in social cognition. Curr Opin Neurobiol. 2008;18:179–184. doi: 10.1016/j.conb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 120.Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- 121.De Dreu CK, Kret ME. Oxytocin conditions intergroup relations through upregulated in-Group empathy, cooperation, conformity, and defense. Biol Psychiatry. 2016;79:165–173. doi: 10.1016/j.biopsych.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 122.Beetz A, Uvnäs-Moberg K, Julius H, Kotrschal K. Psychosocial and psychophysiological effects of human-animal interactions: the possible role of oxytocin. Front Psychol. 2012;3:234. doi: 10.3389/fpsyg.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marlin BJ, Mitre M. D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 126.Mitchell IJ, Gillespie SM, Abu-Akel A. Similar effects of intranasal oxytocin administration and acute alcohol consumption on socio-cognitions, emotions and behaviour: Implications for the mechanisms of action. Neurosci Biobehav Rev. 2015;55:98–106. doi: 10.1016/j.neubiorev.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 127.Smith A. Cognitive empathy and emotional empathy in human behavior and evolution. Psychol Rec. 2006;56:3–21. [Google Scholar]
- 128.Smith A. The empathy imbalance hypothesis of autism: a theoretical approach to cognitive and emotional empathy in autistic development. Psychol Rec. 2009;59:489–510. [Google Scholar]
- 129.Hovey D, Lindstedt M, Zettergren A, Jonsson L, Johansson A, Melke J, et al. Antisocial behavior and polymorphisms in the oxytocin receptor gene: findings in two independent samples. Mol Psychiatry. 2016;21:983–988. doi: 10.1038/mp.2015.144. [DOI] [PubMed] [Google Scholar]
- 130.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The, “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- 131.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 132.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 133.American Psychiatric Association, 1994. Diagnostic and statistical manual of mental disorders (DSM-IV). Washington DC: American Psychiatric Association, 1994.
- 134.Wöhr M, Scattoni ML. Behavioural methods used in rodent models of autism spectrum disorders: current standards and new developments. Behav Brain Res. 2013;251:5–17. doi: 10.1016/j.bbr.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 135.Lukas M, Neumann ID. Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav Brain Res. 2013;251:85–94. doi: 10.1016/j.bbr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 136.Caldwell HK, Aulino EA, Freeman AR, Miller TV, Witchey SK. Oxytocin and behavior: Lessons from knockout mice. Dev Neurobiol. 2017;77:190–201. doi: 10.1002/dneu.22431. [DOI] [PubMed] [Google Scholar]
- 137.Marlin BJ, Froemke RC. Oxytocin modulation of neural circuits for social behavior. Dev Neurobiol. 2017;77:169–189. doi: 10.1002/dneu.22452. [DOI] [PubMed] [Google Scholar]
- 138.Peñagarikano O. Oxytocin in animal models of autism spectrum disorder. Dev Neurobiol. 2017;77:202–213. doi: 10.1002/dneu.22449. [DOI] [PubMed] [Google Scholar]