Abstract
Polyparasitism is common in the developing world. We have previously demonstrated that schistosomiasis-positive (SP) Malian children, aged 4–8 years, are protected from malaria compared to matched schistosomiasis-negative (SN) children. The effect of concomitant schistosomiasis upon acquisition of T cell memory is unknown. We examined antigen-specific T cell frequencies in 48 Malian children aged 4–14 to a pool of malaria blood stage antigens, and a pool of schistosomal antigens, at a time point during a malaria episode and at a convalescent time point ~6 months later, following cessation of malaria transmission. CD4+ T cell-derived memory responses, defined as one or more significant cytokine (IFN-γ, TNF-α, IL-2, and/or IL-17A) responses, was measured to schistoma antigens in 18/23 SP children at one or both time points, compared to 4/23 SN children (P < 0.0001). At the time of malaria infection, 12/24 SN children and 15/23 SP children (P = 0.29) stimulated with malaria antigens demonstrated memory recall as defined by CD4-derived cytokine production. This compares to 7/23 SN children and 16/23 SP children (P = 0.009) at the convalescent timepoint. 46.2% of cytokine-producing CD4+ T cells expressed a single cytokine after stimulation with malaria antigen during the malaria episode. This fell to 40.9% at follow-up with a compensatory rise of multifunctional cytokine secretion over time, a phenomenon consistent with memory maturation. The majority (53.2–59.5%) of responses derived from CD45RA−CD62L− effector memory T cells with little variation in the phenotype depending upon the time point or the study cohort. We conclude that detectable T cell memory responses can be measured against both malaria and schistosoma antigens and that the presence of Schistosoma haematobium may be associated with long-term maintenance of T memory to malaria.
Keywords: malaria, schistosomiasis, coinfection, multifunctional T cells, T cell memory, memory, Plasmodium falciparum
Introduction
Plasmodium falciparum and Schistosoma spp. are co-endemic parasitic diseases with worldwide distribution. Revised estimates suggest that falciparum malaria causes 212 million infections annually, while schistosomiasis affects an estimated 207 million with 92% residing in Africa (1, 2). Both parasitic diseases predominate in sub-Saharan Africa but the host impact of dual infection is understudied. A growing body of evidence suggests that a pre-existing infection can modulate the effects of a second infection within the human host. This can occur indirectly, as is the case in HIV where a reduction in CD4+ T cells results in host susceptibility to viral and parasitic infections. This can also occur directly, such as in helminth infections, where chronicity of infection and the robust response of the immune system results in a background environment that modulates the host response to a secondary infection. The term, “helminth” encompasses a wide range of representative examples including soil transmitted helminthes with limited systemic perturbation, as well as tissue-invasive helminths capable of surviving for years within the human host. Schistosoma haematobium is a long-lived blood fluke capable of exerting a persistent stimulatory effect on the host immune system, chiefly to egg antigens, and modestly protects against clinical uncomplicated P. falciparum malaria in an age-specific manner in West African children (3, 4).
Chronic schistosoma infection, characterized by persistent egg patency, results in an expansion of TH2-mediated responses (5, 6), as well as the induction of regulatory pathways leading to parasite immunomodulation (7). While the immunologic mechanisms involved in exerting clinical protection may be multifactorial, there is evidence of differential immunologic responses to malaria in children with underlying S. haematobium (8–13). These immunologic perturbations may result in an altered clinical response to an acute malaria exposure. A systematic examination of the immune response to malaria among Malian children with asymptomatic S. haematobium demonstrate significantly more memory B cell response to pooled malaria vaccine antigens [apical membrane antigen 1 (AMA1) and merozoite surface protein 1 (MSP1)] (14), altered cytokine patterns characterized by IL-4 and IL-10 TH2-enrichment as well as IL-6 and IFN-γ elevation (8), and suppressed T regulatory cells response (15), compared to age-matched children without underlying schistosomiasis.
Protection against pre-erythrocytic malaria may be mediated by CD4 T cells as evidenced by the ability for circumsporozoite-specific CD4 T cells to mediate clearance of hepatocyte infection in murine models and adoptive transfer of protection in vivo (16, 17). Multifunctionality, a term referring to the ability of T cells to simultaneously produce multiple cytokines (e.g., IFN-γ, IL-2, and TNF-α) has been associated with increased protective responses against some infections and is therefore generally accepted as an indicator of the “quality” of T cell response (18, 19). CD4 T cells may gain effector function with maturation resulting in the ability to secrete multiple cytokines while eventually losing effector function in a linear manner (19). Evidence of durable immunologic memory to malaria antigens is mixed, particularly in young children, where repetitive and ongoing exposure to malaria is required to achieve and maintain immunity (20, 21). Chronic immune activation due to a helminth infection (characterized by a TH2 cytokine production pattern) may alter responses to a secondary stimuli that depend upon TH1 cytokine production, such as malaria antigens (5, 22), as well as affect the induction and maintenance of T cell memory responses (23). For example, it has been reported that underlying filarial infection suppresses malaria-specific TH1 and TH17 responses in co-infected Malian children (24). We report herein, findings that demonstrate maintenance of CD4 T cell memory responses at a convalescent time point following documentation of acute malaria infection in children with underlying S. haematobium compared to helminth-free, age-matched children.
Materials and Methods
Study Population and Clinical Trial Design
Bandiagara (pop. ~13,600) is located in Mali, West Africa, and has intense seasonal transmission (July–December) of P. falciparum malaria. The entomologic inoculation rate at the time of this field study ranged from 20 to 60 infected bites per month at peak transmission and, children experience a mean of 1.54 symptomatic malaria episodes per season (25). S. haematobium and Schistosoma mansoni are endemic to the area (26, 27), with S. haematobium prevalence of 25% in children aged 4–14 years and ~50% in adults (3). Children can be exposed to schistosomes as early as age 2 or 3 years. This study was conducted over two sequential malaria transmission seasons (2002–03) and study details have previously been reported (3, 8). Briefly, children aged 4–14 years of age, diagnosed as having asymptomatic, chronic S. haematobium (SP), were age, gender, and residence-matched to a child without schistosomiasis [schistosomiasis-negative (SN)] prior to malaria transmission. Children were followed weekly over the malaria transmission season (25 weeks) and at a dry season follow-up appointment (~6–9 months after enrollment at a time when standing water pools had dried and schistosoma transmission had ceased). The primary endpoint of the clinical trial was time to first clinical malaria infection. Clinic personnel were available 24 h-a-day throughout study duration to detect, examine, and treat symptomatic malaria episodes. A clinical episode of malaria was defined as P. falciparum parasitemia and axillary temperature ≥37.5°C on active surveillance, or parasitemia and symptoms leading to treatment-seeking behavior in the absence of other clear cause on passive surveillance. All children were pre-treated with albendazole to eliminate concomitant helminth infections and study samples were drawn at the time of their first clinical malaria episode (or at study week 25/Day 175 in the absence of a clinical infection) and again at the final dry season appointment. Children were optimally treated for schistosomiasis with praziquantel at the final appointment.
Ethics
This trial was carried out in accordance with the recommendations of the University of Bamako and the University of Maryland Institutional Review Boards (IRBs) with written consent obtained from all legal guardians. Village permission to conduct research was obtained from village chiefs, government officials and traditional healers prior to study initiation. Individual written informed consent was obtained from the parent or legal guardian of each child prior to screening and enrollment in accordance with the Declaration of Helsinki. All children displaying gross hematuria or symptoms of genitourinary pathology were treated with praziquantel (40 mg/kg) therapy and discharged from the study.
Sample Collection
Patient whole blood (7–10 mL) was collected at the study clinic into sterile eppendorf and EDTA tubes on admission, prior to institution of anti-malarial therapy, and immediately refrigerated. Sera was processed as previously described (8). Blood was processed by density centrifugation, within 2 h of acquisition, utilizing lymphocyte separation medium (ICN Biomedical Inc, Aurora, OH, USA) following standard techniques (28). Peripheral blood mononuclear cells (PBMC) were resuspended in media and linear-rate frozen using isopropyl alcohol containers (Nalgene, USA) to −70°C in the field site before transfer in liquid nitrogen storage containers to the University of Maryland at Baltimore. Samples for the primary experiment were chosen based on the availability of both a transmission and dry season time point and the presence of at least 10 × 106 cells for each child at each time point. A secondary analysis from the same study, which was part of a larger, previously unpublished set of experiments, examined samples with only a single transmission season time point utilizing a second flow cytometry panel of antibodies.
PBMC Stimulation
Each primary experiment consisted of a U.S. malaria-naïve adult control, and paired samples (transmission and dry season) from an SP child and an age-matched SN child and was performed by an investigator blinded to the age and schistosoma status of the samples. Thawed PBMC were rested overnight at 37°C, 5% CO2 and washed. A portion of cells (2.0 × 106) was removed to serve as negative (media) and positive [stimulation with 10 µg/mL Staphylococcus enterotoxin B (SEB); Sigma, St. Louis, MO, USA] controls. The remaining PBMC were split into two aliquots consisting of ~1.0 × 106 cells each and stimulated with a malaria antigen pool [consisting of AMA1 (29) and Merozoite Surface Protein 1 (MSP142)] (30), and S. haematobium antigen pools [soluble egg antigen and soluble worm antigen protein (SWAP)]. Antigen stimulation for the assay was optimized at 5 μg/mL/antigen. The malaria antigens chosen represent two vaccine candidates being tested at the same field site in Mali, while the S. haematobium antigens are commonly used in schistosoma research. The 3D7 stain of malaria is well documented at the Malian site and strain-specific protection against an AMA1 vaccine has been established (31). AMA1 has been shown to elicit cell-mediated immunity (32). Using an optimized protocol (33), all cells were stimulated for 2 h before protein transport was blocked by adding 0.5 μL/tube GolgiPlug (BD Pharmingen) followed by overnight incubation. The secondary analysis examined PBMC stimulated with AMA1 (5 µg/mL) alone and processed in an identical fashion.
Flow Cytometry Staining and Analysis
Primary Panel
Peripheral blood mononuclear cells were stained with fluorochrome-labeled monoclonal mouse anti-human antibodies (mAb) against surface antigens (CD3-Energy Coupled Dye (ECD, Beckman Coulter, clone UCHT1), CD4-Pacific Orange (BD Biosciences, clone SK3), CD8-FITC (BD Biosciences, clone HIT8a), CD19 (Invitrogen, clone SJ25-C1)/CD14 Invitrogen, clone TüK4)/Vivid-Pacific Blue, CD45RA Quantum Dots (Qdot) 655 (clone 5H3), and CD62L APC-EF780 (eBiosciences, clone DREG-56), followed by fixation/permeabilization by using cytofix/cytoperm solution (eBiosciences) and intracellular staining with mAb to IFN-γ-APC (BD Biosciences, clone B27), IL-2-phycoerythrin (PE)-Cy7 (BD Biosciences, clone MQ1-17H12), TNF Alexa Flour 700 (BD Biosciences, clone Mab11), IL-17A PerCP-Cy5.5 (eBiosciences, clone eBio64DEC17), and CD69-PE (eBiosciences, clone FN50).
Secondary Panel
A subset of unpaired PBMC (from the transmission season) were stained with CD3-ECD, CD4-APC-Cy7 (BD Biosciences, clone RPA-T4), CD8-PE-Cy7, CD19/CD14-PacBlue, biotinylated CD45RA (BD Biosciences, clone HI100) PacOrange, CD62L PE-Cy5 (BD Biosciences, clone DREG-56), IFN-γ-PE (BD Biosciences, clone B27), IL-4 Alexa488 (BioLegends, clone M8D4-8), and IL-10 APC (BioLegends, clone JES3-19F1) mAb as described above.
Cells were resuspended in 1% formaldehyde fixation buffer and analyzed using a BD LSR II SORP 4-laser flow cytometer. PBMC from healthy subjects were used as internal controls in the experiments. A total of 244,000–1,000,000 events (mean ~600,000) in the forward and side scatter (FS/SS) lymphocyte gate were collected per sample. List-mode data files were analyzed using WinList 7.1 3D (Verity Software House, Topsham, ME, USA). An amine reactive dye (ViViD, Invitrogen, OR, USA) was used as a dead cell discriminator and B lymphocytes (CD19+) and macrophages/monocytes (CD14+) were excluded from analysis. Doublets/aggregates were subtracted from analysis and gate placement determined with the aid of Fluorescence Minus One controls. Specimens were included in the analysis if (1) the cell viability was >80% after thawing and (2) cells were shown to be functionally active as determined by the production of IFN-γ by at least 0.2% CD3+ cells after stimulation with SEB. A response was considered specific if (1) the differential in the number of positive events in the stimulant pool compared to the media control was significantly increased by χ2 analyses; and (2) the net percentage of cytokine-producing cells was ≥0.1% in stimulant pool as compared to the media control. A response was considered positive if the production of one or more cytokines, meeting the pre-defined criteria, was measured in response to antigen stimulation of PBMC.
Statistics
Statistical analysis was performed on GraphPad Prism 5 (Graphpad Software, Inc., San Diego, CA, USA), and demographic and immunologic data were stratified and evaluated by age group (age 4–8 and 9–14 years). Student t-test (two-tailed) or Mann–Whitney U test were used to compare continuous and/or nonparametric data and χ2 analysis, using Mantel–Haenszel or Fisher Exact (two-tailed) as appropriate, was performed for categorical data. Cellular multifunctionality was assessed using the WinList 3D 7.1 FCOM to enumerate phenotypes. Spearman rank correlation coefficient was calculated utilizing GraphPad Prism 5. A significance level of P < 0.05 was considered statistically significant.
Results
Sample Population
Primary Dataset
Samples from children with >10 × 106 PBMC/time point (n = 48, mean age 7.9 years, range 4–14 years) were thawed and examined. Evaluative data was available from 24 SP children (mean egg count 58 eggs/10 mL urine) and 24 SN children, all but two of who developed 1–4 malaria episodes during the transmission season (Table 1). One SP child who remained malaria-free was age-matched to an SN child who likewise, remained malaria-free over the course of the study period. Samples were excluded if the viability or thawed quantity of PBMC was insufficient (n = 3 time points). SP children had a statistically longer time to first clinical malaria infection and a trend toward a reduced geometric mean parasite density at the time of malaria infection but experienced similar numbers of malaria episodes over the course of a single transmission season (Table 1). There was no age-related difference in the character of the malaria infection in SP children. Children were deemed to be free of S. haematobium based upon 2–3 negative urine examinations and 1–2 stool examinations coupled with follow-up studies performed ~9 months later prior to the dry season follow-up. We have previously demonstrated similar prevalence of soil transmitted helminth infections and hemoglobinopathies (hemoglobin S and C) in these age-matched populations (3).
Table 1.
Demographics.
| Category | SP Mal (n = 24) | SN Mal (n = 24) | P value |
|---|---|---|---|
| Mean age (range) | 7.8 (4–14) | 8.0 (4–14) | 0.73 |
| Female (%) | 9 (37.5) | 15 (62.5) | 0.09 |
| Eggs (range)a | 57.7 (2–466) | 0 | n/a |
| Malaria episodesb (range) | 2.19 (1–4) | 2.23 (1–4) | 0.90 |
| Days to first malariab episode (range) | 40.2 (49–113) | 26.8 (3–40) | 0.006 |
| Parasitemiab,c (range) | 3,803 (750–90,000) | 10,284 (1,025–105,000) | 0.15 |
Demographic characteristics at enrollment and features of subsequent Plasmodium falciparum malaria infections of Schistosoma haematobium-positive (SP Mal) and age-matched S. haematobium-negative (SN Mal) Malian children contributing peripheral blood mononuclear cells for primary immunologic analysis.
aUrinary egg excretion detected in 10 mL of filtered morning (10:00 a.m. to 2:00 p.m.) urine.
bExcluding two children (one SP and one SN) who remained malaria free during study follow-up.
cGeometric mean parasite density per cubic millimeter.
Bold indicates significant value with P ≤ 0.05.
Intracellular Cytokine Expression to Antigenic Stimulation
Malaria Antigen
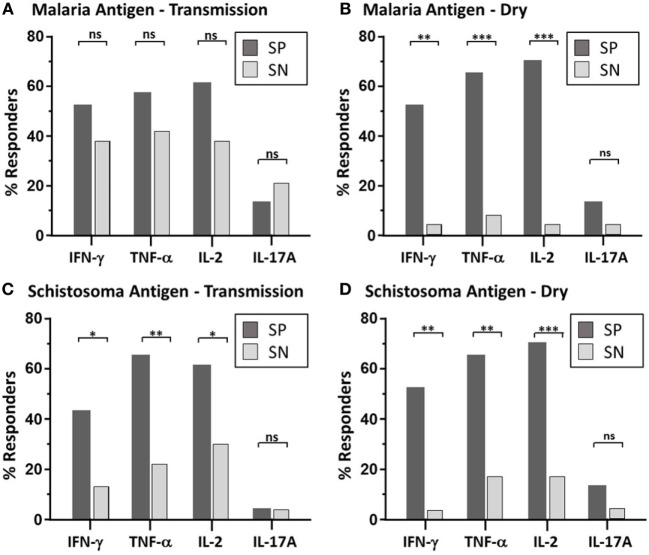
Paired PBMC samples obtained at two time points (malaria transmission (wet) and dry season) were examined via multiparameter flow cytometry (Figure S1 in Supplementary Material). Intracellular cytokine production (IFN-γ, TNFα, IL2, and IL17A) from PBMC stimulated with pooled malaria and schistosoma antigens was measured (Table 2; Figure S2 in Supplementary Material) in 24 SP Mal and 24 SN Mal children (n = 48). The majority of cytokine-producing cells were found to derive from CD3+CD19−CD4+CD8− T cells with a minority (<0.03%) observed from CD8+ T cells. The amount of cytokine measured after malaria antigen stimulation was low, but significant, with a geometric mean value of less than 0.2%. More SP children [15/23 (65%)] had detectable cytokine expression (one or more cytokines) than SN children [12/24 (50%); P = 0.29, Mantel–Haenszel χ2 analysis], during the malaria transmission season at the time of their malaria episode (Figure 1A); however, little difference was noted between the two groups in terms of the geometric mean percent of that cytokine response (Figure S2 in Supplementary Material). A small minority of children [3/23 (13%) SP and 5/24 (21%) SN children] had demonstrable IL-17A cytokine production at the time of an active malaria infection. Contrary to previous immunologic results (14, 15), we found no significant difference in cytokine production to antigen stimulation when stratified by age (Spearman rank correlation coefficient, ρ = −0.17, P = 0.26). When paired dry season samples were analyzed, fewer SN children [7/23 (30.4%)] had a recall response to malaria antigen (defined as one or more cytokine response) as compared to SP children, who not only retained the low-level memory response to malaria antigen but had a higher number of responders [16/23 (70%); P = 0.009, OR = 5.01, 95% confidence interval (CI) (1.27–22.77), Mantel–Haenszel χ2 analysis] Individual cytokines results demonstrate statistically significant results for IFN-γ, TNF-α, and IL-2 (Figure 1B). The overall amount of secreted cytokine (IFN-γ, TNF-α, IL-2, or IL-17A) remained statistically similar to that measured during the transmission season but without high responding outliers (Table 2; Figure S2 in Supplementary Material). Eleven of the 16 SP children with demonstrable malaria antigen recognition during the dry season had detectable cytokine production at the earlier, transmission season time point. Minimal detectable IL-17A was measured at the dry season timepoint, suggesting that this cytokine may be part of the inflammatory response mounted to acute infection but does not play a significant role in memory response post-transmission season. Malaria-naïve U.S. adult controls had no detectable increase of intracellular cytokines to either antigen stimulant.
Table 2.
Intracellular cytokine expression to antigen stimulation.
| Season | Cohort | Aga | No. (IFNγ)b (range) | Pc | No. (TNFα) (range) | Pc | No. (IL-2) (range) | Pc | No. (IL-17A) (range) | Pc |
|---|---|---|---|---|---|---|---|---|---|---|
| Wetd | SP | Pf | 12/23 (0.22) (0.1–1.09) | 0.32 | 13/23 (0.39) (0.1–1.8) | 0.31 | 14/23 (0.32) (0.1–1.7) | 0.11 | 3/23 (0.37) (0.1–0.91) | ns |
| SNc | Pf | 9/24 (0.22) (0.1–0.86) | 10/24 (0.19) (0.1–0.57) | 9/24 (0.29) (0.1–1.04) | 5/24 (0.25) (0.1–0.71) | |||||
| SP | Sh | 12/23 (0.25) (0.1–0.69) | 0.0003 | 15/23 (0.28) (0.1–0.75) | <0.0001 | 16/23 (0.23) (0.1–0.72) | <0.0001 | 3/23 (0.09) (0.1) | 0.28 | |
| SN | Sh | 1/24 (0.15) (n/a) | 2/24 (0.36) (0.15–0.55) | 1/24 (0.50) (n/a) | 1/24 (0.1) (n/a) | |||||
| Dryd | SP | Pf | 10/23 (0.24) (0.1–1.08) | 0.02 | 15/23 (0.29) (0.1–1.89) | 0.003 | 14/23 (0.29) (0.1–1.7) | 0.04 | 1/23 (0.10) (n/a) | ns |
| SN | Pf | 3/23 (0.12) (0.1–0.15) | 5/23 (0.18) (0.11–0.34) | 7/23 (0.15) (0.1–0.32) | 1/23 (0.13) (n/a) | |||||
| SP | Sh | 12/23 (0.25) (0.1–0.57) | 0.0004 | 15/23 (0.33) (0.1–0.81) | 0.001 | 18/23 (0.26) (0.1–1.00) | <0.0001 | 4/23 (0.17) (0.1–0.31) | 0.16 | |
| SN | Sh | 1/23 (0.25) (n/a) | 4/23 (0.40) (0.15–0.72) | 4/23 (0.44) (0.19–0.95) | 1/23 (0.17) (n/a) | |||||
Results of the intracellular cytokine expression after antigenic stimulation of peripheral blood mononuclear cells (PBMC) acquired from age-matched children with [schistosomiasis-positive (SP)] or without Schistosoma Haematobium [schistosomiasis-negative (SN)] during the malaria transmission (wet) season at the time of a malaria episode and again during a dry season follow-up visit. The results represent those individuals with statistically significant increases in the percentage of cytokine expressed compared to media controls after PBMC stimulation and the denominator represents the total number of individual PBMC samples analyzed. The results are depicted in graphical form in Figure 1.
aPBMC were stimulated with a malaria antigen pool (apical membrane antigen 1 and merozoite surface protein 1) or with an S. haematobium pool (soluble egg antigen and soluble worm antigen protein).
bNumber (No.) of individuals with statistically significant cytokine expression, the geometric mean of the net% of cytokine detected (stimulant minus media control) in positive individuals, and the range measured.
cχ2 analysis, using Mantel–Haenszel analysis, was performed between SP vs. SN children with statistically significant quantities of cytokine production after antigen stimulation. P value significance set at <0.05. Not significant, ns.
dThree experiments were excluded due to insufficient or poor viability cell quantities to allow proper interpretation of data.
Bold indicates significant value with P ≤ 0.05.
Figure 1.
Maintenance of parasite-specific memory response—depicted is the bar graph representation of the percent of responders with significant memory response, as defined by CD4 T cell-derived expression to each individual cytokine (IFN-γ, TNF-α, IL2, and IL-17A), after malaria antigen pool (apical membrane antigen 1 and merozoite surface protein 1) (A,B) or Sh antigen pool (soluble egg antigen and soluble worm antigen protein) (C,D) stimulation. Peripheral blood mononuclear cells acquired from schistosoma-positive (SP: dark bar) or -negative (SN: light bar) Malian children aged 4–14 years acquired at the time of acute malaria during the transmission season and again, 6 months later, during the dry season were stimulated with parasite antigen and results were measured by flow cytometry after gating on CD19−CD14−CD3+CD8−CD4+ T cells after live/dead discrimination. χ2 results using Mantel–Haenszel analysis was performed between SP vs. SN children with statistically significant quantities of cytokine production after antigen stimulation. P value significance set at *<0.05; **<0.001; ***<0.0001. Not significant, ns. The dot plot depiction of the individual cytokine net percentage of response is depicted in Figure S2 in Supplementary Material.
S. haematobium (Sh) Antigen
Paired PBMC were stimulated with Sh antigen as described above. Significant production of at least one cytokine was measured after Sh antigen stimulation in the majority of SP children [17/23 (74%)] compared to 2/24 (8.3%) of SN children [P = < 0.0001, 95% CI (4.69–271), χ2 analysis]. As noted with the malaria antigen, the net percentage of cytokine expression, in those that had a demonstrable cytokine response, was not significantly different between groups (Table 2; Figure S2 in Supplementary Material), but the number of children with antigen-specific cytokine production was quite different (Figure 1). At the dry season time point, and prior to administration of praziquantel, 18/23 (78.3%) SP children had detectable PBMC cytokine production compared to 4/23 (17.4%) of SN children [P = < 0.0001, 95% CI (3.3–102), χ2 analysis]. Of the SN children with detectable response to Sh antigen, none had evidence of urinary egg secretion at follow-up. One SN child demonstrated a particularly robust response to antigen at both the wet and dry season time point, without detectable egg secretion, suggesting that this individual may have been previously exposed or developed inherent or putative resistance to urinary schistosomiasis (34). The remaining three children had modest responses at a single time point, which may also represent a sub-clinical infection or a false positive response; however, malaria-naïve U.S. adult controls had no detectable increase of intracellular cytokines to Sh antigen.
Multifunctional T Cell and Memory Subpopulations Analysis
In PBMC found to have antigen recognition to malaria or schistosoma antigen, we measured multifunctional T cell responses (secreting two or more cytokines at a single cell level) at the time of malaria infection and in post-season follow-up (dry season). We also examined major memory subpopulations producing cytokines in PBMC specimens from SP and SN children (Figures 2A,B).
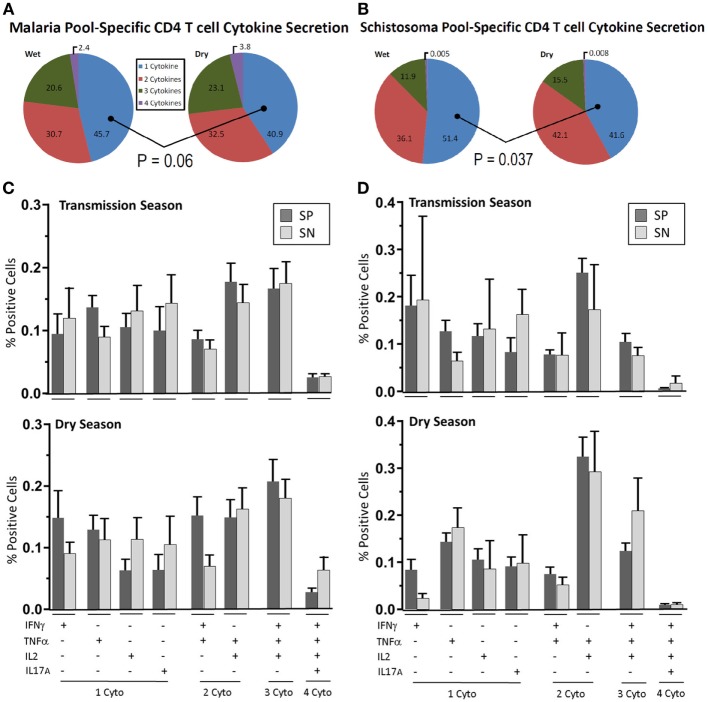
Figure 2.
Parasite-specific multifunctional cytokine expression over time. Depicted are pie-charts (A,B) showing the multifunctional nature, expressed as geometric mean of singlet, doublet, triplet, and quadruplet cytokine (IFN-γ, TNF-α, IL2, and IL-17A) combinations secreted from CD4+ T cells after peripheral blood mononuclear cells (PBMC) stimulation with either (A) malaria antigen pool (apical membrane antigen 1 and merozoite surface protein 1) or (B) schistosoma antigen pool (soluble egg antigen and soluble worm antigen protein). PBMC were acquired from schistosoma-negative (SN) or -positive (SP) Malian children aged 4–14 years at the time of acute malaria during the transmission season and again, 6 months later, during the dry season. The most prevalent cytokine combinations expressed to malaria antigen stimulation (C) or schistosoma antigen stimulation (D) are depicted for each group (SP—dark bar, SN—light bar) during the transmission season and dry season. Results are depicted as the mean percentage of positive cells with SE. The dot plot depiction of individual cytokine net percentage of response is depicted in Figure S3 in Supplementary Material.
Malaria
During the acute malaria episode, 45.7% of cytokine-secreting CD4+ T cells expressed a single cytokine after stimulation with malaria antigen with the remainder expressing more than one cytokine (Figure 2A). The distribution and mean values of the most prevalent combinations of cytokine secretion are depicted in Figure 2C (raw data are depicted in Figure S3 in Supplementary Material). Over the duration of follow-up, single cytokine expression at a cellular level fell to 40.9% (P = 0.06) of the total number of cytokine-producing cells.
Of the multifunctional CD4+ T cells measured, 30.7% were noted to be double-positive and 20.8% were triple-positive. The majority of triple-positive (81.7%) were IFN-γ+TNF-α+IL2+-expressing at the time of an acute malaria episode. A minor population of quadruple-positive CD4+ T cells was also measured (2.4%). This increased slightly to 32.5% double-positive, 23.1% triple-positive and 3.8% quadruple positive at the dry season follow-up with the majority (86.6%) of triple-positive again being IFN-γ+TNF-α+IL2+-expressing. The percentage rise of multifunctional cytokine secretion over time was incremental but persistent across all combinations of cytokine but did not reach statistical significance in any one particular cytokine combination (Figures 2C,D). IL-17A production was measured in the acute malaria stage with expression being significantly reduced at the dry season follow-up. An inverse relationship was noted between IFN-γ and IL-17A. In those children with elevated CD4+ T cell IFN-γ expression to antigen stimulation, low IL-17A levels were measured and vice versa (Spearman rank sum = 0.2515, ρ = 0.04).
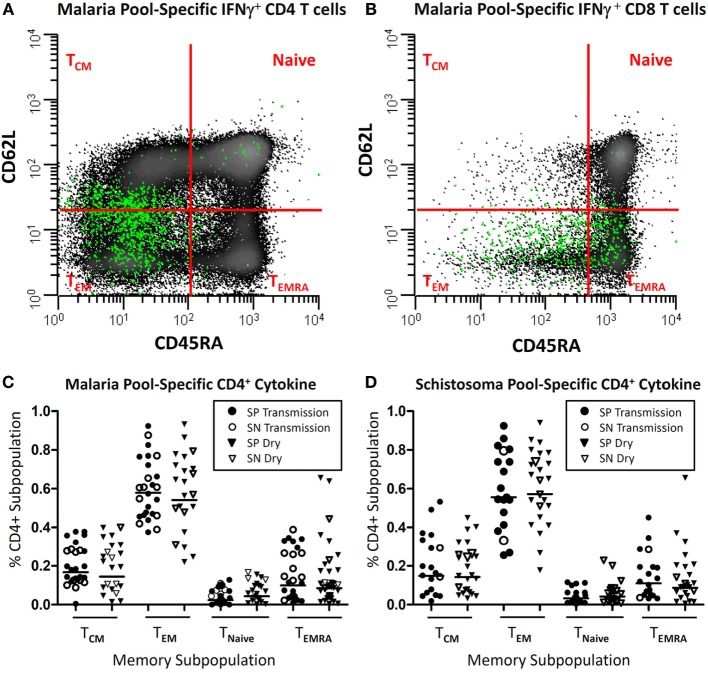
Upon examination of the memory subpopulation from which cytokine-secreting cells derived, it was noted that the majority of inflammatory cytokines (range 55.5–61.4% geometric mean per cohort) derived from CD45RA−CD62L− effector memory T cells (TEM). If there was a detectable memory response, there was little variation depending upon the time point (transmission vs. dry season) or the study cohort (SP vs. SN). Results of SP and SN groups at both time points are depicted (Figure 3). CD45RA−CD62L+ central memory T cells (TCM) accounted for 13.9–16.8% of cytokine secretion. A minority of cytokine derived from CD45RA+ effector memory T cells (TEMRA) (7.7–14%) population and naïve T cells (2.1–4.7%).
Figure 3.
Parasite-specific memory subpopulations. Depicted is a representative example (A,B) of where T cells expressing intracellular IFN-γ (green) after antigen stimulation (malaria pool consisting of apical membrane antigen 1 and merozoite surface protein 1) distribute after being back gated onto the total population CD3+CD19−CD14− cells following live/dead discrimination and stratified as CD4+ (A) or CD8+ (B) from a child with acute Plasmodium falciparum infection and further stratified by memory subpopulation utilizing CD45RA and CD62L surface markers. Panels (C,D) depict the distribution of cytokine-secreting (either IFN-γ, TNF-α, IL2, and/or IL17A), malaria or schistosoma-specific CD4+ T cells, stratified by memory subpopulation, in children with (SP) or without Schistosoma haematobium (SN), during the transmission season and at a later convalescent time point (dry season).
Schistosoma haematobium
Of the children with detectable Sh antigen recognition, 51.4% of all cytokine-secreting cells expressed a single cytokine while the remainder expressed two or more cytokines (Figures 2B–D). The most prevalent cytokine expressed by cytokine-secreting cells was IFN-γ expressed by 18.3% of these cells at the time of the acute malaria episode. This value fell to 7.2% by the dry season convalescent appointment (P = 0.06). TNF-α production remained relatively stable comprising 12.2% of single cytokine-producing cells at the time of acute malaria and 14.9% during the dry season follow-up.
Among the multifunctional CD4+ T cells measured, 36.1% were noted to be double-positive, 11.9% were triple-positive, and a small percentage were quadruple-positive (0.5%) reflective of the low prevalence of IL-17A measured to S. haematobium infection. The majority of triple-positive [10.5/11.9 (88.2%)] were IFN-γ+TNFα+IL2+-expressing at the time of an acute malaria episode. Over time an incremental but persistent increase in multifunctional cell cytokine production was noted across all cytokine combinations (similar to results reported for malaria antigen stimulation, Figure 2B). At the dry season follow-up, the percentage of single-cytokine-producing cells fell to 41.6% (transmission season vs. dry season, P = 0.037, two-tailed Mann–Whitney Rank Sum), 42.1% double-positive (P = 0.27), 15.5% triple-positive (P = 0.38) and 0.8% (P = 0.58) quadruple positive. Raw data can be found in Figure S3 in Supplementary Material.
There was little variation in memory subpopulation in which cytokine production was observed, between seasons or study cohorts. SP and SN results were combined for analysis and reported as geometric mean values (Figure 3). The majority of cytokine (51.4–62.9%) derived from TEM. TCM accounted for 14–22% of cytokine secretion with slightly more TCM noted in the small subset of SN compared to SP population (13.5 vs. 22.5%, P = 0.20). A minority of cytokine was detected from cells of TEMRA (5–11%) population and naïve T cells (2–4%).
Secondary Subset Analysis
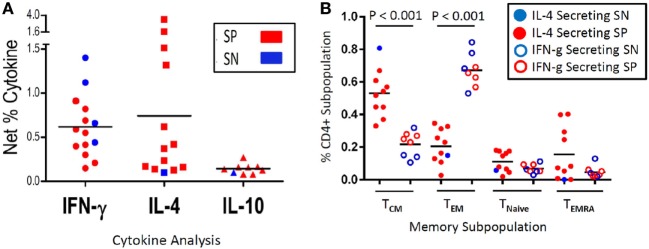
A secondary experiment utilized unmatched transmission season samples from Malian children from this same study (n = 63, mean age 8.3 years, range 4–13 years), which were thawed and examined as part of a previously unpublished data set. Evaluative data were available from 30 SP children and 30 SN children who developed between 1 and 5 malaria episodes during a single transmission season (Table S1 in Supplementary Material). Among PBMC stimulated with malaria antigen AMA1, 4/30 (13.3%) SN vs. 10/30 (33%) SP expressed IFN-γ (P = 0.07, OR 2.5, CI: 0.71–9.99, χ2 analysis); 1/30 (3.3%) SN vs. 12/30 (40%) SP expressed IL-4 (P = 0.002, OR 11.7, CI: 1.8–266.8, Fisher Exact); and 1/30 (3.3%) SN vs. 7/30 (23.3%) SP expressed IL-10 (P = 0.05, OR 6.8, CI: 0.97–163.8, Fisher Exact) (Figure 4A). While PBMC from eight SP children expressed both IFN-γ and IL-4, in all but one case, one cytokine was predominantly expressed (defined as >50%). One child had similar amounts of IL-4 and IFN-γ cytokine expression. We then examined the CD45RA− memory subpopulations, divided into CD62L− T effector memory (TEM) and CD62L+ T central memory (TCM), relative to the cytokine expression profile. Of those children that had an inflammatory expression profile characterized by IFN-γ expression, the percentage of TEM predominated as compared to those children with a dominant IL-4 expression profile (mean TEM: 66.9 vs. 20.4%, P < 0.001). Additionally, those children with an IL-4 expression profile (predominantly SP children) had a higher percentage of TCM as compared to those children with a dominant IFN-γ expression (mean TCM: 53 vs. 22.2%, P < 0.001) (Figure 4B). There was no age-related correlation with cytokine expression between SP and SN children and no change in T naïve or effector memory RA+ (EMRA) populations. However, when SP children were stratified between those expressing an IL-4 profile (n = 9) and those with an IFN-γ profile (n = 4), children expressing IFN-γ were significantly older (7.4 vs. 13 years, P < 0.001).
Figure 4.
Malaria antigen-specific cytokine expression and corresponding memory subpopulation. Depicted is the net percentage of CD4+ T cell-derived IFN-γ, IL-4, or IL-10 cytokine secreted from peripheral blood mononuclear cells (PBMC) acquired from schistosoma-positive (SP) or -negative (SN) Malian children with evidence of antigen recognition after stimulation with malaria apical membrane antigen 1 antigen (A). Cells were gated on CD19−CD14−CD3+CD8−CD4+ after live/dead discrimination. Panel (B) depicts the memory subpopulation distribution from those PBMC that predominantly secreted IL-4 (a representative TH2 cytokine) (left column, solid dots, N = 9 SP, and 1 SN) or IFN-γ (a representative inflammatory TH1 cytokine) (right column, open dots, N = 4 SN and 4 SP) as depicted in Panel A (IL-10 not depicted). PBMC that expressed both cytokines (N = 7 SP and 0 SN) were categorized based on the dominant cytokine (defined as >50%) expressed. The memory subpopulations from one volunteer that secreted equal amounts of IL-4 and IFN-γ was excluded (B). Memory subpopulations were defined as T central memory (TCM—CD62L+CD45RA−), T effector memory (TEM—CD62L−CD45RA−), Naïve T cells (TNaive—CD62L+CD45RA+), or T effector memory RA+ (TEMRA—CD62L−CD45RA+). The mean value is represented as a horizontal bar and statistical significance set at P < 0.05.
Discussion
Very little is known about the immunologic perturbations that one parasitic infection inflicts upon the immune response to another infection when they co-occur in the human host. We have demonstrated an age-specific reduction in P. falciparum malaria acquisition among Malian children, aged ≤9 years, infected with S. haematobium over the course of a single transmission season (3). In this study, we have measured detectable CD4+ T cell memory response against both malaria antigens and Sh antigens and demonstrated multifunctional cellular responses against both antigens during an active malaria transmission season. The responses to the malaria antigens were similar among age-matched children with and without Sh. Lower CD8+ T cell response was noted against either antigen in both populations. At a convalescent, dry season time point after malaria transmission ceased, children with chronic Sh infection appeared to retain their memory responses to malaria antigen whereas uninfected children’s memory response waned considerably (70 vs. 30%). SP children also had similar memory responses to Sh antigens at both time points. The quality of these cellular responses, as measured by cellular multifunctionality, increased over the 6- to 9-month time interval with elevated dual, triple, and quadruple cytokine-secreting cells to malaria and Sh antigens at the convalescent time point.
The role of CD4+ T cells is well established in malaria. However, the presence of CD4+ T cell memory response in chronically infected individuals with schistosomiasis and the kinetics of these cells is poorly understood. While CD4+ T cell memory cells are thought to provide protection against re-infection by schistosomes, the prevailing belief is that chronic helminth infection leads to suppression of the CD4 T cell memory compartment. Moreover, as we have demonstrated, helminth infections can modulate the host adaptive response against itself and other “bystander” antigens (12, 13, 15). Mutapi et al. reported that the CD4+ TEM populations were decreased in schistosoma-infected individuals compared to uninfected individuals and that the CD4+ T cell memory population paradoxically decreased after treatment of the helminth-infected individuals (35). We examined cell populations after antigen stimulation; a notable design difference. This analysis was performed in a subset of children utilizing a flow panel that incorporated the measurement of IL-4 production. We demonstrated enhanced CD4+ TEM in PBMC from Malian children who demonstrated antigen recognition of malaria proteins, which appeared predominantly in children with an inflammatory expression profile characterized by IFN-γ secretion. We observed this expression profile in most SN and some SP children. Of importance, those children with an IL-4 expression dominance (representative of a TH2 cytokine profile and chronic schistosomiasis) had significantly elevated CD4+ TCM populations. Helminth-infected children comprised the majority of those with a dominant IL-4 expression profile, with only one uninfected exception; however, a subset of SP children demonstrated an inflammatory-dominant profile. The subset of SP children with an inflammatory-dominant profile were older than those with an IL-4 dominant profile (13 vs 7.4 years, P < 0.0001). This fact would argue against the inflammatory-dominant profile in SP children being due to an acute Sh infection as Malian children would have been exposed at a much younger age. This is also consistent with the finding that IFN-γ production in schistomiasis-infected individuals is associated with acquisition of anti-helminth immunity (36). Of note, four of the IL-4 producing SP children had detectable IL-10 production as well, which has been associated with helminth-induced anergy and hyporesponsiveness as well as downregulatory responses (37, 38).
While much of the protective immune response to malaria is thought to be due to liver tissue-resident CD8 T cells, IL-4-secreting CD4+ T cells are thought to be critical for mediating CD8 T cell response to malaria liver antigens (39). Additionally, CD8 T cell memory response are dependent upon IL-4 and IL-4 receptor interaction at the level of the liver in the murine model (40). It is well established that cell-mediated immunity to malaria rapidly wanes in children and that acquisition of immunity requires repetitive exposure to malaria. Therefore, it is remarkable that the children with concomitant Sh appeared to retain their memory response to malaria antigen at a time point 6–9 months after their malaria exposure. This was not seen in Sh-negative, age-matched children followed over the same malaria transmission season. It is also possible that the effects simply reflect a previously undescribed cross-reaction between antigens but we have no evidence to support or refute this possibility. Our results provide the first evidence in humans, albeit indirect, that an immunologic milieu rich in IL-4, such as seen in a helminth infection, may contribute to enhanced T cell memory against a bystander antigens, such as those encountered in malaria. Moreover, while numbers were small, older SP children had an inflammatory expression profile analogous to uninfected children. This might provide an immunologic explanation for our field findings that children aged 9 years or less, and infected with Sh, had modest protection against the acquisition of P. falciparum malaria compared to age-matched uninfected children or older children with Sh.
Ethics Statement
This trial was carried out in accordance with the recommendations of the University of Bamako and the University of Maryland Institutional Review Boards (IRBs) with written consent obtained from all legal guardians. Village permission to conduct research was obtained from village chiefs, government officials, and traditional healers prior to study initiation. Individual written informed consent was obtained from the parent or legal guardian of each child prior to screening and enrollment in accordance with the Declaration of Helsinki. All children displaying gross hematuria or symptoms of genitourinary pathology were treated with praziquantel (40 mg/kg) therapy and discharged from the study.
Author Contributions
KL obtained funding; KL, OD, CP, and MS participated in study design; KL, AD, CA, ID, CP, and OD participated in study conduct and regulatory review. KL and MS participated in immunologic assays and interpretation; KL, CP, and MS participated in writing of manuscript; all authors participated in editing of manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
KL is supported by The National Institutes of Health (NIH) (U19 AI110820, U01 AI089342, and R01AI110852), the Vaccine Research Center of NIH and the EMMES Corporation (HHSN272201000049I), The Office of the Surgeon General, Department of the Army (W81XWH-15-R-0034), and the Joint Warfighter Medical Research Program and Sanaria Inc. (W81XWH-JW14843). This work was originally funded by an NIH R01 (AI-067954) and a Doris Duke Clinical Scientist Development Award. The field site was funded under NIH N01-AI85346 and U01AI065683-06. We would like to acknowledge the population of Bandiagara, Mali, for their support and participation in this and many other trials. Additional thanks are extended to our flow cytometric technicians, Ms. Regina Harley and Cathy Storrer, for their input and invaluable talents, and to Dr. Reswan Wahid for his critical editing of the manuscript and Figures. S. haematobium proteins were obtained through the Schistosome Related Reagent Repository (SR3) sponsored by the National Institute of Allergy and Infectious Disease with special thanks to Dr. Fred Lewis. Apical Membrane Antigen 1 was kindly donated by Dr. David Lanar of Walter Reed Armed Institutes of Research (WRAIR). Merozoite Surface Protein 1 (42) was kindly donated by Dr. Evelina Angov of WRAIR.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/articles/10.3389/fimmu.2017.01995/full#supplementary-material.
Gating strategy. Gating strategy for CD4+ memory response depicting (A) representative IFN-γ cytokine expression in response to malaria antigen stimulation from the central memory (CD62L+CD45RA−), naïve (CD62L+CD45RA+), effector memory (CD62L−CD45RA−), and EMRA (CD62L−CD45RA+) subpopulations. The media control and the malaria antigen-stimulated peripheral blood mononuclear cells (PBMC) from schistosoma-positive children and gated on CD19−CD14−CD8−CD4+ T cells after live/dead discrimination (B–G). Panels (B–E) depict CD69+ cytokine expression. Panels (F,G) are from an alternate panel and represent the total cytokine expressed from CD4+ T cells.
Intracellular cytokine expression to antigen stimulation. Depicted is the dot plot representation of the overall CD4+ T cell immune response, expressed as produced cytokine (in net%), to either malaria antigen pool (apical membrane antigen 1 and merozoite surface protein 1) (A,B) or Sh antigen pool (soluble egg antigen and soluble worm antigen protein) (C,D). Peripheral blood mononuclear cells (PBMC) acquired from schistosoma-negative [schistosomiasis-negative (SN)—hollow dot] or -positive [schistosomiasis-positive (SP)—solid dot] Malian children aged 4–14 years at the time of acute malaria during the transmission season and again, 6 months later, during the dry season. PBMC were stimulated with parasite antigen and results were measured by flow cytometry after gating on CD19−CD14−CD3+CD4+CD8− T cells after live/dead discrimination. Only children with positive responses (i.e., significant by χ2 analysis and >0.1% over background; as described in Section “Materials and Methods”) are shown. Horizontal bars represent the geometric mean values of IFN-γ, TNF-α, IL2, and IL-17A for SN and SP children combined.
Seasonal parasite-specific multifunctional cytokine expression. Depicted are the most prevalent cytokine combinations elicited after parasite antigen stimulation (A,B) in individual children [schistosomiasis-positive (SP)—solid, schistosomiasis-negative (SN)—hollow] stratified by number of cytokines expressed during the transmission season and dry season. Peripheral blood mononuclear cells acquired from schistosoma-negative (SN—hollow shapes) or -positive (SP—solid shapes) Malian children aged 4–14 years at the time of acute malaria during the transmission season and again, 6 months later, during the dry season were stimulated with either (A) malaria antigen pool (apical membrane antigen 1 and merozoite surface protein 1) or (B) schistosoma antigen pool (soluble egg antigen and soluble worm antigen protein).
References
- 1.World Malaria Report 2016. World Health Organization. (2016). Available from: http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/
- 2.World Health Organization. Schistosomiasis, Fact Sheet No. 115. (2017). Available from: http://www.who.int/mediacentre/factsheets/fs115/en/
- 3.Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, Coulibaly D, et al. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am J Trop Med Hyg (2005) 73:1124–30. 10.4269/ajtmh.2005.73.1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briand V, Watier L, Le Hesran JY, Garcia A, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: protective effect of schistosomiasis on malaria in Senegalese children? Am J Trop Med Hyg (2005) 72:702–7. 10.4269/ajtmh.2005.72.702 [DOI] [PubMed] [Google Scholar]
- 5.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med (1991) 173:159–66. 10.1084/jem.173.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, Heiny S, et al. Egg deposition is the major stimulus for the production of Th2 cytokines in murine Schistosomiasis mansoni. J Immunol (1991) 146:1322–7. [PubMed] [Google Scholar]
- 7.Baumgart M, Tompkins F, Leng J, Hesse M. Naturally occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J Immunol (2006) 176:5374–87. 10.4049/jimmunol.176.9.5374 [DOI] [PubMed] [Google Scholar]
- 8.Lyke KE, Dabo A, Sangare L, Arama C, Daou M, Diarra I, et al. Effects of concomitant Schistosoma haematobium infection on the serum cytokine levels elicited by acute Plasmodium falciparum malaria infection in Malian children. Infect Immun (2006) 74:5718–24. 10.1128/IAI.01822-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remoue F, Diallo TO, Angeli V, Herve M, De Clercq D, Schacht AM, et al. Malaria co-infection in children influences antibody response to schistosome antigens and inflammatory markers associated with morbidity. Trans R Soc Trop Med Hyg (2003) 97:361–4. 10.1016/S0035-9203(03)90170-2 [DOI] [PubMed] [Google Scholar]
- 10.Diallo TO, Remoue F, Schacht AM, Charrier N, Dompnier JP, Pillet S, et al. Schistosomiasis co-infection in humans influences inflammatory markers in uncomplicated Plasmodium falciparum malaria. Parasite Immunol (2004) 26:365–9. 10.1111/j.0141-9838.2004.00719.x [DOI] [PubMed] [Google Scholar]
- 11.Diallo TO, Remoue F, Gaayeb L, Schacht AM, Charrier N, De CD, et al. Schistosomiasis coinfection in children influences acquired immune response against Plasmodium falciparum malaria antigens. PLoS One (2010) 5:e12764. 10.1371/journal.pone.0012764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartgers FC, Yazdanbakhsh M. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunol (2006) 28:497–506. 10.1111/j.1365-3024.2006.00901.x [DOI] [PubMed] [Google Scholar]
- 13.Hartgers FC, Obeng BB, Kruize YC, Dijkhuis A, McCall M, Sauerwein RW, et al. Responses to malarial antigens are altered in helminth-infected children. J Infect Dis (2009) 199:1528–35. 10.1086/598687 [DOI] [PubMed] [Google Scholar]
- 14.Lyke KE, Wang A, Dabo A, Arama C, Daou M, Diarra I, et al. Antigen-specific B memory cell responses to Plasmodium falciparum malaria antigens and Schistosoma haematobium antigens in co-infected Malian children. PLoS One (2012) 7:e37868. 10.1371/journal.pone.0037868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyke KE, Dabo A, Arama C, Daou M, Diarra I, Wang A, et al. Reduced T regulatory cell response during acute Plasmodium falciparum infection in Malian children co-infected with Schistosoma haematobium. PLoS One (2012) 7:e31647. 10.1371/journal.pone.0031647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renia L, Marussig MS, Grillot D, Pied S, Corradin G, Miltgen F, et al. In vitro activity of CD4+ and CD8+ T lymphocytes from mice immunized with a synthetic malaria peptide. Proc Natl Acad Sci U S A (1991) 88:7963–7. 10.1073/pnas.88.18.7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwenk RJ, Richie TL. Protective immunity to pre-erythrocytic stage malaria. Trends Parasitol (2011) 27:306–14. 10.1016/j.pt.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med (2007) 13:843–50. 10.1038/nm1592 [DOI] [PubMed] [Google Scholar]
- 19.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol (2008) 8:247–58. 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- 20.Wipasa J, Elliott S, Xu H, Good MF. Immunity to asexual blood stage malaria and vaccine approaches. Immunol Cell Biol (2002) 80:401–14. 10.1046/j.1440-1711.2002.01107.x [DOI] [PubMed] [Google Scholar]
- 21.McGregor IA. The development and maintenance of immunity to malaria in highly endemic areas. Clin Trop Med Commun Dis (1986) 1:1–29. [Google Scholar]
- 22.Kullberg MC, Pearce EJ, Hieny SE, Sher A, Berzofsky JA. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol (1992) 148:3264–70. [PubMed] [Google Scholar]
- 23.Elrefaei M, El Sheikh N, Kamal K, Cao H. HCV-specific CD27-CD28- memory T cells are depleted in hepatitis C virus and Schistosoma mansoni co-infection. Immunology (2003) 110:513–8. 10.1111/j.1365-2567.2003.01769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly YI, Diallo AA, et al. Filarial infection suppresses malaria-specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. J Immunol (2011) 186:4725–33. 10.4049/jimmunol.1003778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coulibaly D, Diallo DA, Thera MA, Dicko A, Guindo AB, Kone AK, et al. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am J Trop Med Hyg (2002) 67:604–10. 10.4269/ajtmh.2002.67.604 [DOI] [PubMed] [Google Scholar]
- 26.De Clercq D, Rollinson D, Diarra A, Sacko M, Coulibaly G, Landoure A, et al. Schistosomiasis in Dogon country, Mali: identification and prevalence of the species responsible for infection in the local community. Trans R Soc Trop Med Hyg (1994) 88:653–6. 10.1016/0035-9203(94)90212-7 [DOI] [PubMed] [Google Scholar]
- 27.Corachan M, Ruiz L, Valls ME, Gascon J. Schistosomiasis and the Dogon country (Mali) [see comments]. Am J Trop Med Hyg (1992) 47:6–9. 10.4269/ajtmh.1992.47.6 [DOI] [PubMed] [Google Scholar]
- 28.Lyke KE, Burges RB, Cissoko Y, Sangare L, Kone A, Dao M, et al. HLA-A2 supertype-restricted cell-mediated immunity by peripheral blood mononuclear cells derived from Malian children with severe or uncomplicated Plasmodium falciparum malaria and healthy controls. Infect Immun (2005) 73:5799–808. 10.1128/IAI.73.9.5799-5808.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutta S, Lalitha PV, Ware LA, Barbosa A, Moch JK, Vassell MA, et al. Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infect Immun (2002) 70:3101–10. 10.1128/IAI.70.6.3101-3110.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angov E, Aufiero BM, Turgeon AM, Van HM, Ockenhouse CF, Kester KE, et al. Development and pre-clinical analysis of a Plasmodium falciparum merozoite surface protein-1(42) malaria vaccine. Mol Biochem Parasitol (2003) 128:195–204. 10.1016/S0166-6851(03)00077-X [DOI] [PubMed] [Google Scholar]
- 31.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med (2011) 365:1004–13. 10.1056/NEJMoa1008115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyke KE, Daou M, Diarra I, Kone A, Kouriba B, Thera MA, et al. Cell-mediated immunity elicited by the blood stage malaria vaccine apical membrane antigen 1 in Malian adults: results of a phase I randomized trial. Vaccine (2009) 27:2171–6. 10.1016/j.vaccine.2009.01.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salerno-Goncalves R, Wahid R, Sztein MB. Ex Vivo kinetics of early and long-term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin Vaccine Immunol (2010) 17:1305–14. 10.1128/CVI.00234-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira RR, Figueiredo JP, Cardoso LS, Jabar RL, Souza RP, Wells MT, et al. Factors associated with resistance to Schistosoma mansoni infection in an endemic area of Bahia, Brazil. Am J Trop Med Hyg (2012) 86:296–305. 10.4269/ajtmh.2012.11-0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nausch N, Bourke CD, Appleby LJ, Rujeni N, Lantz O, Trottein F, et al. Proportions of CD4+ memory T cells are altered in individuals chronically infected with Schistosoma haematobium. Sci Rep (2012) 2:472. 10.1038/srep00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jankovic D, Wynn TA, Kullberg MC, Hieny S, Caspar P, James S, et al. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-gamma-dependent effector mechanisms. J Immunol (1999) 162:345–51. [PubMed] [Google Scholar]
- 37.Mahanty S, Mollis SN, Ravichandran M, Abrams JS, Kumaraswami V, Jayaraman K, et al. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J Infect Dis (1996) 173:769–73. 10.1093/infdis/173.3.769 [DOI] [PubMed] [Google Scholar]
- 38.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol (2010) (184):5375–82. 10.4049/jimmunol.0904067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med (2002) 8:166–70. 10.1038/nm0202-166 [DOI] [PubMed] [Google Scholar]
- 40.Morrot A, Hafalla JC, Cockburn IA, Carvalho LH, Zavala F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J Exp Med (2005) 202:551–60. 10.1084/jem.20042463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gating strategy. Gating strategy for CD4+ memory response depicting (A) representative IFN-γ cytokine expression in response to malaria antigen stimulation from the central memory (CD62L+CD45RA−), naïve (CD62L+CD45RA+), effector memory (CD62L−CD45RA−), and EMRA (CD62L−CD45RA+) subpopulations. The media control and the malaria antigen-stimulated peripheral blood mononuclear cells (PBMC) from schistosoma-positive children and gated on CD19−CD14−CD8−CD4+ T cells after live/dead discrimination (B–G). Panels (B–E) depict CD69+ cytokine expression. Panels (F,G) are from an alternate panel and represent the total cytokine expressed from CD4+ T cells.
Intracellular cytokine expression to antigen stimulation. Depicted is the dot plot representation of the overall CD4+ T cell immune response, expressed as produced cytokine (in net%), to either malaria antigen pool (apical membrane antigen 1 and merozoite surface protein 1) (A,B) or Sh antigen pool (soluble egg antigen and soluble worm antigen protein) (C,D). Peripheral blood mononuclear cells (PBMC) acquired from schistosoma-negative [schistosomiasis-negative (SN)—hollow dot] or -positive [schistosomiasis-positive (SP)—solid dot] Malian children aged 4–14 years at the time of acute malaria during the transmission season and again, 6 months later, during the dry season. PBMC were stimulated with parasite antigen and results were measured by flow cytometry after gating on CD19−CD14−CD3+CD4+CD8− T cells after live/dead discrimination. Only children with positive responses (i.e., significant by χ2 analysis and >0.1% over background; as described in Section “Materials and Methods”) are shown. Horizontal bars represent the geometric mean values of IFN-γ, TNF-α, IL2, and IL-17A for SN and SP children combined.
Seasonal parasite-specific multifunctional cytokine expression. Depicted are the most prevalent cytokine combinations elicited after parasite antigen stimulation (A,B) in individual children [schistosomiasis-positive (SP)—solid, schistosomiasis-negative (SN)—hollow] stratified by number of cytokines expressed during the transmission season and dry season. Peripheral blood mononuclear cells acquired from schistosoma-negative (SN—hollow shapes) or -positive (SP—solid shapes) Malian children aged 4–14 years at the time of acute malaria during the transmission season and again, 6 months later, during the dry season were stimulated with either (A) malaria antigen pool (apical membrane antigen 1 and merozoite surface protein 1) or (B) schistosoma antigen pool (soluble egg antigen and soluble worm antigen protein).