Abstract
Critical illness may lead to significant long-term neurological morbidity and patients frequently develop neuropsychological disturbances including acute delirium or memory impairment after intensive care unit (ICU) discharge. Mechanical ventilation (MV) is a risk factor to the development of adverse neurocognitive outcomes. Patients undergoing MV for long periods present neurologic impairment with memory and cognitive alteration. Delirium is considered an acute form of brain dysfunction and its prevalence rises in mechanically ventilated patients. Delirium duration is an independent predictor of mortality, ventilation time, ICU length of stay and short- and long-term cognitive impairment in the ICU survivors. Although, neurocognitive sequelae tend to improve after hospital discharge, residual deficits persist even 6 years after ICU stay. ICU-related neurocognitive impairments occurred in many cognitive domains and are particularly pronounced with regard to memory, executive functions, attentional functions, and processing speed. These sequelae have an important impact on patients’ lives and ICU survivors often require institutionalization and hospitalization. Experimental studies have served to explore the possible mechanisms or pathways involved in this lung to brain interaction. This communication can be mediated via a complex web of signaling events involving neural, inflammatory, immunologic and neuroendocrine pathways. MV can affect respiratory networks and the application of protective ventilation strategies is mandatory in order to prevent adverse effects. Therefore, strategies focused to minimize lung stretch may improve outcomes, avoiding failure of distal organ, including the brain. Long-term neurocognitive impairments experienced by critically ill survivors may be mitigated by early interventions, combining cognitive and physical therapies. Inpatient rehabilitation interventions in ICU promise to improve outcomes in critically ill patients. The cross-talk between lung and brain, involving specific pathways during critical illness deserves further efforts to evaluate, prevent and improve cognitive alterations after ICU admission, and highlights the crucial importance of tailoring MV to prevent adverse outcomes.
Keywords: Mechanical ventilation (MV), brain dysfunction, cognitive impairment, critically ill, intensive care unit (ICU)
Introduction
There is growing evidence that critical illness may lead to significant long-term morbidity. Recent studies have indicated the high prevalence of neurocognitive impairments persisting for years after hospital discharge among patients who have survive an episode of critical illness (1), and have emphasized the need to reduce the neurological morbidity in these patients.
The pathophysiological mechanisms underlying these alterations may originate outside the central nervous system (CNS). The interrelation between predisposing factors (such as advanced age, multiple medical comorbidities and, especially, pre-existing cognitive impairments) and factors directly associated with critical illness such as hypoxemia (2), hypotension (3), sepsis (4), and blood glucose dysregulation (5,6) can contribute to these alterations. Furthermore, other factors associated with clinical management in the intensive care unit (ICU), including medication (7) and mechanical ventilation (MV) (6,8), inflammatory mediators, metabolic disturbances, neurotransmitter imbalances and cholinergic deficiency (9), can result in acute brain damage and should be taken into account. This review will focus specifically in the MV as a factor that may contribute to the development of cognitive alterations.
MV and the brain
Despite being a vital life support tool for many critical patients, MV is not without its complications. It may worsen lung injury or even induce it [a condition termed ventilator-induced lung injury (VILI)] (10). VILI is triggered by the mechanotransduction of mechanical to biological signal at epithelial and endothelial levels in the lung. This leads to a deleterious inflammatory cascade, and inflammatory mediators can promote local tissue injury by a phenomenon termed biotrauma (10), which may even spread to other distal organs and systems, and eventually induce multiorgan failure. MV may also cause bacterial translocation from the lungs into the systemic circulation, thus producing distal organ failure (11,12).
Various experimental studies have suggested that ventilatory strategies which cause overstretching of lung regions or that produce repetitive opening and closing of lung units are harmful (10,13,14). Patients undergoing these ventilator strategies may be at risk of VILI and also of ventilation-induced development of multiorganic systemic failure (15).
Experimental studies have revealed the importance of the brain-lung interaction in the context of MV (14,16,17). Quílez et al. described how MV induced differential c-fos expression in several areas in the brain, depending on the ventilatory pattern, tidal volume (VT) and level of PEEP, thus supporting the hypothesis that an iatrogenic effect of MV may affect the brain (14,18). Chen et al. found that prolonged MV (6 h) in mice induced cognitive decline and increased activation of microgliosis and apoptotic cascades after surgery, thus indicating the detrimental effects of prolonged MV in the brain (19). González-López et al. identified a novel mechanism driven via vagal and dopaminergic pathways that triggers hippocampal apoptosis in response to lung stretch in mice undergoing MV (20).
Lungs can “sense” mechanical stimuli through their mechanoreceptors which communicate this information to the brain by a variety of mechanisms, possibly involving the autonomic nervous system (14). This communication can be mediated via a complex web of signaling events involving neural, inflammatory, immunologic and neuroendocrine pathways. Lung injuries due to inadequate ventilator settings may produce an inflammatory response, releasing pulmonary inflammatory mediators into the bloodstream and triggering a brain response. Systemic endothelial activation and inflammation can also be explained by the activation of sympathetic nerve terminals in organs distal to lung parenchyma (21). Furthermore, MV may impair regional blood flow and brain oxygenation, due to increased mean airway pressure, reduced lymphatic drainage and activation of the autonomic system (22). Patient-ventilator asynchrony results in increased work of breathing and raises the mechanical ventilator load; inspiratory loading has been associated with modified cortical activities, and with activation of the premotor cortical areas, which may have important pathophysiological implications (23).
Whatever the pathway involved, this release of inflammatory mediators associated with VILI may increase functional and metabolic activity in the brain (14), among other organs. Several trials in patient survivors of acute respiratory distress syndrome (ARDS) have described cognitive deterioration, including memory, language and cognitive decline. Patients undergoing MV for long periods present neurologic impairment with memory and cognitive alteration (24,25).
Implications of brain dysfunction for critically ill survivors
Delirium
Delirium is defined by the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-V) as a disturbance of attention, awareness and cognition that tends to fluctuate in severity during the course of a day: these disturbances are not better explained by a pre-existing, established or evolving neurocognitive disorder, and there is evidence that they are a direct physiological consequence of another medical state. This condition is understood as an acute form of brain dysfunction that affects 14–24% of hospital admissions and 15–53% of postoperative patients; it is, manifested by hypoactive and hyperactive states, occurring even in the same patients (26,27). In the critical care context, the prevalence of delirium rises to between 60% and 80% in ICU patients undergoing MV (28-31), and delirium duration has emerged as an independent predictor of mortality, ventilation time, ICU length of stay (28,32,33) and short- and long-term cognitive impairment (34) in critically ill patients and ICU survivors. Its presence has also been associated with a 39% increase in ICU costs (35).
The list of risk factors for delirium in ICU patients is extensive and wide-ranging. They are generally classified into two types: predisposing factors, which refer to the characteristics of the patients and chronic pathology such as advanced age, previous cognitive impairment, respiratory disease or hypertension; and precipitating factors, related to the environment and the acute illness status. The latter are considered more modifiable conditions, and are thus potential targets for preventing ICU delirium. MV, acute physiologic derangements, infection, coma, illness severity and benzodiazepine administration are included in this category (2,36-40).
The pathophysiological mechanisms of ICU delirium are poorly understood, but two of the main triggers that have been proposed are neurotransmitter imbalance and inflammation (41). In fact, both seem to be plausible and may be related. The neurotransmitter imbalance hypothesis arises from the presence of multiple neurotransmitter systems related to the control of cognition, behavior and mood in human beings. Disturbances in those systems, specifically the dopaminergic and cholinergic systems, have been associated with delirium (42,43). On the other hand, critical illness and its management cause inflammation that may lead to multiple organ dysfunction (44). Inflammatory mediators initiate a cascade of events than may produce endothelial damage, microvascular compromise and neuroinflammation (45,46).
The cytokine signal can be transmitted to the brain by, direct neural pathways (via primary autonomic afferents), transport across the blood-brain barrier, or entry via the circumventricular region. Increased TNFα levels in the brain have been associated with microglial activation that may affect astrocytic and neural function. These mechanisms could explain the neurobehavioral manifestation of delirium; moreover, if the microglial activation persists or the cholinergic inhibitory control of microglia is impaired (due to incipient neurodegenerative process, pharmacological treatment, or even advanced normal aging…) the neurotoxicity of this inflammatory response may be associated with further cognitive impairments (47).
Cognitive impairments on discharge
MV is widely used in the ICU, and has been identified as a pervasive risk factor for cognitive dysfunction among ICU survivors. Currently, 22 studies have been published on the incidence of neurocognitive impairments after critical illness (1), 14 of which included only MV patients, and eight mixed MV and non-MV ventilated populations. Most included patients with postoperative complications, acute lung injury, ARDS, COPD exacerbations and trauma conditions. The results of these studies show that MV and its duration predict adverse neurocognitive outcomes after discharge (6,8).
Whether these impairments represent new sequelae of critical illness or worsening of previously existing impairments is difficult to ascertain because baseline data on cognitive functioning are generally unavailable or underestimated. Differences in study populations, definitions of cognitive impairment, neuropsychological tests, and follow-up make it difficult to compare studies; however, it is widely agreed that around three-quarters of critically ill survivors develop new neurocognitive impairments, which correspond to mild-moderate dementia in around one third (7). Neurocognitive sequelae may improve within 6 to 12 months after discharge, but residual deficits tend to become chronic thereafter, persisting in around 47% of survivors after 2 years and in 25% after 6 years (3,48). ICU-related neurocognitive impairments occurred in many cognitive domains and are particularly pronounced with regard to memory, executive functions, attentional functions, and processing speed (2,3,5,7,48-50).
These long-lasting neurocognitive impairments have an important impact on patients’ lives, as they affect ability to perform activities of daily living, impede their ability to return to work, and reduce quality-of-life both for the patients themselves and for their relatives (51,52). Importantly, these neurocognitive impairments also create a major public health burden since ICU survivors often require institutionalization and hospitalization (53).
Respiratory patterns
Analysis of the variability of the respiratory pattern
Breathing in normal conditions is generated by central pattern generators which are special networks of neurons located in specific regions of the CNS. Although breathing is normally treated as a rhythmic process, the variability in cycle-to-cycle measurements is substantial in awake, healthy adult humans (54). Experimental studies with rats (55,56) have shown that alterations in internal (metabolic or physiological) and external conditions, due to the reorganization of the brain stem respiratory network dynamics, creates new rhythmogenic mechanisms in order to adapt and respond to these changes. MV can affect these respiratory networks, and many respiratory variables which can be modified by the ventilatory pattern such as VT, end-expiratory lung volume (EELV), total time respiratory cycle (Ttot) or respiratory rate (RR) may contribute to the significant breath-to-breath variability (54,57). The mechanisms underlying breath-to-breath variability arise from the nonlinear dynamical behavior of neuromechanical reflex loops (58). The central processing of vagal afferent activity is nonlinear and this nonlinear feedback effect may increase the dimension of breathing (59). A variety of methods from nonlinear dynamics and chaos theory can be used to characterize the complexity of the respiratory pattern. The most popular are: (I) phase space plots to discover the phase space trajectory for the system under study, and the short- or long-term relationship between the current state (e.g., the current respiratory cycle) and future states; (II) correlation dimension (Grassberger & Procaccia algorithm), a measure which reflects the extent that an object occupies the space in which it is embedded; (III) entropy measures (60-62) to assess the degree of regularity of the breath-to-breath variability time series; (IV) detrended fluctuation analysis (DFA) (63) to study the fractal properties of time series and the short- and long-term correlations involved in its dynamic; and (V) surrogate data analysis (64) to extract the nonlinear components involved in the dynamics of the breath-to-breath variability.
Dellaca et al. (57) found long-range correlations in the fluctuations of the cycle-to-cycle variations of several respiratory parameters. Using a neuromechanical model, they proposed that correlations in the timing and amplitude of the physiological parameters originated from the brain with the exception of EELV which showed the strongest correlations due to the contribution of the viscoelastic properties.
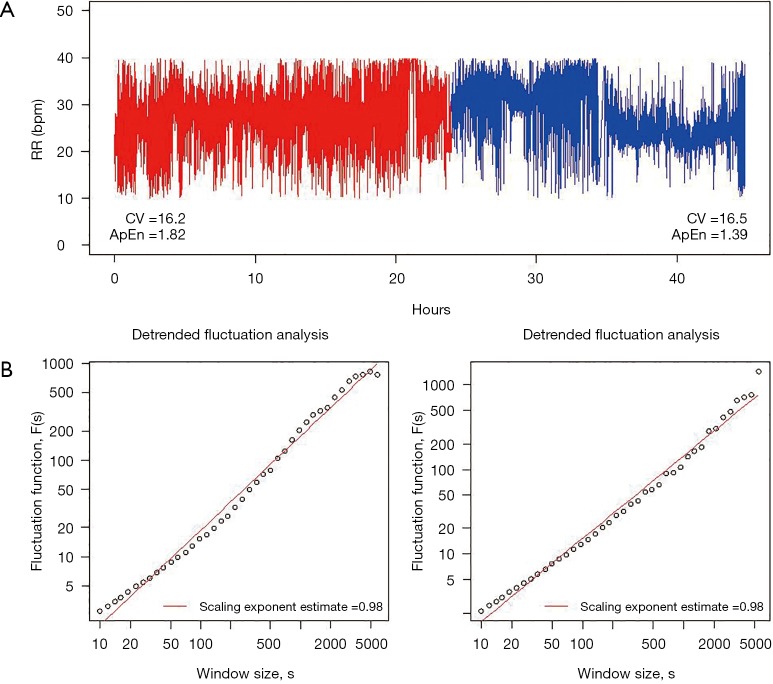
Figure 1 shows a broad attempt to characterize the complexity of the breathing pattern through RR variability time series in a representative tracheotomized patient in our center.
Figure 1.
Complexity of the breathing pattern. This figure shows a broad attempt to characterize the complexity of the breathing pattern through respiratory rate variability time series in a representative tracheotomized patient in our center. (A) RR during the 24 hours before (red trace) and 24 hours after (blue trace) a tracheotomy procedure. The variability was similar in the two periods; this was attributed to the value of the coefficient of variation, CV. However, approximate entropy, ApEn decreased for the post period, suggesting a time series with a less irregular (or more predictable) pattern of fluctuations; (B) the power law relationship between the fluctuation function F(s) and window size s, F(s) ~ sα, appears as a straight line with slope (i.e., the scaling exponent), α, on the log-log graph, exhibiting a 1/f fractal-like behavior in both cases. RR, respiratory rate.
Therapeutics to prevent brain dysfunction
MV to protect the brain
The application of protective ventilation strategies in the care of brain injured patients seems mandatory, especially because of the relative frequent occurrence of ARDS in these patients (65). In fact, Elmer et al. (66) demonstrated an association between high VT ventilation and the development of ARDS after intubation for intracerebral hemorrhage, and more recently Beitler et al. (67) showed that lower VT after out-hospital cardiac arrest is independently associated with favorable neurocognitive outcome, more ventilator-free days, and more shock-free days. Some studies indicate that MV should be implemented in patients with established brain injury, in order to protect both the brain and the lung, but it is no clear how to ventilate the lungs in order to avoid brain injury. Avoiding hypoxemia and maintaining appropriate arterial pressure may have a positive effect on neurological outcome. Some authors have found that PEEP-induced overdistension and an associated elevation of arterial carbon dioxide tension was followed by an increase in intracranial pressure and consequent brain injury (68). The application of protective ventilation strategies, especially those that minimize lung stretch, have been positively evaluated in populations such as ARDS patients, but the effect on the brain was not explored. These strategies may improve outcomes in the general population, avoiding failure of distal organ, including the brain, but may also have harmful effects in patients with intracranial hypertension due to hypercapnia. The effect of the different MV strategies will vary depending on the patient and the pathology. Therefore, it is difficult to make any general recommendations, and individualized treatment is mandatory. Protective MV must provide safe oxygenation and alveolar ventilation and simultaneous must prevent neuroinflammation.
Other therapies
There is some evidence that long-term neurocognitive impairments experienced by critically ill survivors may be mitigated after rehabilitation. In a sample of ICU survivors, Jackson et al. (69) found that combined cognitive and physical therapy improved executive functions and instrumental activities of daily living. However, delaying interventions until after ICU discharge may be less effective, and introducing interventions only when cognitive and physical decline has already appeared seems insufficient in order to reverse deficits completely (70,71). For this reason, early interventions have received increased attention in recent years. Inpatient rehabilitation interventions in ICU promise to improve outcomes in critically ill patients, and may decrease the incidence and duration of delirium, shorten ICU and hospital stay, and ultimately reduce costs while improving cognitive function and quality of life after discharge. The most common rehabilitation strategies during ICU stay have mainly involved physical interventions such as early mobilization (72,73) and occupational therapy (74) aimed to enhance functional recovery, and the early detection of delirium (75). Only recently have early rehabilitation strategies in the ICU been extended beyond physical therapy to include cognitive interventions (76-78). Nevertheless, the results about neurocognitive interventions in the ICU are limited and the issue of whether these interventions can prevent or improve long-term cognitive impairments in ICU survivors has not yet been elucidated.
Conclusions
During critical illness there is always cross-talk between lung, brain and other organs involving specific pathways even when the organs are not apparently impaired. Brain injury predisposes to lung injury and vice versa; therefore, the treatments applied must protect both organs. Today, lung protective MV is an accepted supportive treatment for patients with concomitant brain and lung injury. The evidence suggests that critical illness often results in long-term neurocognitive impairments in one-third of survivors and that these impairments have a significant impact on daily living, quality of life and economic costs. Further efforts must be made to evaluate, prevent and improve cognitive alterations after ICU admission.
Acknowledgements
Funding: This work was carried out as part of the Neurocognition and Critically Ill Patients research line at the University Hospital Parc Taulí, which is co-funded by the Programs of Support to Research: SGR-1320 from the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) Departament d’Empresa I Coneixement de la Generalitat de Catalunya, CIBER de Enfermedades Respiratorias from the Instituto de Salud Carlos III and Fundació Parc Taulí (Ref. CIR 2014/028). This project is part of the research programs PI13/02204 and PI16/01606 in the Spanish Plan Nacional de R+D+I and co-funded by the ISCIII- Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER); Marató TV3 (ref. 112810) and CIBERES-CIBER BIOINGENIERIA BIOMATERIALES Y NANOMEDICINA (CIBER-BBN)(Ref. ES15PINT007).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Wolters AE, Slooter AJ, van der Kooi AW, et al. Cognitive impairment after intensive care unit admission: a systematic review. Intensive Care Med 2013;39:376-86. 10.1007/s00134-012-2784-9 [DOI] [PubMed] [Google Scholar]
- 2.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 2012;185:1307-15. 10.1164/rccm.201111-2025OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2005;171:340-7. 10.1164/rccm.200406-763OC [DOI] [PubMed] [Google Scholar]
- 4.Guerra C, Linde-Zwirble WT, Wunsch H. Risk factors for dementia after critical illness in elderly Medicare beneficiaries. Crit Care 2012;16:R233. 10.1186/cc11901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duning T, van den Heuvel I, Dickmann A, et al. Hypoglycemia aggravates critical illness-induced neurocognitive dysfunction. Diabetes Care 2010;33:639-44. 10.2337/dc09-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins RO, Suchyta MR, Snow GL, et al. Blood glucose dysregulation and cognitive outcome in ARDS survivors. Brain Inj 2010;24:1478-84. 10.3109/02699052.2010.506861 [DOI] [PubMed] [Google Scholar]
- 7.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med 2003;31:1226-34. 10.1097/01.CCM.0000059996.30263.94 [DOI] [PubMed] [Google Scholar]
- 8.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med 2010;182:183-91. 10.1164/rccm.200903-0442OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 2013;21:1190-222. 10.1016/j.jagp.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 10.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294-323. 10.1164/ajrccm.157.1.9604014 [DOI] [PubMed] [Google Scholar]
- 11.Murphy DB, Cregg N, Tremblay L, et al. Adverse ventilatory strategy causes pulmonary-to-systemic translocation of endotoxin. Am J Respir Crit Care Med 2000;162:27-33. 10.1164/ajrccm.162.1.9908110 [DOI] [PubMed] [Google Scholar]
- 12.Blanch L, Quintel M. Lung-brain cross talk in the critically ill. Intensive Care Med 2017;43:557-9. 10.1007/s00134-016-4583-1 [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Aguilar J, Quílez ME, Marti-Sistac O, et al. Early physiological and biological features in three animal models of induced acute lung injury. Intensive Care Med 2010;36:347-55. 10.1007/s00134-009-1695-x [DOI] [PubMed] [Google Scholar]
- 14.Quílez ME, Fuster G, Villar J, et al. Injurious mechanical ventilation affects neuronal activation in ventilated rats. Crit Care 2011;15:R124. 10.1186/cc10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998;157:1721-5. 10.1164/ajrccm.157.6.9709092 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalvo R, Marti-Sistac O, Blanch L, et al. Bench-to-bedside review: brain-lung interaction in the critically ill--a pending issue revisited. Crit Care 2007;11:216. 10.1186/cc5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quílez ME, Lopez-Aguilar J, Blanch L. Organ crosstalk during acute lung injury, acute respiratory distress syndrome, and mechanical ventilation. Curr Opin Crit Care 2012;18:23-8. 10.1097/MCC.0b013e32834ef3ea [DOI] [PubMed] [Google Scholar]
- 18.Quílez ME, Rodriguez-Gonzalez R, Turon M, et al. Moderate Peep After Tracheal Lipopolysaccharide Instillation Prevents Inflammation and Modifies the Pattern of Brain Neuronal Activation. Shock 2015;44:601-8. 10.1097/SHK.0000000000000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Zhang Z, Chen T, et al. Prolonged mechanical ventilation-induced neuroinflammation affects postoperative memory dysfunction in surgical mice. Crit Care 2015;19:159. 10.1186/s13054-015-0882-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-López A, Lopez-Alonso I, Aguirre A, et al. Mechanical ventilation triggers hippocampal apoptosis by vagal and dopaminergic pathways. Am J Respir Crit Care Med 2013;188:693-702. 10.1164/rccm.201304-0691OC [DOI] [PubMed] [Google Scholar]
- 21.Plotz FB, Slutsky AS, van Vught AJ, et al. Ventilator-induced lung injury and multiple system organ failure: a critical review of facts and hypotheses. Intensive Care Med 2004;30:1865-72. 10.1007/s00134-004-2363-9 [DOI] [PubMed] [Google Scholar]
- 22.Pelosi P, Rocco PR. The lung and the brain: a dangerous cross-talk. Crit Care 2011;15:168. 10.1186/cc10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raux M, Ray P, Prella M, et al. Cerebral cortex activation during experimentally induced ventilator fighting in normal humans receiving noninvasive mechanical ventilation. Anesthesiology 2007;107:746-55. 10.1097/01.anes.0000287005.58761.e8 [DOI] [PubMed] [Google Scholar]
- 24.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest 2006;130:869-78. 10.1378/chest.130.3.869 [DOI] [PubMed] [Google Scholar]
- 25.Pustavoitau A, Stevens RD. Mechanisms of neurologic failure in critical illness. Crit Care Clin 2008;24:1-24, vii. 10.1016/j.ccc.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 26.European Delirium Association. American Delirium Society The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med 2014;12:141. 10.1186/s12916-014-0141-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol 2009;5:210-20. 10.1038/nrneurol.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291:1753-62. 10.1001/jama.291.14.1753 [DOI] [PubMed] [Google Scholar]
- 29.Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 2007;33:66-73. 10.1007/s00134-006-0399-8 [DOI] [PubMed] [Google Scholar]
- 30.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma 2008;65:34-41. 10.1097/TA.0b013e31814b2c4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pisani MA, Murphy TE, Van Ness PH, et al. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med 2007;167:1629-34. 10.1001/archinte.167.15.1629 [DOI] [PubMed] [Google Scholar]
- 32.Pisani MA, Kong SY, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med 2009;180:1092-7. 10.1164/rccm.200904-0537OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med 2010;38:2311-8. 10.1097/CCM.0b013e3181f85759 [DOI] [PubMed] [Google Scholar]
- 34.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 2010;38:1513-20. 10.1097/CCM.0b013e3181e47be1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med 2004;32:955-62. 10.1097/01.CCM.0000119429.16055.92 [DOI] [PubMed] [Google Scholar]
- 36.van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 2012;344:e420. 10.1136/bmj.e420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Rompaey B, Elseviers MM, Schuurmans MJ, et al. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care 2009;13:R77. 10.1186/cc7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, et al. Risk factors for intensive care delirium: a systematic review. Intensive Crit Care Nurs 2008;24:98-107. 10.1016/j.iccn.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 39.Vasilevskis EE, Pandharipande PP, Girard TD, et al. A screening, prevention, and restoration model for saving the injured brain in intensive care unit survivors. Crit Care Med 2010;38:S683-91. 10.1097/CCM.0b013e3181f245d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaal IJ, Devlin JW, Peelen LM, et al. A systematic review of risk factors for delirium in the ICU. Crit Care Med 2015;43:40-7. 10.1097/CCM.0000000000000625 [DOI] [PubMed] [Google Scholar]
- 41.Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care 2008;12 Suppl 3:S3. 10.1186/cc6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flacker JM, Cummings V, Mach JR, Jr, et al. The association of serum anticholinergic activity with delirium in elderly medical patients. Am J Geriatr Psychiatry 1998;6:31-41. 10.1097/00019442-199802000-00005 [DOI] [PubMed] [Google Scholar]
- 43.Trzepacz PT. Delirium. Advances in diagnosis, pathophysiology, and treatment. Psychiatr Clin North Am 1996;19:429-48. 10.1016/S0193-953X(05)70299-9 [DOI] [PubMed] [Google Scholar]
- 44.Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med 2001;29:S99-106. 10.1097/00003246-200107001-00032 [DOI] [PubMed] [Google Scholar]
- 45.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med 1999;340:207-14. 10.1056/NEJM199901213400307 [DOI] [PubMed] [Google Scholar]
- 46.Zhang QH, Sheng ZY, Yao YM. Septic encephalopathy: when cytokines interact with acetylcholine in the brain. Mil Med Res 2014;1:20. 10.1186/2054-9369-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 2010;375:773-5. 10.1016/S0140-6736(09)61158-2 [DOI] [PubMed] [Google Scholar]
- 48.Mikkelsen ME, Shull WH, Biester RC, et al. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology 2009;14:76-82. 10.1111/j.1440-1843.2008.01419.x [DOI] [PubMed] [Google Scholar]
- 49.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369:1306-16. 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woon FL, Dunn CB, Hopkins RO. Predicting cognitive sequelae in survivors of critical illness with cognitive screening tests. Am J Respir Crit Care Med 2012;186:333-40. 10.1164/rccm.201112-2261OC [DOI] [PubMed] [Google Scholar]
- 51.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293-304. 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 52.Norman BC, Jackson JC, Graves JA, et al. Employment Outcomes After Critical Illness: An Analysis of the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors Cohort. Crit Care Med 2016;44:2003-9. 10.1097/CCM.0000000000001849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adhikari NK, Fowler RA, Bhagwanjee S, et al. Critical care and the global burden of critical illness in adults. Lancet 2010;376:1339-46. 10.1016/S0140-6736(10)60446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fadel PJ, Barman SM, Phillips SW, et al. Fractal fluctuations in human respiration. J Appl Physiol (1985) 2004;97:2056-64. [DOI] [PubMed] [Google Scholar]
- 55.Rubin JE, Shevtsova NA, Ermentrout GB, et al. Multiple rhythmic states in a model of the respiratory central pattern generator. J Neurophysiol 2009;101:2146-65. 10.1152/jn.90958.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith JC, Abdala AP, Koizumi H, et al. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 2007;98:3370-87. 10.1152/jn.00985.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dellaca RL, Aliverti A, Lo Mauro A, et al. Correlated variability in the breathing pattern and end-expiratory lung volumes in conscious humans. PLoS One 2015;10:e0116317. 10.1371/journal.pone.0116317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruce EN. Temporal variations in the pattern of breathing. J Appl Physiol (1985) 1996;80:1079-87. [DOI] [PubMed] [Google Scholar]
- 59.Bruce EN. Nonlinear Dynamics of Respiratory Reflexes. IFAC Proceedings Volumes 1994;27:497-500. [Google Scholar]
- 60.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E Stat Nonlin Soft Matter Phys 2005;71:021906. 10.1103/PhysRevE.71.021906 [DOI] [PubMed] [Google Scholar]
- 61.Pincus S. Approximate entropy (ApEn) as a complexity measure. Chaos 1995;5:110-7. 10.1063/1.166092 [DOI] [PubMed] [Google Scholar]
- 62.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 2000;278:H2039-49. 10.1152/ajpheart.2000.278.6.H2039 [DOI] [PubMed] [Google Scholar]
- 63.Peng CK, Havlin S, Stanley HE, et al. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995;5:82-7. 10.1063/1.166141 [DOI] [PubMed] [Google Scholar]
- 64.Theiler J, Eubank S, Longtin A, et al. Testing for nonlinearity in time series: the method of surrogate data. Physica D: Nonlinear Phenomena 1992;58:77-94. 10.1016/0167-2789(92)90102-S [DOI] [Google Scholar]
- 65.Mazzeo AT, Fanelli V, Mascia L. Brain-lung crosstalk in critical care: how protective mechanical ventilation can affect the brain homeostasis. Minerva Anestesiol 2013;79:299-309. [PubMed] [Google Scholar]
- 66.Elmer J, Hou P, Wilcox SR, et al. Acute respiratory distress syndrome after spontaneous intracerebral hemorrhage*. Crit Care Med 2013;41:1992-2001. 10.1097/CCM.0b013e31828a3f4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beitler JR, Ghafouri TB, Jinadasa SP, et al. Favorable Neurocognitive Outcome with Low Tidal Volume Ventilation after Cardiac Arrest. Am J Respir Crit Care Med 2017;195:1198-206. 10.1164/rccm.201609-1771OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mascia L, Grasso S, Fiore T, et al. Cerebro-pulmonary interactions during the application of low levels of positive end-expiratory pressure. Intensive Care Med 2005;31:373-9. 10.1007/s00134-004-2491-2 [DOI] [PubMed] [Google Scholar]
- 69.Jackson JC, Ely EW, Morey MC, et al. Cognitive and physical rehabilitation of intensive care unit survivors: results of the RETURN randomized controlled pilot investigation. Crit Care Med 2012;40:1088-97. 10.1097/CCM.0b013e3182373115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandez-Gonzalo S, Turon M, De Haro C, et al. Do sedation and analgesia contribute to long-term cognitive dysfunction in critical care survivors? Med Intensiva 2017. [Epub ahead of print]. 10.1016/j.medin.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 71.Mehlhorn J, Freytag A, Schmidt K, et al. Rehabilitation interventions for postintensive care syndrome: a systematic review. Crit Care Med 2014;42:1263-71. 10.1097/CCM.0000000000000148 [DOI] [PubMed] [Google Scholar]
- 72.Burtin C, Clerckx B, Robbeets C, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med 2009;37:2499-505. 10.1097/CCM.0b013e3181a38937 [DOI] [PubMed] [Google Scholar]
- 73.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 2008;36:2238-43. 10.1097/CCM.0b013e318180b90e [DOI] [PubMed] [Google Scholar]
- 74.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874-82. 10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brummel NE, Girard TD. Preventing delirium in the intensive care unit. Crit Care Clin 2013;29:51-65. 10.1016/j.ccc.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brummel NE, Girard TD, Ely EW, et al. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and Cognitive Therapy in ICU (ACT-ICU) trial. Intensive Care Med 2014;40:370-9. 10.1007/s00134-013-3136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turon M, Fernandez-Gonzalo S, Jodar M, et al. Feasibility and safety of virtual-reality-based early neurocognitive stimulation in critically ill patients. Ann Intensive Care 2017;7:81. 10.1186/s13613-017-0303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turon M, Fernandez-Gonzalo S, Gomez-Simon V, et al. Cognitive stimulation in ICU patients: should we pay more attention? Crit Care 2013;17:158. 10.1186/cc12719 [DOI] [PMC free article] [PubMed] [Google Scholar]