Abstract
Brucellosis is an emerging infectious disease affecting humans and animals. In this study, we investigated the in vitro and in vivo effects of tannic acid (TA) against Brucella abortus infection. After infection, F-actin polymerization and mitogen-activated protein kinases (MAPKs) (ERK 1/2 and p38α) phosphorylation were reduced in TA-treated cells compared with that in control cells. The mice were infected via an intraperitoneal route and were orally given TA or phosphate-buffered saline for 14 days. Spleen weights of the TA-treated and control mice were not different; however, splenic proliferation of B. abortus was significantly reduced in the TA-treated group. Immune response analysis showed that, compared with the control group, non-infected TA-treated mice displayed increased levels of interferon-γ (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), and interleukin-10 at 3 days post-infection and a further increase in IFN-γ and MCP-1 at 14 days post-infection. In contrast, compared with the control group, infected TA-treated mice displayed elevated levels of IFN-γ at 3 days post-infection, which continued to increase at 14 days post-infection, as was also observed for tumor necrosis factor. Taken together, the results showing TA activation of cytokine production and inhibition of bacterial proliferation in the host highlight a potential use of TA treatment in the control of Brucella infection.
Keywords: Brucella abortus, actins, cytokines, mitogen-activated protein kinase, tannins
Introduction
Brucellosis is a contagious disease affecting humans and domestic animals [22]. Due to its significant socioeconomic impact in terms of decreased animal productivity, including decreased milk production, abortion, and infertility, brucellosis is considered an important disease [17,26]. Brucella, the causative agent, binds to distinct phagocytic receptors resulting in zipper-like phagocytosis or phagocytosis through lipid raft microdomains. It is exposed to a harsh environment once inside a host cell but develops multiple strategies to evade the host's immune response mechanisms, resulting in successful persistent infection and replication [1,20]. The mitogen-activated protein kinase (MAPK) cascade is important in the phagocytosis of bacteria via professional phagocytes and remodeling of the actin cytoskeleton [12].
Control programs for animal brucellosis are generally based on vaccination with live attenuated Brucella abortus S19 in cattle and Brucella melitensis in sheep and goats; however, these vaccines may cause abortion, premature birth, and decreased milk yield, hence their use has been eliminated for use in cattle in Korea [7,26]. The use of antibiotics does not seem to have a significant role in brucellosis control programs, particularly in productive animals, due to economic, epidemiological, and public health factors [16]. Furthermore, treatment for brucellosis is complicated due to emerging treatment resistance that leads to high treatment failure or relapse rates [17]. Therefore, it is necessary to find alternative control options that are safe and effective in the treatment of brucellosis.
Tannins are natural products commonly found in almost every plant part and are known for their antimicrobial properties [24]. A specific type of tannin, namely tannic acid (TA), has been reported to inhibit the growth of Aeromonas hydrophila, Aeromonas sobria, Escherichia coli, Klebsiella pneumonia, Listeria monocytogenes, Staphylococcus aureus, Salmonella Typhimurium, Helicobacter pylori, and Cytophaga columnaris [9]. The reported possible mechanism for such inhibition mainly involves the bacterial cell wall, resulting in lysis of complexes within cell wall proteins, membrane disruption metal ion complexation, and binding to adhesions [18]. In our previous study, tannin-derived components of galla rhois (GR), collectively TA, methyl gallate (MG), and gallic acid, were reported to have a bactericidal effect against B. abortus infection when applied as a GR ethanol extract and to have an inhibitory effect on bacterial proliferation in mouse spleen [11]. In addition, we have also demonstrated therapeutic effects of MG against Brucella infection; effects transpiring via induction of cytokine production [21]. Consequently, in this study, we investigated the therapeutic effects of TA, another GR component, on the splenic proliferation of B. abortus and the host immune response in a mouse model.
Materials and Methods
Tannic acid preparation
TA (Sigma-Aldrich, USA) was dissolved in sterile phosphate-buffered saline solution (PBS, pH 7.4) (100 mg/mL) and sterilized via membrane filtration (0.45 µm membranes, Minisart; Sartorius Stedim Biotech, Germany).
Bacterial strains
The B. abortus 544 (ATCC 23448) strain was obtained from the Laboratory of Bacteriology Division in Animal and Plant Quarantine Agency, Korea and cultivated in Brucella broth (Becton Dickinson, USA) at 37℃ with aeration. Routine cultivation was carried out in Brucella broth or agar (1.5%).
Cell culture
Murine RAW 264.7 cells (American Type Culture Collection, USA) were grown at 37℃ with 5% CO2 atmosphere as previously described [20] and were seeded in tissue culture plates at a concentration of 1 × 105 cells per well. Culture medium was changed to fresh medium without antibiotics prior to infection.
Cytotoxicity assay
The cells were incubated with different concentrations of TA (0, 40, 80, 100, and 200 µg/mL) in a 96-well cell culture plate. After 48 h of incubation, cell viability was assessed by using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
F-actin staining and FACS analysis
For F-actin staining, pre-treated macrophages were prepared in 12-well plates with 18-mm diameter glass coverslips and were infected with fluorescein isothiocyanate (FITC)-conjugated B. abortus as previously described [20]. Macrophages were viewed under a laser scanning confocal microscope (Olympus FV1000; Olympus, Japan) and images were processed by using FV10-ASW Viewer software (ver. 3.1; Olympus).
For fluorescence-activated cell sorting (FACS) analysis, cells in six-well plates were harvested after pre-treatment and infection as described above. Lysophosphatidylcholine (20 µg/mL) containing tetramethylrhodamine isothiocyanate (TRITC)-phalloidin (1 µM) was used to permeabilize and stain the infected cells (both purchased from Sigma-Aldrich) followed by incubation for 30 min at 22℃. After final washing, the F-actin content was quantified by using a FACSVerse flow cytometer (BD Biosciences, USA).
Western blotting
Pre-treatment and infection of macrophages was undertaken as previously described [20]. Lysis of cells, measurement of protein concentration, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and western blotting were performed as previously described [21].
Protective effect and immune response analysis
Specific-pathogen-free female ICR mice (six-to eight-week-old mice) were maintained at 23℃ ± 1℃ with a 12 h light/dark cycle and free access to food and water and acclimatized for at least one week prior to use in experiments. The mice were randomly grouped into groups of 4 to 5 mice each; two non-infected groups were orally given PBS (negative control) or TA (80 µg/mL) and two infected groups were orally given PBS (positive control) or TA (80 µg/mL). Infection was performed intraperitoneally with an injection of 2 × 104 colony-forming units (CFUs) of B. abortus. Oral treatment was given three days prior to infection and continued until 14 days post-infection. At three days post-infection, serum samples from the tail vein were collected, and, at 14 days post-infection, the mice were sacrificed. Blood was then collected from the hearts, and spleens were removed and weighed. Spleens of infected mice were homogenized, serially diluted in PBS, and plated on Brucella agar as previously described [11]. The agar plates were incubated at 37℃ for three days and the number of CFUs for each spleen was counted. All of the procedures were approved by the Animal Ethical Committee of Gyeongsang National University (approval No. GNU-120423-M0012).
The levels of interleukin (IL)-12p70, tumor necrosis factor (TNF), interferon-γ (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), IL-10, and IL-6 in the serum samples were analyzed as previously described [13] by using a mouse inflammation cytometric bead array kit (BD Biosciences) according to the manufacturer's instructions. Cytokines were measured by using a FACSVerse flow cytometer (BD Biosciences). Briefly, 50 µL serum samples were added to Falcon tubes with an equal volume of mixed capture beads. Fifty microliters of Mouse Inflammation PE Detection Reagent were added and the mixture incubated for 2 h at room temperature in the dark. One milliliter of wash buffer was then added and the mixture centrifuged at 200 × g for 5 min. The supernatant was carefully removed and 300 µL of wash buffer was added to resuspend the bead pellet. BD CellQuest software was used for data acquisition, and the data were analyzed by Becton, Dickinson and Company (USA).
Statistical analysis
The results for each experiment are expressed as means ± SD. Statistical analysis was performed in GraphPad InStat software (ver. 3; GraphPad Software, USA) with Student's t-test or one-way analysis of variance (ANOVA) applied for statistical comparisons between groups.
Results
Effects on murine macrophage viability
The cytotoxicity of TA was evaluated by incubating RAW 264.7 cells with different concentrations of TA (0, 40, 80, 100, and 200 µg/mL) for 48 h. Cell viability remained at 100% with no change in optical density (OD) values at or below concentrations of 80 µg/mL. The highest concentration (80 µg/mL) at which the OD values did not change was used in subsequent experiments.
Effects on F-actin polymerization and MAPK phosphorylation
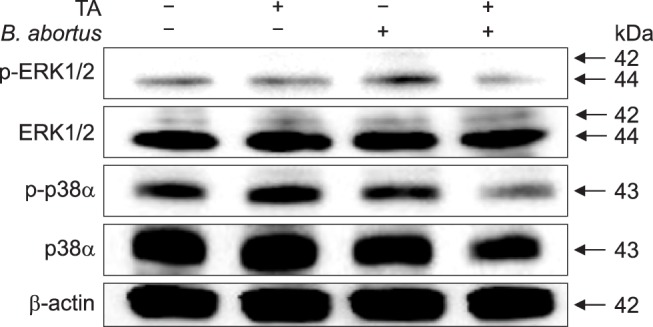
Under fluorescence microscopy, attenuated F-actin polymerization was observed in infected TA-treated cells (panel A in Fig. 1) and was confirmed through the use of a FACS assay, which revealed a 1.11-fold reduction from that of the positive control (panel B in Fig. 1). No significant difference in F-actin fluorescence was observed between the non-infected groups. In our previous study (unpublished data), TA effectively reduced invasion of B. abortus in RAW 264.7 cells, and the results in this study indicate that this inhibition could be due to attenuation of F-actin polymerization, which is essential for the uptake of microbial pathogens by phagocytes. In addition, treatment of cells with TA suppressed phosphorylation of ERK 1/2 and p38α in TA-treated cells by 3.33- and 1.80-fold, respectively, at 30 min post-infection as compared to the positive controls (Fig. 2), indicating a negative effect on bacterial invasion into macrophages.
Fig. 1. Effect of tannic acid (TA) on F-actin polymerization in RAW 264.7 cells. (A) The stained cells were viewed under a laser scanning confocal microscope. (B) F-actin content was quantified using a flow cytometer. The values expressed are means ± SD for each group. A statistically significant difference from the control group is indicated by an asterisk (*p < 0.05). DIC, differential interference contrast; B. abortus, Brucella abortus.
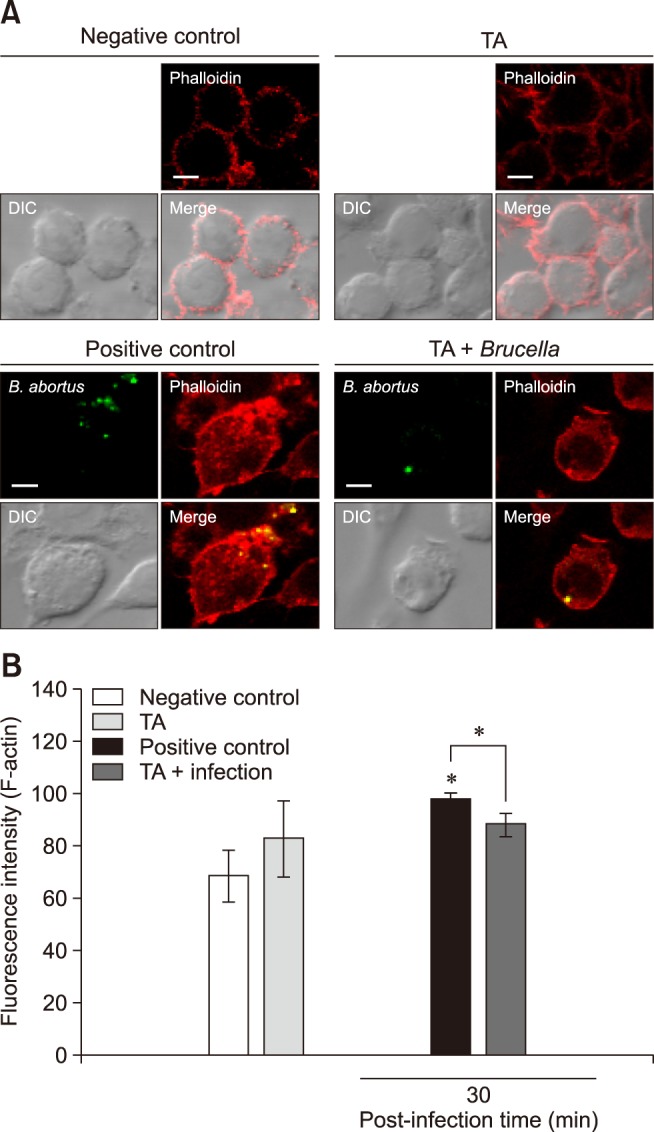
Fig. 2. Effect of tannic acid (TA) on mitogen-activated protein kinase (MAPK) phosphorylation in RAW 264.7 cells. Cells were pre-treated with TA or PBS. The cells were then infected with Brucella abortus and the cell lysates collected. Immunoblot analysis of total RAW 264.7 cell lysates was assessed by using phospho-specific ERK1/2, JNK, and p38α antibodies.
Effects on bacterial proliferation in mice
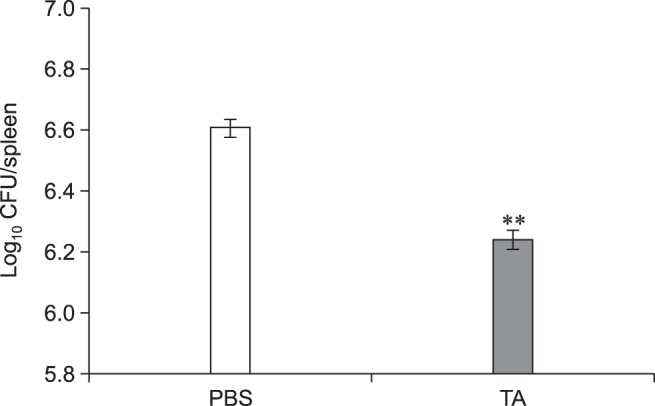
The in vitro inhibitory effects of TA were further confirmed by in vivo TA treatment of a mouse model. In the non-infected groups, the total weights of spleen collected from TA-treated mice were not significantly different from those of the control group. Although slightly lower, the infected TA-treated mice showed no statistically significant difference in total weights of the spleen when compared with the positive control group (Fig. 3). However, the average number of CFUs in the spleens of TA-treated mice was significantly reduced (1.48-fold, p < 0.05) from that of the positive control (Fig. 4). These results indicated that TA treatment in mice did not effectively reduce inflammation of the spleens, but it did successfully reduce the proliferation of B. abortus in spleen.
Fig. 3. Total weight of spleens of mice orally treated with tannic acid (TA) or PBS. Mice were orally given TA or PBS from 3 days prior to infection to 14 days post-infection. The mice were then sacrificed, the spleens harvested and weighed. A statistically significant difference from the control group is indicated by an asterisk (*p < 0.05).
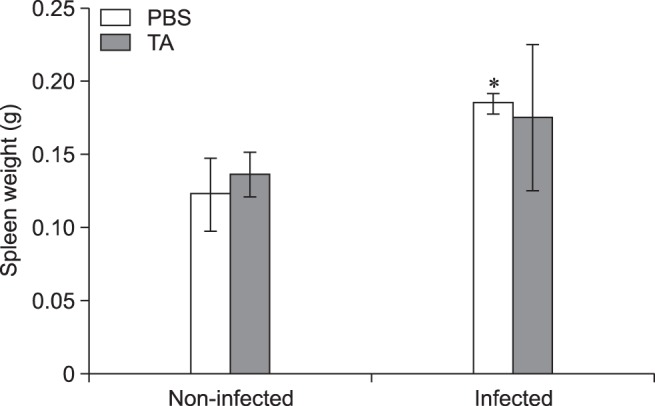
Fig. 4. Proliferation of Brucella abortus in spleens of tannic acid (TA)-treated mice or PBS-treated mice. Mice were orally given TA or PBS from 3 days prior to infection to 14 days post-infection. At 14 days post-infection, mice were sacrificed, and the spleens homogenized and serially diluted in PBS. The number of colony-forming units (CFUs) was counted to assess bacterial proliferation in each spleen. A statistically significant difference from the control group is indicated by an asterisk (**p < 0.01).
Effects on immune response in mice
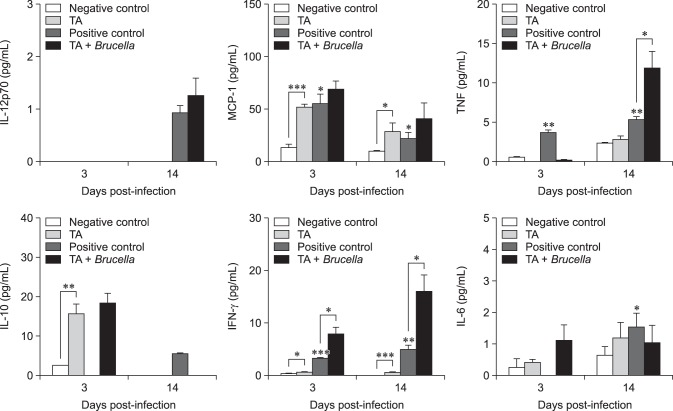
In the non-infected groups and compared to the control group, the TA-treated mice showed elevated levels of IFN-γ (2.83-fold, p < 0.05), MCP-1 (3.80-fold, p < 0.001) and IL-10 (6.36-fold, p < 0.01) at 3 days post-infection, and further increases in IFN-γ (9.05-fold, p < 0.001) and MCP-1 (2.86-fold, p < 0.05) were observed at 14 days post-infection. In the infected groups, compared to the control group, the TA-treated mice displayed an elevated level of IFN-γ (2.50-fold, p < 0.05) at 3 days post-infection, which was observed to continue to increase at 14 days post-infection (3.25-fold, p < 0.05) in concert with an elevated level of TNF (2.22-fold, p < 0.05) (Fig. 5). Taken together, these findings indicated that TA treatment aids in the enhancement of cytokine release in mice, particularly in those cytokines involved in the cellular-mediated immune response.
Fig. 5. Immune response analysis of tannic acid (TA)-treated mice or PBS-treated mice against Brucella abortus infection in mice. Mice were orally given TA or PBS from 3 days prior to infection to 14 days post-infection. At 3 days post-infection, serum samples were collected via tail vein in each animal. At 14 days post-infection, mice were sacrificed, and blood was collected from the heart. Serum samples were analyzed for cytokine release by using a flow cytometer. A statistically significant difference from the control group is indicated by asterisks (*p < 0.05; **p < 0.01; ***p < 0.001). IL, interleukin; MCP-1, monocyte chemoattractant protein-1; TNF, tumor necrosis factor; IFN, interferon.
Discussion
Brucellosis is considered an emerging infectious disease in many areas of the world with an estimate of 500,000 new cases every year in humans worldwide [23]. No current validated vaccine is available for humans, and the control programs for animal brucellosis are based on vaccination, which has several disadvantages. Moreover, the use of antibiotics for brucellosis treatment is controversial due to the emergence of antibiotic resistance, which leads to treatment failure and relapse. In our previous study (unpublished data), TA was shown to directly inhibit the growth of B. abortus in a concentration-dependent manner, as well, TA affected the pathogen's internalization into RAW 264.7 cells. The results of this study suggest that the potential of TA to inhibit B. abortus invasion is contributed through its inhibitory action on F-actin polymerization and MAPKs phosphorylation (ERK 1/2 and p38α), which are essential for phagocytic uptake of microbes [12]. This is similar to the results of a study performed on cell line HSC-2, established from a human oral squamous cell carcinoma, in which pre-treatment of these cells with TA inhibited the MAPK signaling pathways [8]. Since adhesion is one of the initial stages of the infectious process, TA could be used as an anti-adhesion agent to control B. abortus infection and probably other intracellular pathogens.
Brucella is a stealthy pathogen with no classical toxins and its intracellular lifestyle limits its exposure to the host's innate and adaptive immune responses, protects from the effects of antibiotics, and eventually leads to its pathologic features [5,19]. The T helper cell type 1 (Th1) cellular immune response is important for the clearance of Brucella, which mainly resides in macrophages, but the effectiveness of the Th2 humoral immune response remains unclear [5,14]. These two subclasses of effector helper T cells can be distinguished by examining the cytokines they secrete; cytokines are key factors in protection against brucellosis, mediating and directing the immune response [3,5,10]. In this study, the cytokine content in serum samples was analyzed as that content was reflective of the infection status of splenic cells [4]. In the non-infected groups, TA-treated mice displayed showed increased production of IFN-γ, MCP-1 and IL-10 at 3 days post-infection. cytokines IFN-γ and MCP-1 were observed to increase until 14 days post-infection, but the IL-10 level did not increase. IFN-γ activates macrophages and is involved in the control of murine brucellosis. MCP-1 regulates the migration and infiltration of monocytes and lymphocytes and has been implicated in host immunity to Brucella, while IL-10 aids in preventing further tissue damage [5,6,15,25]. Our cytokine analysis suggests that TA could be used as an immunostimulator or an adjuvant. A previous study reported that TA, in addition to β-lactam antibiotics, can be a useful adjuvant agent for S. aureus skin infections [2]. In addition, TA can be a potential adjuvant to improve immune response to co-administered antigens in a vaccine formulation. In Brucella-infected groups, increases in IFN-γ levels were observed at both 3 and 14 days post-infection in TA-treated mice. On the other hand, TNF was observed to increase significantly only at 14 days post-infection. Brucella suis has been reported to inhibit TNF production in human macrophages, suggestive of the pathogen's strategy for intracellular survival [10]. The TNF cytokine maximizes the phagocytic activity of macrophages and is crucial in destroying live Brucella within macrophages [15].
TA treatment in mice did not reduce inflammation of the spleens, but it did significantly reduce proliferation of B. abortus in these organs. TA has been reported to inhibit the growth of several bacterial pathogens as well as the HIV and influenza viruses. In our unpublished data, TA was observed to directly inhibit the growth of B. abortus. Taken together, TA treatment in mice successfully controls B. abortus infection in mice, and such control is attributed to the production of important cytokines, particularly those involved in the Th1 cellular immune response, and probably to its bactericidal effect.
In conclusion, the oral treatment of TA in mice can lead to induction of cytokine production and inhibition of Brucella proliferation in their spleens. The results of this study highlight the potential of using TA as a therapeutic agent for the control and prevention of animal brucellosis.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Korea (HI16C2130).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Ahmed W, Zheng K, Liu ZF. Establishment of chronic infection: Brucella's stealth strategy. Front Cell Infect Microbiol. 2016;6:30. doi: 10.3389/fcimb.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother. 2001;48:487–491. doi: 10.1093/jac/48.4.487. [DOI] [PubMed] [Google Scholar]
- 3.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed. New York: Garland Science; 2002. [Google Scholar]
- 4.Athanassakis I, Iconomidou B. Cytokine production in the serum and spleen of mice from day 6 to 14 of gestation: cytokines/placenta/spleen/serum. Dev Immunol. 1996;4:247–255. doi: 10.1155/1995/42412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol. 2015;185:1505–1517. doi: 10.1016/j.ajpath.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwida M, El-Ashker M, Melzer F, El-Diasty M, El-Beskawy M, Neubauer H. Use of serology and real time PCR to control an outbreak of bovine brucellosis at a dairy cattle farm in the Nile Delta region, Egypt. Ir Vet J. 2016;69:3. doi: 10.1186/s13620-016-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizaki H, Yamada S, Yanagiguchi K, Koyama Z, Ikeda T, Hayashi Y. Pre-treatment with tannic acid inhibits the intracellular IL-8 production by chitosan in a human oral epithelial cancer cell line. Oral Med Pathol. 2008;13:135–141. [Google Scholar]
- 9.Kim TJ, Silva JL, Kim MK, Jung YS. Enhanced antioxidant capacity and antimicrobial activity of tannic acid by thermal processing. Food Chem. 2010;118:740–746. [Google Scholar]
- 10.Ko J, Splitter GA. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin Microbiol Rev. 2003;16:65–78. doi: 10.1128/CMR.16.1.65-78.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JJ, Bae JH, Kim DH, Lim JJ, Kim DG, Lee HJ, Min W, Rhee MH, Chang HH, Park H, Kim S. Intracellular replication inhibitory effects of Galla Rhois ethanol extract for Brucella abortus infection. J Ethnopharmacol. 2011;138:602–609. doi: 10.1016/j.jep.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Lee JJ, Kim DH, Kim DG, Lee HJ, Min W, Rhee MH, Cho JY, Watarai M, Kim S. Toll-like receptor 4-linked Janus kinase 2 signaling contributes to internalization of Brucella abortus by macrophages. Infect Immun. 2013;81:2448–2458. doi: 10.1128/IAI.00403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JJ, Kim DH, Park SB, Lim JJ, Kim DG, Min WG, Lee HJ, Kim DK, Chang HH, Kim S. Redundant effects of ketamine on the pathogenesis and severity of Brucella abortus infection. Comp Immunol Microbiol Infect Dis. 2013;36:71–81. doi: 10.1016/j.cimid.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Lim JJ, Kim DH, Lee JJ, Kim DG, Min W, Lee HJ, Rhee MH, Kim S. Protective effects of recombinant Brucella abortus Omp28 against infection with a virulent strain of Brucella abortus 544 in mice. J Vet Sci. 2012;13:287–292. doi: 10.4142/jvs.2012.13.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J Immunol. 2008;180:1080–1087. doi: 10.4049/jimmunol.180.2.1080. [DOI] [PubMed] [Google Scholar]
- 16.Moreno E. Retrospective and prospective perspectives on zoonotic brucellosis. Front Microbiol. 2014;5:213. doi: 10.3389/fmicb.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motamedi H, Darabpour E, Gholipour M, Seyyed Nejad SM. In vitro assay for the anti-Brucella activity of medicinal plants against tetracycline-resistant Brucella melitensis. J Zhejiang Univ Sci B. 2010;11:506–511. doi: 10.1631/jzus.B0900365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myint KB, Sing LC, Wei Z. Tannic acid as phytochemical potentiator for antibiotic resistance adaptation. APCBEE Procedia. 2013;7:175–181. [Google Scholar]
- 19.Rambow-Larsen AA, Petersen EM, Gourley CR, Splitter GA. Brucella regulators: self-control in a hostile environment. Trends Microbiol. 2009;17:371–377. doi: 10.1016/j.tim.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyes AW, Arayan LT, Simborio HL, Hop HT, Min W, Lee HJ, Kim DH, Chang HH, Kim S. Dextran sulfate sodium upregulates MAPK signaling for the uptake and subsequent intracellular survival of Brucella abortus in murine macrophages. Microb Pathog. 2016;91:68–73. doi: 10.1016/j.micpath.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Reyes AW, Kim DG, Simborio HL, Hop HT, Arayan LT, Min W, Lee JJ, Chang HH, Kim S. Methyl gallate limits infection in mice challenged with Brucella abortus while enhancing the inflammatory response. J Appl Microbiol. 2016;120:552–559. doi: 10.1111/jam.13019. [DOI] [PubMed] [Google Scholar]
- 22.Reyes AW, Simborio HL, Hop HT, Arayan LT, Kim S. Molecular cloning, purification and immunogenicity of recombinant Brucella abortus 544 malate dehydrogenase protein. J Vet Sci. 2016;17:119–122. doi: 10.4142/jvs.2016.17.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo G, Pasquali P, Nenova R, Alexandrov T, Ralchev S, Vullo V, Rezza G, Kantardjiev T. Reemergence of human and animal brucellosis, Bulgaria. Emerg Infect Dis. 2009;15:314–316. doi: 10.3201/eid1502.081025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. [Google Scholar]
- 25.Xavier MN, Winter MG, Spees AM, Nguyen K, Atluri VL, Silva TM, Bäumler AJ, Müller W, Santos RL, Tsolis RM. CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog. 2013;9:e1003454. doi: 10.1371/journal.ppat.1003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon H, Moon OK, Lee SH, Lee WC, Her M, Jeong W, Jung SC, Kim DS. Epidemiology of brucellosis among cattle in Korea from 2001 to 2011. J Vet Sci. 2014;15:537–543. doi: 10.4142/jvs.2014.15.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]