Abstract
The aim of this study was to examine the association between coffee intake and tooth loss. This study hypothesized that the intake of coffee would increase the prevalence of tooth loss in Korean adults. Subject information was obtained from the Korea National Health and Nutrition Examination Survey conducted in 2010–2011. Sociodemographic and lifestyle variables, anthropometric and biochemical status, metabolic health and glucose tolerance status, as well as oral health behaviors were evaluated. The number of remaining teeth was negatively associated with the frequency of coffee intake (p-value < 0.05). Daily coffee consumers had significantly higher levels of body mass index (BMI), waist circumference (WC), total cholesterol, and low density lipoprotein cholesterol (LDL-C) (all p-value < 0.05). Individuals with less than 20 remaining teeth had higher BMI, WC, diastolic blood pressure, and LDL-C (all p-value < 0.05). Finally, participants who drank coffee on a daily basis were more likely to have fewer remaining teeth. The prevalence of having less than 20 remaining teeth was 69% higher in groups with daily coffee intake than those with coffee intake of less than once a month after adjustment for potential covariates (Odds Ratio [95% CI] = 1.69 [1.35, 2.13]). In conclusion, daily coffee consumption is closely associated with tooth loss in Korean adults.
Introduction
Coffee is one of the most popular brewed beverages and is derived from roasted coffee beans1. Coffee is composed of caffeine, chlorogenic acid, trigonelline, diterpenoids, cafestol, and kahweol2. Coffee has been shown to have beneficial effects for mood improvement, cognitive behavior, and endurance throughout extensive exercise3,4. Daily coffee consumption (>2 cups) among Korean adults5 increased by 48% from 2001 to 2011.
Coffee consumption has been reported to be associated with the prevention of several diseases. In the United States, a prospective cohort study revealed that coffee consumption was associated with lower mortality due to cardiovascular disease, chronic respiratory diseases, pneumonia and influenza, and self-injury, but not cancer6. However, there is still controversy about coffee consumption’s effects on some metabolic disorders. Several reports showed that coffee consumption was inversely correlated with the occurrence of metabolic syndrome7,8, even though another study reported that the consumption of instant coffee with high sugar content might increase the risk of metabolic syndrome9. Cohort studies revealed that coffee consumption was inversely associated with type 2 diabetes10,11, whereas, other reports demonstrated that coffee had no effect on insulin sensitivity or protection against diabetes12,13.
Tooth loss is a common problem that is especially found in the elderly. Tooth loss is caused by dental caries, periodontal disease, and trauma14,15. Tooth loss leads to decreased nutritional intake and general weakness which is linked to several medical problems including physical limitations and cognitive impairment16. A review pointed out that tooth loss clearly restricted nutritional and oral health, overall quality of life, and eventually shortened life expectancy17. Recently, a cross-sectional research revealed that periodontitis may be associated with coffee consumption1. However, another study demonstrated that coffee consumption had beneficial effects on periodontal health18.
Therefore, this study aimed to investigate the possible association between coffee consumption and tooth loss among Korean adults. We hypothesized that coffee intake would be associated with the occurrence of tooth loss.
Materials and Methods
Overview of the survey and participants
The data for this study were obtained from the 2010–2011 Korea National Health and Nutrition Examination Survey, a cross-sectional and nationwide survey supervised by the Ministry of Health and Welfare of South Korea. Specially trained investigators inspected a representative population of South Korean adults with well-designed questionnaires including physical inspections, health interviews, and nutritional examinations19.
Initially, 13,306 participants were included in the study. 4,147 participants aged <40, 1,287 participants who had missing values in nutrition, 534 participants who had missing values in dental status, and 39 participants with any other missing values were removed from the study. A final total of 7,299 participants were selected for this study. All the participants provided written informed consent. This study was approved by the Institutional Review Board (IRB) of the Korean Center for Disease Control and Prevention, and was accomplished according to the Ethical Principles for Medical Research Involving Human Subjects based on the Helsinki Declaration. This study was confirmed according to the STROBE guidelines and presented as a flowchart (Fig. 1).
Figure 1.
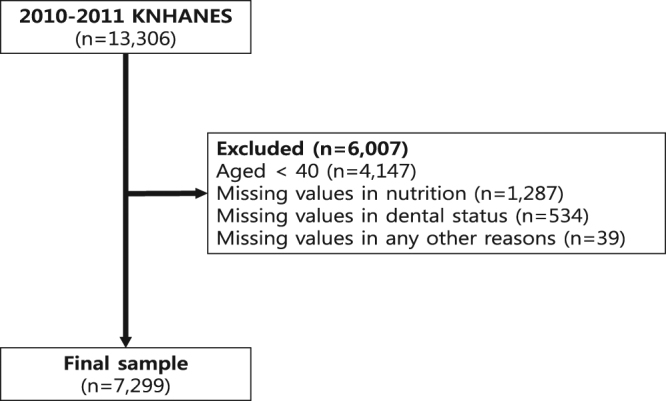
Flowchart of the study according to STROBE guidelines.
Sociodemographic and lifestyle variables
Sociodemographic and lifestyle variables of the participants were collected with a self-administered questionnaire including those for alcohol drinking, cigarette smoking, household income, physical exercise, and education level. Smokers were categorized as ex-smokers, non-smokers, or current smokers. Alcohol users were categorized as heavy drinkers (>30 g/day), mild to moderate drinkers (1–30 g/day), or non-drinkers20. The level of education was classified as either having graduated from high school (≥13 years) or not. The rate of physical exercise was measured based on the International Physical Activity Questionnaire. Participants who performed body exercises for 30 minutes/session at least 5 times/week, or those who engaged in physical exercise for 20 minutes/session at least 3 times/week were categorized as regular exercisers. Household income was divided into quartiles by the amount earned by family members. Stress level was categorized as either “uncontrollable”, “stressful”, “controllable”, or “never”21. The place of residence was subdivided into urban and rural classifications. The presence of a spouse was also considered in the analysis.
Anthropometric measurements
Qualified trained examiners evaluated the data. Height was measured to the nearest 0.1 cm, and body weight was documented using a digital scale to the nearest 0.1 kg in bare feet and lightweight clothing. Waist circumference was assessed to the nearest 0.1 cm at the slimmest mid-point between the costal and the iliac crest margins, over loose clothing at the end of a normal expiration22. BMI was measured by dividing body weight (kg) by the square of height (m2). Hypertension was defined as a systolic and/or a diastolic blood pressure that was consistently higher than 140 and 90 mm Hg, respectively. Pre-hypertension was categorized as elevated blood pressure above normal, but below hypertension as follows: a diastolic pressure 80–89 mm Hg or a systolic blood pressure 120–139 mm Hg. Neither hypertension nor pre-hypertension was categorized as normotension.
Biochemical measurements
Trained personnel collected biochemical samples from the participants. A standard mercury sphygmomanometer (Baumanometer, W. A. Baum Co., Copiague, NY, USA) was used to calculate blood pressure. Systolic and diastolic blood pressures were checked three times at five minute intervals and an average was determined. Blood samples were obtained from the antecubital vein after an eight hour fast for each participant. Samples were stored immediately at −70 °C and then conveyed to a central testing institute (NeoDin Medical Institute, Seoul, South Korea). Low and high-density lipoprotein cholesterol, serum fasting plasma glucose (FPG), total cholesterol, and triglycerides were measured with an automated enzymatic analyzer (Hitachi 7600; Hitachi, Ltd., Tokyo, Japan).
Descriptions of metabolic syndrome
Metabolic syndrome in Asians was defined according to American Heart Association/National Heart, Lung, and Blood Institute criteria23. Participants with at least three of the following 5 medical conditions were defined as having metabolic syndrome: waist circumference ≥ 80 cm for women and ≥90 cm for men, current use of an anti-hypertensive drug or blood pressure ≥130/85 mmHg, use of an anti-diabetic drug or FPG ≥ 100 mg/dL, use of an anti-dyslipidemic drug or fasting triglycerides ≥ 150 mg/dL, and use of an anti-dyslipidemic drug or high-density lipoprotein cholesterol < 50 mg/dL in women and <40 mg/dL in men.
The number of remaining teeth and oral health behaviors
The number of remaining teeth was examined by the trained dental staff member who were under license. Participants reported the frequency of daily tooth brushing, which was defined as the total number of tooth brushing sessions per day. Self-reported oral health status and number of dental visits within a year were also recorded24. Self-reported oral health status was categorized as “good”, “moderate”, or “bad”. Dental pain within the previous year was defined as whether the participant felt pain or discomfort in their dental area during that year.
Statistical analyses
The data are expressed as a percentage (standard error) for categorical variables and mean ± standard error for continuous variables. Rao–Scott Chi-square tests for categorical variables and Student’s t-tests for continuous variables were used. A multiple linear regression analysis was performed examining the frequency of coffee consumption and biochemical parameters after adjustment for covariates including age and gender. The association between number of remaining teeth and frequency of coffee consumption was analyzed using one-way analysis of covariance (ANCOVA). Finally, multiple logistic regression analyses were conducted to determine the odd ratios for having less than 20 remaining teeth according to the frequency of coffee consumption. Model 1 was a model with no adjustment. Model 2 was adjusted for sociodemographic covariates including gender and age. Model 3 was adjusted for components of Model 2 and lifestyle variables including smoking, drinking, physical activity, BMI, education status, and household income. Model 4 was adjusted for the components of Model 3 plus diseases and dental variables (metabolic syndrome, stress level, and number of daily tooth brushing sessions). The SAS statistical software version 9.3 (SAS institute, Cary, NC, USA) was used for all analyses. Statistical significance was defined as p < 0.05.
Results
Table 1 displays the characteristics of the included participants. The participants were divided into two groups by the number of remaining teeth (≥20 or <20). The prevalence of having less than 20 remaining teeth was significantly higher in individuals with high waist circumference, systolic blood pressure, serum triglyceride, and household income in the lowest quartile. Individuals with metabolic syndrome were more likely to have less than 20 remaining teeth (all p-values < 0.05).
Table 1.
Participant characteristics.
| Remaining teeth (n) < 20 | |||
|---|---|---|---|
| No | Yes | p-value | |
| n | 5359 | 1940 | |
| Age (year) | 52.5 ± 0.2 | 66.6 ± 0.37 | <0.001 |
| Gender (M) | 48.3 (0.7) | 46.4 (1.5) | 0.31 |
| BMI (kg/m2) | 24.1 ± 0.05 | 23.6 ± 0.09 | <0.001 |
| BMI ≥ 25 (yes) | 36.5 (0.8) | 32.2 (1.2) | 0.03 |
| WC (cm) | 82.7 ± 0.2 | 83.6 ± 0.27 | 0.01 |
| SBP (mmHg) | 121.9 ± 0.32 | 129.3 ± 0.61 | <0.001 |
| DBP (mmHg) | 79 ± 0.21 | 76.5 ± 0.36 | <0.001 |
| FPG (mg/dL) | 99.7 ± 0.42 | 104.6 ± 0.86 | <0.001 |
| TC (mg/dL) | 194.3 ± 0.65 | 192.6 ± 1.12 | 0.18 |
| HDL-C (mg/dL) | 52.3 ± 0.23 | 50.6 ± 0.39 | <0.001 |
| LDL-C (mg/dL) | 114.7 ± 0.59 | 113.5 ± 0.92 | 0.26 |
| TG* (mg/dL) | 118 (115.4–120.6) | 125 (120.2–130) | 0.01 |
| Present smoker (yes) | 20 (0.8) | 21.4 (1.3) | 0.34 |
| Drinking alcohol monthly (yes) | 55.7 (0.9) | 40.3 (1.3) | <0.001 |
| Regular physical exercise (yes) | 22.2 (0.8) | 17.8 (1.3) | 0.02 |
| Income (lowest quartile) | 15.8 (0.8) | 40.4 (1.6) | <0.001 |
| Education level (>13years) | 61.3 (1.1) | 22.6 (1.4) | <0.001 |
| Place of residence (urban) | 76.8 (2.4) | 61.5 (3.8) | <0.001 |
| Presence of spouse (yes) | 87.5 (0.6) | 70.4 (1.6) | <0.001 |
| MetS (yes) | 33.2 (0.8) | 48 (1.6) | <0.001 |
| WC_Mets (yes) | 39.3 (0.9) | 45.8 (1.3) | <0.001 |
| Daily tooth brushing (n) | <0.001 | ||
| ≤1 | 12.5 (0.7) | 23.2 (1.4) | |
| 2 | 50.9 (1) | 48.6 (1.5) | |
| ≥3 | 36.6 (1.1) | 28.2 (1.7) | |
| Dental visit within a year (yes) | 25.9 (1) | 15.1 (1.3) | <0.001 |
| Self-reported oral health status(yes) | <0.001 | ||
| 1 | 14.4 (0.7) | 7.8 (0.8) | |
| 2 | 40.7 (1) | 20.5 (1.5) | |
| 3 | 44.9 (0.9) | 71.7 (1.7) | |
| Dental pain within a year (yes) | 27.7 (1) | 25 (1.6) | 0.12 |
Data are presented as mean ± standard error for continuous variables and percentage (standard error) for categorical variables. *P-values were obtained by independent t-tests for continuous variables or Chi-square tests for categorical variables. *Geometric mean (95% CI). Hypertension was defined as >140/90 mmHg, prehypertension as the systolic blood pressure 120–139 mm Hg or a diastolic pressure 80–89 mm Hg. Neither hypertension nor prehypertension were designated as normotension. Self-reported oral health statuses were divided into score 1 as good, 2 as moderate, and 3 as bad. Abbreviation: BMI; body mass index, WC; waist circumference, SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglyceride, Mets; metabolic syndrome, WC_Mets; waist circumference which met the inclusion criteria for metabolic syndrome, WC ≥ 90 cm for men, and ≥80 cm for women.
Table 2 shows the associations between the number of remaining teeth, the frequency of coffee intake, and biochemical/anthropometric parameters by one-way ANCOVA which was adjusted for covariates including age and sex. Daily coffee consumers had significantly higher levels of body mass index (BMI), waist circumference (WC), total cholesterol (TC), and low density lipoprotein cholesterol (LDL-C) (all p-value < 0.05) after adjustment for age and gender. Similarly, individuals with less than 20 remaining teeth had significantly higher levels of BMI, WC, diastolic blood pressure (DBP), and LDL-C (all p-value < 0.05).
Table 2.
Association between the number of remaining teeth, coffee intake, and biochemical/anthropometric parameters.
| BMI | WC | SBP | DBP | FPG | TC | HDL-C | LDL-C | TG* | |
|---|---|---|---|---|---|---|---|---|---|
| Coffee intake (n) | |||||||||
| <1/mo | 23.7 ± 0.1 | 82.3 ± 0.3 | 125.1 ± 0.6 | 77.7 ± 0.4 | 102.8 ± 1.4 | 191.3 ± 1.6 | 51.1 ± 0.5 | 111.2 ± 1.3 | 125 (118.3–132) |
| 2/mo–1/wk | 23.9 ± 0.2 | 83.3 ± 0.6 | 125 ± 1 | 77.9 ± 0.6 | 100.6 ± 1 | 191.4 ± 1.7 | 52.3 ± 0.8 | 111.2 ± 1.5 | 122.6 (114.7–130.9) |
| 2–6/wk | 23.7 ± 0.1 | 82.3 ± 0.4 | 124.6 ± 0.7 | 77.1 ± 0.5 | 103.1 ± 1.2 | 193 ± 1.6 | 53 ± 0.6 | 114.1 ± 1.5 | 116.4 (111.1–121.9) |
| Daily | 24.1 ± 0.1 | 83.2 ± 0.2 | 125.2 ± 0.3 | 77.9 ± 0.2 | 100.6 ± 0.4 | 195.7 ± 0.7 | 51.9 ± 0.2 | 116.6 ± 0.6 | 117.5 (114.8–120.3) |
| p-value | 0.01 | 0.01 | 0.9 | 0.36 | 0.19 | 0.01 | 0.11 | <0.001 | 0.1 |
| Remaining teeth (n) | |||||||||
| <20 | 24.1 ± 0.1 | 83.1 ± 0.2 | 125.1 ± 0.3 | 78.1 ± 0.2 | 100.6 ± 0.5 | 195.2 ± 0.7 | 52 ± 0.2 | 115.9 ± 0.6 | 118.2 (115.6–120.8) |
| 20 | 23.6 ± 0.1 | 82.5 ± 0.3 | 125.1 ± 0.6 | 77 ± 0.4 | 102.8 ± 0.9 | 192.6 ± 1.2 | 51.5 ± 0.4 | 113.1 ± 1 | 120.4 (115.4–125.8) |
| p-value | <0.001 | 0.04 | 0.97 | 0.02 | 0.06 | 0.06 | 0.3 | 0.02 | 0.47 |
*Geometric mean (95% CI). *p-value < 0.05 designated statistical significance. Data were analyzed by one-way analysis of covariance (ANCOVA), and adjusted for covariates including age and gender. Abbreviations: WC; waist circumference, BMI; body mass index, SBP; systolic blood pressure, DBP; diastolic blood pressure, FPG; fasting plasma glucose, TC; total cholesterol, HDL-C; high density lipoprotein cholesterol, LDL-C; low density lipoprotein cholesterol, TG; serum triglyceride.
Table 3 represents the mean number of remaining teeth according to the frequency of coffee intake by one-way ANCOVA. The mean number of remaining teeth among participants were negatively associated with the frequency of coffee intake (all p-value < 0.05).
Table 3.
The number of remaining teeth according to the frequency of coffee intake.
| Remaining teeth (n) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Coffee intake (n) | ||||
| <1/mo | 21.9 ± 0.3 | 22.3 ± 0.2 | 22.4 ± 0.2 | 23 ± 0.2 |
| 2/mo–1/wk | 22.4 ± 0.4 | 22.3 ± 0.3 | 22.3 ± 0.3 | 22.9 ± 0.3 |
| 2–6/wk | 22.4 ± 0.3 | 21.9 ± 0.2 | 22.1 ± 0.2 | 22.6 ± 0.2 |
| daily | 23.3 ± 0.2 | 21.6 ± 0.1 | 21.7 ± 0.1 | 22.2 ± 0.1 |
| p-value | <0.001 | 0.02 | 0.02 | 0.004 |
The data are presented as mean ± standard error for continuous variables. *p-value < 0.05 designated statistical significance. Data were analyzed by one-way ANCOVA. MODEL1 was non-adjusted. MODEL2 was adjusted for gender and age. MODEL3 was adjusted for gender, age, drinking, smoking, household income, physical exercise, and education level. MODEL4 was adjusted for gender, age, drinking, smoking, metabolic syndrome, household income, physical exercise, education level, BMI, number of daily tooth brushing sessions, and stress level.
Table 4 shows the prevalence of having less than 20 remaining teeth according to the frequency of coffee intake. The prevalence of having less than 20 remaining teeth was about 69% higher in daily coffee consumers than individuals having coffee less than one time per month after adjustment for covariates (Odds Ratio [95% Confidence Intervals] = 1.69 [1.35, 2.13]).
Table 4.
Prevalence of having less than 20 remaining teeth according to the frequency of coffee intake
| OR (95% CI) | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Coffee intake (n) | ||||
| <1/mo | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 2/mo–1/wk | 0.87 (0.64, 1.17) | 1.02 (0.73, 1.42) | 1.07 (0.78, 1.48) | 1.16 (0.81, 1.64) |
| 2–6/wk | 0.91 (0.73, 1.14) | 1.19 (0.91, 1.55) | 1.18 (0.90, 1.54) | 1.34 (1.02, 1.78) |
| daily | 0.70 (0.59, 0.83) | 1.43 (1.15, 1.77) | 1.48 (1.19, 1.84) | 1.69 (1.35, 2.13) |
Multiple logistic regression analyses were performed. MODEL1 was non-adjusted. MODEL2 was adjusted for gender and age. MODEL3 was adjusted for gender, age, drinking, smoking, household income, physical exercise, and education level. MODEL4 was adjusted for gender, age, drinking, smoking, metabolic syndrome, household income, physical exercise, education level, BMI, number of daily tooth brushing sessions, and stress level.
Finally, the prevalence of having less than 20 remaining teeth by age group were assessed (the elderly 65 or more vs. others). However, we did not find any association between age group (Supplementary information).
Discussion
This study found that daily coffee consumers had significantly higher levels of BMI, WC, TC, and LDL-C. Individuals with metabolic syndrome or its components were more likely to have less than 20 remaining teeth. The mean number of remaining teeth among participants was negatively associated with the frequency of coffee intake. Finally, the prevalence of having less than 20 remaining teeth was 69% higher in daily coffee consumers than those who had coffee less than one time per month after adjustment for covariates.
This study found that coffee consumption may have harmful effects on dental health, leading to tooth loss. An increasing trend of instant coffee consumption in South Korea may explain the relationship between coffee consumption and tooth loss5. A cross-sectional study among Korean adults showed that 76% of the participants were habitual coffee drinkers, most of whom consumed instant coffee mixed with sugar and powdered creamer9. The study showed that instant coffee consumers had an elevated risk of metabolic syndrome and its components including obesity, abdominal obesity, and hypo-HDL-C. Other studies reported that the consumption of instant coffee with high sugar content may increase the risk of metabolic syndrome9. A qualitative systemic review also showed that sugar from instant coffee-mix contributed to increased HDL-C and risk of metabolic syndrome25. Since several studies demonstrated that metabolic syndrome is closely associated with tooth loss26–29, one can expect that the habitual consumption of a sugar containing coffee mix could increase tooth loss.
People who drink coffee can add sugar or syrup to their coffee which can cause tooth structure damage30. Women who drank sweetened coffee either 1–4 or >5 cups per day had higher risk for tooth loss. Sugar contained in coffee may lead to bacterial fermentation which can result in the destruction of the enamel surface31. Similarly, other studies revealed that caries risk increased in cases of coffee consumption with additives including sweeteners and creaming agents, whereas coffee consumption without additives had a caries preventive effect32. They also found that the caries index was 2.9 in individuals who drank black coffee and 5.5 for individuals who drank coffee with additives.
Individuals with periodontitis demonstrated significantly more coffee consumption in Korean male adults. They had a 45% increased prevalence of periodontitis when they consumed coffee 3 or more times per day1. Experimental studies supported this finding. In rodents, the daily consumption of high doses of caffeine provoked ligature-induced periodontitis33,34. Since periodontitis is one of the leading causes of tooth loss, coffee consumption may lead to tooth loss by periodontal breakdown.
Coffee/caffeine consumption may lead to a malfunction in calcium metabolism, reduction in bone mineral density, and delayed bone repair35. Caffeine may inhibit the development of osteoblasts by decreasing the expression of vitamin D receptors on the surface of osteoblasts36, or by causing the upstream mediator cyclic AMP to down-regulate osteoblast proliferation37. Another experimental study showed that daily caffeine consumption may increase osteoclastogenesis38. The intake of coffee was significantly associated with an increased risk for osteoporosis and osteoporotic fracture39. Since caffeine can provoke osteoporosis, tooth loss also increased as osteoporosis risk increased40. Coffee consumption may elevate calcium excretion through urine which may also increase osteoporosis41. Calcium loss may be harmful to the elderly who have less calcium intake. Recent long-term longitudinal cohort studies revealed that a high coffee intake of 4 or more cups daily was associated with a small reduction in bone density42.
Collectively, one can assume that sugar and other ingredients from instant coffee mix may increase the risk of metabolic syndrome and its components. In addition, dietary carbohydrates from sugar components may increase the risk of dental caries or periodontal disease, which eventually lead to tooth loss. Looking from a different point of view, the long-term intake of coffee may evoke catabolic bone metabolism, decreasing the density of alveolar bone, and eventually leading to tooth loss.
This study has some limitations. First, this study cannot clarify the precise causal relationship between tooth loss and coffee consumption because of its cross-sectional observational design. Further longitudinal cohort studies are necessary to clarify the precise effect of coffee consumption on tooth loss. Second, this study did not subdivide the sample by age group, brand, or type of coffee, which could affect the outcome variables. Third, either coffee volume or caffeine content could vary among different size of cups, which might influence the reliability of the study. Fourth, this study didn’t deal with effect of coffee with additives like sugar, milk or cream, which may have different effects on metabolism. This study also has strengths. First, to the best of our knowledge, this is the first study that demonstrated the close association between coffee consumption and tooth loss in South Korean adults. Second, the present study showed that oral and metabolic health could benefit from less coffee consumption. The findings from this study emphasize the oral health issues of coffee consumption as well as metabolic health.
Conclusion
Daily coffee consumption is associated with tooth loss in Korean adults.
Electronic supplementary material
Author Contributions
I.S.S. initiated, design, and wrote the paper. K.H. performed the statistical analyses. J.J.R. contributed to the critical review of the study. Y.J.C. and J.B.P. participated in the design, and review of the paper. All the authors agreed to the final version of the paper. Y.J.C. and J.B.P. are the guarantor of the present work and takes responsibility for the integrity and precision of the data management.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Yeon-Jo Choi and Jun-Beom Park contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20789-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yeon-Jo Choi, Email: yeonjochoi@yahoo.co.kr.
Jun-Beom Park, Email: jbassoonis@yahoo.co.kr.
References
- 1.Han K, Hwang E, Park JB. Association between Consumption of Coffee and the Prevalence of Periodontitis: The 2008–2010 Korea National Health and Nutrition Examination Survey. PLoS One. 2016;11:e0158845. doi: 10.1371/journal.pone.0158845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown L, Poudyal H, Panchal SK. Functional foods as potential therapeutic options for metabolic syndrome. Obes Rev. 2015;16:914–941. doi: 10.1111/obr.12313. [DOI] [PubMed] [Google Scholar]
- 3.Campbell B, et al. International Society of Sports Nutrition position stand: energy drinks. J Int Soc Sports Nutr. 2013;10:1. doi: 10.1186/1550-2783-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temple JL. Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci Biobehav Rev. 2009;33:793–806. doi: 10.1016/j.neubiorev.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Je Y, Jeong S, Park T. Coffee consumption patterns in Korean adults: the Korean National Health and Nutrition Examination Survey (2001–2011) Asia Pac J Clin Nutr. 2014;23:691–702. doi: 10.6133/apjcn.2014.23.4.11. [DOI] [PubMed] [Google Scholar]
- 6.Loftfield E, et al. Association of Coffee Consumption With Overall and Cause-Specific Mortality in a Large US Prospective Cohort Study. Am J Epidemiol. 2015;182:1010–1022. doi: 10.1093/aje/kwv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Kim K, Park SM. Association between the Prevalence of Metabolic Syndrome and the Level of Coffee Consumption among Korean Women. PLoS One. 2016;11:e0167007. doi: 10.1371/journal.pone.0167007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosso G, et al. Association of daily coffee and tea consumption and metabolic syndrome: results from the Polish arm of the HAPIEE study. Eur J Nutr. 2015;54:1129–1137. doi: 10.1007/s00394-014-0789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HJ, Cho S, Jacobs DR, Park K. Instant coffee consumption may be associated with higher risk of metabolic syndrome in Korean adults. Diabetes Res Clin Pract. 2014;106:145–153. doi: 10.1016/j.diabres.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Ranheim T, Halvorsen B. Coffee consumption and human health - beneficial or detrimental? - Mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol Nutr Food Res. 2005;49:274–284. doi: 10.1002/mnfr.200400109. [DOI] [PubMed] [Google Scholar]
- 11.Lin WY, et al. Coffee consumption is inversely associated with type 2 diabetes in Chinese. Eur J Clin Invest. 2011;41:659–666. doi: 10.1111/j.1365-2362.2010.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krebs JD, Parry-Strong A, Weatherall M, Carroll RW, Downie M. A cross-over study of the acute effects of espresso coffee on glucose tolerance and insulin sensitivity in people with type 2 diabetes mellitus. Metabolism. 2012;61:1231–1237. doi: 10.1016/j.metabol.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Akash MSH, Rehman K, Chen SQ. Effects of coffee on type 2 diabetes mellitus. Nutrition. 2014;30:755–763. doi: 10.1016/j.nut.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Aida J, et al. Reasons for permanent tooth extractions in Japan. J Epidemiol. 2006;16:214–219. doi: 10.2188/jea.16.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chestnutt IG, Binnie VI, Taylor MM. Reasons for tooth extraction in Scotland. J Dent. 2000;28:295–297. doi: 10.1016/S0300-5712(99)00069-X. [DOI] [PubMed] [Google Scholar]
- 16.Shimazaki Y, et al. Influence of dentition status on physical disability, mental impairment, and mortality in institutionalized elderly people. J Dent Res. 2001;80:340–345. doi: 10.1177/00220345010800010801. [DOI] [PubMed] [Google Scholar]
- 17.Friedman PK, Lamster IB. Tooth loss as a predictor of shortened longevity: exploring the hypothesis. Periodontol 2000. 2016;72:142–152. doi: 10.1111/prd.12128. [DOI] [PubMed] [Google Scholar]
- 18.Machida T, et al. Severe Periodontitis Is Inversely Associated with Coffee Consumption in the Maintenance Phase of Periodontal Treatment. Nutrients. 2014;6:4476–4490. doi: 10.3390/nu6104476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song IS, Han K, Ryu JJ, Park JB. Association between underweight and tooth loss among Korean adults. Sci Rep. 2017;7:41524. doi: 10.1038/srep41524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song, I. S. et al. Associations between the consumption of carbonated beverages and periodontal disease The 2008–2010 Korea national health and nutrition examination survey. Medicine95 (2016). [DOI] [PMC free article] [PubMed]
- 21.Lee KJ, Kim JI. Relating Factors for Depression in Korean Working Women: Secondary Analysis of the Fifth Korean National Health and Nutrition Examination Survey (KNHANES V) Asian Nurs Res. 2015;9:265–270. doi: 10.1016/j.anr.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Song IS, et al. Severe Periodontitis Is Associated with Insulin Resistance in Non-abdominal Obese Adults. J Clin Endocrinol Metab. 2016;101:4251–4259. doi: 10.1210/jc.2016-2061. [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, et al. Circulation. 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity; pp. 1640–1645. [DOI] [PubMed] [Google Scholar]
- 24.Song IS, Han K, Choi YJ, Ryu JJ, Park JB. Influence of oral health behavior and sociodemographic factors on remaining teeth in Korean adults: 2010–2012 Korea national health and nutrition examination survey. Medicine (Baltimore) 2016;95:e5492. doi: 10.1097/MD.0000000000005492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marventano S, et al. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clin Nutr. 2016;35:1269–1281. doi: 10.1016/j.clnu.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Kim, S. W. et al. Tooth Loss and Metabolic Syndrome in South Korea: The 2012 Korean National Health and Nutrition Examination Survey. Medicine95 (2016). [DOI] [PMC free article] [PubMed]
- 27.Holmlund A, Hulthe J, Lind L. Tooth loss is related to the presence of metabolic syndrome and inflammation in elderly subjects: a prospective study of the vasculature in Uppsala seniors (PIVUS) Oral Health Prev Dent. 2007;5:125–130. [PubMed] [Google Scholar]
- 28.Hyvarinen K, Salminen A, Salomaa V, Pussinen PJ. Systemic exposure to a common periodontal pathogen and missing teeth are associated with metabolic syndrome. Acta Diabetol. 2015;52:179–182. doi: 10.1007/s00592-014-0586-y. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Hollis JH. Associations between the number of natural teeth and metabolic syndrome in adults. J Clin Periodontol. 2015;42:113–120. doi: 10.1111/jcpe.12361. [DOI] [PubMed] [Google Scholar]
- 30.Morabia A, Costanza MC. Tea, coffee, and sweet tooth: towards a Japanese paradox. Prev Med. 2010;50:157–158. doi: 10.1016/j.ypmed.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Das, S. & Rajput, S. S. Toxic Level of Soft Drinks and Sports Drink on Health Status. Int J of Adv in Pharmac Biol Chem2 (2013).
- 32.Namboodiripad PA, Kori S. Can coffee prevent caries? J Conserv Dent. 2009;12:17. doi: 10.4103/0972-0707.53336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezerra JP, da Silva LR, de Alvarenga Lemos VA, Duarte PM, Bastos MF. Administration of high doses of caffeine increases alveolar bone loss in ligature-induced periodontitis in rats. J Periodontol. 2008;79:2356–2360. doi: 10.1902/jop.2008.080204. [DOI] [PubMed] [Google Scholar]
- 34.Bezerra JP, et al. Effects of estrogen deficiency and/or caffeine intake on alveolar bone loss, density, and healing: a study in rats. J Periodontol. 2013;84:839–849. doi: 10.1902/jop.2012.120192. [DOI] [PubMed] [Google Scholar]
- 35.Lacerda SA, et al. Bone quality associated with daily intake of coffee: a biochemical, radiographic and histometric study. Braz Dent J. 2010;21:199–204. doi: 10.1590/S0103-64402010000300004. [DOI] [PubMed] [Google Scholar]
- 36.Rapuri PB, Gallagher JC, Nawaz Z. Caffeine decreases vitamin D receptor protein expression and 1,25(OH)2D3 stimulated alkaline phosphatase activity in human osteoblast cells. J Steroid Biochem Mol Biol. 2007;103:368–371. doi: 10.1016/j.jsbmb.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 37.Kamagata-Kiyoura Y, et al. Combined effects of caffeine and prostaglandin E2 on the proliferation of osteoblast-like cells (UMR106-01) J Periodontol. 1999;70:283–288. doi: 10.1902/jop.1999.70.3.283. [DOI] [PubMed] [Google Scholar]
- 38.Yi JR, et al. Caffeine may enhance orthodontic tooth movement through increasing osteoclastogenesis induced by periodontal ligament cells under compression. Arch Oral Biol. 2016;64:51–60. doi: 10.1016/j.archoralbio.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 40.Nicopoulou‐Karayianni K, et al. Tooth loss and osteoporosis: the OSTEODENT Study. J Clin Periodontol. 2009;36:190–197. doi: 10.1111/j.1600-051X.2008.01365.x. [DOI] [PubMed] [Google Scholar]
- 41.Barrett-Connor E, Chang JC, Edelstein SL. Coffee-associated osteoporosis offset by daily milk consumption. The Rancho Bernardo Study. JAMA. 1994;271:280–283. doi: 10.1001/jama.1994.03510280042030. [DOI] [PubMed] [Google Scholar]
- 42.Hallstrom H, et al. Long-term coffee consumption in relation to fracture risk and bone mineral density in women. Am J Epidemiol. 2013;178:898–909. doi: 10.1093/aje/kwt062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


