Abstract
Introduction
Long non-coding RNA (lncRNA) plays a key role in various disorders. However, its role in keloid is still unclear.
Aim
We explored differentially expressed (DE) lncRNAs and mRNAs between keloid tissue (KT)s and normal tissue (NT)s, as well as keloid fibroblast (KFB)s and normal fibroblast (NFB)s, respectively.
Material and methods
We use KTs and NTs from the chest of 5 patients, and 3 pairs of KFBs and NFBs, to perform microarray respectively. Gene ontology and pathway analyses were conducted by online software DAVID (Database for Annotation, Visualization and Integrated Discovery). The validation of targeted lncRNAs were conducted by qRT-PCR in enlarged samples (79 KTs and 21 NTs).
Results
We identified 3680 DE-lncRNAs in tissue essay, and 1231 DE-lncRNAs in cell essay. Furthermore, we found that many lncRNAs and their relative mRNAs were regulated simultaneously in keloid. We identified that ENST00000439703 and uc003jox.1 were up-regulated in both of the above essays through comparing the results of lncRNA screening between tissue essay and cell essay; the results were confirmed through qRT-PCR in enlarged samples.
Conclusions
Our study demonstrates that numerous lncRNAs are involved in the pathogenesis and development of the keloid.
Keywords: long non-coding RNA, keloid, fibroblast, mRNA, pathway
Introduction
Keloid is a type of benign tumor unique to human skin, and is formed during an abnormal wound healing process [1]. The quality of life of the patients associated with keloid is dramatically impaired [2]. It is characterized by pathological accumulation of extracellular matrix (ECM) such as collagen, fibronectin and α-smooth muscle actin, angiogenesis, and inflammatory cell infiltration [3]. Until now, the pathogenesis of keloid has been unclear. In previous studies, Chen et al. [4] reported that 402 genes were expressed differentially between keloid and normal tissue, including 250 up-regulated and 152 down-regulated genes which involved in the cellular skeleton and movement, cellular cycle modulation, metabolism and signal transduction factor. Additionally, Russell et al. [1] identified that several fibrosis-associated genes were deregulated in keloid. Importantly, several groups discovered that mTOR signaling is closely correlated with keloid formation, and the mTOR inhibitors possess therapeutic efficacy in in vitro and ex vivo studies [5].
Long non-coding RNA (lncRNA) is a subgroup of non-coding RNA, defined as longer than 200 nucleotides with limited or without protein-coding capacity. Recently, some lncRNAs were indentified to involve developmental processes and be associated to various disorders, particularly in cancer [6]. Noticeably, Liang et al. [7] reported 1731 up-regulated lncRNAs and 782 down-regulated lncRNAs in keloid lesions compared with normal skin tissue. They indicate that lncRNA CACNA1G-AS1-regulated mRNA which encodes a subtype of T-type Ca2+ channels may be the important machinery for the keloid formation. In addition, Zhu et al. [8] reported that lncRNA-ATB regulated the secretion of TGF-β2 in keloid fibroblast (KFB)s by partly down-regulating the ZNF217 expression level via miR-200c, and demonstrated that lncRNA-ATB/miR-200c/ZNF217/TGF-β2 signaling axis involved the initiation and progression of keloid. lncRNA H19 was reported to participate in the proliferation of keloid fibroblasts [9]. However the role of lncRNA in keloid remains to be clarified.
Aim
To clarify preliminarily which lncRNAs were involved in keloid and their underlying role in pathology.
Material and methods
Source of samples
This study has been approved by the Ethics Committee Board of the Institute of Dermatology, Chinese Academy of Medical Science and Peking Union Medical College. The written consent was obtained from all the involved patients before the surgery. The patients recruited in this study had not been pretreated for keloid at least 3 months before this operation, from which the keloid and normal tissue samples (adjacent normal tissue of peri-keloid lesion resected together with the keloid lesion) were obtained. The keloid and normal tissue samples were detached and identified by the surgeons who performed the corresponding operation. All the samples of the keloid were confirmed by histopathologic analysis. The adipose tissues of all samples were removed, and then immediately frozen by liquid nitrogen after resection. All samples were stored at –80°C until the performance of the microarray.
We considered the following details in this study design: (i) samples were obtained from the same skin location (chest) of 5 patients; (ii) lesional and normal tissues from individual patients served as the tested samples and the own control respectively; (iii) only the adipose tissue was removed from both samples, and the epidermis was reserved in the final sample for microarray analysis. We removed the adipose tissue but reserved the epidermis, as the fibroblast-keratinocyte cross talk was verified as a crucial mechanism in keloid pathogenesis in previous studies, for example the studies of Funayama et al. [10], Lim et al. [11] and Canady et al. [12], etc.
Cell culture
Keloid fibroblasts were isolated from intralesional tissues of keloid, and normal-fibroblast (NFB)s after the healthy men’s circumcision. The passage 1 (p1) to passage 2 (p2) cells were used in this study. Freshly resected specimens were removed of adipose tissue and washed in PBS three times. Then, the reserved tissues were cut into 3 mm × 8 mm × 5 mm tissue blocks and incubated in 5 mg/ml dispase II (D4693, Sigma-Aldrich, St. Louis, MO, USA) for 4 h at 37°C to exclude epidermis. After washing tissues with PBS again, the blocks were cut into 2 mm × 2 mm × 2 mm and incubated in 3 mg/ml collagenase, type I (17100017, Thermo Fisher Scientific, Waltham, MA, USA) at 2 h at 37°C. Then the tissue blocks were blown, and suspension filtrated through cell strainer. Cells were cultured in a sterile flask with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen Corp., Carlsbad, CA, USA) containing penicillin (100 U/ml), streptomycin (100 mg/ml) and 10% fetal bovine serum within the incubator at 37°C and 5% CO2. The medium was changed every 3 days. When fibroblasts grew to confluent, they were digested with 0.25% trypsin and sub-cultured in fresh medium at a 1 : 2 split radio.
Microarray profiling
Total RNAs were extracted from the samples using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. NanoDrop ND-1000 (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the quality of RNAs. The integrity of RNAs was measured by the standard denaturing agarose gel electrophoresis. The Human LncRNA Expression Microarray version 3.0 (ArrayStar, Inc., Rockville, MD, USA) was used in this study according to the manufacturer’s instructions. The 30,586 lncRNAs and 26,109 coding genes were included in this microarray. The lncRNAs are constructed using the most highly respected public transcriptome databases (Refseq, UCSC knowngenes, Gencode, etc), as well as the landmark publications. Each transcript was represented by a specific splice junction probe or exon probe which can identify accurately unique transcript. Housekeeping genes served as the positive probes, and negative probes were also printed on the array to control the hybridization quality.
RNA labeling and array hybridization
The sample labeling and array hybridization were taken following the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technologies, Santa Clara, CA, USA) with minor modifications. Briefly, the purification of the mRNA from total RNA was performed by removing rRNA (mRNA-ONLY™ Eukaryotic mRNA Isolation Kit, Epicentre Biotechnologies, Madison, WI, USA). Then, all samples were amplified and transcribed into fluorescent cRNA along the entire length of the transcripts without 3’ bias utilizing a random priming method (Agilent p/n 5190-0442). The labeled cRNA was purified by RNeasy Mini Kit (Qiagen p/n 74104, Valencia, CA, USA). NanoDrop ND-1000 was used to assess the concentration and specific activity of the labeled cRNA (pmol Cy3/ugc RNA). Agilent Gene Expression Hybridization Kit (Agilent p/n 5188-5242) was taken for the hybridization. Firstly, 5 ml 10×blocking agent and 1 ml 25× fragmentation buffer were added to 1 mg of each labeled cRNA. Secondly, the mixtures were heated at 60°C for 30 min. Lastly, 25 ml 2 × GE Hybridization buffer was added to dilute the labeled cRNA. The 50 ml of hybridization solution was dispensed into the gasket slides and assembled to the lncRNA microarray slides. The slides were incubated in an Agilent Hybridization Oven (Agilent p/nG2545A) for 17 h at 65°C. After hybridization, washing and fixation, the processed slides were scanned using the Agilent DNA Microarray Scanner G2505C.
Data analysis
The images were processed with Agilent Feature Extraction software (v11.0.1.1). The raw data’s quantile normalization and further data analysis were performed with the GeneSpring GX (v12.1) software package (Agilent Technologies). The quantile normalization of the raw data is to make the distribution of probe intensities identical to each array. The probe quality was marked with ‘present’, ‘marginal’ and ‘absent’ according to their levels of intensity from strong to poor. After the quantile normalization, lncRNAs and mRNAs that have been identified by ‘present’ or ‘marginal’, at least 5 out of 10 samples were chosen for subsequent data processing. The selection criterion of differentially expressed lncRNAs and mRNAs was p-value < 0.05 and fold change ≥ 2. The differentially expressed lncRNAs and mRNAs between keloid and normal tissues were shown through volcano plot filtering and hierarchical clustering analysis (MeV 4.8) was performed to demonstrate the lncRNAs and mRNAs expression patterns among samples. The microarray analysis was assisted by Kangcheng Biology Engineering Co., Ltd., (Shanghai, China).
Currently, lncRNAs were found to exert their function via regulating associated or nearby mRNA. According to genomic location and context, lncRNAs are classified into different subgroups including intergenic, bi-directional, sense and antisense lncRNAs. Long intergenic non-coding RNAs (lincRNAs) and antisense lncRNAs have attracted a lot of attention because of recruiting chromatin modification complexes [13]. In this study, we defined sense lncRNAs, antisense lncRNAs and bidirectional lncRNAs as the genic lncRNAs for analysis of the interplay between lncRNAs and mRNAs. Additionally, lincRNAs and enhancer-like lncRNAs, a class of lncRNAs with an enhancer-like function in activation of gene expression [14], were also screened to detect the interplay between them and their associated mRNAs.
Gene ontology and pathway analysis of differential expression genes
Gene ontology (GO) analysis and pathway analysis were performed to investigate the potential functions of the differentially expressed mRNAs in GO terms or biological pathways. The GO project can provide a controlled vocabulary to describe gene and gene products in any organism. Gene ontology analysis includes biological process (BP), cellular component [15] and molecular function (MF). The Fisher’s exact test is applied for avoiding overlap between the differentially expressed gene list and the GO annotation list. The significance of GO terms enrichment in the differentially expressed gene list is indicated by p-value. Pathway analysis, a functional analysis which maps genes to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways was used to identify the active pathways based on differentially expressed genes. The significance of the pathway associated with the conditions is shown by enrichment scores calculated through p-value (Fisher p-value). Gene ontology and pathway analyses were conducted by online software DAVID (Database for Annotation, Visualization and Integrated Discovery).
Quantitative reverse transcription PCR
The extraction and concentration detection for total RNA were carried out according to the manufacturer’s protocol. Then, the RNA was reverse-transcribed to complementary DNA (cDNA) using the SuperScript TM III Reverse Transcriptase kit (Invitrogen; Thermo Fisher Scientific, Inc., CA, USA). Additionally, quantitative reverse transcription PCR (qRT-PCR) reactions were performed on ViiA 7 Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 10 s and 60°C for 1 min. The expression levels of uc003jox.1, ENST00000439703 and β-actin were detected at least in triplicate. The primers were designed using Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA, USA) and synthesized by Kangcheng Biology Engineering Co., Ltd. The expression levels of both lncRNAs were calculated through 2– Δ CT formula; ΔCT is the difference between the target lncRNA and β-actin.
The primers sequences were as follows
uc003jox.1:
forward 5’-TGGGACACTATCAGCAACTACG-3’
reverse 5’-GGTGAGGTTGGCGATTGTT-3’
ENST00000439703:
forward 5’-AAAGCAGAAGACACTGGTTGAGC-3’
reverse 5’-GCAGGTTTTGTTGCGAAGTG-3’
β-actin:
forward 5’- GTGGCCGAGGACTTTGATTG-3’
reverse 5’- CCTGTAACAACGCATCTCATATT-3’.
Statistical analysis
Each experiment was repeated at least three times, and quantitative data were presented as mean and standard deviation (mean ± SD). Paired t-test was used to analyze the lncRNA expression differences between the keloid tissues and normal tissues; Student’s t-test was used to analyze the lncRNA expression differences between the keloid-derived fibroblasts and normal fibroblasts; Student’s t-test was also used to analyze the expression differences of uc003jox.1, ENST00000439703 and β-actin between keloid tissues and normal tissues in qRT-PCR assay, and p-value < 0.05 was considered as a significant difference. All the analyses were performed using SPSS 16.0 software.
Results
In this study, we firstly explore the differential expression of lncRNAs and mRNAs between keloid tissue (KT) and normal tissue (NT) on the chest from each of 5 patients (Table 1) using high-throughput lncRNA array. The profiling of differentially expressed (DE)-lncRNAs and DE-mRNAs is shown in Figures 1 A–D. From the microarray data, 3680 of DE-lncRNAs and 5448 DE-mRNAs (Supplementary tables) were identified in the KTs compared to NTs (fold change ≥ 2 and p < 0.05). Among the DE-lncRNAs, 2238 were up-regulated, and 1442 were down-regulated.
Table 1.
The table lists the details of clinical samples, including keloid tissue and normal tissue (KT and NT respectively)
| Patient | Status | Sex | Age [years] | Location |
|---|---|---|---|---|
| KT1 | Keloid tissue | M | 53 | Chest |
| KT2 | Keloid tissue | F | 61 | Chest |
| KT3 | Keloid tissue | F | 23 | Chest |
| KT4 | Keloid tissue | M | 23 | Chest |
| KT5 | Keloid tissue | M | 16 | Chest |
| NT1 | Normal tissue | M | 53 | Chest |
| NT2 | Normal tissue | F | 61 | Chest |
| NT3 | Normal tissue | F | 23 | Chest |
| NT4 | Normal tissue | M | 23 | Chest |
| NT5 | Normal tissue | M | 16 | Chest |
Figure 1.
Hierarchical clustering analysis of differentially expressed lncRNAs (A) and mRNAs (C) between KT and NT. In the heat map, the column indicates a sample, and the row indicates a gene. The samples are arranged into the groups based on the expression level. The green and red colors are referred to the low and high expression levels of the genes, respectively. The expression profile of lncRNA (B) and mRNA (D) in the KT compared with the NT are shown in the volcano plot. The horizontal green line represents p-value of 0.05 and the vertical green lines represent 2.0-fold up and down. The red points represent differentially expressed lncRNA with statistical significance
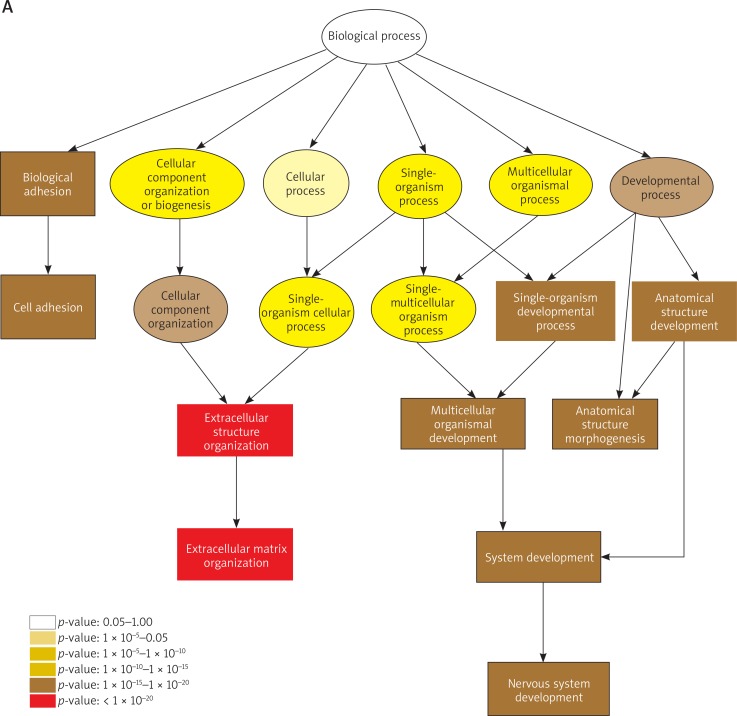
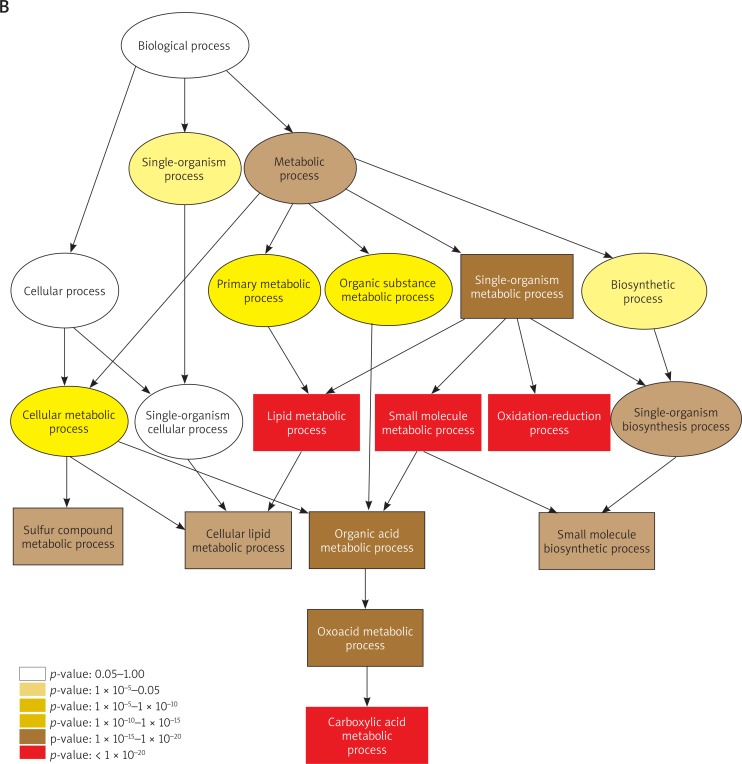
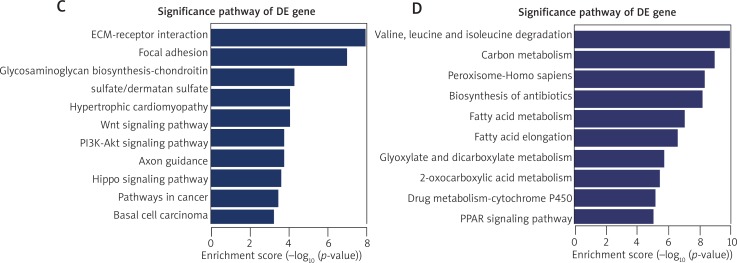
Furthermore, 2526 up-regulated and 2922 down-regulated DE-mRNAs were confirmed in the KT compared to NT (Figure 1 D). First, GO analysis was performed to identify the potential collective genes which contribute to keloid formation. The results of GO analysis in the biological process are shown in Figures 2 A, B. The most significantly up-regulated transcripts were involved in the extracellular structure organization and extracellular matrix organization. Meanwhile, the most significantly down-regulated transcripts were involved in the lipid metabolic process, small molecule metabolic process, oxidation-reduction process and carboxylic acid metabolic process. Secondly, the pathway analysis was performed to survey the systematic protein coding gene function. Our data revealed that 53 and 60 pathways contained up-regulated and down-regulated DE-mRNAs, respectively. The top 10 active pathways in up-regulated and down-regulated pathways are listed in Figures 2 C and D, respectively. The most enriched pathways include ECM-receptor interaction, and valine, leucine and isoleucine degradation respectively. Data on GO analysis and pathway analysis are approximately consistent with the previous study [7].
Figure 2.
Gene ontology (GO) analysis of the up-regulated (A) and down-regulated (B) differentially expressed mRNAs was performed in the biological process (BP). Significant GO terms are classified into the various colored circles or squares in the BP tree according to the p-value. Each color represents a range of p-value. Pathway analysis was performed in the up-regulated (C) and down-regulated (D) DE-mRNAs and the top 10 active pathways are listed. The horizontal axis indicates the –log10 (p-value) and the vertical axis corresponds to the pathway category. The lower the p-value, the more significant is the pathway
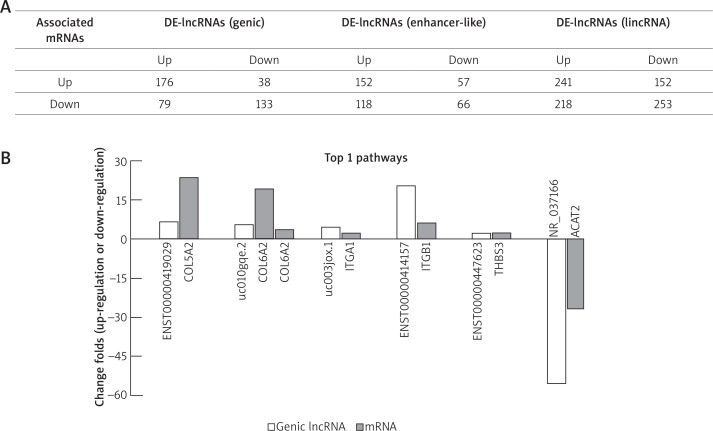
In this study, we defined sense lncRNAs, antisense lncRNAs and bidirectional lncRNAs as the genic lncRNAs. In the DE-lncRNAs database, 1628 genic lncRNAs have their coding genes, and we discovered that 426 of these coding genes remarkably altered expression levels in keloid. The 72.5% genic lncRNAs were regulated synergistically with their associated mRNAs, and the rest was in the opposite direction. Meanwhile, our data showed the synergetic differential expression of 393 enhancer-like lncRNAs and 864 long intergenic non-coding RNA (lincRNA)s with their nearby genes (Figure 3 A). Additionally, we screened the expression change folds of the mRNAs and their relative lncRNAs in the top 1 of active pathways in up-regulation and down-regulation. In the former group, COL5A2, COL6A2, ITGA1, ITGB1 and THBS3 mRNAs were reported to have relative lncRNA, including ENST00000419029, uc010gqe.2, uc003jox.1, ENST00000414157 and ENST00000447623, respectively, whereas, in the latter group, only ACAT2 has been identified to have its own one relative lncRNA, NR_037166. Intriguingly, we found that the mRNAs and their lncRNAs were up-regulated or down-regulated simultaneously (Figure 3 B).
Figure 3.
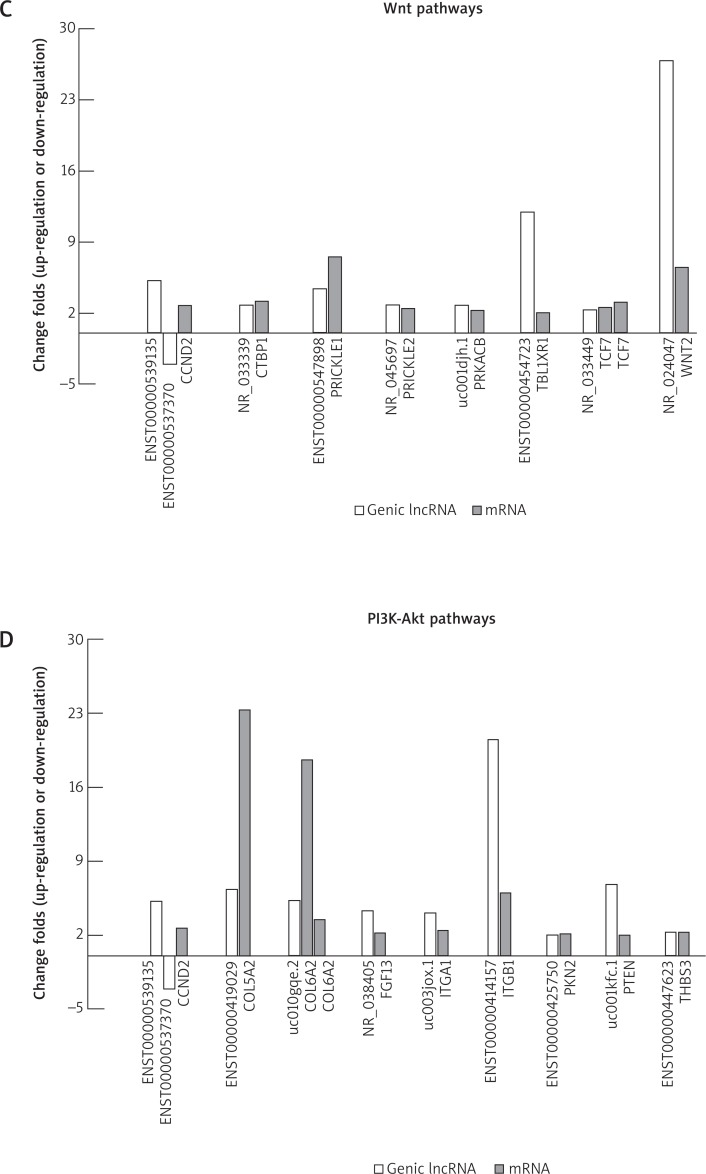
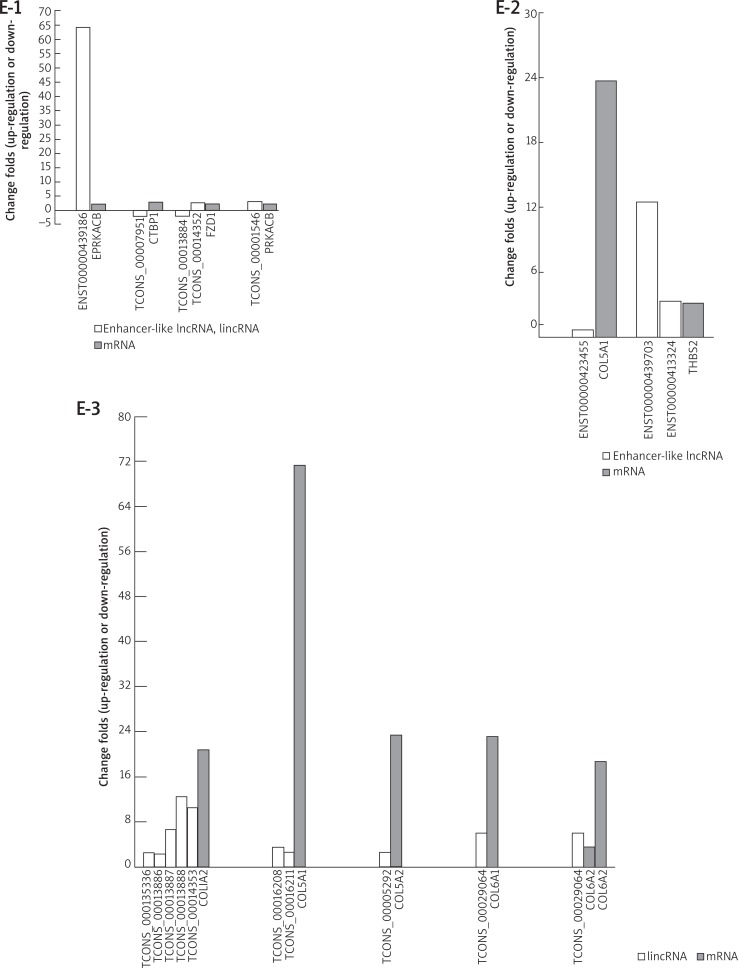
A – The genic lncRNAs and their associated mRNAs are identified. The genic lncRNAs includes exon sense, intron sense, intronic antisense, natural antisense and bidirectional lncRNA. The table lists the expression relationship of the 426 mRNAs which have been screened out and their associated genic lncRNAs (in the same or opposite direction). The correlations of enhancer-like lncRNAs or lincRNAs with their nearby mRNAs are also shown in this table. The relationships of DE-genic lncRNA-mRNA in top 1 active pathway (both of the up-regulated and down-regulated pathway), Wnt pathway and PI3K-Akt pathway are exhibited in (B), (C) and (D), respectively. In Wnt pathway, DE-mRNAs and relative differentially expressed enhancer-like lncRNAs and lincRNAs are shown in (E-1). In PI3K-Akt pathway, DE-mRNAs and their associated DE-enhancer-like lncRNAs and DE-lincRNAs are shown in (E-2) and (E-3), respectively. In the histogram, the vertical axis represents fold-change of up-regulation or down-regulation in the DE-genes between the KT and NT. Positive values and negative values present the up-regulation and down-regulation, respectively
Our findings showed that 8 kinds of DE-mRNAs in the Wnt pathway were changed along with the differential expression of their relative genic-lncRNAs, including NR_024047, ENST00000454723, etc. In the PI3K-Akt pathway, 9 kinds of DE-mRNAs have the change of their associated genic-lncRNAs such as ENST00000414157, uc001kfc.1, etc. (Figures 3 C, D). The relationships of DE-mRNAs and relative differentially expressed enhancer-like lncRNAs and lincRNAs in the Wnt pathway and the PI3K-Akt pathway are shown in Figures 3 E 1–3, suggesting that lncRNAs might contribute to the deregulation of the Wnt and PI3K-Akt-mTOR pathways in the keloid formation.
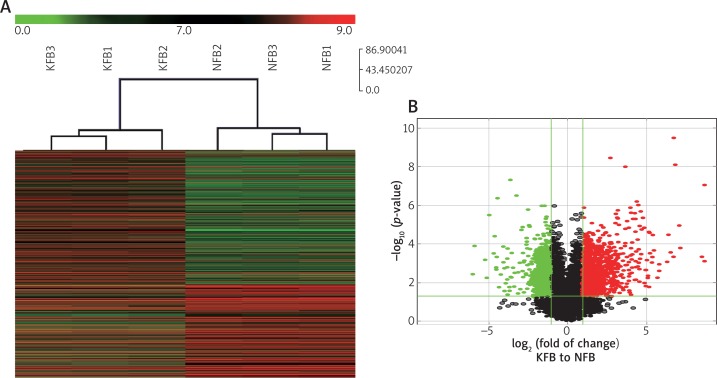
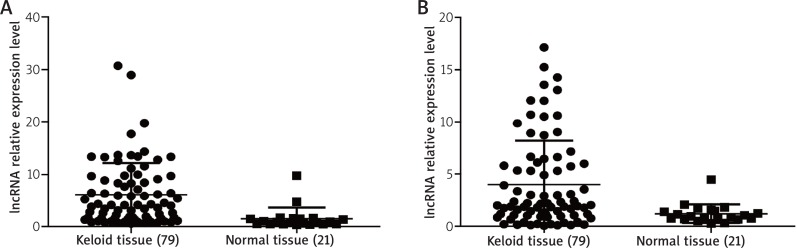
In addition, we detected differentially expressed lncRNAs in KFBs and NFBs through lncRNA microarray (Figure 4 A). Three KFBs and three NFBs were included during passages 1–2. We identified 738 up-regulated DE-lncRNAs and 493 down-regulated DE-lncRNAs in KFBs compared to NFBs (fold change ≥ 2 and p < 0.05) (Figure 4 B and Supplementary table). In comparison with the results of tissue microarray, 71 overlapped DE-lncRNAs were identified in the accordant regulation, suggesting that the deregulations of these lncRNAs were the stable events in the formation and development of keloid due to their permanent detection in both tissue and cell level. Among them, 59 were up-regulated; 12 were down-regulated (Table 2). Interestingly, some of overlapped DE-lncRNAs, such as uc003jox.1, ENST00000414157 and ENST00000439703, which are tightly associated with the PI3K-Akt pathway, were in accordance with the results of tissue assay. Furthermore, the up-regulation of uc003jox.1 and ENST00000439703 was validated by qRT-PCR in other samples of 79 KTs and 21 NTs (Figure 5 A, B).
Figure 4.
Expression profiles of differentially expressed lncRNAs in KFBs and NFBs. (A) Hierarchical clustering analysis of DE-lncRNAs was performed on 3 samples of KFBs and NFBs. From right to left there are samples of NFB1, NFB3, NFB2, KFB2, KFB1 and KFB3. The green and red colors are referred to the low and high expression levels of the genes, respectively. (B) In the volcano plot, the horizontal green line reveals p-value of 0.05 and the vertical green lines reveals 2.0-fold up and down. The red and green points refer to up-regulated and down-regulated DE-lncRNAs, respectively with statistical significance
Table 2.
Fold change of overlapped DE-lncRNAs between tissue microarray and cell microarray. The seqname, regulation result, regulated fold change in tissue samples and regulated fold change in cell samples are listed respectively
| Seqname | Regulation | Fold change (tissue) | Fold change (cell) |
|---|---|---|---|
| ENST00000419703 | Up | 6.6279183 | 29.5223065 |
| NR_015406 | Up | 6.127057 | 2.1462103 |
| ENST00000486545 | Up | 2.1611805 | 26.0017106 |
| NR_029408 | Up | 4.4565784 | 2.7067804 |
| NR_024277 | Up | 2.4244666 | 2.6440482 |
| NR_026880 | Up | 3.801088 | 2.2751523 |
| NR_033997 | Up | 149.2830172 | 5.8316748 |
| ENST00000478294 | Up | 9.4379243 | 4.4005833 |
| ENST00000567422 | Up | 2.4006793 | 2.5924725 |
| ENST00000458111 | Up | 5.4592087 | 3.7915979 |
| ENST00000544710 | Up | 3.7497856 | 3.36143 |
| ENST00000440688 | Up | 4.363394 | 2.0548057 |
| NR_040001 | Up | 12.0982995 | 3.4556154 |
| ENST00000538640 | Up | 5.3177373 | 3.4447074 |
| ENST00000439703 | Up | 37.5996003 | 2.7942808 |
| ENST00000502253 | Up | 4.1488472 | 8.4714346 |
| NR_026812 | Up | 3.2063431 | 7.2126622 |
| ENST00000510240 | Up | 6.5481183 | 2.8280585 |
| NR_024584 | Up | 2.6485533 | 2.1350618 |
| NR_024376 | Up | 30.7304332 | 7.5323829 |
| ENST00000428777 | Up | 2.6279418 | 2.2848405 |
| NR_037676 | Up | 5.8588119 | 3.1231713 |
| ENST00000425358 | Up | 5.4738734 | 2.4136129 |
| TCONS_00001798 | Up | 10.2987539 | 3.1307256 |
| ENST00000454470 | Up | 6.2799806 | 13.253207 |
| ENST00000568587 | Up | 2.4663634 | 3.8557262 |
| TCONS_00021754 | Up | 7.4893816 | 2.4426713 |
| uc001tgo.1 | Up | 3.2070159 | 2.1708516 |
| ENST00000435858 | Up | 4.8845392 | 2.3302421 |
| ENST00000565685 | Up | 2.2082968 | 13.6576358 |
| ENST00000441110 | Up | 18.7701319 | 6.6895967 |
| ENST00000442449 | Up | 4.5210899 | 2.1229493 |
| ENST00000519680 | Up | 3.8296695 | 41.6543223 |
| TCONS_00022133 | Up | 4.1459333 | 4.3731873 |
| uc001jxr.1 | Up | 13.9935921 | 2.2261205 |
| ENST00000555011 | Up | 2.4487763 | 3.0459493 |
| NR_045484 | Up | 2.5344549 | 2.3011443 |
| ENST00000419223 | Up | 2.1002016 | 3.9448574 |
| uc001elb.4 | Up | 2.9022957 | 3.0025765 |
| ENST00000522970 | Up | 13.354998 | 6.5806575 |
| NR_002835 | Up | 3.4703051 | 2.6761612 |
| NR_033203 | Up | 2.9062432 | 12.4014734 |
| ENST00000414157 | Up | 20.5191141 | 3.3653084 |
| ENST00000420762 | Up | 4.4536074 | 11.8442702 |
| uc003whs.1 | Up | 8.3941298 | 16.8886215 |
| ENST00000568976 | Up | 14.6488443 | 9.2075056 |
| NR_024606 | Up | 3.374096 | 4.3664496 |
| uc003jox.1 | Up | 4.1420907 | 2.2874195 |
| ENST00000415236 | Up | 2.0476512 | 2.1363844 |
| ENST00000503006 | Up | 2.990847 | 2.6190821 |
| uc001vvu.3 | Up | 11.8209182 | 34.8693887 |
| ENST00000562983 | Up | 4.5531966 | 2.4191555 |
| ENST00000520024 | Up | 2.4325546 | 3.3738865 |
| ENST00000562575 | Up | 20.1567271 | 4.6671655 |
| NR_024511 | Up | 2.7578358 | 2.6654016 |
| NR_024366 | Up | 10.8398681 | 8.0423688 |
| AL049990 | Up | 5.1490601 | 17.3778352 |
| ENST00000583262 | Up | 2.1512159 | 2.2956871 |
| ENST00000563647 | Up | 17.5689738 | 2.3271887 |
| ENST00000441146 | Down | 2.2789757 | 2.3304502 |
| NR_024420 | Down | 4.3697563 | 3.4260364 |
| ENST00000519013 | Down | 2.3895936 | 2.3447347 |
| NR_026914 | Down | 2.6868538 | 2.086728 |
| TCONS_00005763 | Down | 4.1855274 | 3.4774865 |
| ENST00000578585 | Down | 2.3118484 | 4.4383912 |
| ENST00000433310 | Down | 2.2115708 | 22.4760384 |
| ENST00000436033 | Down | 3.1542324 | 2.5350929 |
| uc004afc.3 | Down | 2.2409343 | 2.3957733 |
| uc021pbg.1 | Down | 4.3768514 | 5.8841137 |
| ENST00000517495 | Down | 3.1341935 | 15.7814628 |
| ENST00000431557 | Down | 2.9071502 | 2.4842267 |
Figure 5.
The validation of lncRNA ENST00000439703 (A) and uc003jox.1 (B) in keloid lesions and normal tissues through qRT-PCR. The y-axis represents a relative expression level of lncRNA, b-actin serves as the housekeeping gene (p < 0.05)
Discussion
In this study, we have shown a number of lncRNAs and mRNAs differentially expressed between keloid and control in either tissue microarray or cell microarray, and 71 overlapped DE-lncRNAs were identified in accordant regulation in both assays. Importantly, the 72.5% genic lncRNAs were regulated synergistically with their associated mRNAs, and the rest was in the opposite direction in keloid tissue. Finally, the up-regulation of PI3K-Akt pathway associated lncRNAs uc003jox.1 and ENST00000439703, which were screened out through tissue microarray and cell microarray, were validated by qRT-PCR in other samples of 79 KTs and 21 NTs.
Recently, quantities of lncRNAs have been reported to involve cancer formation and development [16]. For instance, Sun et al. [17] reported that lncRNA HOXA11-AS promoted proliferation of gastric cancer through modulating chromatin modification factors. The studies of Liang et al. [7], Zhu et al. [8] and Zhang et al. [9] already indicated that some lncRNAs play an important role in keloid. In addition, Li et al. [18] reported that lncRNA8975-1 was up-regulated fibroblast from hypertrophic scar and involved collagen expression. Based on our findings that a great deal of lncRNAs was deregulated in KT and KFBs, our study demonstrates that numerous lncRNAs are involved in the pathogenesis and development of the keloid. However, the actual functions of numerous lncRNAs are still elusive. Therefore, the mechanism of lncRNA-mRNA interaction in keloid is needed to be clarified, especially the interplay between lncRNAs and mRNAs in the pathways closely related with keloid.
Our data showed that the Wnt and PI3K-Akt signaling pathways were included in high-enrichment pathways of up-regulation; it coincides with the previous report of Smith et al. [19] that multiple genes of the Wnt pathway were misexpressed in fibroblast detached from fibroblast lesion. Sato also observed an increase in β-catenin protein, the crucial modulator of Wnt signaling, in KTs [20]. Moreover, involvement of PI3K-Akt-mTOR axis was identified in keloid pathogenesis. For example, treatment of mTOR complex 1 and 2 inhibitors resulted in suppression of Akt and mTOR signaling and inhibition of the proliferation, migration and invasion of the keloid cell [5]. Additionally, Zhang et al. [21] also found that the green tea extract inhibited type I collagen production by interfering with the PI3K-Akt-mTOR pathway in KFBs.
In this preliminary study, there are the following limitations. We have not performed any functional study about specific lncRNA. In addition, the actual mechanism by which lncRNAs participated in the pathogenesis and development of keloid remains to be clarified.
Conclusions
Our study demonstrates that numerous lncRNAs are involved in the pathogenesis and development of the keloid, and most of them (e.g. uc003jox.1 and ENST00000439703) were deregulated in a synergism with their associated mRNAs. Our study provides a new sight to the role of lncRNA in pathogenesis of keloid.
Acknowledgments
Acknowledgments
This study was supported by CAMS Innovation Fund for Medical Sciences (2017-12M-1-017 and 2016-12M-1-005).
This work was supported by grants from the National Natural Science Foundation of China (No. 81371755, 81673083), the PhD Programs Foundation of Ministry of Education of China (20131106120046), the Jiangsu Provincial Special Program of Medical Science (BL2012003) and the Jiangsu Province Natural Science Foundation (No. BK20131064) to Heng Gu. Wenbo Bu, Xu Chen and Song Xu are supported by the PUMC Youth Fund and Fundamental Research Funds for the Central Universities (3332015116, 3332014008, 3332015026, 2016RC320005).
Conflict of interest
The authors declare no conflict of interest.
Supplementary (web page, Internet)
Lists of differential expressed lncRNAs in tissue and cell
Differentially expressed lncRNAs in tissue and cell. P-value presented the expression differences of lncRNA normalized intensities between keloid tissues and normal tissues, keloid-derived fibroblasts and normal fibroblasts, respectively, and p-value < 0.05 referred to have significant differences. FDR was a modified p-value which was calculated from Benjamini Hochberg FDR. Fold change indicated the absolute ratio of lncRNA normalized intensities between keloid tissues and normal tissues, keloid-derived fibroblasts and normal fibroblasts, respectively. Regulation column: up represents up-regulation and vice versa. The seqname referred to the lncRNA name based on the most highly respected public transcriptome databases (Refseq, UCSC knowngenes, Gencode, etc.), as well as the landmark publications.
References
- 1.Russell SB, Russell JD, Trupin KM, et al. Epigenetically altered wound healing in keloid fibroblasts. J Investig Dermatol. 2010;130:2489–96. doi: 10.1038/jid.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinholz M, Poetschke J, Schwaiger H, et al. The dermatology life quality index as a means to assess life quality in patients with different scar types. J Eur Acad Dermatol Venereol. 2015;29:2112–9. doi: 10.1111/jdv.13135. [DOI] [PubMed] [Google Scholar]
- 3.Andrews JP, Marttala J, Macarak E, et al. Keloid pathogenesis: potential role of cellular fibronectin with the EDA domain. J Investig Dermatol. 2015;135:1921–4. doi: 10.1038/jid.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Fu X, Sun X, et al. Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray. J Surg Res. 2003;113:208–16. doi: 10.1016/s0022-4804(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 5.Syed F, Sanganee HJ, Singh S, et al. Potent dual inhibitors of TORC1 and TORC2 complexes (KU-0063794 and KU-0068650) demonstrate in vitro and ex vivo anti-keloid scar activity. J Investig Dermatol. 2013;133:1340–50. doi: 10.1038/jid.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–69. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang X, Ma L, Long X, et al. LncRNA expression profiles and validation in keloid and normal skin tissue. Int J Oncol. 2015;47:1829–38. doi: 10.3892/ijo.2015.3177. [DOI] [PubMed] [Google Scholar]
- 8.Zhu HY, Bai WD, Li C, et al. Knockdown of lncRNA-ATB suppresses autocrine secretion of TGF-beta2 by targeting ZNF217 via miR-200c in keloid fibroblasts. Sci Rep. 2016;6:24728. doi: 10.1038/srep24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Liu CY, Wan Y, et al. Long non-coding RNA H19 promotes the proliferation of fibroblasts in keloid scarring. Oncol Letters. 2016;12:2835–9. doi: 10.3892/ol.2016.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funayama E, Chodon T, Oyama A, et al. Keratinocytes promote proliferation and inhibit apoptosis of the underlying fibroblasts: an important role in the pathogenesis of keloid. J Investig Dermatol. 2003;121:1326–31. doi: 10.1111/j.1523-1747.2003.12572.x. [DOI] [PubMed] [Google Scholar]
- 11.Lim CP, Phan TT, Lim IJ, et al. Cytokine profiling and Stat3 phosphorylation in epithelial-mesenchymal interactions between keloid keratinocytes and fibroblasts. J Investig Dermatol. 2009;129:851–61. doi: 10.1038/jid.2008.337. [DOI] [PubMed] [Google Scholar]
- 12.Canady J, Arndt S, Karrer S, et al. Increased KGF expression promotes fibroblast activation in a double paracrine manner resulting in cutaneous fibrosis. J Investig Dermatol. 2013;133:647–57. doi: 10.1038/jid.2012.389. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:925–33. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orom UA, Derrien T, Beringer M, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cathcart P, Lucchesi W, Ottaviani S, et al. Noncoding RNAs and the control of signalling via nuclear receptor regulation in health and disease. Best Pract Res Clin Endocrinol Metabol. 2015;29:529–43. doi: 10.1016/j.beem.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Sun M, Nie F, Wang Y, et al. LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76:6299–310. doi: 10.1158/0008-5472.CAN-16-0356. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Chen L, Cao C, et al. The long non-coding RNA Lnc-RNA8975-1 is upregulated in hypertrophic scar fibroblasts and controls collagen expression. Cell Physiol Biochem. 2016;40:326–34. doi: 10.1159/000452548. [DOI] [PubMed] [Google Scholar]
- 19.Smith JC, Boone BE, Opalenik SR, et al. Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J Investig Dermatol. 2008;128:1298–310. doi: 10.1038/sj.jid.5701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–7. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Kelly AP, Wang L, et al. Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J Investig Dermatol. 2006;126:2607–13. doi: 10.1038/sj.jid.5700472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.