Abstract
Signaling mediated by G protein-coupled receptors (GPCRs) is essential for the migration of cells toward chemoattractants. The recruitment of neutrophils to injured tissues in zebrafish larvae is a useful model for studying neutrophil migration and trafficking in vivo. Indeed, the study of this process led to the discovery that PI3Kγ is required for the polarity and motility of neutrophils, features that are necessary for the directed migration of these cells to wounds. However, the mechanism by which PI3Kγ is activated remains to be determined. Here we show that signaling by specifically the heterotrimeric G protein subunit Gβ1 is critical for neutrophil migration in response to wounding. In embryos treated with small-molecule inhibitors of Gβγ signaling, neutrophils failed to migrate to wound sites. Although both the Gβ1 and Gβ4 isoforms are expressed in migrating neutrophils, only deficiency for the former (morpholino-based knockdown) interfered with the directed migration of neutrophils towards wounds. The Gβ1 deficiency also impaired the ability of cells to change cell shape and reduced their general motility, defects that are similar to those in neutrophils deficient for PI3Kγ. Transplantation assays showed that the requirement for Gβ1 in neutrophil migration is cell autonomous. Finally, live imaging revealed that Gβ1 is required for polarized activation of PI3K, and for the actin dynamics that enable neutrophil migration. Collectively, our data indicate that Gβ1 signaling controls proper neutrophil migration by activating PI3K and modulating actin dynamics. Moreover, they illustrate a role for a specific Gβ isoform in chemotaxis in vivo.
Keywords: Cell migration, Neutrophil, G protein, Gβ1, Imaging
1. Introduction
Directed cell migration along a chemoattractant gradient, i.e., chemotaxis, is critical for many physiological and pathological processes, including embryonic development, wound healing, immune responses and cancer metastasis (Swaney et al., 2010). Many signaling molecules serve as chemoattractants, acting on cell surface receptors to regulate chemotaxis. G protein-coupled receptors (GPCRs) are such receptors. They transmit chemotactic signals through heterotrimeric G proteins, which consist of multiple isoforms of α, β and γ subunits. Upon stimulation, the Gα subunit and Gβγ dimer dissociate from one another, and each activates downstream effectors in a particular signaling pathway (Dupre et al., 2009; Hamm, 1998; Smrcka, 2008a). G proteins are divided into four classes based on their α subunit: Gs, Gi, Gq and G12/13 (Simon et al., 1991). Studies using Dictyostelium discoideum and leukocytes have implicated Gα12/13 and the free Gβγ released by Gi in the regulation of chemotaxis via distinct mechanisms: Gα12/13 activate the Rho guanine exchange factor (RhoGEF) and RhoA to facilitate retraction at the cell posterior; and Gβγ activates phosphatidylinositol 3-kinase (PI3K) in the leading region of the cell to establish an intracellular gradient of signal that is critical for cell polarity and directed migration (Wang, 2009).
The importance of G protein signaling in cell migration in vivo is being increasingly recognized (Bussmann and Raz, 2015). In particular, signaling mediated by the chemokine Cxcl12 (also known as stromal cell-derived factor-1, or SDF-1) and its cognate receptor Cxcr4, a GPCR, has been implicated in the migration of a wide range of cell types, including primordial germ cells (Boldajipour et al., 2011; Doitsidou et al., 2002; Knaut et al., 2003), cells of the lateral line primordium (LLP) (Haas and Gilmour, 2006), endodermal progenitors (Mizoguchi et al., 2008; Nair and Schilling, 2008), and endothelial cells of vascular and lymphatic vessels (Cha et al., 2012; Harrison et al., 2015; Ivins et al., 2015; Siekmann et al., 2009). Our work has shown that G proteins are involved in cell migration in vivo, with: Gα12/13 regulating convergence and extension movements, as well as epiboly, during gastrulation (Lin et al., 2009, 2005); Gβγ signaling enabling PGC migration by regulating Rac1 activity (Xu et al., 2012); and Gβ1 promoting LLP migration, probably in response to Cxcr12a/Cxcr4b signaling (Xu et al., 2014).
Neutrophil migration in zebrafish has proven useful for studying cell migration in vivo at high resolution, owing to the transparency of the zebrafish embryos and the ability to genetically label neutrophils with GFP (Deng and Huttenlocher, 2012; Mathias et al., 2006; Renshaw et al., 2006). In response to wounding, the neutrophils are rapidly recruited to sites of injury to clear inflammation (Mathias et al., 2006; Renshaw et al., 2006). Additionally, neutrophils in the head mesenchyme display spontaneous high motility (Yoo et al., 2010). It is not entirely clear which signals generated by tissue injury trigger neutrophil migration. A gradient of hydrogen peroxide (H2O2) induced by wounding provides a rapid signal that recruits neutrophils to the wound region (Niethammer et al., 2009). However, such H2O2 production is not necessary for the recruitment of neutrophils towards bacterial infection (Deng et al., 2012), indicating that neutrophil migration utilizes diverse mechanisms in response to various stimuli. On the other hand, recent studies showed that expression of the chemokine Cxcl8 is increased in response to bacterial and chemical insult (Oehlers et al., 2010), as well as tissue injury (de Oliveira et al., 2013), and that the signaling mediated by Cxcl8 and its receptor Cxcr2 is required for the recruitment of neutrophils to sites of bacterial infection (Deng et al., 2013) and transection injury (de Oliveira et al., 2013). Additionally, the Cxcl12-Cxcr4 signaling axis has been shown to be involved in neutrophil motility and recruitment to wounds (Walters et al., 2010). These findings indicate that GPCR signaling plays a vital role in neutrophil migration in vivo.
Direct evidence in support of GPCR signaling in neutrophils comes from live imaging studies, which have shown that activated PI3K [evident from the production of phosphatidylinositol (3,4,5)P3-phosphatidylinositol (3,4)P2, PI(3,4,5)P3-PI(3,4)P2] accumulates at the leading edge of the migrating neutrophil (Yoo et al., 2010). Importantly, it has also been shown that PI3Kγ, the major downstream effector of Gβγ signaling, is required for the production of PI(3,4,5)P3-PI(3,4)P2 in neutrophils, and for their motility and directed migration (Yoo et al., 2010). However, the mechanism responsible for the activation of PI3Kγ is not known.
In this study, we show that Gβγ signaling is required for the directed migration of neutrophils in response to wounding, as well as for their general motility. We found that neutrophils express both Gβ1 and Gβ4, yet only Gβ1 is essential for their migration, indicating that Gβ function is isoform specific. Additionally, ratiometric imaging revealed that signaling by Gβ1 is needed for polarized activation of PI3K, and for the actin dynamics that drive neutrophil migration. These findings reveal an additional mechanism that neutrophils utilize for their migration.
2. Materials and methods
2.1. Zebrafish strains and husbandry
WT (Tubingen, Tubingen/AB) and transgenic Tg(mpx:GFP) (Renshaw et al., 2006), Tg(actb2: Lifeact-GFP) and Tg(actb1:GFP-utrCH) (Behrndt et al., 2012) strains of zebrafish were used and maintained as described previously (Xu et al., 2011). Embryos were obtained by natural mating and staged according to hours post fertilization (hpf) at 28.5 °C, using standard methods.
2.2. Morpholino (MO) and DNA injection
Previously validated MOs (obtained from Gene Tools, LLC) that target the translation (the nucleotides that target the ATG initiation codon are underlined) or RNA slicing of gnb1 and gnb4 (Hippe et al., 2009; Xu et al., 2012, 2014) were injected into embryos at the one-cell stage at the indicated doses. In all cases, a p53 MO (2 ng) was co-injected to reduce MO-triggered general apoptosis (Robu et al., 2007). Two sets of MOs targeting gnb1 (gnb1a and gnb1b) were used: gnb1a MO1 (2 ng, 5′-GAGTTCGCTCATTTTCTTCTGCTTC) plus gnb1b MO1 (2 ng, 5′-CTGGTCCAGTTCACTCATTTTCCTC) (Xu et al., 2012, 2014); and gnb1a MO2 (3 ng, 5′-CTGGTCGAGTTCGCTCATTTTCTTC) (Hippe et al., 2009) plus gnb1b MO2 (8 ng, 5′-AATTAGGTGGTT-ACCTGTGATAGT, targets the splice site at the junction between the first exon and the following intron) (Xu et al., 2014). One set of MOs targeting gnb4 (gnb4a and gnb4b) was used: gnb4a MO1 (4 ng, 5′-CCGCAACTGCTC CAGCTCACTCATG-3′) plus gnb4b MO1 (4 ng, 5′-GACGCAACTGCTCCAACTCACTCAT) (Xu et al., 2014). The efficiency of these MOs was demonstrated by Western blotting (in the case of gnb1a MO2, Fig. 3 of Hippe et al. (2009); for all others, in Supplementary Fig. 1 of Xu et al. (2014)). The mpx:PHAKT-EGFP and mpx:DsRed plasmids (25 pg), together with the Tol2 transposase mRNA (30 pg), were injected into embryos at the one-cell stage (Yoo et al., 2010).
2.3. Tailfin wounding assay and drug treatment
Wounding assays were carried out as described previously (Mathias et al., 2006). Briefly, a small portion of the tail fin was excised from embryos at 48 or 72 hpf, and the embryos were then embedded immediately in 1% low melting-point agarose (LMA) for live imaging. For drug treatment, embryos were pretreated with M158C (25 μM), M158D (25 μM) or M119 (100 μM) in 1% DMSO for 30 min at 28 °C before wounding. After wounding, the embryos were embedded in LMA containing the same drug, and during live imaging they were covered with Danieau buffer also containing the drug. In some cases, 2 h after wounding the embryos were fixed with 4% PFA and the number of neutrophils located at the wound site were counted.
2.4. Whole-mount immunofluorescence
After wounding, the embryos were fixed in 4% PFA overnight at 4 °C. Whole-mount immunohistochemistry was performed as described previously (Lin et al., 2005; Xu et al., 2014). The following antibodies were used: pan-Gβ (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) and anti-Gβ1 (1:200, GeneTex, Irvine, CA).
2.5. Transplantation
Tg(mpx:GFP) embryos served as donors, and wild-type embryos as hosts. Approximately 50 donor cells at the sphere stage were transplanted into host embryos at the equivalent stage, as described previously (Xu et al., 2014). Host embryos were screened for the presence of GFP-positive neutrophils at 2 dpf. Wounding and time-lapse imaging were performed as described.
2.6. Microscopy, time-lapse imaging and analysis
For time-lapse imaging, embryos were embedded in 1% low melting-point agarose using glass-bottom dishes. Epifluorescence time-lapse imaging was performed using a Leica DMI 6000 microscope; samples were imaged on a stage heated to 28.5 °C as described previously (Xu et al., 2014). For the analysis of neutrophil migration, images were taken using a 10×/NA 0.3 objective at 5-min intervals for 2 h, and refocusing was performed throughout the imaging period. For the analysis of changes in cell morphology, neutrophils in the head were imaged using a 20×/NA 0.7 objective, at 15-s intervals for 30 min. Confocal time-lapse imaging was performed using a laser-scanning confocal inverted microscope (LSM700, Carl Zeiss, Inc.) with a 20×/ NA 0.8 objective, and Z-stack images were taken at 1–2 μm intervals (the entire group of neutrophils was covered) every 30 s for 15 min, using the following settings: 1024 × 1024 pixels, speed 9, two averaging and a 1 air unit pinhole. For still confocal imaging, embryos were mounted in 75% glycerol/PBS and Z-stack images covering all neutrophils were acquired using an LD C-Apo 40×/NA 1.1 water objective.
All time-lapse images were initially analyzed using the Metamorph or Fiji software. Cell tracking was analyzed using the manual tracking plug-in of the Fiji software. Data were exported to Excel, where the speed, path, and direction of cell migration were determined as reported previously (Lin et al., 2005). The direction of neutrophil movement was determined at 2–3 min intervals, and the resulting angles relative to anterior-posterior body axis were plotted as rose diagrams using PAST software (https://folk.uio.no/ohammer/past/) (Hammer et al., 2001; Williams et al., 2014). For assessment of changes in cell morphology, neutrophils of interest were outlined, and the length (L) and width (W) and of the cell at each time point were determined using Fiji. Changes in the length-to-width ratio (ΔLWR) between two time points Tn and Tn-1 [ΔLWR/s = (LWRn-LWRn-1)/(Tn-Tn-1)] were calculated.
For analysis of the distribution of PHAKT-GFP, mpx:DsRed and mpx:PHAKT-GFP plasmid DNAs were co-injected into WT embryos; for monitor actin dynamics in neutrophils, mpx:DsRed plasmid DNA was injected into Tg(actb2: Lifeact-GFP) or Tg(actb1:GFP-utrCH) embryos, in which all cells were labeled with Lifeact-GFP or GFP-utrCH. The DsRed signal was used to determine the effects on cytoplasmic volume and the cell membrane of neutrophils. Ratiometric GFP/DsRed images (in which the GFP signal is divided by the DsRed signal) were used to determine the distributions of PHAKT and actin in migrating neutrophils in vivo, using methods described previously (Kardash et al., 2010; Xu et al., 2012). Briefly, all images were converted into 32-bit images using the ImageJ software. “Threshold” was applied using the “auto” default settings of Fiji, and the background was set to NaN. The median value of the entire individual neutrophil was measured, and was used to normalize the image. The ratiometric GFP/DsRed images were generated.
To quantify the ratio of the GFP/DsRed intensities in different regions of neutrophils, we reviewed the time-lapse movies and identified time points at which neutrophils did not have many pseudopods. We then oriented the images of the neutrophils in the direction of migration of a cell, selected that cell (using the rectangle tool to span the leading and trailing edges of the cell), and performed “plot profile” analysis. The resulting measurements represent the relative GFP/DsRed intensities along the long axis of the cell body axes, which was then divided into quarters. The average GFP/DsRed intensity in each quarter of the cell was calculated, with the first quarter representing the trailing region, and the fourth quarter the leading region (Fig. 6G).
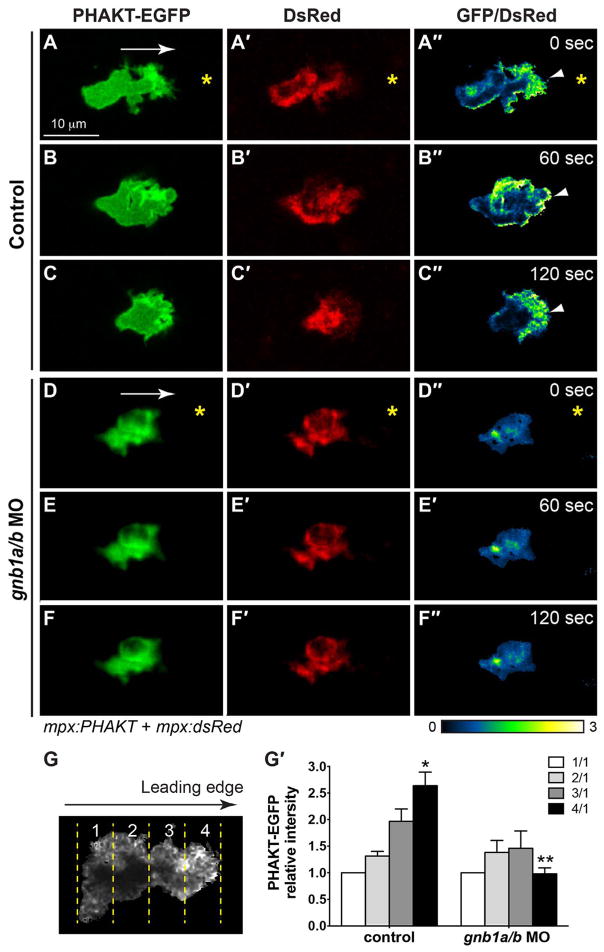
Fig. 6. Gβ1 signaling regulates directed neutrophil migration by activating PI3K.
(A–F″) Snapshots from confocal time-lapse imaging (Supplementary Movie 6) of migrating neutrophils expressing PHAKT-EGFP and DsRed in control (A–C) and gnb1a/b MO1-injected (D–F) embryos following wounding of tailfin. (A–F) PHAKT-EGFP. (A′-F′) DsRed. (A″-F″) Ratiometric images of PHAKT-EGFP/DsRed. Yellow asterisks: side nearest wound; white arrowheads: PHAKT-GFP enrichment at neutrophil leading front; white arrows: direction of migration. (G–G′) Quantification of ratios of intensity of PHAKT-EGFP/DsRed. (G) Illustration of the method. Neutrophils were selected for “plot profile” analysis using the rectangle tool to span the leading and trailing edges of the cells. The measurements were grouped by quarter and the average GFP/DsRed intensity in each quarter was calculated, with the first quarter representing the trailing region and the fourth quarter representing the leading region. (G′) Graph showing the ratio of intensities of PHAKT-GFP/DsRed in each quarter relative to that in the first quarter in control (5 neutrophils in 5 embryos) and gnb1a/b MO1-injected (7 neutrophils in 7 embryos) embryos. * p = 0.003 vs 1/1 in control, ** p = 0.0001 vs 4/1 in control by t-test, two-way ANOVA; p < 0.0001 among groups in control embryos, by one-way ANOVA; p = 0.587 among groups in gnb1a/b MO1-injected embryos, by one-way ANOVA.
Because the leading region is enriched for PHAKT-GFP, the intensity of signal in the first quarter was used as the baseline in determining the relative intensity of signal in the other regions. For GFP-utrCH imaging, the intensity in the fourth quarter (the leading region) was used as the baseline in determining the relative intensity of the other regions.
All images of the same type were acquired using the same settings, processed using the Metamorph or Fiji software, and edited and compiled using Adobe Photoshop® and Adobe Illustrator software. Graphs were generated using Graphic Prism software.
2.7. Statistical analysis
Data were compiled from at least two independent experiments and are presented as the mean ± SEM. Statistical analyses were performed using the Prism 7 software (GraphPad). For two groups of data, unpaired 2-tailed Student’s t-tests with unequal variance and two–way ANOVA were used; for multiple groups of data, one-way ANOVA analysis was performed. The number of cells and embryos analyzed in each experiment is indicated in the figure legends.
3. Results
3.1. Gβγ signaling is required for the migration of neutrophils to wounds
To determine if Gβγ signaling is required for the migration of neutrophils in response to wounding, we utilized small-molecule inhibitors that disrupt Gβγ signaling by binding competitively to the functional “hotspots” of βγ G in complex with downstream signaling proteins, including PI3Kγ (Bonacci et al., 2006). We used Tg(mpx:GFP) embryos, in which GFP expression is driven by the neutrophil-specific myeloperoxidase (mpx) promoter, and monitored the migration of GFP-expressing neutrophils (Renshaw et al., 2006). Embryos were pre-treated with the Gβγ inhibitor M158C or its inactivated form, M158D, for 30 min (Bonacci et al., 2006), and then the tailfin was injured as described previously (a small portion was excised) (Mathias et al., 2009, 2006; Renshaw et al., 2006), and the embryos were immediately monitored for neutrophil migration. As shown previously (Mathias et al., 2009, 2006; Renshaw et al., 2006; Yoo et al., 2010), in control embryos treated with 1%DMSO, the wound attracted neutrophils over the course of 2 h (Fig. 1A–A′, Supplementary Movie 1). In embryos treated with the Gβγ inhibitor M158C, very few neutrophils migrated to the wound during this period (Fig. 1C–C′, Supplementary Movie 1), whereas in counterparts treated with M158D, they migrated to the wound normally (Fig. 1B–B′, Supplementary Movie 1). Quantitation of neutrophil migration 2 h after wounding revealed that on average the number of neutrophils that had migrated in control embryos was ~ 4 (4.4 ± 0.4, 30 embryos), in M158D-treated embryos ~ 5 (5.1 ± 0.4, 28 embryos, p = 0.29, t-test), and in M158C-treated embryos < 1 (0.6 ± 0.2 neutrophils, 28 embryos; p < 0.0001 vs control neutrophils, t-test and two–way ANOVA)(Fig. 1D).
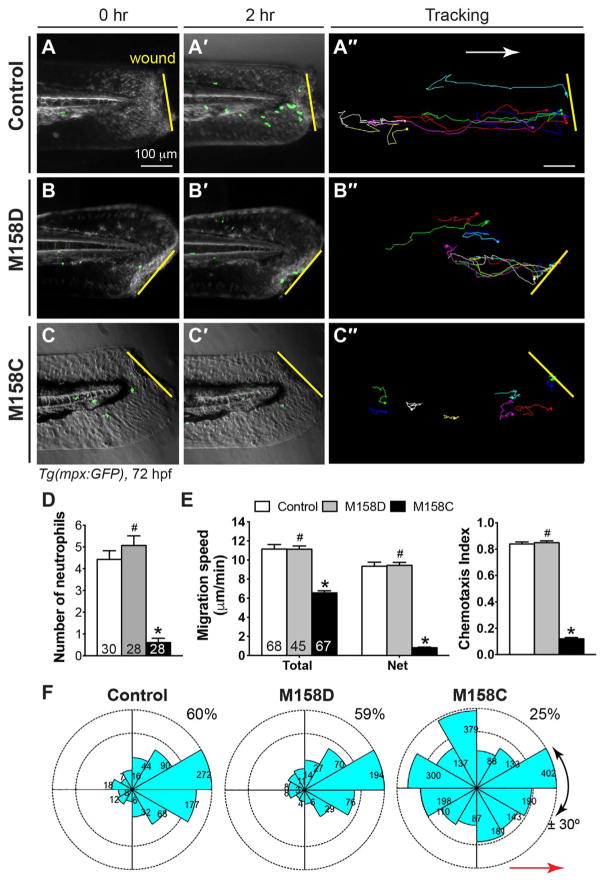
Fig. 1. Gβγ signaling is required for wound-induced neutrophil migration in vivo.
72-hpf Tg(mpx:GFP) embryos pretreated for 30 min with the small-molecule Gβγ inhibitor M158C (25 μM), its inactive compound M158D (25 μM), or 1% DMSO only (control), were wounded (a portion of the tail fin was excised). (A–C′) Snapshots from epifluorescence and bright-field movies of the tail region (right) immediately after wounding (0 h, A–C) and 2 h after wounding (2 h, A′–C′). (A″–C″) Representative tracks (multiple colors) delineate routes of migration of individual neutrophils. (D) Number of neutrophils that reach the wound site within 2 h (the number of embryos analyzed is shown). (E) Total and net speeds of migration, and chemotaxis index, of neutrophils in 1% DMSO- (68 neutrophils, 7 embryos), M158D- (45 neutrophils, 5 embryos), and M158C- (67 neutrophils, 9 embryos) treated embryos. (F) Rose diagrams showing the directions of neutrophil movement events relative to the direction of migration (along anteroposterior axis, red line with arrowhead). The direction of migration was analyzed every 2–3 min throughout the time-lapse period, and were grouped into 30° sectors, with the number of events in each sector indicated. The fraction of migration events oriented at an angle of ± 30° with respect to the direction of migration is 60% of 750 events in 1% DMSO-treated neutrophils, 59% of 453 events in M158D-treated neutrophils, and 25% of 453 events in M158C-treated neutrophils (p < 0.001, by t-test, two-way ANOVA, for M158C vs DMSO; p < 0.001, by one-way ANOVA for all three groups). Yellow lines: areas of wounding; Red line with arrowhead: the direction of migration. #: p > 0.05 vs control, *: p < 0.001 vs control.
Supplementary material related to this article can be found online at http://dx.doi.org/10.1016/j.ydbio.2017.05.024.
To further investigate the migration behavior of neutrophils, we performed time-lapse experiments in DMSO-treated control (68 neutrophils, 7 embryos), M158D-treated (45 neutrophils, 5 embryos) and M158C-treated (67 neutrophils, 9 embryos) embryos. Analysis of total cell speed, accounting for movements in all directions, revealed that the DMSO- and M158D-treated neutrophils migrated at similar speeds (11.15 ± 0.47 μm/min and 11.15 ± 0.33 μm/min respectively, p = 0.49, t-test), whereas the M158C-treated neutrophils migrated more slowly (6.56 ± 0.23 μm/min, p = 4.73×1015, vs control neutrophils, t-test and ANOVA) (Fig. 1E). One-way ANOVA analysis indicated that the speed of neutrophil migration in the M158C-treated group differed significantly from that in the others (p < 0.0001). An analysis of net speed, in which actual movements during the time-lapse period were accounted for, showed that DMSO- and M158D-treated neutrophils migrated at 9.35 ± 0.44 μm/min and 9.44 ± 0.32 μm/min, respectively (p > 0.05, t-test), and revealed the chemotaxis indexes (CIs; ratios of net migration to total migration) to be 0.84 ± 0.12 and 0.85 ± 0.08, respectively. This finding suggests that these neutrophils migrated efficiently toward the area of wounding. Notably, the net speed of the M158C-treated neutrophils was strongly compromised (0.81 ± 0.07 μm/min; p < 0.001, by t-test and ANOVA), and the CI was low (0.08 ± 0.01; p < 0.001, by t-test and ANOVA) (Fig. 1E), suggesting that these neutrophils underwent little directed migration. Consistent with these findings, tracking of cell migration paths revealed that whereas the DMSO- and M158D-treated neutrophils took fairly direct paths along the anterior-posterior axis towards the wound (Fig. 1A″–B″), the M158C-treated neutrophils migrated along circuitous paths (Fig. 1C″). Analysis of the direction of cell movement at 2–3 min intervals throughout the time-lapse period revealed that DMSO-and M158D-treated neutrophils migrated largely towards the area of wounding, with 60% (total 750 events) and 59% (total 453 events) of migration events occurring at ± 30° of the anterior-posterior axis (Fig. 1F). Strikingly, M158C-treated neutrophils displayed a random migration pattern (no obvious directionality), and only 25% of migration events (total 2354) occurred at ± 30° of the anterior-posterior axis (Fig. 1F; p < 0.0001, by t-test and ANOVA). Results for embryos treated with the Gβγ inhibitor M119 were similar to those for embryos treated with M158C (not shown). Thus, inhibiting Gβγ function impaired the directed migration of neutrophils, affecting both the direction of migration and migration speed following wounding. Thus, Gβγ signaling is required for proper neutrophil migration in zebrafish embryos.
3.2. Gβ1, but not Gβ4, is required for the direct migration of neutrophils
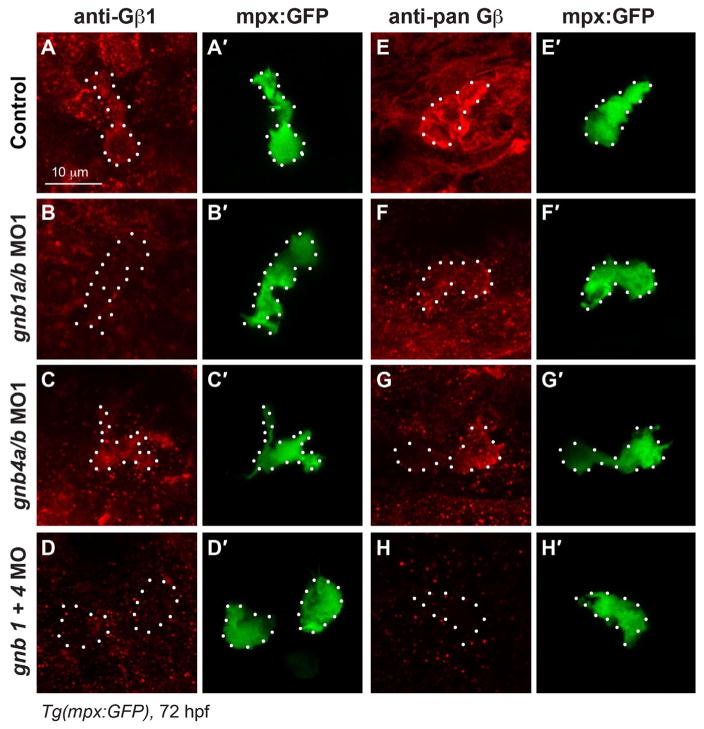
In our further investigation of the functions of Gβγ in neutrophil migration, we focused on Gβ, as it has fewer isoforms than Gγ. We previously identified nine zebrafish genes that encode five Gβ isoforms (all except Gβ2 are duplicated), and found that only the Gβ1 and Gβ4 isoforms (gnb1a, gnb1b, gnb4a, and gnb4b) are ubiquitously expressed throughout gastrulation and day 1 (Xu et al., 2012, 2014), the period during which neutrophils develop. Here, we examined the expression of Gβ1 and Gβ4 in neutrophils of Tg(mpx:GFP) zebrafish at 72 hpf, the time at which neutrophil migration had been assessed, using an antibody specific for the β1 isoform, and a pan-Gβ antibody that recognizes most Gβ isoforms including both Gβ1 and Gβ4. We found that Gβ was expressed in neutrophils, which are labeled with GFP in this zebrafish line (Fig. 2A–A′ and E–E′). We confirmed this expression by injecting previously validated antisense morpholino oligonucleotides (MOs) targeting gnb1a, gnb1b, gnb4a, and gnb4b (Xu et al., 2014). Injecting the gnb1a/b MOs almost completely eliminated the expression of Gβ1 in neutrophils (Fig. 2B–B′), but only reduced the expression Gβ detected by the pan-Gβ antibody (Fig. 2F–F′), suggesting that Gβ1 and other Gβ isoforms are expressed in neutrophils. Injecting the MOs targeting gnb4a and gnb4b (gnb4a/b MOs) did not change the expression of Gβ1 (Fig. 2C–C′), but reduced the total levels of Gβ detected by the pan-Gβ antibody (Fig. 2G–G′), suggesting that Gβ4 is also expressed in neutrophils. Finally, injecting MOs targeting all gnb1 and gnb4 isoforms eliminated not only the expression of Gβ1 (Fig. 2D–D′), but also that of total Gβ (Fig. 2H–H′). Together, these data indicate that Gβ1 and Gβ4 are expressed in neutrophils and that the MOs used efficiently inhibit the expression of their targeted genes in neutrophils.
Fig. 2. Both Gβ1 and Gβ4 are expressed in neutrophils.
(A–H) Confocal images of neutrophils showing the expression of Gβ1 (A–D) and total Gβ (E–H), as detected by immunostaining in 72 hpf-Tg(mpx:GFP) embryos, with or without injection of the indicated MOs. (A′–H′) Corresponding GFP-expressing neutrophils. White dots outline neutrophils.
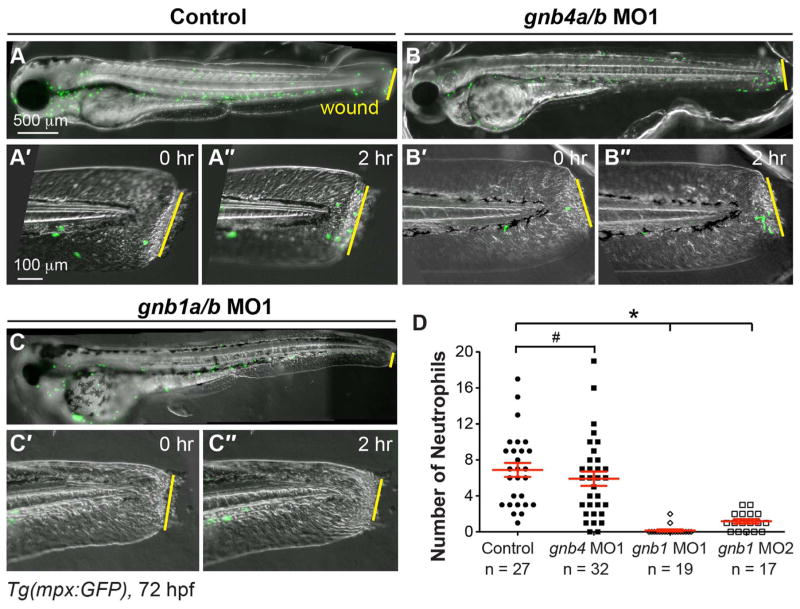
We then assessed the effects of inhibiting Gβ expression on the migration of neutrophils in Tg(mpx:GFP) embryos following wounding. We found that injecting gnb1a/b or gnb4a/b MOs did not change either overall embryo morphology or GFP expression at 72 hpf (Fig. 3A–C). Time-lapse imaging of the wounded tailfin showed that mpx-GFP expressing neutrophils in control, uninjected and gnb4a/b MOs-injected embryos migrated towards the wound, whereas very few neutrophils in gnb1a/b MOs-injected embryos did so (Fig. 3A′–C″ and D, Supplementary Movie 2). Quantification of the number of neutro-phils at the wound site 2 h after injury revealed 6 neutrophils (5.9 ± 0.8, 32 embryos) in gnb4a/b-depleted embryos, similar to the number observed in control embryos (6.9 ± 0.8, 27 embryos, P = 0.39). In contrast, on average less than 1 neutrophil was present in gnb1a/b-depleted embryos (0.2 ± 0.1, 19 embryos, P < 0.0001) (Fig. 3D). In addition, assessment of the total number of neutrophils revealed that there was no significant difference in control and gnb1 MO1-injected embryos (106 ± 4 and 95 ± 4 respectively, 8 embryos in each group, P = 0.18, t-test). Thus, the injection of gnb1 MO1 did not affect the overall number of neutrophils. Similarly, injection of another previously validated set of MOs (gnb1a/b MO2) (Hippe et al., 2009; Xu et al., 2014) significantly inhibited the recruitment of neutrophils towards wound sites after 2 h (1.1 ± 0.25, 17 embryos; Fig. 3D and data not shown). Collectively, theses data indicated that Gβ1, but not Gβ4, is required for the directed migration of neutrophils.
Fig. 3. Gβ1, but not Gβ4, is required for wound-induced neutrophil migration.
Epifluorescence time-lapse experiments tracking neutrophil migration for 2 h following wounding of the indicated 72 hpf-Tg(mpx:GFP) embryos. (A–C″) Overlay of epifluorescence and bright-field images of the whole embryo (A–C) and the tail region, immediately after wounding (0 h, A′–C′) and 2 h after wounding (2, A″–C″). Yellow lines: areas of wounding. (D) Number of neutrophils that reach the wound sites within 2 h of wounding (the number of embryos analyzed is indicated).
Supplementary material related to this article can be found online at http://dx.doi.org/10.1016/j.ydbio.2017.05.024.
3.3. Gβ1 in neutrophils directly regulates their migration in response to wounding
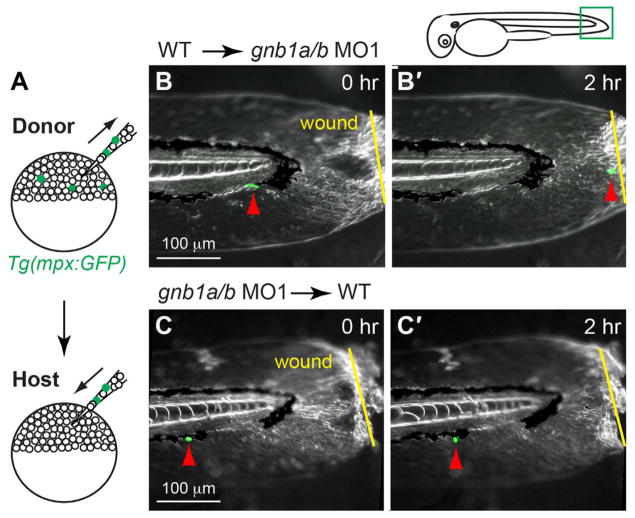
As Gβ1 is expressed ubiquitously in embryos, Gβ1 in either the neutrophils themselves or the surrounding cells could regulate neutrophil migration. To determine which is the case, we performed genetic mosaic experiments, transplanting cells from Tg(mpx:GFP) embryos into host wild-type embryos in which neutrophils were not labeled (Fig. 4A). Under these conditions, any mpx:GFP expressing neutrophils in the host embryos were derived only from the donor embryos.
Fig. 4. Gβ1 function in neutrophils is required for wound-induced neutrophil migration.
(A) Schematic diagram depicting transplantation procedure. Cells from 72 hpf-Tg(mpx:GFP) embryos (donor) were transplanted into wildtype (WT) embryos (host) at the sphere stage. (B–C) Snapshots from epifluorescence and bright-field time-lapse movie (Supplementary Movie 3), showing positions of neutrophils and wound sites immediately (0 h, B, C) and 2 h (2 h, B′, C′) after wounding. (B–B′) gnb1ab MO1-treated host embryos transplanted with cells from control Tg(mpx:GFP) embryo, with wound site (yellow arrows) and nearest GFP-expressing neutrophil (red arrows) indicated. In 6 out of 7 host embryos, transplanted mpx-GFP expressing neutrophils migrated to the wound. (C–C′) Control host embryos transplanted with cells from gnb1a/b MO1-injected Tg(mpx:GFP) embryo, with wound site (yellow line) and nearest GFP-expressing neutrophil (red arrows) indicated. In 6 host embryos, none of the transplanted mpx-GFP expressing neutrophils migrated to the wound. Drawing at top shows wound position and area imaged (green box).
We found that only 1% (10 in1000) host embryos contained GFP-expressing neutrophils from donor embryos. In these embryos, the number of GFP-expressing neutrophils found was typically less than three, and in some embryos 1–2 of these cells was located in the region near the tail. We screened host embryos for mpx-GFP expressing neutrophils in the tail region, injured the tailfin, and performed time-lapse imaging to monitor the migration of these cells following wounding. Thus, in most cases, we imaged one neutrophil per embryo. When wild-type neutrophils were transplanted into gnb1a/b-depleted embryos, in 6 out of 7 embryos mpx-GFP expressing neutrophils migrated to the wound (Fig. 4B–B′, Supplementary Movie 3). However, when cells from embryos injected with gnb1a/b-MOs were transplanted into wild-type embryos, the mpx-GFP expressing neutrophils failed to migrate to the wound site (Fig. 4C–C′, 6 embryos, Supplementary Movie 3). These data indicate that Gβ1 in neutrophils directly regulates their migration in response to wounding, whereas Gβ1 in the surrounding tissue does not affect this directed migration.
Supplementary material related to this article can be found online at http://dx.doi.org/10.1016/j.ydbio.2017.05.024.
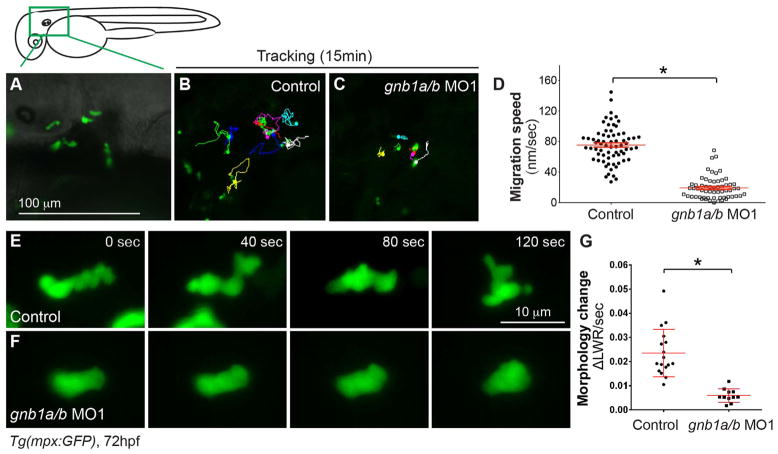
3.4. Gβ1 signaling is critical for random neutrophil motility
In addition to the directed migration triggered by tissue wounding, neutrophils in mesenchymal tissues of the head exhibit migration that lacks obvious directionality and seems to be spontaneous, and this is defined as random motility (Mathias et al., 2006; Yoo et al., 2010). Given that both the directed and random migration of neutrophils are regulated by PI3Kγ (Yoo et al., 2010), we postulated that random neutrophil motility is also regulated by Gβ1 signaling. To test this, we monitored neutrophil migration in the head at 3 dpf by time-lapse imaging as described previously (Fig. 5A) (Yoo et al., 2010). This tracking of neutrophil movement showed that, compared to neutrophils in control embryos (69 neutrophils, 6 embryos), those in embryos injected with the gnb1a/b MO1 displayed much less movement and the total distance, and the velocity of their migration were significantly reduced (60 neutrophils, 6 embryos P < 0.0001) (Fig. 5B–D, Supplementary Movie 4). This impaired random motility resembled that in embryos treated with the PI3Kγ inhibitor LY294002 (Yoo et al., 2010), suggesting that Gβ1 and PI3Kγ act in the same pathway. Furthermore, although the morphology of neutrophils during random migration changes rapidly, the shape of gnb1a/b-depleted neutrophils did not change much (Fig. 5E–F, Supplementary Movie 5). To quantify the morphological changes in neutrophils, we took time-lapse images at 20X and measured the length (L) and width (W) and of the neutrophils at each time point. We then used these numbers to calculate the change in ratio of width-to-length (ΔLWR) between time points Tn and Tn-1 (ΔLWR/s = (Ln/Wn-Ln-1/Wn-1)/ (Tn-Tn-1). Our results showed that gnb1a/b-depleted neutrophils experienced a significant reduction in the speed of change in morphology (17 neutrophils in 2 control embryos; 11 neutrophils in 4 gnb1a/b MO1-injected embryos, P < 0.0001) (Fig. 5G). Together, our results indicate that Gβ1 signaling is required not only for directed migration of neutrophils to a wound, but also for their random motility.
Fig. 5. Gβ1 signaling is critical for neutrophil motility.
Epifluorescence time-lapse experiments were performed on neutrophils in the head region (green box) of 72 hpf-Tg(mpx:GFP) embryos, using ×5 (A–D) and ×20 (E–G) objectives. (A) Representative image showing GFP-expressing neutrophils in the head region. (B–C) 15-min tracks of neutrophil migration, from time-lapse movies of control (B) and gnb1ab MO1-injected (C) Tg(mpx:GFP) embryos (Supplementary Movie 4). (D) Speed of neutrophil migration in the indicated embryos (69 neutrophils in 6 control embryos, 60 neutrophils in 6 gnb1ab MO1-injected embryos). (E–F) Snapshots from 2-min movies of control (E) and gnb1a/b MO1-injected (F) Tg(mpx:GFP) embryos, showing the morphological changes in neutrophils (Supplementary Movie 5). (G) Changes in length-width ratio (LWR) of neutrophils over time (17 neutrophils in 2 control embryos and 11 neutrophils in 4 gnb1ab MO1-injected embryos were analyzed, *P < 0.0001).
Supplementary material related to this article can be found online at http://dx.doi.org/10.1016/j.ydbio.2017.05.024.
3.5. Gβ1 is required for polarization of PI3K activation in neutrophils
PI3Kγ is one of the major downstream components of Gβγ signaling in regulating cell migration (Dupre et al., 2009; Smrcka, 2008a, 2008b). The striking phenotypic similarities between embryos in which Gβγ or PI3Kγ function is inhibited suggest that these proteins act in the same genetic pathway, and that Gβγ signaling regulates neutrophil movement by activating PI3Kγ. To test this, we assessed the activity of PI3K in vivo by monitoring the localization of PHAKT-GFP, a probe that has been widely used to detect PI(3,4,5)P3-PI(3,4)P2, a product of PI3K activity. As in other cell types that have been studied in vitro and in vivo, activation of PI3K was limited to the leading edge of the pseudopod, a condition that is critical for neutrophil migration in zebrafish (Yoo et al., 2010). We co-injected embryos with plasmid DNAs encoding mpx:PHAKT-GFP and mpx:DsRed, which leads to the expression of PHAKT-GFP and mpx:DsRed specifically in neutrophils, as reported in a previous study (Yoo et al., 2010). The injected embryos were screened for the labeling of GFP and DsRed, and the tailfins were injured to induce neutrophil migration. Subsequently, confocal time-lapse imaging was performed to monitor the dynamics of PHAKT-GFP, as well as that of DsRed (marks the neutrophil cytoplasm). Ratiometric imaging (whereby the GFP signal is divided by that of DsRed) was used to determine the distribution of PHAKT. As reported previously, in control embryos the leading edge was enriched for PHAKT (Fig. 6A–C″, Supplementary Movie 6). This was supported by quantitative “plot profile” analysis, in which the average ratio of GFP/DsRed intensity in each quarter of the cell was calculated (the first quarter representing the trailing region, and the fourth quarter the leading region) (Fig. 6G). Using the intensity in the first quarter as the baseline, we found that the relative PHAKT-GFP/DsRed signal increased gradually towards the leading region, and the highest ratio was observed in the leading region (4/1). This value was about 2.6 ± 0.2, and was significantly different from that in the trailing region (1/1) (Fig. 6G′, p < 0.001, t-test and ANONA). In contrast, there was no significant enrichment of the PHAKT-GFP/DsRed ratio in gnb1a/b-depleted neutrophils (Fig. 6D–F″). Quantification showed that the PHAKT-GFP/DsRed ratios in different regions of cells relative to the trailing region (1/1) did not differ significantly (Fig. 6G′). The ratio in the leading region was 0.96 ± 0.11, which was not significantly different from that in trailing region (1 in the “1” region) (Fig. 6G′, p > 0.05, t-test and ANONA). These results are consistent with the notion that these neutrophils lack polarity. Together, these data indicate that PI3K activity was reduced in neutrophils in the context of reduced Gβ1 signaling, supporting the hypothesis that Gβ1 activates PI3K to regulate neutrophil migration.
Supplementary material related to this article can be found online at http://dx.doi.org/10.1016/j.ydbio.2017.05.024.
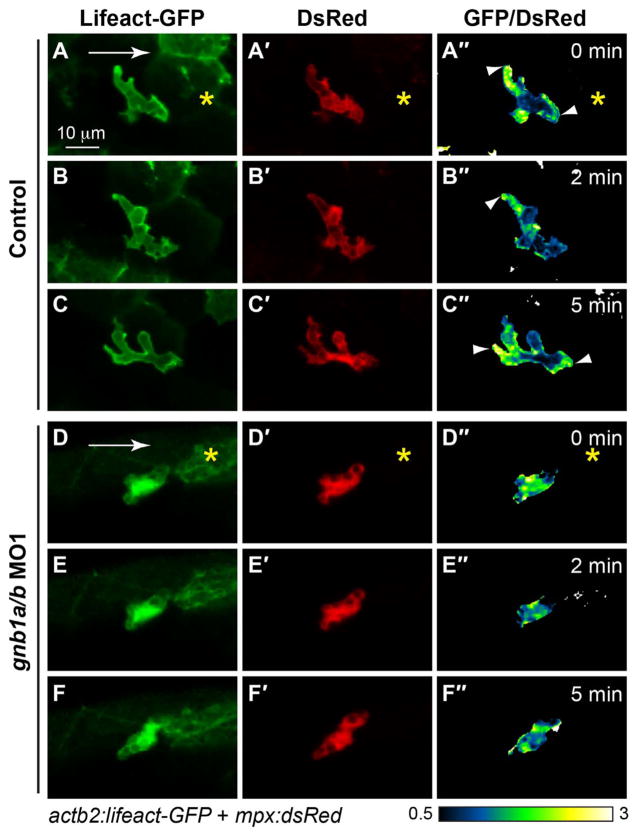
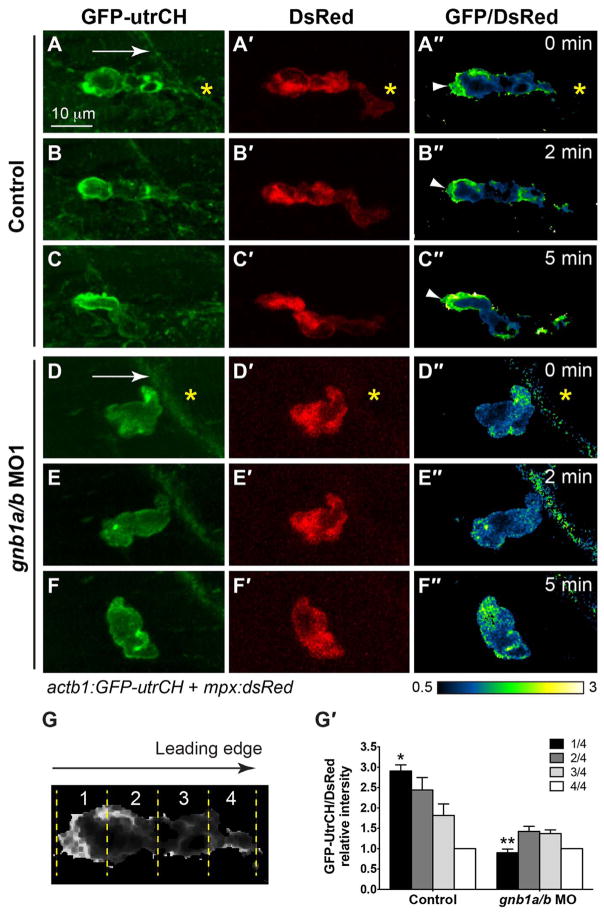
3.6. Gβ1 is required for the polarization and accumulation of F-actin
Actin assembly and polymerization are critical for the formation of cell protrusions that drive cells to migrate. Previous studies showed that F-actin in neutrophils is polarized during directed migration, with F-actin remaining stable (as reveled by GFP-Utrophin labeling) in the tail but undergoing dynamic restructuring (as revealed by Lifeact-GFP labeling) at the leading edge (Yoo et al., 2010). Interestingly, PI3Kγ is required not only for promoting actin polymerization.
at the leading edge, but also for maintaining the anteroposterior polarity of stable F-actin at the trailing edge (Yoo et al., 2010). Our previous studies showed that in zebrafish Gβγ signaling regulates remodeling of the actin cytoskeleton, controlling both the migration of individual primordial germ cells and collective migration of the lateral line primordium (Xu et al., 2014).
Based on the findings described above, we postulated that Gβγ signaling likely activates PI3Kγ to control actin dynamics, and to enable neutrophil migration. To test this, we employed a strategy similar to that for PHAKT-GFP labeling, performing confocal ratiometric imaging on Tg(actb2: Lifeact-GFP) and Tg(actb1:GFP-utrCH) embryos injected with plasmid DNA encoding mpx:DsRed. Consistent with previous findings, in control neutrophils responding to wounding, Lifact-GFP was present at both the front and back of the cell (Fig. 7A–C″, Supplementary Movie 7). However, no clear polarity of Lifact-GFP enrichment was observed in gnb1a/b-depleted neutrophils (Fig. 7D–F″, Supplementary Movies 7), supporting the idea that Gβγ signaling is required to control PI3Kγ-mediated actin polymerization and the formation of protrusions at the leading region of the cell. Furthermore, in control embryos, stable actin (as revealed by GFP-utrCH labeling) was present only in the back of the cell (Fig. 8A–C″, G′; Supplementary Movie 8). We again performed ratiometric quantification, as in the case of PHAKT-GFP (Fig. 8G), but using the intensity of the fourth quarter (leading region) as the baseline (Fig. 8G′). We found that GFP-utrCH levels were highest in the trailing region (2.91 ± 0.15), and significantly higher than in the leading region (4/4) (Fig. 8G′, p < 0.001, t-test and ANONA). By contrast, in gnb1a/b-depleted neutrophils, GFP-utrCH labeling was not polarized but rather diffuse (Fig. 8D–F″). This result was supported by quantification analysis similar to that used for PHAKT-GFP (Fig. 8G). We found that in the trailing region (# 1) the ratio of GFP-utrCH/DsRed signal relative to the leading region (# 4) was 0.9 ± 0.1; this did not differ significantly from that in the leading region (p > 0.05, t-test and ANONA) but did differ significantly from that in control cells (Fig. 8G′, p < 0.001, t-test and ANONA). Thus, similar to PI3Kγ signaling, Gβγ signaling regulates neutrophil migration by controlling not only the leading protrusions, but also F-actin polarization in the cell. Collectively, our data indicate that signaling mediated by Gβ1 activates PI3Kγ to control neutrophil migration by balancing signaling at the back and front of the cell.
Fig. 7. Gβ1 signaling regulates F-actin dynamics in neutrophils.
Snapshots from confocal time-lapse imaging (Supplementary Movies 7 and 8) of migrating neutrophils expressing Lifeact-GFP in control and gnb1a/b MO1-injected embryos (3 cells in 3 embryos for each group) following wounding. (A–F) Lifeact-GFP. (A′–F′) DsRed. (A″–F″) Ratiometric images of Lifeact-GFP/DsRed. Yellow asterisks: side nearest wound; white arrowheads: sites of Lifeact-GFP enrichment in leading and trailing fronts of neutrophils.
Fig. 8. Gβ1 regulates the polarity of stable F-actin accumulation in neutrophils.
(A–F) Snapshots from confocal time-lapse imaging (Supplementary Movie 8) of migrating neutrophils expressing GFP-utrCH in control and gnb1a/b MO1-injected embryos following wounding. (A–F) GFP-utrCH. (A′–F′) DsRed. (G″–L″) Ratiometric images of GFP-utrCH/ DsRed. Yellow asterisks: side nearest wound; white arrowheads: GFP-utrCH enrichment in neutrophil trailing region; white arrows, direction of migration. (G–G′) Quantification of GFP-utrCH/DsRed. (G) Illustration of the method used to quantify ratio of intensities of PHAKT-EGFP/DsRed, as described in Fig. 6G. (G′) Graph showing the ratio of intensities of GFP-utrCH/DsRed in each quarter of the cell relative to that in the fourth quarter (the leading region), in control (5 neutrophils in 5 embryos) and gnb1a/b MO1-injected (9 neutrophils in 4 embryos) embryos. * p = 0.0001 vs 1/4 in control, ** p < 0.0001 vs 1/4 in control, by t-test, two-way ANOVA; p < 0.0001 among groups in control embryos, by one-way ANOVA; p = 0.0003, among groups in gnb1a/b MO1-injected embryos, by one-way ANOVA.
Supplementary material related to this article can be found online at http://dx.doi.org/10.1016/j.ydbio.2017.05.024.
4. Discussion
In this study, we found that Gβ1 is essential for directed migration and motility of neutrophils in zebrafish. Our finding that neutrophils failed to migrate to injured tissue in embryos in which Gβγ signaling had been disrupted (Fig. 1, Supplementary Movie 1) or that were depleted of Gβ1 (Fig. 3, Supplementary Movie 2) indicates that Gβγ is critical for the chemotactic response of neutrophils in vivo. Additionally, the failure of neutrophils defective for Gβ1 to become polarized and to change shape efficiently, as well as the significant reduction in their motility (Fig. 5, Supplementary Movies 4 and 5), are reminiscent of phenotypes observed in embryos in which PI3Kγ function was disrupted (Yoo et al., 2010). This suggests that Gβ1 may act through PI3Kγ to promote neutrophil migration. This possibility was further supported by the discoveries that in migrating neutrophils defective for Gβ1, PI3K activation was not limited to the leading region (Fig. 6, Supplementary Movie 6) and the anteroposterior polarization of actin during cell migration was not maintained (Fig. 7, Supplementary Movies 7 and 8). Collectively, these results indicate that Gβγ signaling might regulate the PI3Kγ activation that is needed for the proper migration and polarity of neutrophil in zebrafish embryos. PI3Kγ can activate Rac to promote the remodeling of the actin cytoskeleton that is required for cell polarization and directional migration (Andrews et al., 2007; Wang, 2009). Our previous data showed that Gβγ signaling regulates Rac activation to control the polarization and migration of PGCs (Xu et al., 2012). Thus, it is possible that Gβγ regulates PI3Kγ, and then the activation of Rac, to control neutrophil migration.
The Gβγ complexes of heterotrimeric G proteins play critical roles in GPCR-mediated signal transduction (Dupre et al., 2009; Khan et al., 2013; Smrcka, 2008a). The human and mouse genomes have 5 distinct Gβ and 12 distinct Gγ subunits (Smrcka, 2008b). At the amino acid sequence level, the Gβ1–4 subunits share 79–98% similarity, whereas the Gγ subunits share only 25–76% similarity. Different Gβ and Gγ isoforms can pair to form distinct Gβγ complexes and, although the functional consequences have not been tested comprehensively, in some cases no specific pairings are required for their function (Block et al., 2016). Nevertheless, some Gβγ complexes interact with specific Gα isoforms and transmit cellular signals triggered by particular GPCRs. For example, the deletion of Gγ7 impairs signaling mediated by β-adrenergic receptors, but not that mediated by the GPCR PGE1 (Wang et al., 1997). Similarly, C5a-stimulated chemotaxis of the mouse macrophage cell line J774A.1 is mediated primarily by Gβ2, although both Gβ1 and Gβ2 are expressed in these cells (Hwang et al., 2004). Furthermore, the roles of Gβ1 and Gβ2 in neutrophils are distinct: whereas Gβ2 regulates the direction of migration of primary mouse neutrophils, Gβ1 is needed for phagocytosis and the killing of bacteria (Zhang et al., 2010).
Like mammals, zebrafish have 5 Gβ isoforms and their expression patterns during embryonic development are distinct (Xu et al., 2012). Among these isoforms, only Gβ1 and Gβ4 are expressed in embryos after day 1 post fertilization (Xu et al., 2012, 2014). Notably, although both Gβ1 and Gβ4 are expressed in migrating neutrophils (Fig. 2) and share 90% amino acid sequence identity and 96% similarity (Xu et al., 2012), only Gβ1 contributes to the regulation of neutrophil migration (Fig. 3, Supplementary Movie 2). Similarly, our previous finding that Gβ1, but not Gβ4, is required for migration of cells of the pLLP (Xu et al., 2014). The reason for the difference in involvement of Gβ1 and Gβ4 in neutrophil migration is unclear. One possibility is that the abilities of Gβ1 and Gβ4 to bind to Gγ or Gα may differ. Indeed, Gβ1 has been shown to be unique among Gβ subunits with respect to being able to interact with all Gγ subunits (Iniguez-Lluhi et al., 1992; Schmidt et al., 1992). Here, our data not only provide evidence that the function of Gβγ signaling is critical in vivo, but also illustrate that Gβ isoforms play specific roles in controlling developmental processes.
Our transplantation experiment indicates that the requirement for Gβ1 in the migration of neutrophils is cell autonomous (Fig. 4, Supplementary Movie 3), suggesting that G protein-dependent signal transduction within these cells is essential for chemotactic responses. Exactly which GPCRs are expressed in neutrophils is not known, although signaling mediated by two GPCR axes (Cxcl8-Cxcr2, Cxcl12-Cxcr4) has been implicated in neutrophil migration. In the future, it will be of interest to determine which GPCRs in neutrophils are responsible for their recruitment. Notably, the expression of dominant gain-of-function mutant forms of Cxcr4 that are associated with warts, hypo-gammaglobulinemia, infections, and myelokathexis (WHIM) syndrome reduces the motility and directed migration of neutrophils (Walters et al., 2010), suggesting that this GPCR plays a role in neutrophil migration. It is well known that the Gβγ dimer transmits Cxcr4b signal in regulating the chemotaxis of many other cell types (Peracino et al., 1998; Rickert et al., 2000). In addition, our studies indicate that Cxcr4 functions through Gβγ, both to promote the metastasis of breast cancer in nude mice (Tang et al., 2011) and to regulate pLLP migration in live zebrafish embryos (Xu et al., 2014). Thus, Gβγ probably contributes to the signaling in neutrophils that leads to WHIM syndrome. Further studies of the role of Gβγ signaling in this context are expected to shed light on this disorder, as well as on other syndromes caused by immunodeficiency.
Another implication of our discovery that Gβ1 signaling is involved in neutrophil recruitment is that stimuli released by wounding activate GPCRs on neutrophils to trigger the directed migration of these cells. Previously, H2O2 generated in response to injury was shown to trigger the recruitment of neutrophils to wound sites (Niethammer et al., 2009). Our results reveal an additional mechanism that contributes to neutrophil migration. Given that neutrophil recruitment and migration are critical for inflammation, the immune response, and the host defense, our study has significant implications for understanding these conditions.
Supplementary Material
Acknowledgments
This study is supported by grants: NIH/NCRR K99R00RR024119 and National Science Foundation IOS-1354457 (to FL); AHA10GRNT3620015 and NIH R01GM094255 (to SC). We thank John Rawls (Duke University) and Stephen A. Renshaw (University of Sheffield) for providing the Tg(mpx:GFP) line; Carl-Philipp Heisenberg (Institute of Science and Technology Austria) for the Tg(actb2: Lifeact-GFP) and Tg(actb1:GFP-utrCH) lines; and Anna Huttenlocher (University of Wisconsin-Madison) for the mpx:PHAKT-GFP, mpx:DsRed plasmids.
Footnotes
Author contributions
F.L., W.K., and D.Y. conceived the ideas and designed experiments; W.K., D.Y., and K.M performed the experiments; F.L. wrote the manuscript.
References
- Andrews S, Stephens LR, Hawkins PT. PI3K class IB pathway in neutrophils. Sci STKE: Signal Transduct Knowl Environ. 2007;2007:cm3. doi: 10.1126/stke.4072007cm3. [DOI] [PubMed] [Google Scholar]
- Behrndt M, Salbreux G, Campinho P, Hauschild R, Oswald F, Roensch J, Grill SW, Heisenberg CP. Forces driving epithelial spreading in zebrafish gastrulation. Science. 2012;338:257–260. doi: 10.1126/science.1224143. [DOI] [PubMed] [Google Scholar]
- Block H, Stadtmann A, Riad D, Rossaint J, Sohlbach C, Germena G, Wu D, Simon SI, Ley K, Zarbock A. Gnb isoforms control a signaling pathway comprising Rac1, Plcbeta2, and Plcbeta3 leading to LFA-1 activation and neutrophil arrest in vivo. Blood. 2016;127:314–324. doi: 10.1182/blood-2015-06-651034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldajipour B, Doitsidou M, Tarbashevich K, Laguri C, Yu SR, Ries J, Dumstrei K, Thelen S, Dorries J, Messerschmidt EM, Thelen M, Schwille P, Brand M, Lortat-Jacob H, Raz E. Cxcl12 evolution - subfunctionalization of a ligand through altered interaction with the chemokine receptor. Development. 2011;138:2209–2914. doi: 10.1242/dev.068379. [DOI] [PubMed] [Google Scholar]
- Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- Bussmann J, Raz E. Chemokine-guided cell migration and motility in zebrafish development. EMBO J. 2015;34:1309–1318. doi: 10.15252/embj.201490105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YR, Fujita M, Butler M, Isogai S, Kochhan E, Siekmann AF, Weinstein BM. Chemokine signaling directs trunk lymphatic network formation along the preexisting blood vasculature. Dev Cell. 2012;22:824–836. doi: 10.1016/j.devcel.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira S, Reyes-Aldasoro CC, Candel S, Renshaw SA, Mulero V, Calado A. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J Immunol. 2013;190:4349–4359. doi: 10.4049/jimmunol.1203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Harvie EA, Huttenlocher A. Distinct signalling mechanisms mediate neutrophil attraction to bacterial infection and tissue injury. Cell Microbiol. 2012;14:517–528. doi: 10.1111/j.1462-5822.2011.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Huttenlocher A. Leukocyte migration from a fish eye’s view. J Cell Sci. 2012;125:3949–3956. doi: 10.1242/jcs.093633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Sarris M, Bennin DA, Green JM, Herbomel P, Huttenlocher A. Localized bacterial infection induces systemic activation of neutrophils through Cxcr2 signaling in zebrafish. J Leukoc Biol. 2013;93:761–769. doi: 10.1189/jlb.1012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- Dupre DJ, Robitaille M, Rebois RV, Hebert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell. 2006;10:673–680. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001 http://palaeo-electronica.org/2001_2001/past/issue2001_2001.htm.
- Harrison MR, Bussmann J, Huang Y, Zhao L, Osorio A, Burns CG, Burns CE, Sucov HM, Siekmann AF, Lien CL. Chemokine-guided angiogenesis directs coronary vasculature formation in zebrafish. Dev Cell. 2015;33:442–454. doi: 10.1016/j.devcel.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe HJ, Wolf NM, Abu-Taha I, Mehringer R, Just S, Lutz S, Niroomand F, Postel EH, Katus HA, Rottbauer W, Wieland T. The interaction of nucleoside diphosphate kinase B with Gbetagamma dimers controls heterotrimeric G protein function. Proc Natl Acad Sci USA. 2009;106:16269–16274. doi: 10.1073/pnas.0901679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JI, Fraser ID, Choi S, Qin XF, Simon MI. Analysis of C5a-mediated chemotaxis by lentiviral delivery of small interfering RNA. Proc Natl Acad Sci USA. 2004;101:488–493. doi: 10.1073/pnas.0307549100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez-Lluhi JA, Simon MI, Robishaw JD, Gilman AG. G protein beta gamma subunits synthesized in Sf9 cells. Functional characterization and the significance of prenylation of gamma. J Biol Chem. 1992;267:23409–23417. [PubMed] [Google Scholar]
- Ivins S, Chappell J, Vernay B, Suntharalingham J, Martineau A, Mohun TJ, Scambler PJ. The CXCL12/CXCR4 axis plays a critical role in coronary artery development. Dev Cell. 2015;33:455–468. doi: 10.1016/j.devcel.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardash E, Reichman-Fried M, Maitre JL, Boldajipour B, Papusheva E, Messerschmidt EM, Heisenberg CP, Raz E. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat Cell Biol. 2010;12:47–53. doi: 10.1038/ncb2003. (sup pp 41-11) [DOI] [PubMed] [Google Scholar]
- Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC, Miller GJ, Hebert TE. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev. 2013;65:545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- Knaut H, Werz C, Geisler R, Nusslein-Volhard C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279–282. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- Lin F, Chen S, Sepich DS, Panizzi JR, Clendenon SG, Marrs JA, Hamm HE, Solnica-Krezel L. Galpha12/13 regulate epiboly by inhibiting E-cadherin activity and modulating the actin cytoskeleton. J Cell Biol. 2009;184:909–921. doi: 10.1083/jcb.200805148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Sepich DS, Chen S, Topczewski J, Yin C, Solnica-Krezel L, Hamm H. Essential roles of G{alpha}12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J Cell Biol. 2005;169:777–787. doi: 10.1083/jcb.200501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JR, Dodd ME, Walters KB, Yoo SK, Ranheim EA, Huttenlocher A. Characterization of zebrafish larval inflammatory macrophages. Dev Comp Immunol. 2009;33:1212–1217. doi: 10.1016/j.dci.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Verkade H, Heath JK, Kuroiwa A, Kikuchi Y. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development. 2008;135:2521–2529. doi: 10.1242/dev.020107. [DOI] [PubMed] [Google Scholar]
- Nair S, Schilling TF. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science. 2008;322:89–92. doi: 10.1126/science.1160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers SH, Flores MV, Hall CJ, O’Toole R, Swift S, Crosier KE, Crosier PS. Expression of zebrafish cxcl8 (interleukin-8) and its receptors during development and in response to immune stimulation. Dev Comp Immunol. 2010;34:352–359. doi: 10.1016/j.dci.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Peracino B, Borleis J, Jin T, Westphal M, Schwartz JM, Wu L, Bracco E, Gerisch G, Devreotes P, Bozzaro S. G protein beta subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J Cell Biol. 1998;141:1529–1537. doi: 10.1083/jcb.141.7.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108:3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 2000;10:466–473. doi: 10.1016/s0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Thomas TC, Levine MA, Neer EJ. Specificity of G protein beta and gamma subunit interactions. J Biol Chem. 1992;267:13807–13810. [PubMed] [Google Scholar]
- Siekmann AF, Standley C, Fogarty KE, Wolfe SA, Lawson ND. Chemokine signaling guides regional patterning of the first embryonic artery. Genes Dev. 2009;23:2272–2277. doi: 10.1101/gad.1813509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Smrcka AV. G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008a;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka AV. G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008b;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney KF, Huang CH, Devreotes PN. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu Rev Biophys. 2010;39:265–289. doi: 10.1146/annurev.biophys.093008.131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Sun Z, Runne C, Madsen J, Domann F, Henry M, Lin F, Chen S. A critical role of G{beta}{gamma} in tumorigenesis and metastasis of breast cancer. J Biol Chem. 2011 doi: 10.1074/jbc.M110.206615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116:2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. The signaling mechanisms underlying cell polarity and chemotaxis. Cold Spring Harb Perspect Biol. 2009;1:a002980–a002980. doi: 10.1101/cshperspect.a002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Mullah B, Hansen C, Asundi J, Robishaw JD. Ribozyme-mediated suppression of the G protein gamma7 subunit suggests a role in hormone regulation of adenylylcyclase activity. J Biol Chem. 1997;272:26040–26048. doi: 10.1074/jbc.272.41.26040. [DOI] [PubMed] [Google Scholar]
- Williams M, Yen W, Lu X, Sutherland A. Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Dev Cell. 2014;29:34–46. doi: 10.1016/j.devcel.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Echemendia N, Chen S, Lin F. Identification and expression patterns of members of the protease-activated receptor (PAR) gene family during zebrafish development. Dev Dyn. 2011;240:278–287. doi: 10.1002/dvdy.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Kardash E, Chen S, Raz E, Lin F. Gbetagamma signaling controls the polarization of zebrafish primordial germ cells by regulating Rac activity. Development. 2012;139:57–62. doi: 10.1242/dev.073924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ye D, Behra M, Burgess S, Chen S, Lin F. Gbeta1 controls collective cell migration by regulating the protrusive activity of leader cells in the posterior lateral line primordium. Dev Biol. 2014;385:316–327. doi: 10.1016/j.ydbio.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tang W, Jones MC, Xu W, Halene S, Wu D. Different roles of G protein subunits beta1 and beta2 in neutrophil function revealed by gene expression silencing in primary mouse neutrophils. J Biol Chem. 2010;285:24805–24814. doi: 10.1074/jbc.M110.142885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.