Abstract
Nance-Horan syndrome is a rare X-linked recessive inherited disease with clinical features including severe bilateral congenital cataracts, characteristic facial and dental abnormalities. Data from Chinese Nance-Horan syndrome patients are limited. We assessed the clinical manifestations of a Chinese Nance-Horan syndrome pedigree and identified the genetic defect. Genetic analysis showed that 3 affected males carried a novel small deletion in NHS gene, c.263_266delCGTC (p.Ala89TrpfsTer106), and 2 female carriers were heterozygous for the same variant. All 3 affected males presented with typical Nance-Horan syndrome features. One female carrier displayed lens opacities centered on the posterior Y-suture in both eyes, as well as mild dental abnormalities. We recorded the clinical features of a Chinese Nance-Horan syndrome family and broadened the spectrum of mutations in the NHS gene.
Introduction
Nance-Horan syndrome (NHS)[MIM 202350], characterized by congenital cataracts, distinctive dental and facial abnormalities, is a rare X-linked recessive inherited disease first described by Nance and Horan in 19741,2.While its prevalence is still unknown, to date, Nance-Horan syndrome has been reported in Caucasian, Turkish, Tunisian, Arabian, Indian and Chinese ethnic groups1–9. Affected males have severe bilateral congenital dense cataracts, distinctive dental and craniofacial abnormalities10. Microcornea, microphthalmia, hand and foot malformations and mild to moderate intellectual disability have also been recorded in some families11,12. Female carriers have lens opacities involving posterior Y-suture, with milder extra-ocular manifestations5.
The NHS gene, located on Xp22.13, has been linked with this rare disorder13. NHS is abundantly expressed during the development of embryonic tissues, particularly in lens, brain, craniofacial mesenchyme, and primordial teeth10. At least 4 isoforms can result from alternative splicing. Isoform A (NHS-A), the major isoform, encodes a 1630-amino acid protein which is located in the epithelial cell membrane and may interact with the tight junction protein zona occludens-1(ZO-1)14,15. A further study indicated that the NHS protein is a novel regulator of actin remodeling and cell morphology16. The actual function, regulation and interaction of NHS proteins remain not fully understood. About 40 causative mutations in NHS have been reported (HGMD Database; http://www.hgmd.cf.ac.uk), most of which are nonsense mutations or small deletions.
Though approximately 60 Nance-Horan syndrome families or cases have been identified worldwide1,3–8,10,12,17–29, only 4 were Chinese4,9,12,18. This may be due to the lack of awareness of this rare syndrome. In the present study, we characterized the clinical features of a Chinese pedigree with Nance-Horan syndrome and a novel molecular variant in NHS.
Results
Clinical evaluation
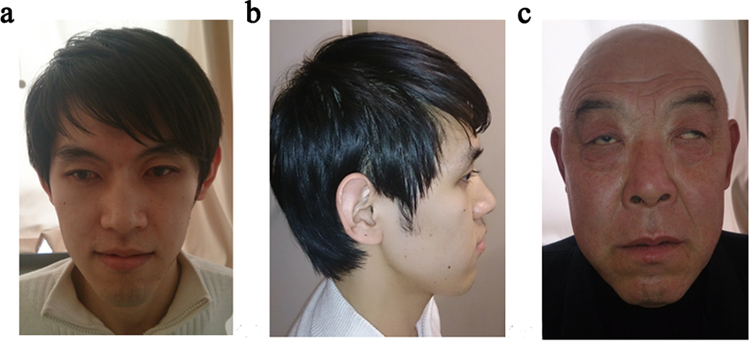
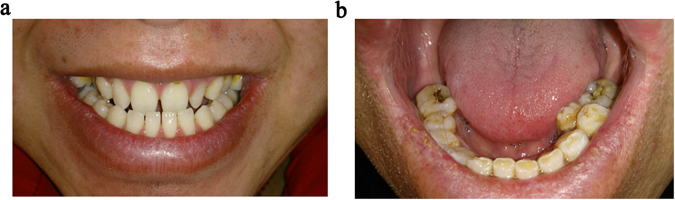
The proband, his brother and one of his uncles were affected while the female members of the family were unaffected. The pedigree suggested an X-linked recessive pattern of inheritance (Fig. 1). All the affected males had poor vision since birth. They were diagnosed with bilateral congenital cataracts and cataract surgery was performed at an early age. Seven years later, the affected brothers (III1 and III2) developed bilateral glaucoma. Although they underwent glaucoma surgeries and medical treatment, intraocular pressure (IOP) was poorly controlled, being around 50 mmHg. II1 complained of intermittent swelling pain of the left eye when he was around age 20, but he had never visited a doctor. When the affected males were referred to our clinic, the best corrected visual acuity (BCVA) ranged from no light perception (NLP) to 0.05. Bilateral cornea cloudy opacity with the appearance of calcium in central cornea(band keratopathy) was present in III1 and III2. Their IOPs were unmeasurably high (>50 mmHg). The IOP of II1 was within normal limits. He had band keratopathy in the left eye. Microcornea (cornea diameter = 9 mm), exotropia and nystagmus were present in all the patients. They all had a long-narrow face (Fig. 2a), large anteverted and mild enlarged pinnae (Fig. 2b). II1 displayed a bulbous nose (Fig. 2c). Dental abnormalities were identified in all individuals, and included screw-driver like incisors (Fig. 3a), mulberry-like molars, and various examples of dental agenesis (Fig. 3b).
Figure 1.
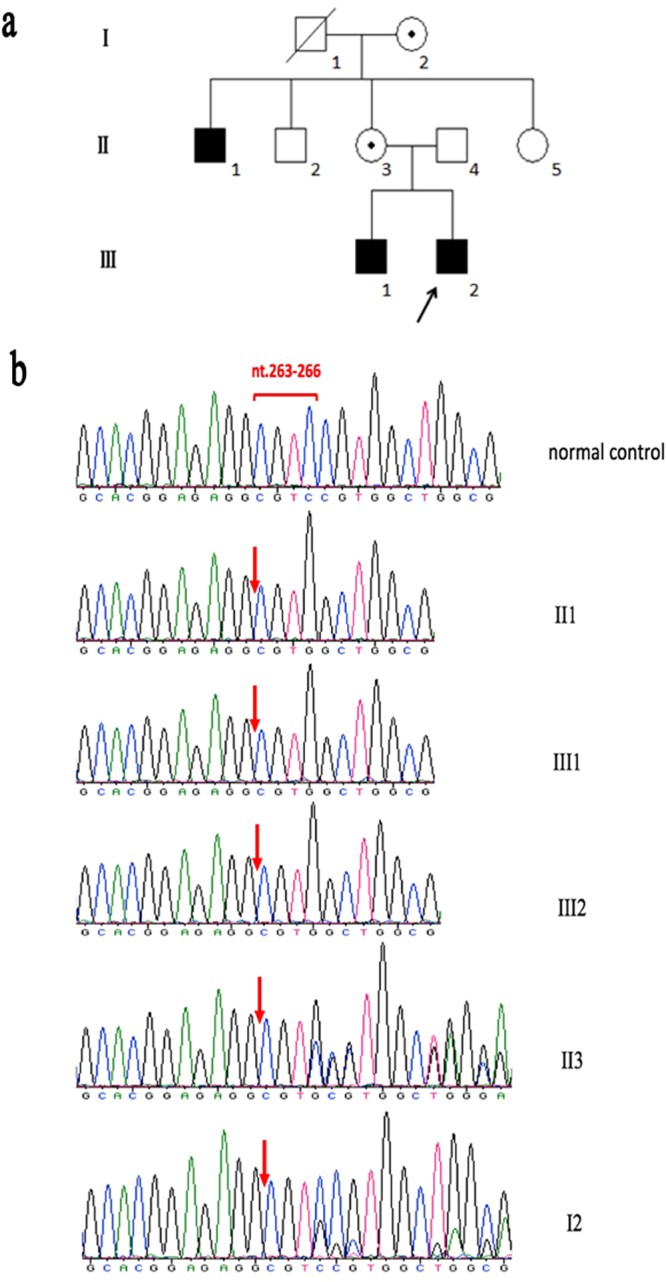
The pedigree and sequencing results of the Nance-Horan syndrome family. (a) The pedigree exhibited an X-linked recessive inheritance mode. Solid symbols indicate affected individuals; open symbols indicate normal subjects; symbols with a dot inside indicate mutant allele carriers; slashed symbols indicate deceased individuals; a square represents a male and a circle, a female individual. An arrow marks the proband. (b) The sequencing results show a 4-base-pair deletion (CGTC) at nucleotide 263 causing a frameshift in codon 89 and a premature termination of translation (p.Ala89TrpfsTer106). The female carriers were heterozygous for the same mutation. nt. refers to nucleotide.
Figure 2.
Representative facial dysmorphology of the NHS patients. (a) The frontal view of III2. III2 had a long and narrow face. (b) The lateral view of III2. III2 had large, anteverted and mild enlarged pinnae. (c)Frontal view of II1. II1 had a bulbous nose.
Figure 3.
Representative dental abnormalities of the NHS patients. (a) Screw-driver like incisors of III1. (b) Mulberry-like molars, crowded premolars and missing of the second molars of II1.
The proband’s mother(II3) was a 50-year-old female with no history of ocular disease except for high myopia. The cornea diameter of both eyes was 12 mm. Her BCVA was 0.15 OD and 0.4 OS. Slit-lamp examination of the both eyes revealed lens opacities centered on the posterior Y-suture (Fig. 4a). She had a normal facial gestalt and ears. Her incisors exhibited mild screw-driver shape and mulberry-like molars. Her left central incisor appeared “normal” according to her description and was subsequently broken accidentally (Fig. 4b). The proband’s grandmother (I2) was unavailable for examination. According to her medical history, she was diagnosed as bilateral cataracts at the age of 58, and had already received surgical treatment.
Figure 4.
Phenotypes of the female carrier (II3). (a) Slit-lamp photograph of II3. Lens opacities centered on the posterior suture, both eyes. (b) Dental abnormalities of II3. Her teeth exhibited mild screw-driver shape incisors and mulberry-like molars. Her left central incisor appeared “normal” according to her description and was subsequently broken accidentally.
No hand and foot abnormality, or intellectual disability were observed among all our subjects. Detailed clinical evaluation of the affected males and the female carriers is summarized in Table 1.
Table 1.
Clinical features of the affected males and the female carriers.
| Affected male patients | Female carriers | ||||
|---|---|---|---|---|---|
| II1 | III1 | III2 | II3 | I2 | |
| Age/gender | 54/M | 24/M | 22/M | 50/F | 80/F |
| BCVA | 0.05/LP | NLP/NLP | LP/LP | 0.15/0.4 | NA |
| Ocular features | |||||
| Congenital cataracts | + | + | + | Y suture opacity | + |
| Cataract surgery | + | + | + | − | + |
| Glaucoma | + | + | + | − | NA |
| Strabismus | + | + | + | − | NA |
| Nystagmus | + | + | + | − | NA |
| Others | band keratopathy | cornea cloudy opacity, band keratopathy | cornea cloudy opacity, band keratopathy | high myopia | NA |
| Facial abnormalities | |||||
| Long-narrow face | + | + | + | − | NA |
| Bulbous nose | + | − | − | − | NA |
| Anteverted and mild enlarged pinnae | + | + | + | − | NA |
| Dental abnormalities | |||||
| Screw-driver shaped incisors | + | + | + | + | NA |
| Mulberry-like molars | + | + | + | + | NA |
| Dental agenesis | missing of the second molars | − | crowded premolars, and missing of the second molars | broken left central incisor | NA |
| Hand and foot abnormalities | − | − | − | − | NA |
| Intellectual disability | − | − | − | − | NA |
BCVA, best corrected visual acuity; LP, light perception; NLP, no light perception; NA, not available.
Genetic analysis
We identified a novel disease-causing NHS mutation in the family. The sequencing results showed a 4-base-pair deletion (CGTC) at nucleotide 263–266 in exon 1 of NHS gene, which would cause a frameshift from codon 89 and a premature termination of translation. (p.Ala89TrpfsTer106). The variant sequence was confirmed by segregation in the pedigree (Fig. 1).
Discussion
Congenital cataract is the leading cause of irreversible blindness in childhood30. Approximately half of congenital cataracts are inherited, either with and without other ocular anomalies or as part of multisystem genetic disorders. Inherited cataracts are most frequently inherited as autosomal dominant traits, but also could be inherited in an autosomal recessive or X-linked pattern31. Nance-Horan syndrome is one of the few syndromes with cataract that is inherited as an X-linked trait. In contrast to other well-studied subgroups of genes associated with congenital cataract such as the crystallins32, NHS was speculated to play a vital role in cell-to-cell tight conjunction formation together with the tight junction protein, ZO-115. NHS-A protein was normally localized at cellular periphery of various tissues, especially lens epithelium16,25,33. Mutant NHS-A protein was found in cytoplasm25. Normally functioned intercellular junctions are important in lens development and maintaining lens homeostasis34,35. The dislocation of the NHS protein and altered intercellular contacts are likely to underlie cataract formation in Nance–Horan syndrome. A novel small deletion (c.263_266delCGTC) was identified in our study that would result in a truncated protein (p.Ala89TrpfsTer106). Nonsense mutations and small deletions are the most common mutations in NHS3. Small insertions, large deletions, large insertions, splice site mutations and missense mutations have also been reported (HGMD Database; http://www.hgmd.cf.ac.uk). Currently, only 4 mutations in Chinese patients with Nance-Horan syndrome have been identified, including a nonsense mutation (p.E108X), a small deletion (c.852delG), splice site mutation (c.1045 + 2 T > A) and a large deletion at Xp22.134,9,12,18.
Congenital cataract is the most prominent feature of Nance-Horan syndrome and leads to profound vision loss and greatly affects the quality of life. The three affected males in our study had congenital bilateral dense nuclear cataracts and received cataract surgery at an early age. The BCVA of our NHS patients ranged from NLP to 0.05. Almost all NHS patients undergo cataract extraction but the overall prognosis for vision is still poor, mostly NLP to 0.35,8,9,18,24. Delayed surgical intervention, amblyopia and glaucoma may contribute to the poor visual outcomes. All affected males in our study developed glaucoma.According to previous reports, about 10% of NHS patients exhibited secondary glaucoma5,7,26. This number might be underestimated because some NHS patients were first diagnosed by dentists or they did not visit doctor to check IOP, like II1. Poorly developed anterior chamber angles in NHS was reported26, which was suggested that the abnormal aqueous humor drainage system contribute to recurrent secondary glaucoma. None of the other reported Chinese NHS patients exhibited glaucoma. In addition to congenital cataracts and glaucoma, our patients also manifested other common ocular features of NHS, including microcornea, strabismus and nystagmus.
Multi-systemic abnormalities in Nance-Horan syndrome are easily overlooked by ophthalmologists, which may lead to inaccurate diagnosis. The affected males in the present study displayed long-narrow face, anteverted and mild enlarged pinnae. II1 exhibited a bulbous nose. All of them presented screw-driver shaped incisors, mulberry-like molars and various dental agenesis including crowded premolars and missing second molars. Long-narrow face, anteverted and mild enlarged pinnae and bulbous nose are the characteristic facial features of NHS. The severity of these phenotypes varied from case to case3,6,8,12,18. Screw-driver shaped incisors and mulberry-like molars are the most distinctive dental abnormalities of NHS24. A spectrum of other dental abnormalities, such as diastema, supernumenary teeth, and dental agenesis were commonly observed24. Spontaneous dental abscess was recorded in one case36. None of our patients exhibited hand and foot abnormalities, and all had normal intellect. Most common hand and foot abnormalities are broad or short fingers and brachymetacarpalia5,12. Sandal gap and partial syndactyly of toes were reported in one Turkish NHS family3. About 30% of affected males have mild to moderate intellectual disability11. Three of the four reported Chinese cases manifest typical NHS features as in the present study, including congenital cataracts, distinctive dental and craniofacial abnormalities12,18,37. The NHS patients in Liao’s study also displayed hand and foot abnormalities, psychomotor retardation, and cryptorchidism, probably as the microdeletion encompasses the REPS2, NHS, SCML1 and RAI2 genes4. The cytogenetic abnormalities involving the flanking genes in Xp22.13 may contribute to the variability of phenotypes such as cryptorchidism and tetralogy of Fallot4,21. However, intellectual disability can present in patients with only NHS gene mutation, which indicated that NHS play a vital role in mental development. Three of the four reported Chinese cases were diagnosed by next generation sequencing, systemic manifestations were not stated in two of them9,12,18. These reports indicate that this rare disorder can be unrecognized, especially when the phenotype is not typical. When checking patients with congenital cataracts, a comprehensive medical history is vital to determine if other organs are involved. A basic assessment of facial, dental, skeletal, genitourinary and neurological abnormalities are important for accurate diagnosis.
Like many other X-linked inherited ocular diseases, such as X-linked retinitis pigmentosa and choroideremia37,38, female carriers of Nance-Horan syndrome can have variable mild phenotypes. It’s mainly due to skewed X inactivation39. II3 experienced declined vision, with a BCVA of 0.15 OD and 0.4 OS. Female carriers of NHS may remain normal vision5,18. II3 displayed bilateral lens opacities centered on the posterior Y-suture. I2 developed bilateral cataracts that required surgery in her late 50′s. It is speculated that the cataracts in I2 were more severe than II3. Y-suture opacity is considered to be a sensitive and specific clinical sign for female carriers of Nance-Horan syndrome5. Nuclear opacity or clear lens was also reported in a small number of cases3,4,18. Besides the typical Y-suture opacity feature, II3 exhibited mild dental abnormalities including screw-driver shape incisors and mulberry-like molars. Heterozygous females of Nance-Horan syndrome may often manifest similar but less pronounced extraocular features than affected males6,29.
In conclusion, our study identifies a novel pathogenic NHS gene mutation in a Chinese pedigree clinically diagnosed with Nance-Horan syndrome. Our findings broaden the spectrum of mutations associated with Nance-Horan syndrome in Chinese population, and shed light on the diagnosis of this rare disease.
Methods
Clinical evaluation
Two male patients with congenital cataracts were identified at the Ophthalmic Genetic Clinic at Peking Union Medical College Hospital (PUMCH), Beijing, China. After checking their uncle and mother, X-linked recessive inheritance was suspected. Detailed medical and family histories were taken. Three affected males and 1 female carrier underwent ophthalmic evaluations, including BCVA according to the decimal Snellen E chart, intraocular pressure (IOP), cornea diameter measurement and slit-lamp biomicroscopy. Non-ocular features were documented including the morphology of teeth, face, ears, hands and feet. The study protocol was approved by the Institutional Review Board of PUMCH and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all subjects for both study participation and publication of identifying images.
Genetic analysis
Genomic DNA was isolated from peripheral blood with the QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Polymerase chain reactions (PCR) were designed to amplify the NHS exons and splice-site sequences. Primers were synthesized according to sequences published previously25. For exon 2 to 8, the final volume of 50 μl contained 40ng DNA, 10pmol of each primer, and 25 μl 2× Taq PCR Master Mix (Biomed Technologies, Beijing, China). For exon1, the final volume of 50 μl contained 2 × PCR buffer, 5× Q solution, dNTP mix(10 mM of each),10pmol of each primer, deionized and distilled water, and HotStarTaq DNA Polymerase (Qiagen, Hilden, Germany). The amplification was performed under the following conditions: 95 °C for 5 min, followed by 33 cycles at 95 °C for 30 s, 60 °C for 30 s, 72 °C for 45 s, with a final extension at 72 °C for 7 min. The PCR products were purified using EasyPure PCR purification Kit (Transgen Biotech, Beijing, China). The amplicons were sequenced using forward and reverse primers on an ABI 3730 Genetic Analyzer (ABI, Foster City, CA). The sequences were assembled and analyzed using Lasergene SeqMan software (DNASTAR, Madison, WI). All available family members were Sanger sequenced in order to confirm segregation of the mutation.
Acknowledgements
We gratefully acknowledge all participating patients and their family members. This work is supported by grants from the Ministry of Science and Technology of the People’s Republic of China (Grant No: 2010DFB33430), the Foundation Fighting Blindness USA (CD-CL-0214-0631-PUMCH), the National Natural Science Foundation of China (81470669), Beijing Natural Science Foundation (7152116) and CAMS Innovation Fund for Medical Sciences (CIFMS 2016-12M-1-002).
Author Contributions
H.L. and R.S. designed the study. R.S., H.L. and L.Y. collected clinical information. H.L., L.Y., Z.S., Z.Y. and S.W. performed the experiments and analyzed the data. H.L. and R.S. wrote the manuscript. All authors reviewed and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nance WE, Warburg M, Bixler D, Helveston EM. Congenital X-linked cataract, dental anomalies and brachymetacarpalia. Birth defects original article series. 1974;10:285–291. [PubMed] [Google Scholar]
- 2.Horan MB, X-linked FA. cataract and Hutchinsonian teeth. Aust.Paediatr.J. 1974;10:98–102. [Google Scholar]
- 3.Tug E, Dilek NF, Javadiyan S, Burdon KP, Percin FE. A Turkish family with Nance-Horan Syndrome due to a novel mutation. Gene. 2013;525:141–145. doi: 10.1016/j.gene.2013.03.094. [DOI] [PubMed] [Google Scholar]
- 4.Liao HM, et al. Identification of a microdeletion at Xp22.13 in a Taiwanese family presenting with Nance-Horan syndrome. Journal of human genetics. 2011;56:8–11. doi: 10.1038/jhg.2010.121. [DOI] [PubMed] [Google Scholar]
- 5.Khan AO, Aldahmesh MA, Mohamed JY, Alkuraya FS. Phenotype-genotype correlation in potential female carriers of X-linked developmental cataract (Nance-Horan syndrome) Ophthalmic genetics. 2012;33:89–95. doi: 10.3109/13816810.2011.634881. [DOI] [PubMed] [Google Scholar]
- 6.Florijn RJ, et al. New mutations in the NHS gene in Nance-Horan Syndrome families from the Netherlands. European journal of human genetics: EJHG. 2006;14:986–990. doi: 10.1038/sj.ejhg.5201671. [DOI] [PubMed] [Google Scholar]
- 7.Coccia M, et al. X-linked cataract and Nance-Horan syndrome are allelic disorders. Human molecular genetics. 2009;18:2643–2655. doi: 10.1093/hmg/ddp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramprasad VL, et al. Truncating mutation in the NHS gene: phenotypic heterogeneity of Nance-Horan syndrome in an asian Indian family. Investigative ophthalmology & visual science. 2005;46:17–23. doi: 10.1167/iovs.04-0477. [DOI] [PubMed] [Google Scholar]
- 9.Li A, et al. Identification of a novel NHS mutation in a Chinese family with Nance-Horan syndrome. Current eye research. 2015;40:434–438. doi: 10.3109/02713683.2014.959606. [DOI] [PubMed] [Google Scholar]
- 10.Burdon, K. P. et al. Mutations in a novel gene, NHS, cause the pleiotropic effects of Nance-Horan syndrome, including severe congenital cataract, dental anomalies, and mental retardation. American journal of human genetics73 (2003). [DOI] [PMC free article] [PubMed]
- 11.Toutain A, Ayrault AD, Moraine C. Mental retardation in Nance-Horan syndrome: clinical and neuropsychological assessment in four families. American journal of medical genetics. 1997;71:305–314. doi: 10.1002/(SICI)1096-8628(19970822)71:3<305::AID-AJMG11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Tian Q, et al. A novel NHS mutation causes Nance-Horan Syndrome in a Chinese family. BMC medical genetics. 2017;18:2. doi: 10.1186/s12881-016-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks S, et al. Refinement of the X-linked cataract locus (CXN) and gene analysis for CXN and Nance-Horan syndrome (NHS) Ophthalmic genetics. 2004;25:121–131. doi: 10.1080/13816810490514360. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, et al. Nance-Horan syndrome protein, NHS, associates with epithelial cell junctions. Human molecular genetics. 2006;15:1972–1983. doi: 10.1093/hmg/ddl120. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, et al. NHS-A isoform of the NHS gene is a novel interactor of ZO-1. Experimental cell research. 2009;315:2358–2372. doi: 10.1016/j.yexcr.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Brooks SP, et al. The Nance-Horan syndrome protein encodes a functional WAVE homology domain (WHD) and is important for co-ordinating actin remodelling and maintaining cell morphology. Human molecular genetics. 2010;19:2421–2432. doi: 10.1093/hmg/ddq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, S. K. et al. ITGB6 loss-of-function mutations cause autosomal recessive amelogenesis imperfecta. Human molecular genetics (2013). [DOI] [PMC free article] [PubMed]
- 18.Hong N, et al. Identification of a novel mutation in a Chinese family with Nance-Horan syndrome by whole exome sequencing. Journal of Zhejiang University. Science. B. 2014;15:727–734. doi: 10.1631/jzus.B1300321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, A. et al. Identification of a Novel NHS Mutation in a Chinese Family with Nance-Horan Syndrome. Current eye research, 1–5 (2014). [DOI] [PubMed]
- 20.van Dorp DB, Delleman JW. A family with X-chromosomal recessive congenital cataract, microphthalmia, a peculiar form of the ear and dental anomalies. Journal of pediatric ophthalmology and strabismus. 1979;16:166–171. doi: 10.3928/0191-3913-19790501-08. [DOI] [PubMed] [Google Scholar]
- 21.Van Esch H, Jansen A, Bauters M, Froyen G, Fryns JP. Encephalopathy and bilateral cataract in a boy with an interstitial deletion of Xp22 comprising the CDKL5 and NHS genes. American journal of medical genetics. Part A. 2007;143:364–369. doi: 10.1002/ajmg.a.31572. [DOI] [PubMed] [Google Scholar]
- 22.Huang KM, et al. Identification of three novel NHS mutations in families with Nance-Horan syndrome. Molecular vision. 2007;13:470–474. [PMC free article] [PubMed] [Google Scholar]
- 23.Franco E, Hodgson S, Lench N, Roberts GJ. Nance-Horan syndrome: a contiguous gene syndrome involving deletion of the amelogenin gene? A case report and molecular analysis. Oral diseases. 1995;1:8–11. doi: 10.1111/j.1601-0825.1995.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 24.Gjorup H, Haubek D, Jacobsen P, Ostergaard JR. Nance-Horan syndrome-The oral perspective on a rare disease. American journal of medical genetics. Part A. 2017;173:88–98. doi: 10.1002/ajmg.a.37963. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, et al. Novel causative mutations in patients with Nance-Horan syndrome and altered localization of the mutant NHS-A protein isoform. Molecular vision. 2008;14:1856–1864. [PMC free article] [PubMed] [Google Scholar]
- 26.Ding X, Patel M, Herzlich AA, Sieving PC, Chan CC. Ophthalmic pathology of Nance-Horan syndrome: case report and review of the literature. Ophthalmic genetics. 2009;30:127–135. doi: 10.1080/13816810902822021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reches A, et al. Prenatal detection of congenital bilateral cataract leading to the diagnosis of Nance-Horan syndrome in the extended family. Prenatal diagnosis. 2007;27:662–664. doi: 10.1002/pd.1734. [DOI] [PubMed] [Google Scholar]
- 28.Chograni M, Rejeb I, Jemaa LB, Chaabouni M, Bouhamed HC. The first missense mutation of NHS gene in a Tunisian family with clinical features of NHS syndrome including cardiac anomaly. European journal of human genetics: EJHG. 2011;19:851–856. doi: 10.1038/ejhg.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bixler D, Higgins M, Hartsfield J., Jr. The Nance-Horan syndrome: a rare X-linked ocular-dental trait with expression in heterozygous females. Clinical genetics. 1984;26:30–35. doi: 10.1111/j.1399-0004.1984.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 30.Apple DJ, Ram J, Foster A, Peng Q. Elimination of cataract blindness: a global perspective entering the new millenium. Survey of ophthalmology. 2000;45(Suppl 1):S1–196. [PubMed] [Google Scholar]
- 31.Pichi F, Lembo A, Serafino M, Nucci P. Genetics of Congenital Cataract. Developments in ophthalmology. 2016;57:1–14. doi: 10.1159/000442495. [DOI] [PubMed] [Google Scholar]
- 32.Santana A, Waiswo M. The genetic and molecular basis of congenital cataract. Arquivos brasileiros de oftalmologia. 2011;74:136–142. doi: 10.1590/S0004-27492011000200016. [DOI] [PubMed] [Google Scholar]
- 33.Dave A, Craig JE, Sharma S. The status of intercellular junctions in established lens epithelial cell lines. Molecular vision. 2012;18:2937–2946. [PMC free article] [PubMed] [Google Scholar]
- 34.Berthoud VM, Minogue PJ, Osmolak P, Snabb JI, Beyer EC. Roles and regulation of lens epithelial cell connexins. FEBS letters. 2014;588:1297–1303. doi: 10.1016/j.febslet.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dave A, et al. Epha2 Mutations Contribute To Congenital Cataract Through Diverse Mechanisms. Molecular vision. 2016;22:18–30. [PMC free article] [PubMed] [Google Scholar]
- 36.Hibbert S. A previously unreported association between Nance-Horan syndrome and spontaneous dental abscesses. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2005;99:207–211. doi: 10.1016/j.tripleo.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, et al. Genetic and phenotypic characteristics of three Mainland Chinese families with choroideremia. Molecular vision. 2012;18:309–316. [PMC free article] [PubMed] [Google Scholar]
- 38.Parmeggiani F, et al. Novel variants of RPGR in X-linked retinitis pigmentosa families and genotype-phenotype correlation. European journal of ophthalmology. 2017;27:240–248. doi: 10.5301/ejo.5000879. [DOI] [PubMed] [Google Scholar]
- 39.Fahim AT, Daiger SP. The Role of X-Chromosome Inactivation in Retinal Development and Disease. Advances in experimental medicine and biology. 2016;854:325–331. doi: 10.1007/978-3-319-17121-0_43. [DOI] [PMC free article] [PubMed] [Google Scholar]