Abstract
Distribution of diazotrophs and their nitrogen fixation activity were investigated in the northern South China Sea (nSCS) and the Kuroshio from July 16th to September 1st, 2009. N2 fixation activities in whole seawater and <10 μm fraction at the surface were measured by acetylene reduction assay. Higher activities were observed at the East China Sea (ECS) Kuroshio and the nSCS shelf. The nSCS basin showed a low N2 fixation activity. The <10 μm fractions (unicellular diazotrophs) contributed major portion to the whole-water activity in the survey time, indicating that nanoplanktonic cyanobacterias were the major diazotrophs in the survey area. Daily N2 fixation rates of Trichodesmium ranged from 0.11 to 9.83 pmolNtrichome−1 d−1 with an average of 4.03 pmolNtrichome−1 d−1. The Luzon Strait and the ECS Kuroshio had higher N2 fixation rates of Trichodesmium than the nSCS shelf and basin. Calculated activities of Trichodesmium at most stations were moderately low compared with that of the whole-water. The contribution of N2 fixation by the whole-water to primary production ranged from 1.7% to 18.5%. The estimated amount of new nitrogen introduced by Trichodesmium contributed up to 0.14% of the total primary production and 0.41% of the new production in the Luzon Strait.
Introduction
N2 fixation is an important process in adding “new” nitrogen to oligotrophic ocean ecosystems1. The current focus in assessing the global role of the upper ocean in sequestering atmospheric CO2 has elevated the importance of quantifying marine N2 fixation2. The filamentous non-heterocystous cyanobacteria Trichodesmium and diatom-diazotroph associations containing the cyanobiont Richelia intracellularis were traditionally considered to be the most important contributors to open-ocean N2 fixation. Trichodesmium mainly inhabits stratified, oligotrophic, tropical and subtropical oceans which are characterized by low nutrient concentrations, clear waters, and deep light penetration3–5. N2 fixation from Trichodesmium may supply up to half of the N required to sustain the rate of the annual particulate N (PN) export from the euphotic zone at Sta. ALOHA (22°45′N, 158°00′W) in the subtropical North Pacific Ocean6. However, the discovery of two unicellular diazotrophic cyanobacteria [UCYN group A (UCYN-A) and Crocosphaera watsonii (group B)], whose abundances and N2 fixation rates can be equal to or greater than those of Trichodesmium, demands the scientists to make a reassessment of the N inputs to the global ocean via N2 fixation7–9.
The South China Sea (SCS) is the second largest marginal sea in the world. It is located in the tropical-subtropical western North Pacific and has a surface area of about 3.5 × 106 km2. Because of the effects of the monsoons and eddies, the northern SCS (nSCS) displays a complex hydrological and biological characters. The surface circulation of the nSCS changes seasonally in response to the prevalent monsoons. Overall seasonal circulation in the SCS is cyclonic in winter and anti-cyclonic in summer10. Upwellings and downwellings are forced by the interaction of monsoons and eddies11. The nSCS exhibits strong variations of temperature and salinity from coastal region to the open sea. The surface water of the nSCS and the Kuroshio is generally warm, stratified and oligotrophic which is supposedly an ideal habitat for N-fixers especially in summer4. The Kuroshio and nSCS are two neighboring and interacting waterbodies. The Kuroshio spans a wide range of latitudes from tropical 15°N to temperate 40°N. The upstream Kuroshio intrudes into the SCS through the Luzon Strait which is the deepest passage between the western Pacific and the SCS. It is suggested that the Kuroshio has a branch intruding into the nSCS in winter, but it sometimes intrudes into the nSCS in summer mostly in the form of “loop” and “extend”12,13. After passing southeastern Taiwan, the Kuroshio water flows northward and exchange their energy and matter with the East China Sea. The Kuroshio and the nSCS show different nutrient dynamics. Gong et al.14 observed that nutricline in the northern SCS is shallower than the Kuroshio14. Chen et al.15 also observed that in summer nitracline is shallower in the SCS than in the Kuroshio15.
Most published studies of marine nitrogen fixation were focused on the North Pacific8,16–19 and North Atlantic20–23. Compared with previous studies in the East China Sea24, the SCS4,15,25 and the Kruoshio26 have been studied deficiently. The present study was conducted to investigate the horizontal and vertical variation of Trichodesmium abundance and it’s in situ nitrogen fixation rates in the nSCS and the Kuroshio. Size fractionation experiments were also conducted to compare the relative importance of the activity between different size classes of diazotrophs in the Kuroshio and nSCS. In addition, environmental factors were measured to determine the main ecological factors that control the nitrogen fixation.
Results
Hydrographic conditions
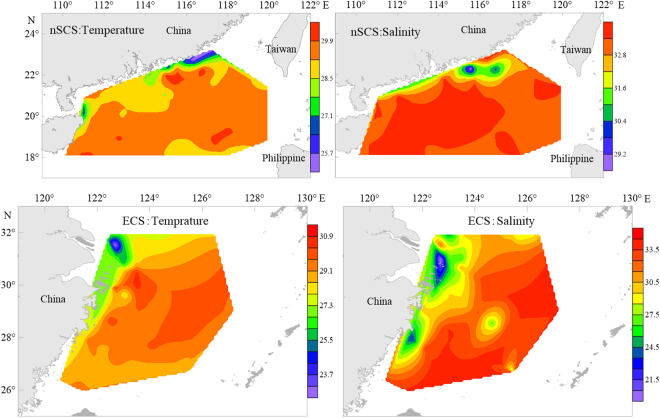
In nSCS, sea surface temperature (SST) ranged between 25.3 °C and 30.5 °C with an average of 29.3 ± 0.8 °C. Surface sea salinity ranged from 28.4 to 33.8 with an average of 33.2 ± 0.7. Most regions in nSCS were characterized by high temperature and high salinity (Fig. 1). In East China Sea, regions that heavily influenced by the Kuroshio also had high temperature and high salinity (Fig. 1). In nSCS during survey time, surface NO3− + NO2−, SRP, and silicate concentrations were lower than 0.15 μmol L−1, 0.1 μmol L−1, and 3 μmol L−1 respectively. The nutrient concentrations of most stations at the survey region were undetectable, especially at the Luzon Strait. In the East China Sea Kuroshio region, surface NO3− + NO2−, SRP, and silicate concentrations were also very low or undetectable.
Figure 1.
Surface sea temperature and salinity in the northern SCS and East China Sea. Note for software: Golden Software Surfer 11 (http://www.goldensoftware.com/).
Horizontal distribution of the N2 fixation activity
N2 fixation rate of whole-water at the surface ranged from 1.14 to 10.40 nmol N L−1d−1 with an average of 4.89 nmol N L−1d−1. N2 fixation rate of the <10 μm fraction at the surface ranged from 0.63 to 7.61 nmol N L−1d−1 with an average of 3.67 nmol N L−1d−1. N2 fixation rate of whole-water changed a lot among different survey stations. High N2 fixation of whole-water at the surface was observed in the ECS Kuroshio and the nSCS shelf. The nSCS basin showed low N2 fixation activity. These rates were in the same order of magnitude as those measured under non-bloom conditions in the East China Sea, the southern Yellow Sea and the nSCS coastal upwelling27,28. The high N2 fixation rates in the nSCS shelf were probably attributed to diazotrophic blooms in sampling stations. N2 fixation activity in the <10 μm fraction exhibited a similar distribution to that of the whole-water in survey region (Fig. 2). Our experiments showed that unicellular diazotrophs contributed significantly to N2 fixation in both the SCS and the Kuroshio.
Figure 2.
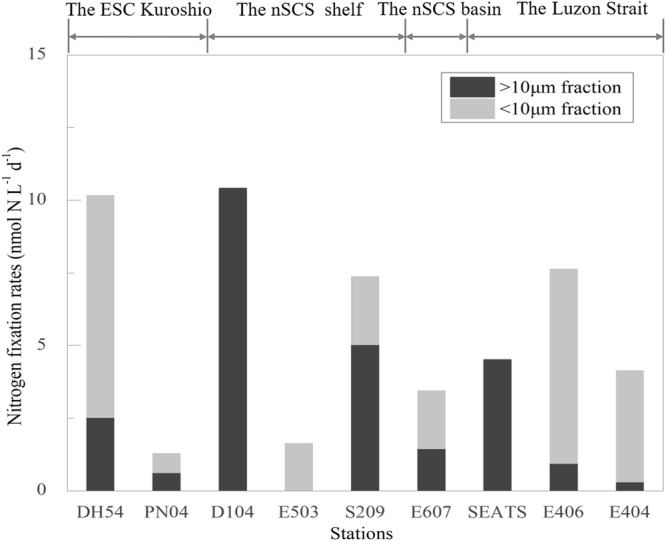
Distributions of N2 fixation activity of the whole-water sample (open circle) and <10 μm fractions (solid circle) at the surface. Note for software: Origin 8.5 PRO (http://www.originlab.com/).
Vertical distribution of the N2 fixation activity
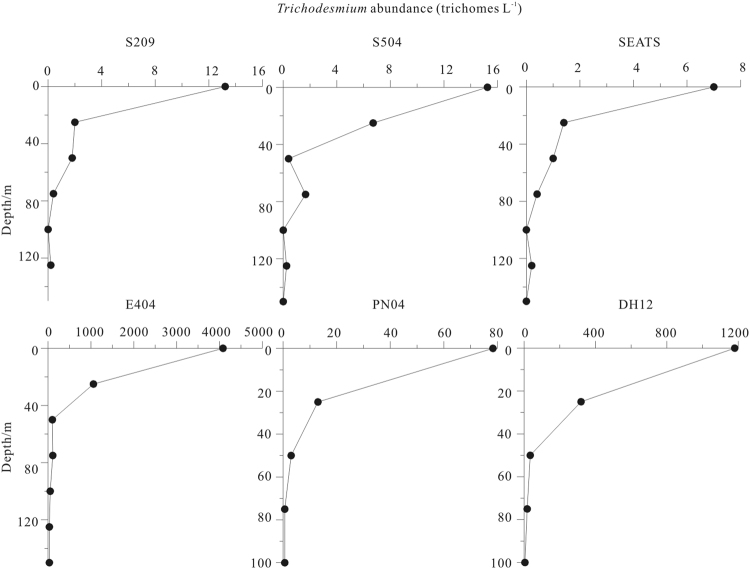
The whole-water N2 fixation rate at Sta. SEATS ranged from 0.76 to 8.50 nmolNL−1d−1 and the average was 4.33 nmolNL−1d−1 (Fig. 3). The >10 μm fractions N2 fixation rates decreased with the light intensity except for the 10% light depth, while the <10 μm fractions N2 fixation rates increased with the light intensity in the middle three light depth. Light intensity is an important factor that determines the distribution of diazotrophs in the water column. Trichodesmium usually prefers the top sunlight of 75 m, but unicellular cyanobacteria tend to appear at deeper layers and down up to ~150 m29. The <10 μm fractions N2 fixation rate of 100% and 1% light depth were undetectable in our study. The rare occurrence of unicellular cyanobacteria at the SEATS station has been verified by molecular study30. The growth of unicellular cyanobacteria in this station could be limited by iron resources due to the competition from non-diazotrophs. The maximum whole-water N2 fixation rate was observed in the 10% light depths in our study, and the result was different from the previous study that the maximum N2 fixation was in the surface layer and decreasing with depth in the lower euphotic zone31. Notably, the main factor resulted in this situation was the N2 fixation rate by >10 μm fractions in the 10% light depths were larger than other depths extraordinarily. The same phenomenon was also observed in the nSCS15 and North Pacific32. A possible reason generated for this phenomenon probably due to the result of the downward flux of Trichodesmium spp33.
Figure 3.
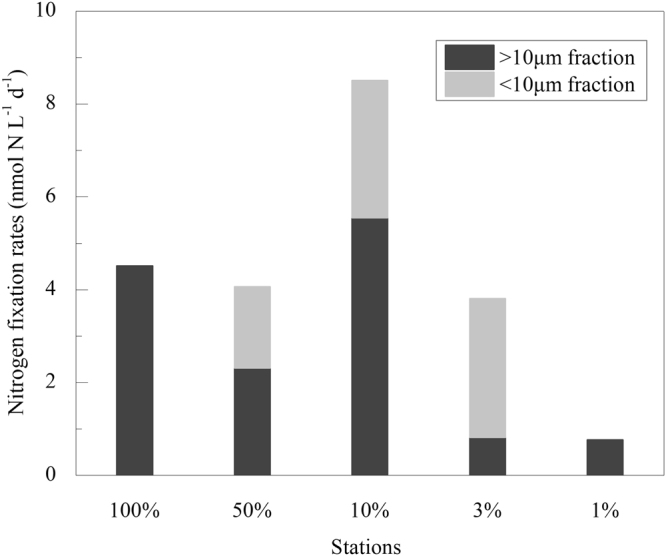
Vertical distribution of N2 fixation rate at Sta. SEATS. Note for software: Origin 8.5 PRO (http://www.originlab.com/).
Trichodesmium fixation rate
Hourly N2 fixation rates of Trichodesmium were converted to daily rates with day length, assuming that Trichodesmium fixed N2 only during the daytime34. Daily N2 fixation rates of Trichodesmium ranged from 0.11 to 9.83 pmolNtrichome−1 d−1 with an average of 4.03 pmolNtrichome−1 d−1. The maximum rate of 9.83 pmolNtrichome−1 d−1 was recorded in Sta. E404 in the Luzon Strait for an experiment containing longitudinal colonies (cultivated from 8:00 am to 2:00 pm). The second maximum rate of 9.64 pmolNtrichome−1 d−1 was also recorded in Sta. E404 for an experiment containing radial colonies (cultivated from 8:00 to 10:00 am). In general, the Luzon Strait and ECS Kurosio had higher N2 fixation rates by Trichodesmium than the nSCS shelf and basin. The variations of N2 fixation rates by Trichodesmium were consistent with that of the Trichodesmium abundances (Fig. 4). In the ECS Kuroshio, Sta. PN04 showed a low N2 fixation rate by Trichodesmium because the experiment was conducted at night (11:00 pm to 1:00 am of the next day).
Figure 4.
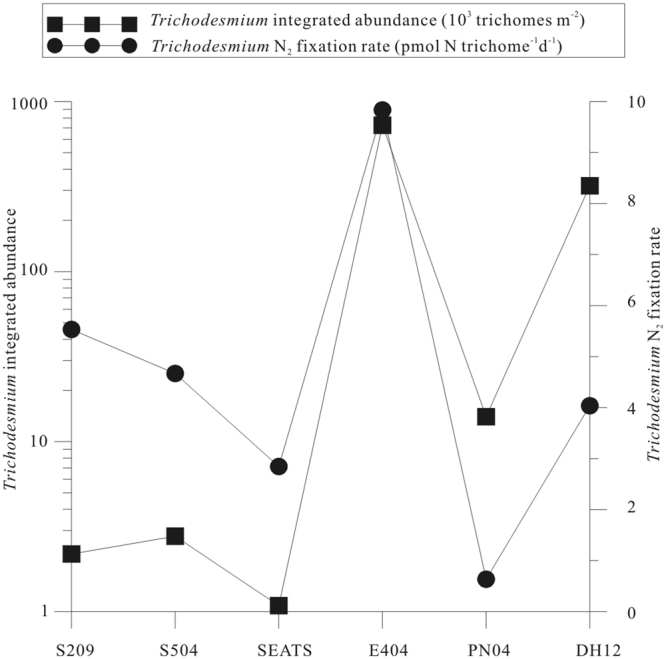
Trichodesmium integrated concentrations and daily N2 fixation rate in survey region. Note for software: Origin 8.5 PRO (http://www.originlab.com/).
A time-series assay was also conducted at Sta. E404. N2 fixation rates by Trichodesmium radial colonies were measured at 8:00–10:00, 10:00–14:00, 14:00–16:00 and 16:00–18:00. The maximum N2 fixation rate occurred in the 8:00 to 10:00 am. The N2 fixation rate by Trichodesmium decreased sharply from 8:00 am to 6:00 pm, and the rate dropped off rapidly to near zero prior to the onset of the dark period. The N2 fixation rate during 4:00 to 6:00 pm was almost undetectable (Fig. 5). The results generated from field incubation were consistent with previous study that high N2 fixation rates were measured for ~6 hours surrounding the middle of the photoperiod and N2 fixation declined sharply in the latter light period35.
Figure 5.
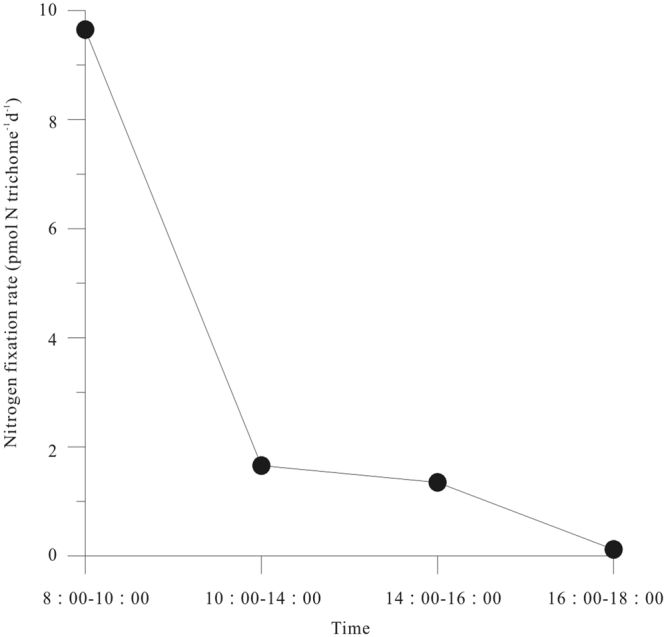
Daily variations of N2 fixation rate by Trichodesmium at Sta. E404. Note for software: Origin 8.5 PRO (http://www.originlab.com/).
Trichodesmium standing crop and their contribution to total Chl a
In most instances, the individual free trichomes were the absolute dominant population in global ocean4. These free trichomes could form macroscopic colonies or aggregates which known as ‘puffs’ and ‘tuffs’ in extreme oligotrophic conditions36. In the present study, three living forms of Trichodesmium were observed during the survey time: the free trichome, the radial colony and the longitudinal colony. Likewise, the primary form of Trichodesmium in most stations were free living trichomes rather than colonies. This trait was in better agreement with previous study of Trichodesmium in the SCS and southern ECS4,37.
The average number of trichomes per radial colony and longitudinal colony in all stations were 80 and 24, respectively. The average number of cells per trichome was 60. This data was typically higher than previous reports in the SCS that colonial forms of Trichodesmium were usually composed of less than 10 trichomes per colony37. Our values were nevertheless lower than other oceans such as tropical North Atlantic Ocean (mean = 98 ± 11, range = 5–153 spp./colony), station ALOHA in subtropical eastern North Pacific Ocean (mean = 182, range = 10–375 spp./colony) and subtropical eastern North Atlantic gyre (mean = 112 ± 47 for puffs and 49 ± 16 for tufts, range = 57–156 for puffs and 31–63 for tufts spp./colony)38–40. Trichodesmium integrated concentrations ranged from 2.18 to 726.17 × 103 trichomes m−2 and the average was 177.65 × 103 trichomes m−2. The Luzon Strait and the ECS Kuroshio had higher Trichodesmium abundances than the nSCS shelf and basin. Vertical distribution of Trichodesmium showed that from the surface to the bottom the abundances of Trichodesmium decreased dramatically (Figs 5 and 6).
Figure 6.
Vertical distribution of Trichodesmium. Note for software: Origin 8.5 PRO (http://www.originlab.com/).
According to the Trichodesmium integrated abundances and Chl a concentration per trichome measured at the sampling stations, estimated Chl a contributed by Trichodesmium varied from 0.1% to 58.5% of the total Chl a (Fig. 7). The values in the ECS Kuroshio and the Luzon Strait were significantly higher than that in the nSCS. Likewise, obvious spatial difference was also observed in another ocean. For example, literature have reported that Trichodesmium to account for, on average, about 61%, 18% and 5% of total Chl a in the Caribbean Sea, the eastern North Pacific Ocean and the Sargasso Sea, respectively39,41. The difference of the contribution in different regions might be caused by the variation of their dominant phytoplankton5.
Figure 7.
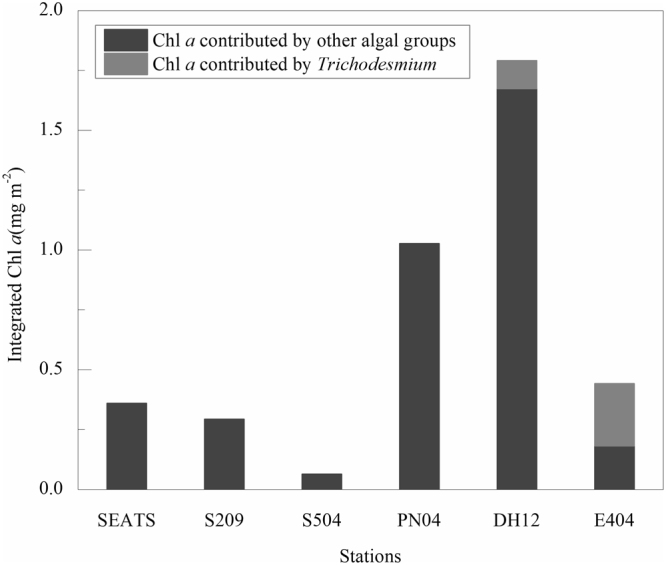
Chl a concentration contributed by Trichodesmium. Note for software: Origin 8.5 PRO (http://www.originlab.com/).
Comparison of N2 fixation rate of Trichodesmium and other diazotrophs and their contributions to the primary production
Calculated activities of Trichodesmium at most stations were quite low in the whole-water (<0.1 nmolNL−1d−1) except at Sta. E404 where the N2 fixation rate by Trichodesmium was as high as 57.7 nmolNL−1d−1. On the contrary, unicellular diazotrophs were accounted for a great part of the whole-water activity at most stations in the survey time. N2 fixation was converted to carbon production using the Redfield ratio (C:N = 6.6:1) for comparison with the primary production. N2 fixation by the whole-water was calculated to contribute to 1.7% to 18.5% of the primary production (Table 1). According to the Trichodesmium integrated abundances and nitrogen fixation rates per trichome observed at the sampling stations, estimated integrated nitrogen fixation rates from Trichodesmium varied from 0.0031 to 7.0 μmol N m−2 d−1. Calculated N2 fixation rate by Trichodesmium showed it could contribute up to 0.14% of the primary production (Table 2).
Table 1.
Estimated whole-water N2 fixation rate and its contribution to primary production at surface water.
| Station | N2 fixation rate (μmolNm−3d–1) | Primary production (mgCm−3d−1) | Contributions of N2 fixation to primary production (%) |
|---|---|---|---|
| DH27b | 1.14 | 5.24 | 1.7 |
| S209 | 7.36 | 4.18 | 13.9 |
| E607 | 3.43 | 1.47 | 18.5 |
| SEATS | 4.50 | 9.44 | 3.8 |
| LE09 | 2.22 | 7.30 | 2.4 |
| E404 | 4.12 | 8.73 | 3.7 |
Table 2.
Calculated Trichodesmium N2 fixation rate and its contribution to the primary production.
| Station | N2 fixation rate (μmolNm−2d−1) | Primary production (mgCm−2d−1) | Contributions of N2 fixation to primary production (%) |
|---|---|---|---|
| S504 | 0.013 | 94.2 | 0.0011 |
| E404 | 7.0 | 381.7 | 0.14 |
| S209 | 0.012 | 242.7 | <0.001 |
| SEATS | 0.0031 | 445.1 | <0.001 |
| DH12 | 1.3 | 400.3 | 0.025 |
Discussion
In the summer, most regions of the nSCS have high SST except some regions which are influenced by the upstream. The stratified water of the nSCS results in the low supplement of the nutrient from deeper water. Originating from the southeast of Taiwan and east of the Luzon Strait, the Kuroshio is characterized by high temperature, high salinity, high transparency and low nutrients concentrations. As a result, most regions of the nSCS and the ECS Kuroshio were warm, stratified and oligotrophic which favored the growth of the diazotrophs. As an important diazotraph in the oligotrophic ocean and the Kuroshio water species indicator, Trichodesmium’s distribution and nitrogen fixation rate in the nSCS and neighboring Kuroshio play an important role in the biological production, especially the new production of the SCS and the Kuroshio. Although conditions in both the SCS and the Kuroshio seem favorable for Trichodesmium growth, the Kuroshio had a higher abundance of Trichodesmium and nitrogen fixation rate than the nSCS. The standing crop and nitrogen fixation rate of Trichodesmium in the SCS basin is very low (Fig. 4 and Table 2). Shiozaki et al.26 suggested the high abundance of Trichodesmium in the ESC Kuroshio were ascribable to the supply of Trichodesmium spp. and other diazotrophs from the surrounding islands26. The Kuroshio is a western boundary current which originating from the southeast of Taiwan and east of the Luzon Strait, and enters the East China Sea (ECS) northeast of Taiwan. During our investigations, the surface abundance of Trichodesmium in Sta.E404 in the Luzon Strait was two orders of magnitude higher than stations in the nSCS and several times higher than stations in the ESC Kuroshio (Fig. 6). These results proved Trichodesmium spp. would likely to be transported to the mainstream of the Kuroshio from the Luzon Strait.
Trichodesmium spp. were traditionally perceived to be the most abundant cyanobacteria in the marine, and it has been demonstrated to contribute the vast majority of N2 fixation rates and primary production to the ocean3,42. However, increasing studies have shown that unicellular diazotrophs often appear to be of equal or greater abundance to the other known N2 fixers due to the revolution use of polymerase chain reaction (PCR) techniques7,31. A DIC budget analysis at the SEATS in the SCS basin demonstrated that unicellular diazotrophs contributed enormously to the N2 fixation, which were thus an indispensable part of the N cycle study in the SCS43. Chen et al.31 observed that unicellular diazotrophs contributed 65% and 50% of the total N2 fixation in the SCS and the Kuroshio, respectively31. According to the results of size fractionation experiments in the present study, unicellular diazotrophs were important N2 fixers in the surface of the nSCS and the Kuroshio. However, investigation on the distribution of the unicellular diazotrophs and their phylogenetic relationships with the unicellular diazotrophs from the tropical North Atlantic and Pacific oceans remain insufficient. Similar situation happens to Richelia intracellularis. Further studies of the other diazotrophs including their distribution and nitrogen fixation rate are needed in order to estimate the role of the N2 fixation in the nutrient and carbon cycling in SCS and the Kuroshio.
In the open ocean, many factors can influence the activity and diversity of diazotrophs. Water temperature is a major factor controlling the distribution of diazotrophs44. Trichodesmium is distributed where temperatures are between 20 °C and 30 °C and thrives when temperature is 25 °C or warmer45,46. By comparison, unicellular diazotrophs often present in deeper and colder waters than Trichodesmium29. In the present study, however, the correlation between size-fractioned N2 fixation and temperature was not significant in both the nSCS and the Kuroshio. This might be attributed to the unusually strong wind and highly turbulent surface during summer cruise31. Diazotrophs, especially Trichodesmium, usually thrives in relatively stable environment. Previous works had demonstrated the importance of wind as a driving force for promoting the growth of Trichodesmium and diatoms47,48. Generally it has been found that highest concentrations of Trichodesmium occur during prolonged calm wind (<10 knots ≈ 5.14 m/s) conditions whereas the highest diatom concentrations occur during windy (>15 knots ≈ 7.72 m/s) conditions. Calm waters with low turbulence benefit bundle formation and tend to enhance N2 fixation4. During the survey time, influenced by the typhoon “Swan” and “Morakot”, a much lower abundance of Trichodesmium was detected at Sta. SEATS (1.08 × 103 trichomes m−2) compared with the previous study4. Besides, diazotrophs growth rates are presumably limited by the availability of non-N nutrients, iron and phosphate are key factors limiting diazotrophs N2 fixation and growth rates49. Iron is a important cofactor of nitrogenase, and it plays an crucial role in the synthesis and expression of nitrogenase in diazotroiphs50. According to literatures, dissolved Fe (dFe) in surface waters ranged from 0.2–0.3 nmol L−1 in the SCS basin stations and 1.8–43.2 nmol L−1 in the northwest SCS coastal stations28,37. It was suggested that Fe availability may limit the rates of N2 fixation in the SCS basin, while it was not happened in the nSCS coastal stations. As for the phosphate, it is a major component of cells which could also limit the growth of diazotroiphs29,51. In the present study, the phosphate (P) concentration in most of the areas was very low or undetectable. However, both Trichodesmium and unicellular diazotrophs had form a number of mechanisms to overcome P limitation. A key characteristic of Trichodesmium is the presence of gas vesicles, which could provide buoyancy and help them migrate vertically in the water column to optimize light intensity or to obtain P and other nutrients from deep waters3. Unlike with Trichodesmium, unicellular diazotrophs could not migrate vertically in the water column, but they had been proved to own a robust capacity for scavenging phosphorus in oligotrophic systems due to the presence of high-affinity phosphate scavenging systems52.
In addition, nitrogenase is instantaneously and irreversibly inactivated by oxygen, N2 fixation is therefore a strictly anaerobic process53. However, many studies have demonstrated that both natural populations and laboratory cultures of Trichodesmium could fix N2 exclusively in the daylight hours when photosynthetic activity takes place simultaneously54,55. Our result in Sta. PN04 and Sta. E404 also proved that Trichodesmium presented to be a daytime N2 fixer. There are several hypotheses which aims to explain this phenomenon, but it is still an enigma how Trichodesmium can evolve O2 while simultaneously fixing N2. The most popular hypothesis is that Trichodesmium can use a combination of spatial and temporal separation strategy of N2 fixation and photosynthesis within the photoperiod35,42,55. Staal et al.55 pointed that light stimulation of nitrogenase activity was most occurred at low O2 concentration, while light stimulation became gradually less important at high O2 concentration55. This result suggested the reduction of N2 fixation in the latter stages of the light period was primarily attributed to the high net production of O2 in the latter stages. Furthermore, nitrogenase activity can be recovered after a dark incubation when the cells were subsequently incubated in the light. Similarly, low O2 concentration is in favor of the recovery of nitrogenase activity55.
Our results were comparable with global ocean, and higher than other study in the same area which might be caused by the discrepancy between the two methods (Table 3). On the basis of a C: N = 6.6, N2 fixation by the whole-water was calculated to contribute to 1.7% to 18.5% of the primary production in surface waters, suggesting that N2 fixation plays a very important role in supporting surface phytoplankton N demand in some stations. Such values were exceeded the previous studies in the nSCS, and were comparable with N input by biological N2 fixation in other oceans27,28,56. The variation of N2 fixation rate by Trichodesmium was consistent with that of the standing crop of Trichodesmium. According to the result of Chen et al.4 new production from Trichodesmium N2 fixation was higher in the Kuroshio than the nSCS in summer, we also found a higher N2 fixation rate by Trichodesmium in the Kuroshio (Table 2)4. Chen et al.4 found the ratios of N2-fixation-based new production by Trichodesmium to NO3-N plus N2-fix were usually <0.14. Based on f-ratio 0.34 at the Kuroshio and 0.22 at nSCS reported by Chen et al.4, Trichodesmium could contributed up to 0.4% of the new primary production at the Luzon Strait and <0.1% of the new primary production at the nSCS basin4. Compared with the previous studies, we got lower N2 fixation rate and abundance of Trichodesmium (Table 4).
Table 3.
Surface N2 fixation rates and dominant diazotroph (s) of different Oceanic Region. ARA = acetylene reduction assay.
| Location | Surface N2 fixation rate (nmol N L−1d−1) | Method | Dominant Diazotroph (s) | Reference |
|---|---|---|---|---|
| nSCS and ECS Kuroshio | 1.14–10.40 | ARA | Unicellular diazotrophs | This study |
| nSCS coastal upwelling | 0.1–5.6 | 15N2 gas bubble | Unknow | 28 |
| SCS (Mekong River plume) | 0.59–22.77 | 15N2 gas bubble | Trichodesmium spp. and DDAs | 63 |
| Western North Pacific | ≈0.3–22 | ARA | Unicellular diazotrophs | 64 |
| North Pacific | 0.360–3.05 | 15N2 gas bubble | Heterotrophic bacteria | 65 |
| Northeast Atlantic | <0.4 | ARA | Unicellular diazotrophs | 66 |
| Eastern North Atlantic | 0–151.2 | 15N2 gas bubble | Trichodesmium and UCYN-A | 67 |
| Arabian Sea | 0.8–225 | 15N2 gas dissolution | Trichodesmium bloom | 68 |
| Indian Ocean | 0.18–1.27 | 15N2 gas bubble | Heterotrophic bacteria | 69 |
Table 4.
Calculated Trichodesmium N2 fixation rate in the South China Sea and the Kuroshio with literature reports of estimated N2 fixation in the adjacent area.
| Location | N2 fixation rate (μmolNm−3d−1) | Method | Primary production (mgCm−2d−1) | Nitrate-uptake-based new production | Reference |
|---|---|---|---|---|---|
| Southeastern ECS | 126 | ARA | 70 | ||
| Kuroshio | 55 | 70,71 | |||
| ECS (Trichodesmium erythraeum) | 279 | ARA | 71 | ||
| ECS (Trichodesmium thiebautii) | 685 | ||||
| Kuroshio winter | 2.4 | 15N2 gas bubble | 530 | 270 | 4 |
| Kuroshio spring | 34.7 | 540 | 140 | ||
| Kuroshio summer | 168.1 | 510 | 160 | ||
| SCS winter | 1.2 | 620 | 230 | ||
| SCS spring | 7.3 | 500 | 210 | ||
| SCS summer | 12.6 | 360 | 80 | ||
| SCS autumn | 5.0 | 540 | 250 | ||
| ECS Kuroshio | 1.29 | ARA | 400.3 | This study | |
| Luzon Strait | 7.01 | 381.7 | |||
| SCS basin | 0.0031 | 445.1 | |||
| SCS shelf | 0.012 | 242.7 |
ARA = acetylene reduction assay.
Conclusion
In conclusion, the abundance of Trichodesmium were higher in the Luzon Strait and ECS Kuroshio than the nSCS shelf and basin, and so does the N2 fixation by Trichodesmium. It was suggested that the high abundance of Trichodesmium in the ECS Kuroshio were ascribable to the supply of Trichodesmium spp. and other diazotrophs from the surrounding islands. Nevertheless, it is still poorly understood and additional research is needed to resolve this question. According to the size-fractioned N2 fixation rates, unicellular diazotrophs also contributed significantly to N2 fixation in both the SCS and the Kuroshio. Further research should focus on the nitrogen and carbon biogeochemical cycles of unicellular diazotrophs in the SCS and the Kuroshio. Trichodesmium presented to be a daytime N2 fixer with the maximum N2 fixation rate in the 8:00 to 10:00 am and the rate decreased sharply as time goes by. Free trichomes were the absolute dominant population in the study area, and the radial colony and the longitudinal colony were also observed during the survey time which could be caused by the shortage of nutrient or trace element. The average number of trichomes per colony in present study was lower than values reported in other oceans, and the contribution of Trichodesmium to total Chla presented spatial difference which might be caused by the variation of their dominant phytoplankton. N2 fixation by the whole-water in some stations contributed a lot to primary production in surface waters, suggesting that N2 fixation plays a very important role in supporting surface phytoplankton N demand in these areas.
Materials and Methods
Station locations and sampling
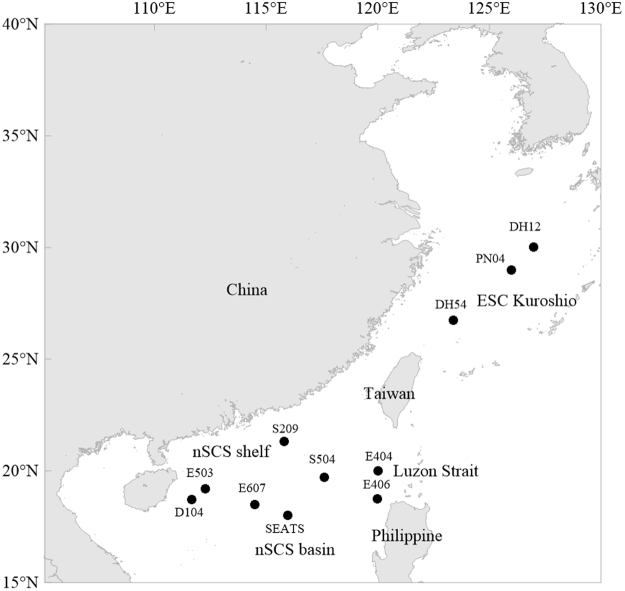
The study area was located between 15°N to 35°N and 105°E to 130°E (Fig. 8). A total of 13 sampling stations were studied during the summer of 2009. Surface water was collected using an HCl-rinsed bucket at all the stations. Vertical seawater was collected by using 30 L Go-Flo bottles attached to a rosette multi-sampler, on which the conductivity, temperature, and depth (CTD) probes were installed (Seabird SBE 9/11). Temperature and salinity were profiled vertically by the CTD profiler.
Figure 8.
Sampling stations of N2 fixation rate. Note for software: Golden Software Surfer 11 (http://www.goldensoftware.com/).
Nutrients and Chl a measurements
Water samples for determination of nutrients were collected into 100 ml HCl-rinsed bottles and stored in 4 °C until analysis. Soluble reactive phosphate (SRP) concentrations were measured using standard molybdenum blue procedure57 or a home-made ship-board C18 enrichment-flow injection analysis system58,59. Nitrate and nitrite concentrations (NO3− + NO2−) were measured by reducing NO3− to NO2− with a Cd column and then determining NO2− using the standard pink azo dye method and a flow injection analyzer60.
Chl a samples were collected in 1 L bottles and then vacuum filtered (<10 mm Hg) through a 25 mm GF/F filter. Filters were kept in 10 mL vials and pigments were extracted with 90% acetone for 24 h inside a freezer at 4 °C. Chlorophyll concentrations of the extraction were then determined based on the fluorescence technique using a Trilogy (CHL NA, Model # 046) fluorometer on board.
Trichodesmium enumeration and its contribution to Chl a
Water samples for determining the vertical distribution of Trichodesmium were collected from 25 m, 50 m, 75 m, 100 m and 150 m depths in each sampling station. Specimens for enumeration of Trichodesmium were prepared on board by filtering a 1–10 L water samples onto a 20 μm polycarbonate membrane filter (25 mm in diameter). Concentrations of the samples were fixed with 1% neutralized formaldehyde and preserved in the dark until analysis. Trichomes and colonies on the entire filter were counted under an inverted microscope. In order to determine the contribution of Trichodesmium to the total Chl a, 30–50 Trichodesmium colonies were picked up and washed in the filtered sea water. Then the chlorophyll concentrations of the colonies were measured using fluorescence method as described above.
Primary production
Primary production (PP) was conducted according to Parsons et al.61 by 14C incubation method61. Seawater samples were collected from surface (Table 1) or five depths of the euphotic layer (Table 2), then prescreened through 200 μm mesh and filled into 250 mL acid-cleaned polycarbonate carboy (Nalgene; USA). Each sample was inoculated with 10 μCi NaH14CO3 before incubation, and carboys which used for incubate subsurface samples were covered with the neutral density filter to simulate the in situ irradiance. Carboys were incubated in an on-deck incubator cooled by running water pumped up from a 5 m depth. After 4 h incubation, seawater samples were filtered through 25 mm diameter GF/F filters, and then the filters were placed in the aluminum foil bags and stored in the −20 °C freezer until analysis. In the laboratory, filters were fumed with HCl to remove residual inorganic carbon before they were moved into the scintillation vials, next the 10 mL scintillation cocktail (Ultima Gold; PerkinElmer; USA) were added. The activity of radioactive was counted using a Tri-Carb 2800TR liquid scintillation counter (Perkin-Elmer; USA).
Size-fractioned N2 fixation rates measurements
N2 fixation activity was measured by acetylene reduction assay3. Sampling stations were grouped into four sectors according to their bottom depths and locations (Fig. 8): the nSCS shelf (Sta. S209, E503 and D104), the nSCS basin (Sta. E607 and SEATS), the ECS Kuroshio (Sta. DH54 and PN04) and the Luzon Strait (Sta. E404 and E406). All experimental incubations were run in triplicate. 500 ml of surface seawater samples were introduced into a 600 mL HCl-rinsed polycarbonate bottles. After sealing with a butyl rubber stopper, 20 mL of acetylene was injected by replacing the same volume of headspace. Seawater samples that passed through a 10 μm mesh were also prepared for incubation of the surface samples in the same manner as whole seawater samples. Samples were incubated for 24 h in an on-deck incubator cooled by running water pumped up from a 5 m depth. At Sta. SEATS, subsurface samples were also obtained from 100%, 50%, 10%, 3% and 1% light depths relative to that of the surface to determine N2 fixation at subsurface layers. The light intensity of the subsurface samples was adjusted to the in situ density using neutral density filters.
N2 fixation by Trichodesmium was also measured by acetylene reduction assay. A total of 6 sampling stations were occupied to determine the N2 fixation rate of Trichodesmium (Fig. 8). Two sites were located in the ECS Kuroshio (Sta. PN04 and DH12), one site in the nSCS shelf (Sta. S209), one site in the Luzon Strait (Sta. E404) and two sites in the nSCS basin (Sta. SEATS and S504). Trichodesmium colonies were collected by a 76 μm mesh phytoplankton net towing gently in the surface water. Trichodesmium colonies were manually picked immediately by using a plastic bacteriological transfer loop and placed into a glass container with GF/F filtered seawater. Approximately 30–50 colonies were transferred to a 25 ml glass vial with 15 ml filtered seawater. The vials were sealed with butyl rubber stoppers. After injection of 2 ml acetylene, vials containing Trichodesmium colonies were incubated for 4–6 h in the daytime. To evaluate N2 fixation of Trichodesmium, their activity at each station was calculated from their numerical abundance and their N2 fixation activity per trichome.
At the end of the incubation, the gas phase of the vials was sampled with a 100 μl gastight syringe (Agilent) and manually injected into a flame ionization gas chromatograph (GC6890N, Agilent). The temperatures of injector, detector and oven were set at 250 °C, 200 °C and 60 °C respectively. The carrier gas was helium at the highest purity available at a flow rate of 9 mlmin−1. The supply of H2 and air for the FID were 30 mlmin−1 and 300 mlmin−1, respectively. The column was 20m-long wide-bore fused silica (0.53 mm inner diameter) packed with Porapak U (Agilent). The ethylene production during the incubation was calculated as Capone62. The produced ethylene was converted to fixed nitrogen with a molar ratio of 4:1. After the assay, the incubated samples were fixed with 1% neutralized formaldehyde for the later enumeration of Trichodesmium.
Acknowledgements
The authors greatly acknowledge the help on GC operation by Dr. Qingyun Nan and Ms. Qing He, and Prof. Tiegang Li at Institute of Oceanology of Chinese Academy of Sciences for sharing the GC. This study was supported by the National Natural Science Foundation of China (no. 41676112), the National Basic Research Program (973 Program) of China (2015CB954002), and the Program for Changjiang Scholars to Jun Sun. We gratefully acknowledge the crew of the R/V Dongfanghong II for their assistance and all the participants for their input and contributions at sea.
Author Contributions
J.S. planned the project and designed the experimental scheme, article framework and did the manuscript. C.W. accomplished the paper writing. F.X.F., T.S. and P.L. gave some useful suggestions to modify the paper. All the authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dugdale RC, Goering JJ. Uptake of New and Regenerated Forms of Nitrogen in Primary Productivity. Limnol. Oceanogr. 1967;12:196–206. doi: 10.4319/lo.1967.12.2.0196. [DOI] [Google Scholar]
- 2.Levitan O, et al. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Global Change Biol. 2007;13:531–538. doi: 10.1111/j.1365-2486.2006.01314.x. [DOI] [Google Scholar]
- 3.Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter EJ. Trichodesmium, a Globally Significant Marine Cyanobacterium. Science. 1997;276:1221–1229. doi: 10.1126/science.276.5316.1221. [DOI] [Google Scholar]
- 4.Chen YLL, Chen HY, Tuo SH, Ohki K. Seasonal Dynamics of New Production from Trichodesmium N2 Fixation and Nitrate Uptake in the Upstream Kuroshio and South China Sea Basin. Limnol. Oceanogr. 2008;53:1705–1721. doi: 10.4319/lo.2008.53.5.1705. [DOI] [Google Scholar]
- 5.Jiang Z, et al. Diazotrophic cyanobacterium Trichodesmium spp. in China marginal seas: Comparison with other global seas. Acta Ecol. Sin. 2015;35:37–45. doi: 10.1016/j.chnaes.2015.01.003. [DOI] [Google Scholar]
- 6.Karl D, et al. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature. 1997;388:533–538. doi: 10.1038/41474. [DOI] [Google Scholar]
- 7.Zehr JP, et al. Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean. Nature. 2001;412:635–638. doi: 10.1038/35088063. [DOI] [PubMed] [Google Scholar]
- 8.Montoya JP, et al. High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature. 2004;430:1027–1032. doi: 10.1038/nature02824. [DOI] [PubMed] [Google Scholar]
- 9.Moisander PH, et al. Unicellular cyanobacterial distributions broaden the oceanic N2 fixation domain. Science. 2010;327:1512–1514. doi: 10.1126/science.1185468. [DOI] [PubMed] [Google Scholar]
- 10.Liu KK, et al. Monsoon-forced chlorophyll distribution and primary production in the South China Sea: observations and a numerical study. Deep-Sea Res. Pt. I: Oceanographic Research Papers. 2002;49:1387–1412. doi: 10.1016/S0967-0637(02)00035-3. [DOI] [Google Scholar]
- 11.Chai, F., Xue, H. & Shi, M. Formation and distribution of upwelling and downwelling in South China Sea. In: Oceanography in China 13, research paper compilation authored by Xue H. J. et al. Ocean Press, Beijing, pp. 117–128 (2001).
- 12.Hu J, Kawamura H, Hong H, Qi Y. A Review on the Currents in the South China Sea: Seasonal Circulation, South China Sea Warm Current and Kuroshio Intrusion. J. Oceanogr. 2000;56:607–624. doi: 10.1023/A:1011117531252. [DOI] [Google Scholar]
- 13.Xue H, et al. Kuroshio intrusion and the circulation in the South China Sea. J. Geophys. Res.: Oceans. 2004;109:C02017. [Google Scholar]
- 14.Gong GC, Liu KK, Liu CT, Pai SC. Chemical hydrography of the South China Sea and a comparison with the West Philippine Sea. Terres. Atmos. Ocean. 1992;3:587–602. doi: 10.3319/TAO.1992.3.4.587(O). [DOI] [Google Scholar]
- 15.Chen YLL, Chen HY, Lin YH. Distribution and downward flux of Trichodesmium in the South China Sea as influenced by the transport from the Kuroshio Current. Mar. Ecol. Prog. Ser. 2003;259:47–57. doi: 10.3354/meps259047. [DOI] [Google Scholar]
- 16.Goebel NL, Edwards CA, Church MJ, Zehr JP. Modeled contributions of three diazotrophs to nitrogen fixation at Station ALOHA. Isme J. 2007;1:606–619. doi: 10.1038/ismej.2007.80. [DOI] [PubMed] [Google Scholar]
- 17.Needoba JA, Foster RA, Sakamoto C, Zehr JP, Johnson KS. Nitrogen Fixation by Unicellular Diazotrophic Cyanobacteria in the Temperate Oligotrophic North Pacific Ocean. Limnol. Oceanogr. 2007;52:1317–1327. doi: 10.4319/lo.2007.52.4.1317. [DOI] [Google Scholar]
- 18.Fong AA, et al. Nitrogen fixation in an anticyclonic eddy in the oligotrophic North Pacific Ocean. Isme J. 2008;2:663–676. doi: 10.1038/ismej.2008.22. [DOI] [PubMed] [Google Scholar]
- 19.Satoshi K, Ken F, Fuminori H, Shigenobu T, Jota K. Latitudinal distribution of diazotrophs and their nitrogen fixation in the tropical and subtropical western North Pacific. Limnol. Oceanogr. 2009;54:537–547. doi: 10.4319/lo.2009.54.2.0537. [DOI] [Google Scholar]
- 20.Carpenter EJ, Mccarthy JJ. Nitrogen Fixation and Uptake of Combined Nitrogenous Nutrients by Oscillatoria (Trichodesmium) thiebautii in the Western Sargasso Sea. Limnol. Oceanogr. 1975;20:389–401. doi: 10.4319/lo.1975.20.3.0389. [DOI] [Google Scholar]
- 21.Falcón LI, Carpenter EJ, Cipriano F, Bergman B, Capone DG. N2 Fixation by Unicellular Bacterioplankton from the Atlantic and Pacific Oceans: Phylogeny and In Situ Rates. Appl. Environ. Microb. 2004;70:765–770. doi: 10.1128/AEM.70.2.765-770.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capone, D. G. et al. Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Glob. Biogeochem. Cycles19, 10.1029/2004GB002331 (2005).
- 23.Benavides M, Moisander PH, Daley MC, Bode A, Arístegui J. Longitudinal variability of diazotroph abundances in the subtropical North Atlantic Ocean. J. Plankton. Res. 2016;38:662–672. doi: 10.1093/plankt/fbv121. [DOI] [Google Scholar]
- 24.Chang J, Chiang KP, Gong GC. Seasonal variation and cross-shelf distribution of the nitrogen-fixing cyanobacterium, Trichodesmium, in southern East China Sea. Cont. Shelf Res. 2000;20:479–492. doi: 10.1016/S0278-4343(99)00082-5. [DOI] [Google Scholar]
- 25.Dong J, Wang Y, Zhang Y. Spatial and seasonal variations of Cyanobacteria and their nitrogen fixation rates in Sanya Bay, South China Sea. Sci. Mar. 2008;72:239–251. doi: 10.3989/scimar.2008.72n2239. [DOI] [Google Scholar]
- 26.Shiozaki T, et al. Why is Trichodesmium abundant in the Kuroshio? Biogeosciences. 2015;12:6931–6943. doi: 10.5194/bg-12-6931-2015. [DOI] [Google Scholar]
- 27.Zhang R, et al. Nitrogen fixation in the East China Sea and southern Yellow Sea during summer 2006. Mar. Ecol. Prog. Ser. 2012;447:77–86. doi: 10.3354/meps09509. [DOI] [Google Scholar]
- 28.Zhang R, et al. Physical‐biological coupling of N2 fixation in the northwestern South China Sea coastal upwelling during summer. Limnol. Oceanogr. 2015;60:1411–1425. doi: 10.1002/lno.10111. [DOI] [Google Scholar]
- 29.Benavides M, Voss M. Five decades of N2 fixation research in the North Atlantic Ocean. Front. Mar. Sci. 2015;2:1–40. doi: 10.3389/fmars.2015.00040. [DOI] [Google Scholar]
- 30.Zhang Y, Zhao Z, Sun J, Jiao NZ. Diversity and distribution of diazotrophic communities in the South China Sea deep basin with mesoscale cyclonic eddy perturbations. FEMS Microbiol. Ecol. 2011;78:417–427. doi: 10.1111/j.1574-6941.2011.01174.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen YLL, et al. The relative contributions of unicellular and filamentous diazotrophs to N2 fixation in the South China Sea and the upstream Kuroshio. Deep-Sea Res. Pt. I: Oceanographic Research Papers. 2014;85:56–71. doi: 10.1016/j.dsr.2013.11.006. [DOI] [Google Scholar]
- 32.Church MJ, Short CM, Jenkins BD, Karl DM, Zehr JP. Temporal patterns of nitrogenase gene (nifH) expression in the oligotrophic North Pacific Ocean. Appl. Environ. Microbiol. 2005;71:5362–5370. doi: 10.1128/AEM.71.9.5362-5370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong LL, Jing HM, Kataoka T, Sun J, Liu HB. Phylogenetic diversity and spatio-temporal distribution of nitrogenase genes (nifH) in the northern South China Sea. Aquat. Microb. Ecol. 2011;65:15–27. doi: 10.3354/ame01531. [DOI] [Google Scholar]
- 34.Berman-Frank I, Lundgren P, Falkowski P. Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res. Microbiol. 2003;154:157–164. doi: 10.1016/S0923-2508(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 35.Berman-Frank I, et al. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science. 2001;294:1534–1537. doi: 10.1126/science.1064082. [DOI] [PubMed] [Google Scholar]
- 36.Zehr JP. Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 2011;19:162–173. doi: 10.1016/j.tim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Wu JF, et al. Dissolved inorganic phosphorus, dissolved iron, and Trichodesmium in the oligotrophic South China Sea. Global Biogeochem. Cy. 2003;17:8–1. [Google Scholar]
- 38.Ricardo ML, David MK. Role of Trichodesmium spp. in the productivity of the subtropical North Pacific Ocean. Mar. Ecol. Prog.Ser. 1996;133:263–273. doi: 10.3354/meps133263. [DOI] [Google Scholar]
- 39.Carpenter EJ, Subramaniam A, Capone DG. Biomass and primary productivity of the cyanobacterium Trichodesmium spp. in the tropical N Atlantic ocean. Deep-Sea Res. Pt. I: Oceanographic Research Papers. 2004;51:173–203. [Google Scholar]
- 40.Taboada FG, Gil RG, Höfer J, González S, Anadón R. Trichodesmium spp. population structure in the eastern North Atlantic subtropical gyre. Deep-Sea Res. Pt. I: Oceanographic Research Papers. 2010;57:65–77. [Google Scholar]
- 41.Carpenter EJ, Price CC. Nitrogen fixation, distribution, and production of Oscillatoria (Trichodesmium) spp. in the western Sargasso and Caribbean Seas. Limnol. Oceanogr. 1977;22:60–72. doi: 10.4319/lo.1977.22.1.0060. [DOI] [Google Scholar]
- 42.Bergman B, Sandh G, Lin S, Larsson J, Carpenter EJ. Trichodesmium – a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS. Microbiol. Rev. 2013;37:286–302. doi: 10.1111/j.1574-6976.2012.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voss M, Bombar D, Loick N, Dippner JW. Riverine influence on nitrogen fixation in the upwelling region off Vietnam, South China Sea. Geophys. Res. Lett. 2006;330:872–879. [Google Scholar]
- 44.Luo YW, Lima ID, Karl DM, Doney SC. Data-based assessment of environmental controls on global marine nitrogen fixation. Biogeosciences. 2014;11:691–708. doi: 10.5194/bg-11-691-2014. [DOI] [Google Scholar]
- 45.Breitbarth E, Oschlies A, Laroche J. Physiological constraints on the global distribution of Trichodesmium – effect of temperature on diazotrophy. Biogeosciences. 2007;4:53–61. doi: 10.5194/bg-4-53-2007. [DOI] [Google Scholar]
- 46.Fu FX, et al. Differing responses of marine N2-fixers to warming and consequences for future diazotroph community structure. Aquat. Microb. Ecol. 2014;72:33–46. doi: 10.3354/ame01683. [DOI] [Google Scholar]
- 47.Bell PRF, Elmetri I. Ecological Indicators of Large-Scale Eutrophication in the Great Barrier Reef Lagoon. Ambio. 1995;24:208–215. [Google Scholar]
- 48.Revelante N, Gilmartin M. Dynamics of phytoplankton in the Great Barrier Reef Lagoon. J. Plankton Res. 1982;4:47–76. doi: 10.1093/plankt/4.1.47. [DOI] [Google Scholar]
- 49.Walworth NG, et al. Mechanisms of increased Trichodesmium fitness under iron and phosphorus co-limitation in the present and future ocean. Nat. Commun. 2016;7:12081. doi: 10.1038/ncomms12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohm JA, Webb EA, Capone DG. Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 2011;9:499–508. doi: 10.1038/nrmicro2594. [DOI] [PubMed] [Google Scholar]
- 51.Church, M. J. & Böttjer, D. Diversity, Ecology, and Biogeochemical Influence of N2-Fixing Microorganisms in the Sea, P.608-625. In S. A. Levin [ed.], Encyclopedia of biodiversity, 2nd ed. Academic Press (2013).
- 52.Dyhrman ST, Haley ST. Phosphorus Scavenging in the Unicellular Marine Diazotroph Crocosphaera watsonii. Appl. Environ. Microb. 2006;72:1452–1458. doi: 10.1128/AEM.72.2.1452-1458.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zehr, J. P. & Bombar, D. Marine Nitrogen Fixation: Organisms, Significance, Enigmas and Future Directions. Biological Nitrogen Fixation. John Wiley & Sons, Inc, 855–872 (2015).
- 54.Fu FX, Bell PRF. Factors affecting N2 fixation by the cyanobacterium Trichodesmium sp. GBRTRLI101. FEMS Microbiol. Ecol. 2003;45:203–209. doi: 10.1016/S0168-6496(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 55.Staal M, Rabouille S, Stal LJ. On the role of oxygen for nitrogen fixation in the marine cyanobacterium Trichodesmium sp. Environ. Microbiol. 2007;9:727–736. doi: 10.1111/j.1462-2920.2006.01195.x. [DOI] [PubMed] [Google Scholar]
- 56.Luo YW, et al. Database of diazotrophs in global ocean: abundance, biomass and nitrogen fixation rates. Earth System Science Data Discussions. 2012;4:47–73. doi: 10.5194/essd-4-47-2012. [DOI] [Google Scholar]
- 57.Pai SC, Yang CC, Riley JP. Effects of acidity and molybdate concentration on the kinetics of the formation of the phosphoantimonylmolybdenum blue complex. Anal. Chim. Acta. 1990;229:115–120. doi: 10.1016/S0003-2670(00)85116-8. [DOI] [Google Scholar]
- 58.Ma J, Yuan D, Liang Y. Sequential injection analysis of nanomolar soluble reactive phosphorus in seawater with HLB solid phase extraction. Mar. Chem. 2008;111:151–159. doi: 10.1016/j.marchem.2008.04.011. [DOI] [Google Scholar]
- 59.Ma J, Yuan D, Liang Y, Dai M. A modified analytical method for the shipboard determination of nanomolar concentrations of orthophosphate in seawater. J. Oceanogr. 2008;64:443–449. doi: 10.1007/s10872-008-0037-x. [DOI] [Google Scholar]
- 60.Pai SC, Yang CC, Riley JP. Formation kinetics of the pink azo dye in the determination of nitrite in natural waters. Anal. Chim. Acta. 1990;232:345–349. doi: 10.1016/S0003-2670(00)81252-0. [DOI] [Google Scholar]
- 61.Parsons, T. R., Maita, Y., Lalli, C. M. A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, UK,173 pp (1984).
- 62.Capone, D. G. Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure. In: Handbook of methods in aquatic microbial ecology, edited by: Kemp, P. F., Sherr, B. F. & Cole, J. J., CRC Press LLC, Boca Raton, 621–631 (1993).
- 63.Bombar D, et al. Distribution of diazotrophic microorganisms and nifH gene expression in the Mekong River plume during intermonsoon. Mar. Ecol. Prog. Ser. 2011;424:39–52. doi: 10.3354/meps08976. [DOI] [Google Scholar]
- 64.Kitajima S, Furuya K, Hashihama F, Takeda S, Kanda J. Latitudinal distribution of diazotrophs and their nitrogen fixation in the tropical and subtropical western North Pacific. Limnol. Oceanogr. 2009;54:537–547. doi: 10.4319/lo.2009.54.2.0537. [DOI] [Google Scholar]
- 65.Shiozaki T, et al. Basin scale variability of active diazotrophs and nitrogen fixation in the North Pacific, from the tropicsto the subarctic Bering Sea. Glob. Biogeochem. Cycles. 2017;31:996–1009. doi: 10.1002/2017GB005681. [DOI] [Google Scholar]
- 66.Benavides M, Agawin NSR, Arístegui J, Ferriol P, Stal LJ. Nitrogen fixation by Trichodesmium and small diazotrophs in the subtropical northeast Atlantic. Aquat. Microb. Ecol. 2011;65:43–53. doi: 10.3354/ame01534. [DOI] [Google Scholar]
- 67.Turk KA, et al. Nitrogen fixation and nitrogenase (nifH) expression in tropical waters of the eastern North Atlantic. ISME J. 2011;5:1201–1212. doi: 10.1038/ismej.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmed A, et al. Nitrogen fixation rates in the eastern Arabian Sea. ESTUAR. COAST. SHELF. S. 2017;191:74–83. doi: 10.1016/j.ecss.2017.04.005. [DOI] [Google Scholar]
- 69.Shiozaki T, Ijichi M, Kodama T, Takeda S, Furuya K. Heterotrophic bacteria as major nitrogen fixers in the euphotic zone of the Indian Ocean. Glob. Biogeochem.Cycles. 2014;28:1096–1110. doi: 10.1002/2014GB004886. [DOI] [Google Scholar]
- 70.Saino, T. Biological nitrogen fixation in the ocean with emphasis on the nitrogen fixation blue-green alga Trichodesmium and its significance in the nitrogen cycling in the low latitude sea areas. Ph.D. Theis, Ocean Research Institute, Univercity of Tokyo, pp. 1–153 (1977).
- 71.Saino, T. & Hattori, A. Nitrogen fixation by Trichodesmium and its significance in nitrogen cycling in the Kuroshio area and adjacent waters. In: Takenouti A Y. (ed.) The Kuroshio IV. Saikon Publishing, Tokyo, pp. 697–709 (1979).