Key Clinical Message
This case highlights the management and diagnostic evaluation of a dog with two individually rare conditions (lung lobe torsion and vena cava aneurysm) that ultimately resulted in fatal pulmonary thromboembolism.
Keywords: Blood clot, congenital, embolism, vascular anomaly
Introduction
Congenital abdominal vascular malformations with subsequent formation of caudal vena cava aneurysms are rare in humans and even less commonly reported in dogs 1, 2, 3, 4, 5, 6, 7, 8. Most often such abnormalities are found incidentally during imaging studies 6. However, there have been several reports of azygos continuation of the caudal vena cava as well as other vascular anomalies resulting in deep vein thrombosis in addition to thrombi in other locations in people 9, 10, 11. To the authors' knowledge, pulmonary thromboembolism (PTE) secondary to aneurysmal dilation of an abdominal vascular anomaly has never been reported in dog.
We report a case of catastrophic PTE in a young dog with a lung lobe torsion and azygos continuation of the caudal vena cava with a segmental aneurysm. We propose this dog's aneurysm predisposed it to PTE formation and may or may not have been associated with the lung lobe torsion.
Case Summary
A 2‐year‐6‐month‐old 18.23‐kg (40.1 lb) castrated male whippet/shar‐pei cross presented to an urban veterinary specialty and emergency care facility as a referral for suspected lung lobe torsion based on thoracic radiographs (Fig. 1). The dog had a several‐week history of partial anorexia and lethargy and an abnormal respiratory pattern characterized by paroxysms of breathing very heavily immediately followed by coughing with a terminal retch. Additionally, the dog had an approximately two‐month history of intermittent exercise intolerance followed by vomiting.
Figure 1.
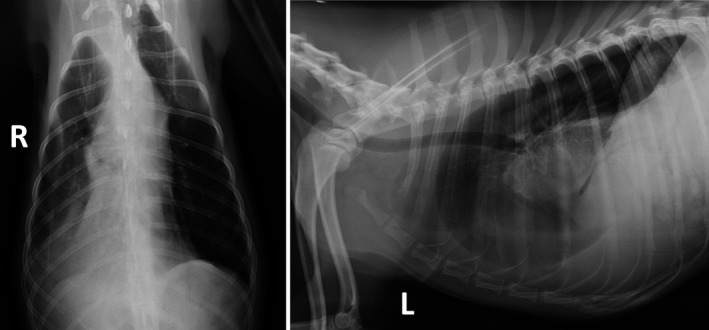
Ventrodorsal and left lateral thoracic radiographs, illustrating lung lobe torsion. Note the abnormal position of the right middle lung lobe along with a vesicular gas pattern and inability to visualize the right middle lobar bronchus. These findings are typical for lung lobe torsion.
On presentation, the dog had a temperature of 39.4°C (103°F), the pulse of 110 beats per minute, and a respiratory rate of 40 breaths per minute. He was bright, alert, and responsive, appeared adequately hydrated, had pink mucous membranes with a capillary refill time of <1 sec, and had a body condition score of 5/9. No murmur or arrhythmia was auscultated. His lung sounds were normal. The rest of his physical examination was unremarkable.
Initial diagnostics included a point of care panel with electrolytes (NOVA CCP, Biomedical, Waltham, MA) that revealed a hyperlactatemia (3.60 mmol/L; reference interval: 0.70–2.80 mmol/L) and a hypochloremia (109.6 mmol/L; reference interval: 112.7–118.3 mmol/L). A complete blood count and chemistry panel were submitted. Abnormal results are reported in Table 1. The leukogram was suggestive of chronic inflammation.
Table 1.
Select abnormal or key biochemical values obtained during hospitalization
| Parameter units (SI units) | Preoperative value (SI value) | Postoperative value (SI value) | Recheck value 12 days postoperative (SI value) | Reference range (SI reference range) |
|---|---|---|---|---|
| WBC number ×103/μL or ×109/L | 19.2 | 17 | 6.0–13.4 | |
| Neutrophil ×103/μL or ×109/L | 14.6 | 13.4 | 3.3–10.1 | |
| Monocyte ×103/μL or ×109/L | 2.6 | 1.4 | 0.1–0.9 | |
| Hemoglobin g/dL (g/L) | 13.9 (139) | 11.5 (115) | 14.3–21.1 (143–211) | |
| Hematocrit % (L/L) | 40.4 (0.40) | 34.9 (0.34) | 41.7–58.1 (0.41–0.58) | |
| Aspartate aminotransferase U/L | 71 | 49 | 14–49 | |
| Alanine aminotransferase U/L | 16 | 12 | 22–74 | |
| Alkaline phosphatase U/L | 129 | 58 | 12–116 | |
| Sodium mmol/L | 144 | 147 | 145–151 | |
| Chloride mmol/L | 102 | 107 | 106–117 | |
| Magnesium mg/dL (μmol/L) | 2.6 (291.72) | 1.8 (201.96) | 1.7–2.4 (190.74–269.28) | |
| Cholesterol mg/dL (mmol/L) | 421 (10.9) | 289 (7.48) | 130–339 (3.37–8.78) | |
| Platelet ×103/μL or ×109/L | 289 | 100 | 161–513 | |
| Reticulocyte percent % (L/L) | 0.5 (0.005) | 4.1 (0.04) | ||
| Absolute reticulocyte count ×109/L | 28 | 196 | ||
| Corrected reticulocyte count % (L/L) | 0.4 (0.004) | 3.1 (0.03) | ||
| Potassium mmol/L | 3.9 | 3.6 | 3.7–5.1 | |
| Prothrombin time seconds | 18.1 | 7.3–11.8 | ||
| Activated partial thromboplastin time seconds | 25.4 | 10.6–14.8 | ||
| PaO2 mmHg | 97.9 | 38.0–65.6 | ||
| Oxygen saturation % | 99.4 | 46.3–89.7 | ||
| NOVA Lactate mmol/L | 3.6 | 0.4 | 0.70–2.80 | |
| NOVA Chloride mmol/L | 109.6 | 112.1 | 112.7–118.3 | |
| NOVA Potassium mmol/L | 3.82 | 3.46 | 3.62–4.60 | |
| NOVA Ionized Calcium mmol/L | 1.32 | 1.42 | 1.15–1.34 | |
| NOVA Ionized Magnesium mmol/L | 0.6 | 0.3–0.5 | ||
| NOVA Glucose mg/dL (mmol/L) | 78 | 124 | 75–116 |
Computed tomography (CT) of the thorax was performed under sedation with dexmedetomidine (Dexmedetomidine hydrochloride, Orion Co., Espoo, Finland) (0.5 mcg/kg, IV), fentanyl (Fentanyl citrate, Hospira, Inc., Lake Forest, IL) (5 mcg/kg, IV), and lidocaine (Lidocaine, Distributed by Henry Schein Animal Health, Dublin, OH) (2 mg/kg, IV), revealing an abnormal right middle lung lobe position, with the apex directed caudally and the base directed cranially. The lobar bronchus was abruptly truncated after it branched from the main stem bronchus. The parenchyma of the right middle lung lobe was generally consolidated but with a distinct vesicular gas pattern. A moderate volume of bilateral pleural effusion was present, which was likely secondary to lung lobe torsion 12. Atelectasis of the right caudal lung lobe was also identified, most likely secondary to the abnormal position of the right middle lung lobe. No other abnormalities were noted. Intravenous contrast media was not administered, as the nonenhanced CT was diagnostic for lung lobe torsion.
The dog underwent a thoracotomy immediately following the CT scan. General anesthesia was induced with propofol (Propofol, Zoetis Inc., Kalamazoo, MI) (4 mg/kg IV given to effect), and the dog was orotracheally intubated. Anesthesia was maintained on isoflurane gas as well as a combination of a fentanyl (Fentanyl citrate, Hospira, Inc.) continuous rate infusion (CRI) (0.1–0.2 mcg/kg/min, IV) and lidocaine (Lidocaine, Distributed by Henry Schein Animal Health, Dublin, OH) CRI (50 mcg/kg/min, IV). Plasmalyte (Plasma‐lyte, Baxter Healthcare Pty Ltd., Old Toongabbie, NSW) was administered IV at 7 mL/kg/h. The anesthetic event including drug selection was overseen by a board‐certified anesthesiologist.
A right‐sided fifth intercostal thoracotomy was performed. The right middle lung lobe was grossly enlarged, solid, and dark with a discrete stalk at the hilus where torsion of the vessels was appreciated. The lobe was resected using a TA 30 V3 cartridge (Autosuture TA‐90 stapler, Covidien, MedWOW Ltd., 100 Arch. Makarios III, P.C. 2460, Nicosia, Cyprus) and was leak checked using 0.9% NaCl flush. No leaks were observed. The rest of the thoracic contents appeared grossly normal. A 16‐Fr thoracostomy tube was placed. The routine closure was performed using 0 PDS (Polydioxanone suture (PDS), ETHICON, Inc., Mexico) in a circumcostal simple interrupted pattern, 0 PDS (Polydioxanone suture (PDS), ETHICON, Inc.) in a simple continuous pattern of the deep and superficial muscle layers, 2‐0 PDS (Polydioxanone suture (PDS), ETHICON, Inc.,) in a simple continuous pattern of the deep and superficial subcutaneous tissues, and finally, surgical skin staples to appose the skin. A DIFF6 Mila wound catheter (Diffusion catheter/wound catheter, Mila International, Inc., Erlanger, KY) was placed prior to closure of the wound. The chest tube was secured with 2‐0 Nylon (Monofilament Nylon, Covidien, Dominican Republic) in a finger trap and purse‐string pattern while the wound catheter (Diffusion catheter/wound catheter, Mila International, Inc., Erlanger, KY) was secured using the supplied catheter wings.
The dog recovered uneventfully from surgery and anesthesia. The packed‐cell volume (PCV) and total protein (TP) immediately postoperatively were 24% and 6.2 g/dL, respectively. The drop in PCV/TP was suspected to be from a combination of anesthesia, fluid resuscitation, and mild blood loss 13. The dog was recovered in an oxygen cage with a set fraction of inspired oxygen of 40%. Analgesia was provided by a Fentanyl (Fentanyl citrate, Hospira, Inc.) CRI at 3 mcg/kg/h and maintenance crystalloid fluids (Lactated Ringers Solution (Hospira Inc., Lake Forest, IL) with 40 mEq KCl/L added). Two hours postoperatively, an arterial blood gas panel with electrolytes (NOVA CCP, Biomedical, Waltham, MA) was performed after being off oxygen for five minutes and is reported in Table 1. This revealed no significant abnormalities, and the A‐a gradient was 7.9 mmHg. The previously elevated lactate had normalized (0.40 mmol/L). PCV and TP were performed at the same time, which were 34% and 7.1 g/dL. He was kept in an oxygen cage overnight to ensure ongoing comfort and in the event hypoventilation developed on the fentanyl CRI. The chest tube was aspirated every 6 h, and the amount of air and fluid was recorded. Bupivacaine (Bupivacaine HCl, Hospira, Inc., Lake Forest, IL) (28 mg) was infused through a wound soaker catheter every 6 h for additional pain control.
The patient appeared comfortable the following morning. A total of 0.06 mL/kg/h of air and 0.15 mL/kg/h of serosanguineous fluid were removed from the thoracostomy tube over a 12‐h period. Based on the minimal volume of fluid production, the chest tube was pulled and supplemental oxygen discontinued. His analgesia was changed to buprenorphine (Buprenorphine hydrochloride, Hospira, Inc., Lake Forest, IL) (0.01 mg/kg, IV every 8 h) and carprofen (Carprofen (Rimadyl), Zoetis Inc., Kalamazoo, MI) (2 mg/kg, PO every 12 h) when eating. Later that evening, he began eating well. Buprenorphine (Buprenorphine hydrochloride, Hospira, Inc.) was discontinued, and Tramadol (Tramadol hydrochloride, Manufactured by Sun Pharmaceutical Industries Ltd, Survey No. 259/15, Dadra‐396 191, (U.T. of D and NH), India; Distributed by Sun Pharmaceutical Industries, Inc., Cranbury, NJ 08512) (2.68 mg/kg, PO every 8 h) was added. He was discharged from the hospital on day two postoperatively. Histopathology of the lung lobe revealed hemorrhagic infarction characterized by diffuse parenchymal necrosis with marked hemorrhage, edema, and fibrin deposition. Microscopic findings were consistent with vascular compromise associated with lung lobe torsion.
Twelve days postoperatively, the owner reported that the dog had seemed lethargic for the last two days. It was recommended that the dog return for suture removal and reevaluation at this time. The skin staples were removed, and the incision appeared well healed.
The owner reported that over the last two days, the dog had had some episodes of tachypnea, similar to those observed prior to surgery. He was partially anorexic and was lethargic. The owner reported that he was 100% himself immediately following the surgery, with more energy and normal appetite. Physical examination for this visit revealed a temperature of 39.9°C (103.8°F), pulse 120 beats per minute, and respiratory rate of 40 breaths per minute. His weight had increased slightly to 19 kg (42 pounds). Normal heart and lung sounds were auscultated, and the dog appeared to exhibit normal respiratory effort. A focused thoracic ultrasound of the chest revealed pleural effusion (1.47 cm in width at the narrowest point just caudal to incision at the costochondral junction, widest was 2.7 cm). A sample was obtained that had a pink and cloudy appearance, most consistent with chylous effusion. Fluid analysis revealed a WBC count of 11.6 K/μL and RBC count of 0.18 mol/L/μL; however, triglyceride levels were not measured. Abdominal focused assessment with sonography for trauma (AFAST) revealed what was thought to be an abdominal mass in the dorsal abdomen or retroperitoneal space. The patient was admitted to the hospital for further evaluation of the abdominal mass, elevated temperature, and tachypnea.
Abnormalities noted in the evaluation of a complete blood count, chemistry panel, and PT/aPTT are reported in Table 1. Pleural fluid analysis and cytology revealed probable chylous effusion with erythrophagia. A fluid triglyceride concentration was not performed. The hematocrit and TP were not reported due to lipemic nature of the effusion. The PT and aPTT were prolonged at 18.1 sec (7.3–11.8 sec) and 25.4 sec (10.6–14.8 sec), respectively. Given the thrombocytopenia and anemia, the prolongation in coagulation times was attributed to a consumptive coagulopathy.
The thoracic radiographs revealed increased soft tissue opacity within the pleural space, with rounding and retraction of the lungs and an alveolar pattern characterized by air bronchograms within the ventral aspect of the right cranial lung lobe that was thought to be most consistent with right cranial lung lobe pneumonia, atelectasis, or hemorrhage. Pleural effusion was also noted and thought to be secondary to the recent thoracotomy versus inflammatory effusion or hemorrhage.
The abdominal ultrasound was performed by a board‐certified radiologist. A very large, thin‐walled, fluid‐filled structure was identified in the right dorsal abdomen, communicating directly with the terminal portion of the caudal vena cava. Echogenic, heterogeneous material was identified centrally within this large fluid‐filled structure. This was suspected to represent a large aneurysmal dilation of the caudal vena cava, and given the absence of a visible connection to the intrahepatic portion of the vena cava, the most likely explanation for the vascular anomaly was segmental aplasia of the caudal vena cava. The echogenic, heterogeneous material identified within the structure was suspected to be a thrombus within the aneurysmal dilation.
Thoracocentesis was performed to remove as much effusion as possible prior to discharge. The dog was discharged with Clavamox (Amoxicillin trihydrate/clavulanate potassium (Clavamox), Zoetis Inc., Kalamazoo, MI) (18.75 mg/kg PO every 12 h) for possible pneumonia as well as Clopidogrel (Clopidogrel, manufactured for Accord Healthcare, Inc., Durham, NC 27703; manufactured by Intas Pharmaceuticals Limited, Ahmedabad, India) (2 mg/kg PO every 24 h) due to concerns for ongoing thrombosis. This drug was chosen over low molecular weight heparin due to concerns that the PT and aPTT were already prolonged. Thrombectomy was discussed with the owner, but given the coagulopathy and the relative clinical stability of the patient, the decision was made to reevaluate the patient in 72 h to assess the PT/aPTT, as well as reevaluate the suspected thrombus. The dog appeared stable at home for the next 2 days until he had an episode of acute respiratory distress while on a short walk (14 days post‐op).
On presentation, the dog was in severe respiratory distress characterized by tachypnea (100 breaths per minute) with abdominal effort and an orthopneic posture. A moderate volume of pleural effusion was appreciated on TFAST; however, only 77 mL of chylous‐appearing fluid was obtained via thoracocentesis. An acute care venous blood gas revealed an elevated lactate (4.10 mmol/L; reference interval 0.70–2.80) and PCV and TP of 45% and 7.3 g/dL. A single lateral thoracic radiograph confirmed a moderate volume pleural effusion as well as irregularly marginated soft tissue opacity caudal to the carina, caudodorsal to the heart, and silhouetting with the diaphragm that was not previously noted. Differentials included pneumonia, vasculitis, PTE, atelectasis, or neoplasia. The dog was admitted to the hospital and placed in 40% oxygen as well as started on dalteparin sodium (Dalteparin sodium (Fragmin), Pfizer Labs, Division of Pfizer Inc., New York), a low molecular weight heparin (155 IU/kg SC every 8 h), methadone (Methadone hydrochloride, Mylan Institutional LLC, Rockford, IL) (0.1 mg/kg, IV every 4 h), combination ampicillin and sulbactam (Unasyn (Ampicillin and Sulbactam), Manufactured by MITIM S.R.L. 25125 Brescia, Italy; Distributed by West‐Ward Pharmaceuticals, Eatontown, NJ) (50 mg/kg, IV every 6 h), maropitant citrate (Cerenia (maropitant citrate), Zoetis Inc., Kalamazoo, MI 49007, Made in Brazil) (1 mg/kg, IV every 24 h), and Lactated Ringers Solution (Hospira Inc.) (80 mL/kg/day). A pulse oximetry was not able to be obtained due to dyspnea, patient noncompliance, and pigmentation.
By the following day, the dog looked mildly improved with regard to respiratory rate and effort, but remained oxygen‐dependent. The plan was to pursue a CT of the chest and abdomen once the dog was stabilized; however, later in the day, the dog's mucous membranes became gray, and his breathing became labored with orthopnea. The dog was euthanized at the owner's request.
A necropsy was performed within 24 h of euthanasia. The abdominal segment of the caudal vena cava had marked aneurysmal dilation (Fig. 2) immediately caudal to the diaphragm, extending a length of 8 cm, which was filled with coagulated blood and fibrin. The aneurysmal vessel was stenotic at the level of the diaphragm and continued through the aortic hiatus along the right ventral aspect of the vertebral column as the right azygos vein, to join the cranial vena cava prior to entering the heart. The thoracic portion of the caudal vena cava could be traced from the right atrium, through the diaphragm (at the level of the caval hiatus), to the liver, but no tributaries from the abdominal cavity could be identified caudal to the liver.
Figure 2.
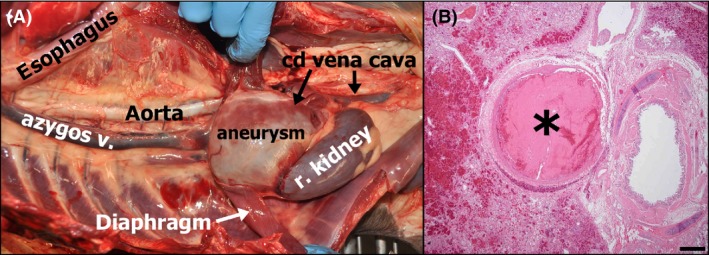
(A) Gross photograph of the thoracoabdominal cavity with most viscera removed, illustrating continuation of the abdominal caudal vena cava (with aneurysm) through the diaphragm as the azygos vein. The thoracic portion of the caudal vena cava, which terminated at the level of the liver, is not pictured as it was removed with the heart. (B) Photomicrograph of the lung depicting an occlusive pulmonary arterial thrombus (asterisk). A bronchus is located to the right of the thrombosed vessel, and hemorrhage is apparent in adjacent parenchyma to the left. Hematoxylin and eosin. Bar = 500 microns.
Grossly and microscopically in all remaining lung lobes, fibrin thrombi occluded pulmonary arterial branches (Fig. 2). Some affected vessels had spindle cell proliferation into the vascular lumen suggesting chronicity.
Discussion
Anomalies of the caudal vena cava in both humans and dogs are rare and are typically incidental findings appreciated during a diagnostic workup for an unrelated problem. The embryologic basis for azygos continuation of the caudal vena cava with a segmental aneurysm has been described in detail elsewhere 9, 14. Briefly, it is thought that the adult caudal venous system, specifically the caudal vena cava and azygos vein, develops after a series of changes in three embryonic veins: umbilical, vitelline, and caudal cardinal. Disruption of this embryologic process can change the communication of the five different segments of the caudal vena cava (prehepatic, hepatic, posthepatic, prerenal, and renal), and in this case, it is likely that the prehepatic portion did not form appropriately. Another embryonic vessel was then likely retained, forming the azygos vein continuation 9, 14. The diagnosis in this dog was made by means of an abdominal ultrasound with necropsy confirmation. The typical imaging modality for diagnosis of vascular anomalies in humans is CT angiography 5, 15.
In general, vascular anomalies are thought to be asymptomatic; however, there are a number of reports of an association between deep vein thrombosis and caudal vena cava anomalies in people as well as a single report of a PTE associated with the same vascular anomaly 14. There is a single case report of a dog with a similar vascular anomaly developing ascites that resolved after thrombectomy and anticoagulant therapy 16. The relationship between abdominal vascular anomalies and thrombus formation is thought to stem from a predilection toward venous stasis 17. In this dog, there was evidence of a large thrombus within the aneurysm. Given its downstream location, it seems reasonable that the aneurysmal thrombus was the source of subsequent PTE formation. It is also possible that this dog's recent surgical and anesthetic events lead to the formation of PTE, as surgery has been reported as a predisposing risk factor for development of PTE in dogs 18.
Lung lobe torsions are also quite unusual in dogs. They typically result from increased mobility of a particular lung lobe, usually the right middle, and twisting of the lobe at its bronchovascular pedicle 19. This results in enlargement secondary to congestion of the lobe from the continued arterial blood supply and complete venous obstruction 20. Radiographic signs usually include pleural effusion, enlargement and consolidation of the twisted lung lobe, presence of air bronchograms, and/or a stippled or liver‐like appearance resulting from trapped gas within the alveoli 12, 19, 20. Air bronchograms are often only appreciated early in the disease process. The trapped air can reabsorb over time, and/or the bronchi can fill with fluid, sometimes making a definitive radiographic diagnosis challenging 20. Radiographs from the primary care veterinarian in this case exhibited some of the common findings of lung lobe torsion including pleural effusion, vesicular gas pattern, and consolidation of the twisted lung lobe (Fig. 1).
It was suspected in this case that the lung lobe torsion was chronic. This is supported by the chronic nature of the clinical signs and the presence of granulation tissue on histopathology of the excised lung lobe. Common microscopic findings for twisted lung lobes in dogs include hemorrhage, necrosis, and thrombosis, all of which were present in this case 21. While it is possible given the chronicity of some of the thrombi seen postmortem, there is no way to know whether the dog's lung lobe torsion was secondary to thrombus formation and the subsequent anatomic changes (deflation, necrosis, and development of effusion) that would facilitate the torsion of an organ in a confined space. Lung lobe torsion secondary to thrombosis has not been reported in the human or dog.
Reported causes and predisposing factors for lung lobe torsions in dogs include trauma, previous thoracic or abdominal surgery, pleural effusion, pneumothorax, pneumonia, and body conformation 21. However, in many cases, the lung lobe torsion is spontaneous and idiopathic 21. This patient had a deep‐chested conformation supporting the theory of an idiopathic etiology. Given the similarity of clinical signs both prior to and twelve days following surgery, it is possible that the patient's clinical signs were attributed to PTEs the entire time and that the lung lobe torsion was unrelated. The presence of multiple chronic and acute pulmonary thrombi in multiple lung lobes postmortem as well as vascular compromise of the excised twisted lung lobe, however, may suggest the possibility that prior thrombosis contributed to the circumstances that led to the lung lobe torsion. Further workup for thrombotic risk factors prior to surgical correction of lung lobe torsion can be considered. Following the logic of Virchow's triad, evaluating patients for regions of blood stasis (i.e., vascular anomalies, cardiac disease, splenic disease, or prior thrombosis), hypercoagulable state (i.e., the presence of neoplasia, hypothyroidism, protein‐losing nephropathy, and hyperadrenocorticism), or endothelial damage (i.e., prior trauma or vasculitis) is reasonable so that steps can be taken to ameliorate thrombotic risk as surgery in itself may increase the odds of catastrophic thrombosis as occurred in the case presented here 18, 22, 23, 24, 25.
In general, the long‐term prognosis for lung lobe torsion is fair 21. Outcomes of twenty‐two dogs with lung lobe torsions were investigated, and it was found that all dogs undergoing surgery survived the procedure, but only half of those dogs had an uneventful recovery with four of the twenty‐two dying or being euthanized within fourteen days of surgery 21.
Conclusion
This is the first documented case of lung lobe torsion and pulmonary thromboembolism associated with azygos continuation of the caudal vena cava with a segmental aneurysm in a dog. This case suggests possible consideration of a complete diagnostic evaluation for risk factors of thrombosis in cases of lung lobe torsion, specifically in unusual breeds, with the hope that identifying such risk factors would allow for treatment that might prevent a life‐threatening complication such as pulmonary thromboembolism.
Authorship
AL: conceived and designed the research, analyzed and interpreted the data, and drafted the manuscript and revised critically. VS‐S: conceived and designed the research, analyzed and interpreted the data, and drafted the manuscript and revised critically. PM: analyzed and interpreted the data, revised critically, captured gross anatomic and histologic images. ST: analyzed and interpreted the data and revised critically.
Conflict of Interest
None declared.
Clinical Case Reports 2018; 6(2): 363–369
The results of this article have not been presented at a scientific meeting.
References
- 1. Alicioglu, B. , Kaplan M., and Ege T.. 2009. Absence of infrarenal inferior vena cava is not a congenital abnormality. Bratisl. Lek. Listy 110:304–306. [PubMed] [Google Scholar]
- 2. May, N. D. S. 1960. Absence of the prerenal segment of the posterior vena cava of the dog. Aust. Vet. J. 36:67–68. [Google Scholar]
- 3. Laborda, J. , Gimeno M., Dominguez L., and Gill J.. 1996. Anomalous caudal vena cava in the dog. Vet. Rec. 138:20–21. [DOI] [PubMed] [Google Scholar]
- 4. Barthez, P. Y. , Siemens L. M., and Koblik P. D.. 1996. Azygos continuation of the caudal vena cava in a dog: radiographic and ultrasonographic diagnosis. Vet. Radiol. Ultrasound. 37:354–356. [Google Scholar]
- 5. Schwarz, T. , Rossi F., Wray J. D., Ablad B., Beal M. W., Kinns J., et al. 2009. Computed tomographic and magnetic resonance imaging features of canine segmental caudal vena cava aplasia. J. Small Anim. Pract. 50:341–349. [DOI] [PubMed] [Google Scholar]
- 6. Hunt, G. B. , Bellenger C. R., Borg R., Youmans K. R., Tisdall P. L., and Malik R.. 1998. Congenital interruption of the portal vein and caudal vena cava in dogs: six case reports and a review of the literature. Vet. Surg. 27:203–215. [DOI] [PubMed] [Google Scholar]
- 7. Fischetti, A. J. , and Kovak J.. 2008. Imaging diagnosis: Azygous continuation of the caudal vena cava with and without portocaval shunting. Vet. Radiol. Ultrasound 49:573–576. [DOI] [PubMed] [Google Scholar]
- 8. Weir, E. C. 1970. Venous Anomalies in the Abdomen of a Dog. Vet. Rec. 86:582. [DOI] [PubMed] [Google Scholar]
- 9. Konopka, C. L. , Salame M., Padulla G. A., Muradás R. R., and Batistella J. C.. 2010. Agenesis of inferior vena cava associated with deep venous thrombosis. J. Vasc. Bras. 9:196–199. [Google Scholar]
- 10. Hamoud, S. , Nitecky S., Engel A., Goldsher D., and Hayek T.. 2000. Hypoplasia of the inferior vena cava with azygous continuation presenting as recurrent leg deep vein thrombosis. Am. J. Med. Sci. 319:414–416. [DOI] [PubMed] [Google Scholar]
- 11. Lambert, M. , Marboeuf P., Midulla M., Trillot N., Beregi J. P., Mounier‐Vehier C., et al. 2010. Inferior vena cava agenesis and deep vein thrombosis: 10 patients and review of the literature. Vasc. Med. 15:451–459. [DOI] [PubMed] [Google Scholar]
- 12. Davies, J. A. , Snead E. C., and Pharr J. W.. 2011. Tussive syncope in a pug with lung‐lobe torsion. Can. Vet. J. 52:656. [PMC free article] [PubMed] [Google Scholar]
- 13. Dhumeaux, M. P. , Snead E. C., Epp T. Y., Taylor S. M., Carr A. P., Dickinson R. M., et al. 2012. Effects of a standardized anesthetic protocol on hematologic variables in healthy cats. J. Feline Med. Surg. 14:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho, B. C. , Choi H. J., Kang S. M., Chang J., Lee S. M., Yang D. G., et al. 2004. Congenital absence of inferior vena cava as a rare cause of pulmonary thromboembolism. Yonsei Med. J. 45:947–951. [DOI] [PubMed] [Google Scholar]
- 15. Bass, J. E. , Redwine M. D., Kramer L. A., Huynh P. T., and Harris J. H. Jr. 2000. Spectrum of congenital anomalies of the inferior vena cava: cross‐sectional imaging findings. Radiographics 20:639–652. [DOI] [PubMed] [Google Scholar]
- 16. Harder, M. A. , Fowler D., Pharr J. W., Tyron K. A., and Shmon C.. 2002. Segmental aplasia of the caudal vena cava in a dog. Can. Vet. J. 43:365–368. [PMC free article] [PubMed] [Google Scholar]
- 17. Chee, Y. L. , Culligan D. J., and Watson H. G.. 2001. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br. J. Haematol. 114:878–880. [DOI] [PubMed] [Google Scholar]
- 18. Goggs, R. , Benigni L., Fuentes V. L., and Chan D. L.. 2009. Pulmonary thromboembolism. J. Vet. Emerg. Crit. Care. 19:30–52. [DOI] [PubMed] [Google Scholar]
- 19. White, R. N. , and Corzo‐Menendez N.. 2000. Concurrent torsion of the right cranial and right middle lung lobes in a whippet. J. Small Anim. Pract. 41:562–565. [DOI] [PubMed] [Google Scholar]
- 20. Breton, L. , DiFruscia R., and Oliveri M.. 1986. Successive torsion of the right middle and left cranial lung lobes in a dog. Can. Vet. J. 27:386–388. [PMC free article] [PubMed] [Google Scholar]
- 21. Neath, P. J. , Brockman D. J., and King L. G.. 2000. Lung lobe torsion in dogs: 22 cases (1981‐1999). J. Am. Vet. Med. Assoc. 217:1041–1044. [DOI] [PubMed] [Google Scholar]
- 22. Williams, T. P. , Shaw S., Porter A., and Berkwitt L.. 2017. Aortic thrombosis in dogs. J. Vet. Emerg. Crit. Care. 27:9–22. [DOI] [PubMed] [Google Scholar]
- 23. Elliott, M. A. , Pardanani A., Lasho T. L., Schwager S. M., and Tefferi A.. 2010. Thrombosis in myelofibrosis: prior thrombosis is the only predictive factor and most venous events are provoked. Haematologica 95:1788–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cushman, M. 2007. Epidemiology and risk factors for venous thrombosis. Semin. Hematol. 44:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomasson, G. , Monach P. A., and Merkel P. A.. 2009. Thromboembolic disease in vasculitis. Curr. Opin. Rheumatol. 21:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


