Abstract Abstract
Amphilochus manudens and Amphilochopsis hamatus are redescribed based on specimens from the BioIce, Mareano, and IceAGE programmes. The new species Amphilochus anoculus sp. n. is described based on material from the IceAGE programme and the preceding BioIce programme; it is separated from the closely related Amphilochus manudens by the absence of eyes, a symmetrically bilobed labrum, four setae on the maxilla 2 outer plate, a rounded corner of epimeral plate 3, and a robust seta at the tip of the telson. There are also clear differences in depth and temperature ranges. Amphilochopsis hamatus is shown to be closely related to Amphilochus manudens and A. anoculus and transferred to Amphilochus s. str.
Keywords: Amphilochus, Amphipoda, BioIce, IceAGE, Mareano, new species, North Atlantic, taxonomy
Introduction
The amphipod family Amphilochidae consists today of 15 genera, of which several are monotypic. There are ninety species, of which most are assigned to the possibly paraphyletic (Hoover and Bousfield 2001) genera Gitanopsis and Amphilochus (Horton et al. 2017). The family is cosmopolitan with the small genera seemingly restricted to specific geographic areas. Historically, the definition of Amphilochidae has been much like what Barnard and Karaman (1991) use as their diagnosis: ”Coxa 4 immensely broadened, coxae 2-4 with contiguous overlapping, not rabbeted, coxa 2 not hidden; coxa 1 very small and hidden by coxa 2. Peduncle of uropod 3 elongate. Telson entire, elongate.”
During the sorting of Amphilochidae material from the BioIce programme for a Master thesis in 2000, it became apparent that three groups of specimens had an anterodistal tooth on the propodus of pereopod 2. Amphilochus manudens and Amphilochopsis hamatus were already known from the literature (Sars 1890–95; Stephensen 1925; Gurjanova 1951), but the last group of specimens; with an anterodistal tooth and seemingly no eyes did not fit any of the described species. Specimens with the same morphological traits have since been found by the authors in amphipod material from Spitsbergen, the Faroe Islands, the Norwegian coast and in newly collected Icelandic material from the follow-up programme to BioIce: IceAGE (for information on IceAGE amphipod collections, see Brix et al. 2014; 2018). We therefore find it timely to describe a new species for the observed morphotype with the anterodistal tooth and no visible eyes. To be able to fully distinguish the new species from the known species it most resembles, morphological redescriptions of these are included, and the three species are genetically barcoded (COI-gene, Folmer et al. 1994) to show a clear separation of species both collected from Iceland (Jażdżewska et al. (2018)) and Norway (Boldsystems.org).
Materials and methods
The material examined in this study comes from the programme BioIce in the years 1991–1997, the IceAGE-programme, and material in the collections of the University museums of Tromsø and Bergen, Norway. A few additional specimens derive from environmental monitoring studies around the Faroe Islands. For information on the collection of the material for BioIce, see Berge and Vader (1997), for the collection of IceAGE material, see Brix et al. (2014, 2018). Most of the new material at the University museum of Bergen comes from the Mareano programme; for collection of this material, see Buhl-Mortensen et al. (2015). The Amphilochidae-material from BioIce was sorted and described in Tromsø for an MSc-thesis (Tandberg 2000). Sample individuals were dissected using a binocular and mounted in rose-bengal-stained polyvinyl-lactophenol for examination under a light microscope. Pencil-drawings were made using a microscope fitted with a drawing tube; drawings were traced with ink and scanned. Digital inking on scanned hand-inked drawings followed procedures described by Coleman (2003, 2009). All scales on drawings are 0.1 mm unless otherwise stated.
Material from IceAGE and the collections from the University Museum of Bergen were identified and dissected for illustration of appendages using a Leica MZ12.5 stereo microscope. Temporary glycerine mounted and permanently mounted appendages (Faure medium) were drawn using a Leica 2500 compound microscope fitted with a camera lucida, and scanned pencil drawings were digitally inked in Adobe Illustrator following the method described by Coleman (2003, 2009). Animals used for COI-sequencing in Norway were photographed using a Leica DFC425 camera fitted with a motorised stacker on a Leica M205 binocular, and the Leica LAS 3.8 software for taking photos. Compilation of stacked photos into a single photo has been performed using Zerene Stacker 1.04 (setting P-max).
Further material for Amphilochus anoculus sp. n. comes from a survey in the Faroe-Shetland Channel (Mannvik et al. 2002), the Norwegian Sea and from the polar basin north of Spitsbergen (Tromsø Museum collections). Ecological data for Amphilochus manudens and Amphilochopsis hamatus were also gathered from the BioFar program (Nørrevang et al. 1994).
Sequencing of COI was performed through IceAGE (for details see Jażdżewska et al. 2018) and NorBOL (The Norwegian Barcode of Life, for details see Lörz et al. 2018).
BioIce material is held at the National Museum of Iceland, Reykjavik, Iceland (IINH-numbers).
IceAGE material is held at the Zoological Museum University of Hamburg, Centre of Natural History (CeNak), Germany (ZMH K-numbers).
NorAmph and other University of Bergen material is held at the University Museum of Bergen, Natural History Collections, Norway (ZMBN-numbers).
Material from University Museum of Tromsø is held at the Natural Collections University of Tromsø, Tromsø, Norway (TSZCr-numbers).
The material from the environmental studies performed by AkvaplanNIVA was kept for five years before it was destroyed: the identification of the amphipods of the survey was performed by the first author.
Results
Taxonomy
Order AMPHIPODA Latreille, 1816
Suborder GAMMARIDEA Latreille, 1802
Family AMPHILOCHIDAE Boeck, 1871
Genus Amphilochus Spence Bate, 1862
Amphilochus Spence Bate, 1862: 107; Stebbing 1906: 149; Barnard and Karaman 1991: 96
Callimerus Stebbing, 1876: 445
Amphilochus anoculus sp. n.
http://zoobank.org/AD3ED2F5-F13B-4885-BD81-492F173B4EA1
Material examined.
from Icelandic (BioIce and IceAGE), Norwegian coastal and arctic (Svalbard) and Faroese waters. (For an extensive list of examined material see Table 1.).
Table 1.
List of stations for examined species of Amphilochus anoculus sp. n., A. manudens, and A. hamatus. Asterisk * after museum-number indicates holo- and paratypes.
| Species | Station name | Sampling programme | Collection number | Latitude (dec) | Longitude (dec) | Depth (m) | Temp (C) | BOLD-accension number | Note |
|---|---|---|---|---|---|---|---|---|---|
| Amphilochus anoculus sp. n. | BioIce 2087 | BioIce | 67,257, -17,446 | 735,0 | -0,40 | ||||
| BioIce 2088 | BioIce | 67,239, -17,857 | 617,0 | -0,40 | |||||
| BioIce 2094 | BioIce | 67,034, -17,570 | 303,0 | 1,70 | |||||
| BioIce 2100 | BioIce | 68,001, -19,421 | 1141,0 | -0,60 | |||||
| BioIce 2107 | BioIce | 67,836, -19,555 | 905,0 | -0,60 | |||||
| BioIce 2136 | BioIce | 66,726, -18,953 | 417,0 | 0,60 | |||||
| BioIce 2149 | BioIce | 66,749, -20,086 | 293,0 | 3,00 | |||||
| BioIce 2318 | BioIce | IINH37886 (wet), IINH37916 (slide) | 64,070, -9,030 | 996,0 | |||||
| BioIce 2325 | BioIce | 63,750, -10,183 | 555,0 | ||||||
| BioIce 2367 | BioIce | IINH37888, IINH37914*, IINH37915* | 64,380, -9,430 | 719,0 | Paratype (slides) | ||||
| 3-1 | Akvaplan NIVA Faroe project | 60,348, -5,167 | 1088,0 | ||||||
| 8-1 | Akvaplan NIVA Faroe project | 60,591, -5,309 | 825,0 | ||||||
| 9-1 | Akvaplan NIVA Faroe project | 60,538, -5,206 | 921,0 | ||||||
| 13-2 | Akvaplan NIVA Faroe project | 60,483, -4,932 | 1022,0 | ||||||
| 15-1 | Akvaplan NIVA Faroe project | 60,553, -4,937 | 1055,0 | ||||||
| 15-3 | Akvaplan NIVA Faroe project | 60,553, -4,937 | 1055,0 | ||||||
| 81 03211 | Tromsø Museum Collection tours | TSZCr 15516 | 63,167, 4,817 | 860,0 | |||||
| 14968 | Tromsø Museum Collection tours | TSZCr 14968 | 70,850, 15,383 | 2100,0 | |||||
| JM 369-05 | UNIS AB321-2005 | TSZCr 14338* | 80,531, 10,578 | 819,0 | Paratypes (wet) | ||||
| R405 RP59 | Mareano | ZMBN_111537 | 72,140, 15,346 | 902,4 | -0,41 | AMPNB487-17 | |||
| R479 RP156 | Mareano | 68,653, 10,301 | 2744,2 | -0,82 | |||||
| R573 RP28 | Mareano | 70,872, 16,933 | 916,5 | -0,64 | |||||
| R642 RP104 | Mareano | ZMBN_104532 | 68,241, 9,243 | 2346,6 | -0,84 | AMPNB354-15 | |||
| R653 RP108 | Mareano | 67,608, 8,392 | 1750,7 | -0,84 | |||||
| R671 RP111 | Mareano | ZMBN_104531 | 67,891, 9,875 | 777,2 | -0,52 | AMPNB353-15 | |||
| R1180 RP86 | Mareano | 71,609, 32,992 | 304,9 | 2,84 | |||||
| Amphilochus anoculus sp. n. | R1200 RP90 | Mareano | 70,854, 32,507 | 248,9 | 3,74 | ||||
| R1225 RP112 | Mareano | ZMBN121953 * | 70,475, 31,734 | 401,4 | 5,45 | Paratype (slide) | |||
| R1225 RP112 | Mareano | ZMBN121959, ZMBN121960 | 70,475, 31,734 | 401,4 | 5,45 | slides | |||
| IceAGE 1010 | IceAGE | ZMH K-47220 | 62,552, -20,395 | 1384,8 | |||||
| IceAGE 1010 | IceAGE | ZMH K-47221 | 62,552, -20,395 | 1384,8 | |||||
| IceAGE 1054 | IceAGE | ZMH K-47222 | 61,603, -31,377 | 2537,3 | |||||
| IceAGE 880 | IceAGE | ZMBN121954 | 63,389, -8,157 | 686,0 | |||||
| IceAGE 880 | IceAGE | ZMH K-47223 | 63,389, -8,157 | 686,0 | AMPIV181-17 | ||||
| IceAGE 1010 | IceAGE | ZMBN121955 | 62,552, -20,395 | 1384,8 | |||||
| IceAGE 1010 | IceAGE | ZMH K-47224 | 62,552, -20,395 | 1384,8 | AMPIV188-17 | ||||
| IceAGE 1057 | IceAGE | ZMH K-47225* | 61,642, -31,356 | 2504,7 | Holotype (slide) | ||||
| IceAGE 1168 | IceAGE | ZMH K-47226 | 67,606, -7,001 | 2372,6 | |||||
| IceAGE 1123 | IceAGE | ZMH K-47227 | 67,214, -26,208 | 716,5 | |||||
| IceAGE 1172 | IceAGE | ZMH K-47228 | 67,578, -6,935 | 2422,4 | |||||
| IceAGE 1181 | IceAGE | ZMBN121956 | 67,658, -12,227 | 1827,0 | |||||
| IceAGE 1119 | IceAGE | ZMBN121957 | 67,214, -26,242 | 696,9 | |||||
| IceAGE 871 | IceAGE | ZMBN121958 | 62,737, -0,946 | 1577,4 | |||||
| IceAGE 1168 | IceAGE | ZMH K-47229 | 67,606, -7,001 | 2372,6 | |||||
| IceAGE 1123 | IceAGE | ZMH K-47230 | 67,214, -26,208 | 716,5 | |||||
| IceAGE 1172 | IceAGE | ZMH K-47231 | 67,578, -6,935 | 2422,4 | |||||
| IceAGE 1172 | IceAGE | ZMH K-47232 | 67,578, -6,935 | 2422,4 | DNA-voucher: ZMH K-47232 | ||||
| IceAGE 1054 | IceAGE | ZMH K-47233 | 61,603, -31,377 | 2537,3 | DNA-voucher: ZMH K-47233 | ||||
| IceAGE 1159 | IceAGE | ZMH K-47234 | 69,111, -9,917 | 2202,8 | |||||
| IceAGE 868 | IceAGE | ZMH K-47235 | 62,152, 0,259 | 587,4 | |||||
| IceAGE 1123 | IceAGE | ZMH K-47236 | 67,214, -26,208 | 716,5 | |||||
| IceAGE 1010 | IceAGE | ZMH K-47237 | 62,552, -20,395 | 1384,8 | DNA-voucher: ZMH K-47237 | ||||
| IceAGE 1006 | IceAGE | ZMBN121952* | 62,551, -20,375 | 1386,8 | Paratype (slide) | ||||
| Amphilochus manudens Spence Bate, 1862 | BioIce 2096 | BioIce | 67,018, -17,578 | 300,0 | 1,70 | ||||
| BioIce 2207 | BioIce | IINH37889 | 67,011, -22,596 | 81,0 | 8,30 | ||||
| BioIce 2213 | BioIce | 64,155, -23,971 | 260,0 | 7,00 | |||||
| BioIce 2215 | BioIce | IINH37887 | 64,157, -24,261 | 213,0 | 6,90 | ||||
| BioIce 2221 | BioIce | 63,917, -25,273 | 240,0 | 6,50 | |||||
| BioIce 2236 | BioIce | 63,450, -24,680 | 293,0 | 6,90 | |||||
| BioIce 2237 | BioIce | IINH37885 | 63,270, -24,408 | 293,0 | 6,90 | ||||
| BioIce 2273 | BioIce | 63,140, -24,983 | 313,0 | 7,00 | |||||
| BioIce 2288 | BioIce | 62,387, -22,677 | 1390,0 | 3,40 | |||||
| BioIce 2308 | BioIce | 63,250, -22,790 | 263,0 | 7,10 | |||||
| BioIce 2314 | BioIce | 63,703, -23,058 | 139,0 | 7,60 | |||||
| BioIce 2352 | BioIce | 63,783, -11,817 | 350,0 | ||||||
| BioIce 2358 | BioIce | 63,167, -11,533 | 318,0 | ||||||
| BioIce 2720 | BioIce | 64,430, -26,403 | 304,0 | 5,60 | |||||
| R405 RP59 | Mareano | 72,137, 15,341 | 899,6 | -0,41 | |||||
| R423 RP69 | Mareano | 71,872, 17,142 | 355,3 | 5,53 | |||||
| R474 RP154 | Mareano | 71,073, 18,543 | 251,0 | 7,52 | |||||
| R503 RP51 | Mareano | 71,772, 25,975 | 321,1 | 4,42 | |||||
| R534 RP60 | Mareano | 70,675, 18,622 | 364,6 | 6,34 | |||||
| R608 RP87 | Mareano | 70,958, 21,120 | 149,0 | 6,57 | |||||
| R613 RP90 | Mareano | 70,769, 20,818 | 246,8 | 6,42 | |||||
| R618 RP91 | Mareano | 70,701, 21,025 | 258,8 | 6,38 | |||||
| R621 RP93 | Mareano | 70,673, 20,852 | 195,6 | 7,28 | |||||
| R631 RP99 | Mareano | 70,805, 19,702 | 178,6 | 6,87 | |||||
| R636 RP102 | Mareano | 70,622, 20,104 | 289,7 | 6,98 | |||||
| R657 RP109 | Mareano | 67,343, 8,638 | 849,6 | -0,84 | |||||
| R721 RP126 | Mareano | 67,841, 11,809 | 183,2 | 6,87 | |||||
| R733 RP128 | Mareano | ZMBN_94864 | 67,720, 10,272 | 219,2 | 7,34 | AMPNB115-14 | |||
| R754 RP132 | Mareano | 67,803, 9,685 | 823,5 | -0,56 | |||||
| R786 RP10 | Mareano | 67,953, 9,589 | 1315,4 | -0,84 | |||||
| Amphilochus manudens Spence Bate, 1862 | R782 RP11 | Mareano | 68,059, 9,468 | 1712,0 | -0,81 | ||||
| R821 RP13 | Mareano | ZMBN_104481 | 67,021, 8,223 | 556,0 | AMPNB303-15 | ||||
| R870 RP19 | Mareano | 67,387, 11,622 | 128,3 | 6,29 | |||||
| R849 RP21 | Mareano | 67,401, 10,822 | 179,6 | 7,51 | |||||
| R1137 RP77 | Mareano | 72,574, 32,386 | 272,3 | 2,09 | |||||
| R1146 RP80 | Mareano | 72,103, 34,287 | 288,9 | 2,18 | |||||
| R1150 RP82 | Mareano | 72,093, 33,701 | 249,5 | 2,83 | |||||
| R1174 RP85 | Mareano | 71,618, 32,225 | 296,5 | 3,39 | |||||
| R1180 RP86 | Mareano | 71,609, 32,992 | 304,9 | 2,84 | |||||
| R1186 RP87 | Mareano | 71,421, 32,859 | 281,5 | 4,47 | |||||
| R1196 RP89 | Mareano | 71,187, 32,243 | 226,0 | 4,27 | |||||
| R1200 RP90 | Mareano | 70,854, 32,507 | 248,9 | 3,74 | |||||
| R1205 RP92 | Mareano | 70,574, 32,273 | 297,3 | 4,22 | |||||
| R1213 RP93 | Mareano | 70,771, 30,785 | 376,1 | 4,62 | |||||
| R1230 RP95 | Mareano | 70,117, 31,350 | 303,9 | ||||||
| IceAGE 868 | IceAGE | ZMH K-47238 | 62,152, 0,259 | 587,4 | |||||
| IceAGE 1082 | IceAGE | ZMH K-47239 | 63,702, -26,394 | 724,4 | |||||
| IceAGE 1017 | IceAGE | ZMH K-47240 | 62,931, -20,774 | 891,7 | |||||
| IceAGE 1032 | IceAGE | ZMH K-47241 | 63,309, -23,158 | 289,4 | |||||
| IceAGE 878 | IceAGE | ZMH K-47242 | 61,897, -10,230 | 781,4 | |||||
| IceAGE 868 | IceAGE | ZMH K-47243 | 62,152, 0,259 | 587,4 | |||||
| IceAGE 1086 | IceAGE | ZMH K-47244 | 63,709, -26,384 | 698,1 | |||||
| IceAGE 1219 | IceAGE | ZMH K-47245 | 66,289, -12,347 | 579,1 | |||||
| IceAGE 1086 | IceAGE | ZMH K-47246 | 63,709, -26,384 | 698,1 | |||||
| IceAGE 876 | IceAGE | ZMH K-47247 | 60,406, -6,615 | 554,3 | |||||
| IceAGE 876 | IceAGE | ZMH K-47248 | 60,406, -6,615 | 554,3 | AMPIV183-17 | ||||
| IceAGE 878 | IceAGE | ZMH K-47249 | 61,897, -10,230 | 781,4 | |||||
| IceAGE 878 | IceAGE | ZMH K-47250 | 61,897, -10,230 | 781,4 | |||||
| IceAGE 878 | IceAGE | ZMH K-47251 | 61,897, -10,230 | 781,4 | |||||
| IceAGE 1168 | IceAGE | ZMH K-47252 | 67,606, -7,001 | 2372,6 | |||||
| Amphilochus manudens Spence Bate, 1862 | IceAGE 1104 | IceAGE | ZMH K-47253 | 66,643, -24,533 | 118,8 | ||||
| IceAGE 1194 | IceAGE | ZMH K-47254 | 67,078, -13,055 | 1573,5 | |||||
| IceAGE 1172 | IceAGE | ZMH K-47255 | 67,578, -6,935 | 2422,4 | |||||
| IceAGE 867 | IceAGE | ZMH K-47256 | 61,997, 0,507 | 302,5 | |||||
| IceAGE 866 | IceAGE | ZMH K-47257 | 61,427, 1,351 | 169,1 | |||||
| IceAGE 870 | IceAGE | ZMH K-47258 | 62,329, -0,102 | 1058,4 | |||||
| IceAGE 867 | IceAGE | ZMH K-47259 | 61,997, 0,507 | 302,5 | |||||
| IceAGE 1123 | IceAGE | ZMH K-47260 | 67,214, -26,208 | 716,5 | |||||
| IceAGE 1082 | IceAGE | ZMH K-47261 | 63,702, -26,394 | 724,4 | DNA-voucher: ZMH K-47261 | ||||
| IceAGE 866 | IceAGE | ZMH K-47262 | 61,427, 1,351 | 169,1 | |||||
| IceAGE 867 | IceAGE | ZMH K-47263 | 61,997, 0,507 | 302,5 | |||||
| IceAGE 868 | IceAGE | ZMH K-47264 | 62,152, 0,259 | 587,4 | |||||
| IceAGE 1086 | IceAGE | ZMH K-47265 | 63,709, -26,384 | 698,1 | DNA-voucher: ZMH K-47265 | ||||
| IceAGE 867 | IceAGE | ZMH K-47266 | 61,997, 0,507 | 302,5 | |||||
| IceAGE 867 | IceAGE | ZMH K-47267 | 61,997, 0,507 | 302,5 | DNA-voucher: ZMH K-47267 | ||||
| Amphilochus hamatus (Stephensen, 1925) | BioIce 2077 | BioIce | IINH37894 | 67,405, -17,104 | 1048,0 | -0,50 | |||
| BioIce 2087 | BioIce | 67,257, -17,446 | 735,0 | -0,40 | |||||
| BioIce 2088 | BioIce | IINH37892 | 67,239, -17,857 | 617,0 | -0,40 | ||||
| BioIce 2090 | BioIce | IINH37893 | 67,222, -17,816 | 539,0 | -0,40 | ||||
| BioIce 2096 | BioIce | IINH37891 | 67,018, -17,578 | 300,0 | 1,70 | ||||
| BioIce 2100 | BioIce | 68,001, -19,421 | 1141,0 | -0,60 | |||||
| BioIce 2107 | BioIce | IINH37890 | 67,836, -19,555 | 905,0 | -0,60 | ||||
| BioIce 2136 | BioIce | IINH37896 | 66,726, -18,953 | 417,0 | 0,60 | ||||
| BioIce 2149 | BioIce | 66,749, -20,086 | 293,0 | 3,00 | |||||
| BioIce 2213 | BioIce | IINH37897 | 64,155, -23,971 | 260,0 | 7,00 | ||||
| BioIce 2236 | BioIce | IINH37898 | 63,450, -24,680 | 293,0 | 6,90 | ||||
| BioIce 2237 | BioIce | 63,270, -24,408 | 293,0 | ||||||
| Amphilochus hamatus (Stephensen, 1925) | BioIce 2317 | BioIce | IINH37889 | 64,117, -9,050 | 996,0 | ||||
| BioIce 2318 | BioIce | IINH37900 | 64,070, -9,030 | 996,0 | |||||
| BioIce 2319 | BioIce | IINH37901 | 64,017, -9,617 | 776,0 | |||||
| BioIce 2340 | BioIce | IINH37902 | 62,133, -13,333 | 1302,0 | |||||
| BioIce 2367 | BioIce | IINH37903 | 64,380, -9,430 | 719,0 | |||||
| BioIce 2410 | BioIce | IINH37904 | 62,860, -21,735 | 1074,0 | 4,00 | ||||
| BioIce 2707 | BioIce | IINH37905 | 63,922, -28,270 | 1407,0 | 3,70 | ||||
| BioIce 2719 | BioIce | IINH37906 | 64,428, -26,403 | 300,0 | 5,60 | ||||
| R671 RP111 | Mareano | ZMBN_104542 | 67,891, 9,875 | 777,2 | -0,52 | AMPNB364-15 | |||
| R877 RP3 | Mareano | ZMBN_104479 | 68,475, 9,785 | 2561,4 | -0,80 | AMPNB301-15 | |||
| R776 RP4 | Mareano | 68,186, 10,354 | 799,9 | -0,74 | |||||
| IceAGE 869 | IceAGE | ZMH K-47268 | 62,270, 0,020 | 846,4 | |||||
| IceAGE 1006 | IceAGE | ZMH K-47269 | 62,551, -20,375 | 1386,8 | |||||
| IceAGE 1006 | IceAGE | ZMH K-47270 | 62,551, -20,375 | 1386,8 | |||||
| IceAGE 1019 | IceAGE | ZMH K-47271 | 62,939, -20,744 | 913,6 | |||||
| IceAGE 1132 | IceAGE | ZMH K-47272 | 67,641, -26,755 | 318,1 | |||||
| IceAGE 1172 | IceAGE | ZMH K-47273 | 67,578, -6,935 | 2422,4 | |||||
| IceAGE 1123 | IceAGE | ZMH K-47274 | 67,214, -26,208 | 716,5 | |||||
| IceAGE 1119 | IceAGE | ZMH K-47275 | 67,214, -26,242 | 696,9 | |||||
| IceAGE 1119 | IceAGE | ZMH K-47276 | 67,214, -26,242 | 696,9 | DNA-voucher: ZMH K-47276 | ||||
| IceAGE 1010 | IceAGE | ZMH K-47277 | 62,552, -20,395 | 1384,8 | |||||
| IceAGE 1010 | IceAGE | ZMH K-47278 | 62,552, -20,395 | 1384,8 | DNA-voucher: ZMH K-47278 | ||||
| IceAGE 1172 | IceAGE | ZMH K-47279 | 67,578, -6,935 | 2422,4 | DNA-voucher: ZMH K-47279 | ||||
| IceAGE 869 | IceAGE | ZMH K-47280 | 62,270, 0,020 | 846,4 | |||||
| IceAGE 1010 | IceAGE | ZMH K-47281 | 62,552, -20,395 | 1384,8 | |||||
Holotype: IceAGE ZMH K-47225, female 3 mm (slide).
Paratypes: Slides: BioIce 2367 male, 3 mm IINH37914; BioIce 2367 female, 3 mm IINH37915; MareanoR1225-RP112 female 4 mm ZMBN121953; IceAGE 1006 male, 3 mm ZMBN121952. Wet-sample: TSZCr 14338 (8 specimens).
Type locality.
ZMH K-47225: IceAGE station 1057 (61.6417, -31.3562) (2504m).
Paratype localities.
IINH37914, IINH37915: BioIce station 2367 (64.3800, -9.4300) (719m); TSZCr 14338: UNIS course-station JM 369-05 (80.5313, 10.5777) (819 m); ZMBN121953: Mareano station R1225-RP112 (70.4748, 31.7340) (401 m); ZMBN121952: IceAGE station 1006 (62.5508, -20.3750) (1386 m).
Distribution.
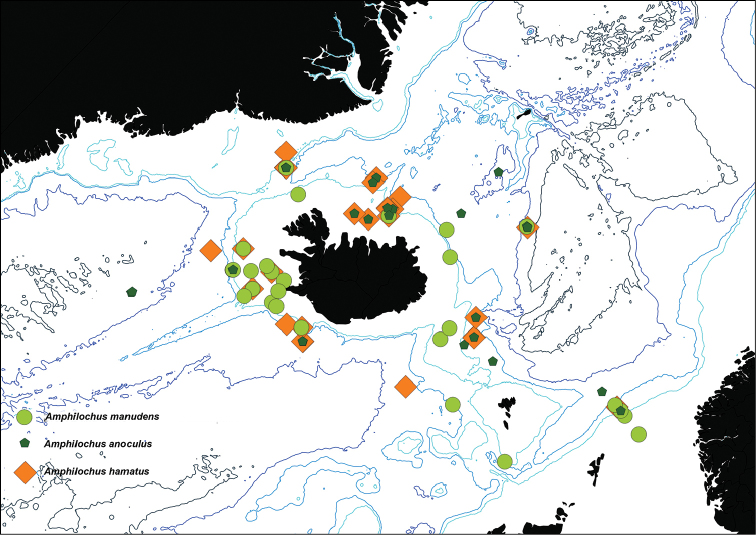
This species is known from BioIce/IceAGE stations in deep and cold waters north and east of Iceland, from deep stations in the Faroe-Shetland Channel, several deep stations north in the Norwegian Sea and from one deep station in the polar basin. It appears to be confined to cold and deep waters (see Fig. 1).
Figure 1.
Map showing the Icelandic distribution of Amphilochus anoculus sp. n., Amphilochus manudens and Amphilohus hamatus (based on BioIce and IceAGE material).
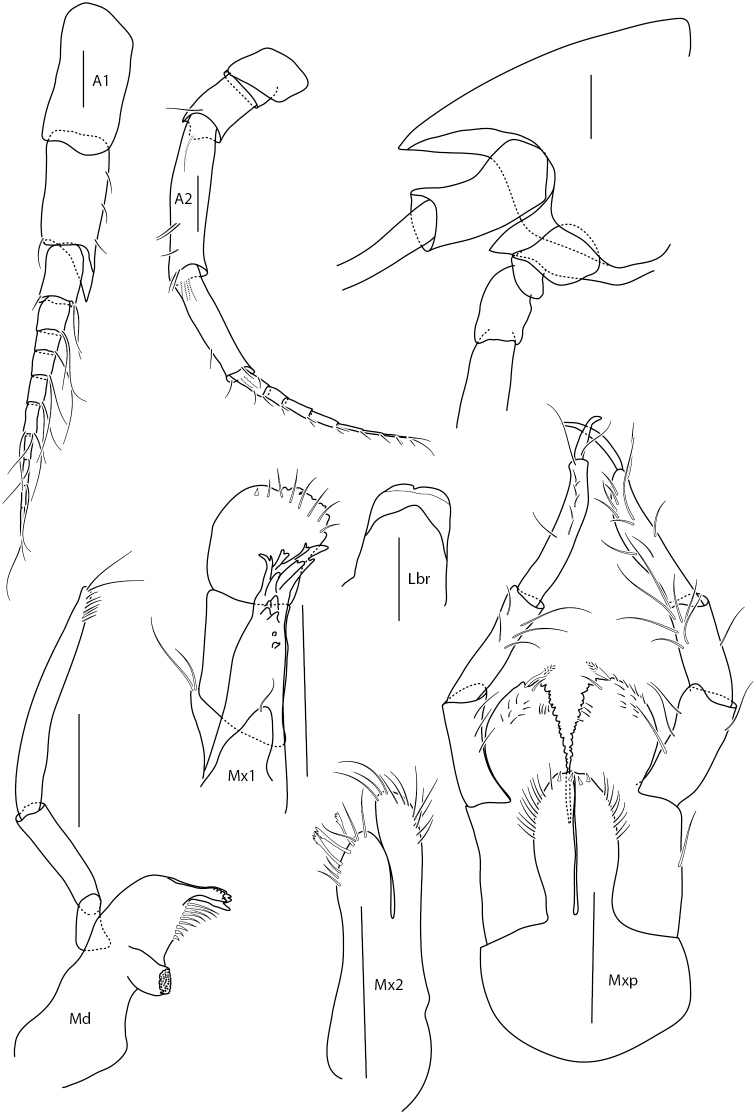
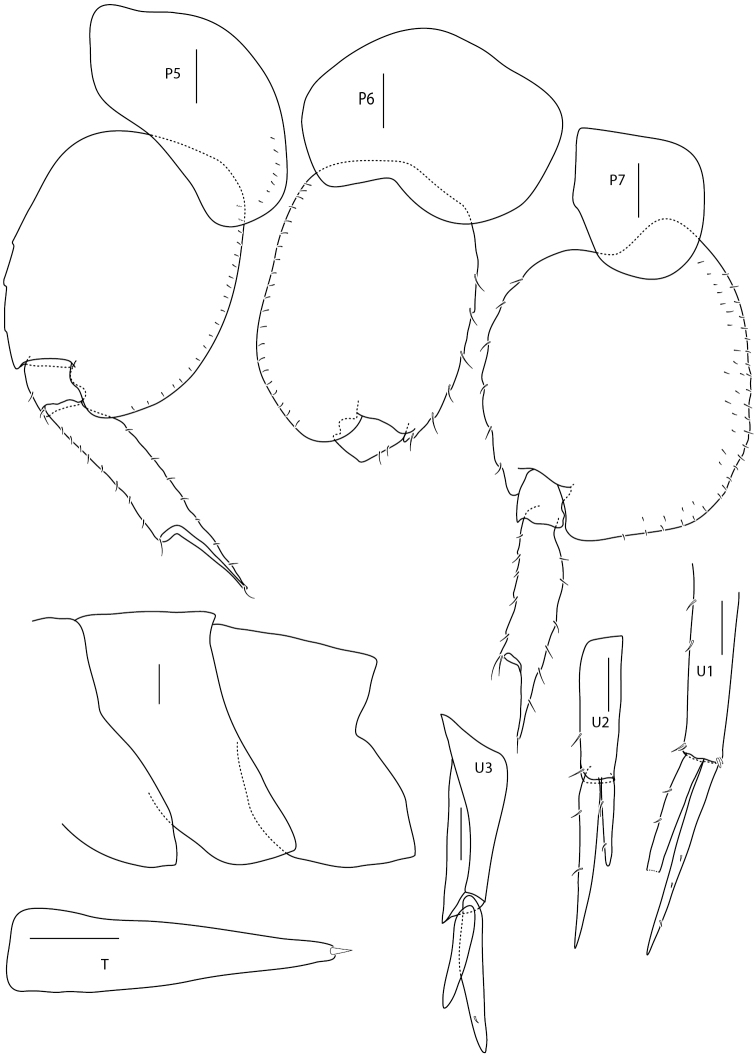
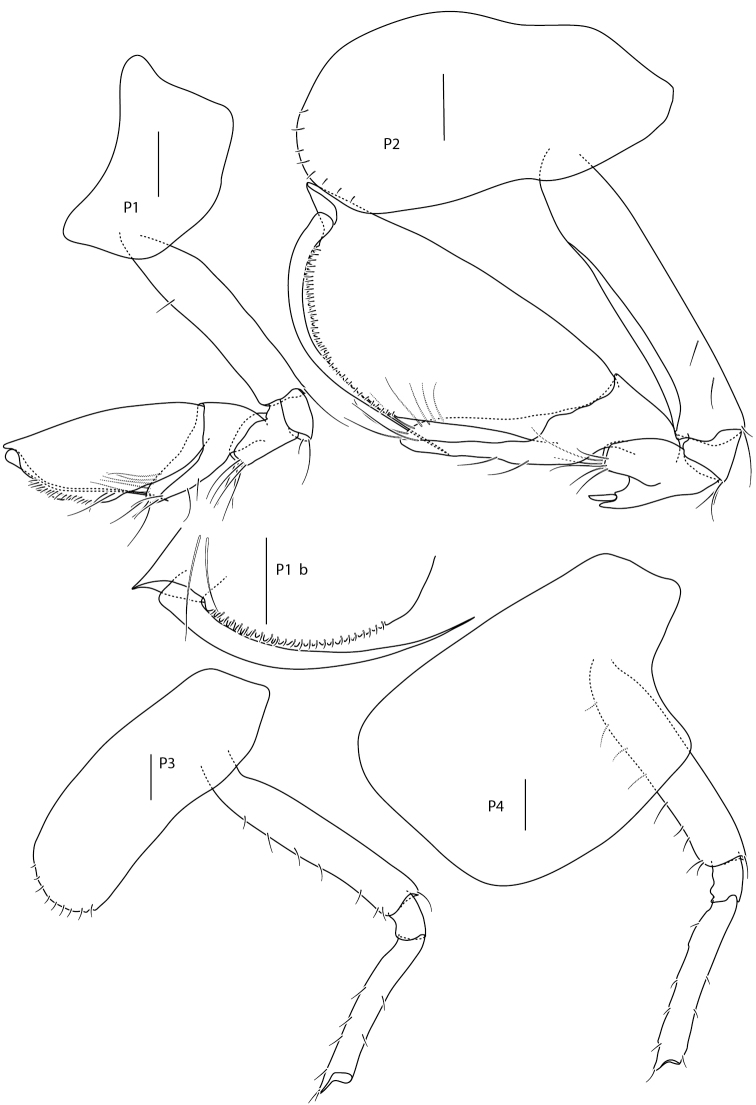
Illustrations are all from paratypes: Figs 2–4 of ZMBN121952, except for Fig. 3 pereopod 1 dactylus (1b) which is from ZMH K-47225 and Fig. 4 uropod 3 and telson that are both from BioIce station 2367.
Figure 2.
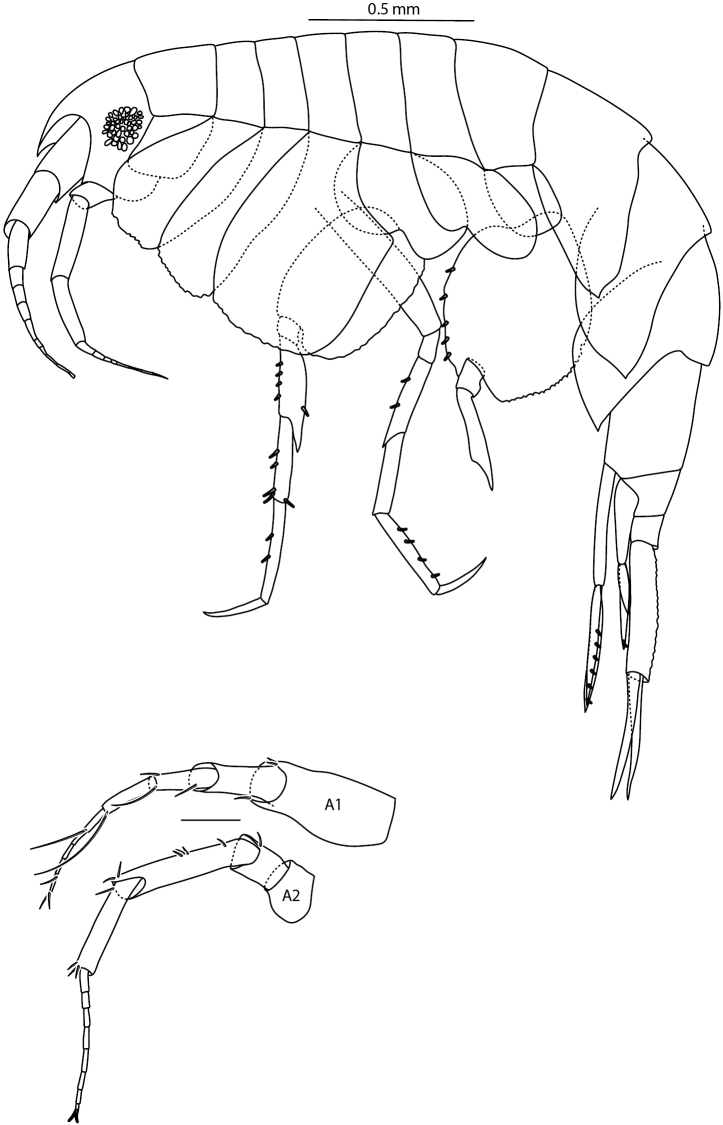
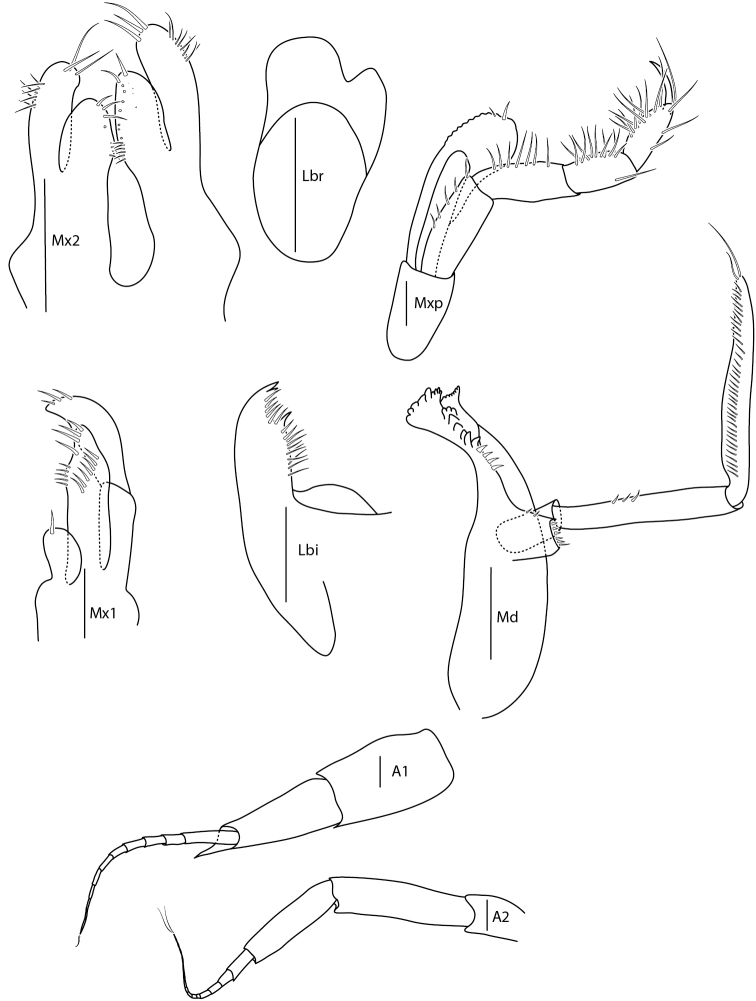
Amphilochus anoculus sp. n. Head and mouthparts. ZMBN121952. Scale bars: 0.1 mm.
Figure 4.
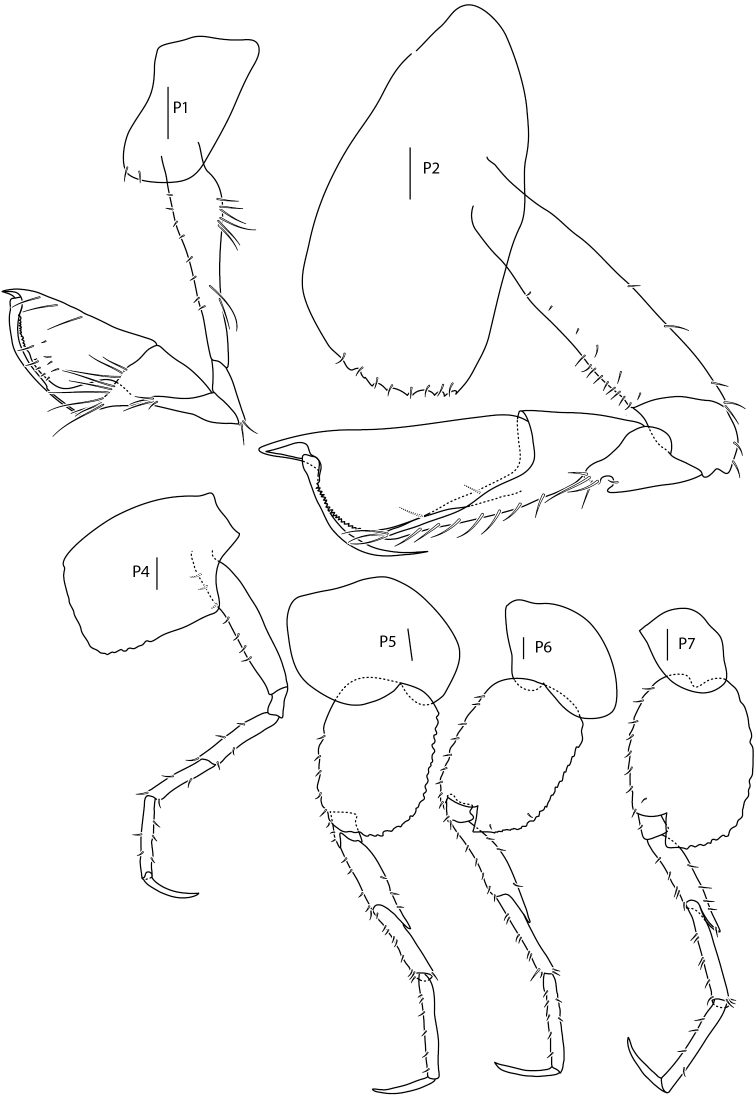
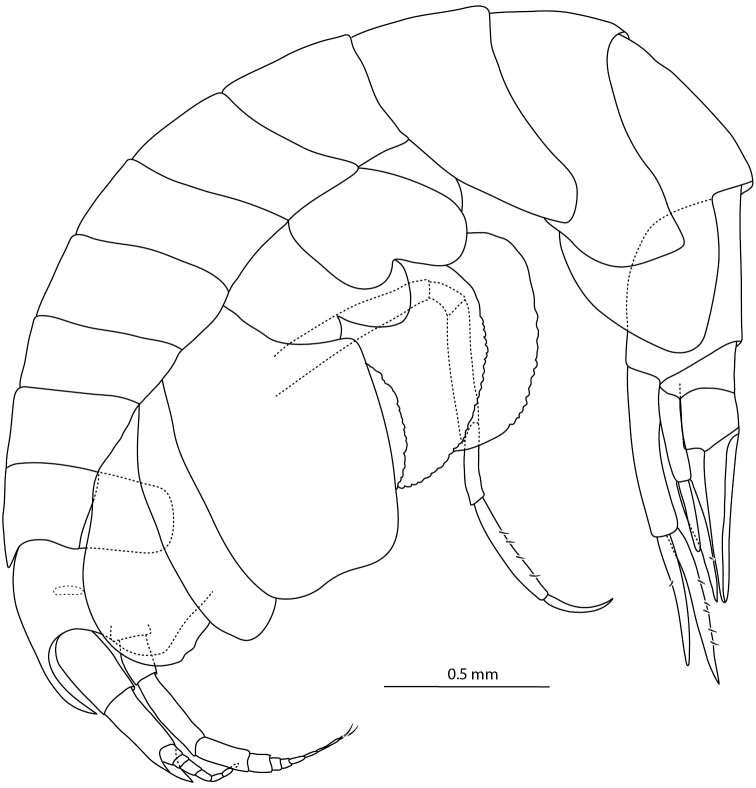
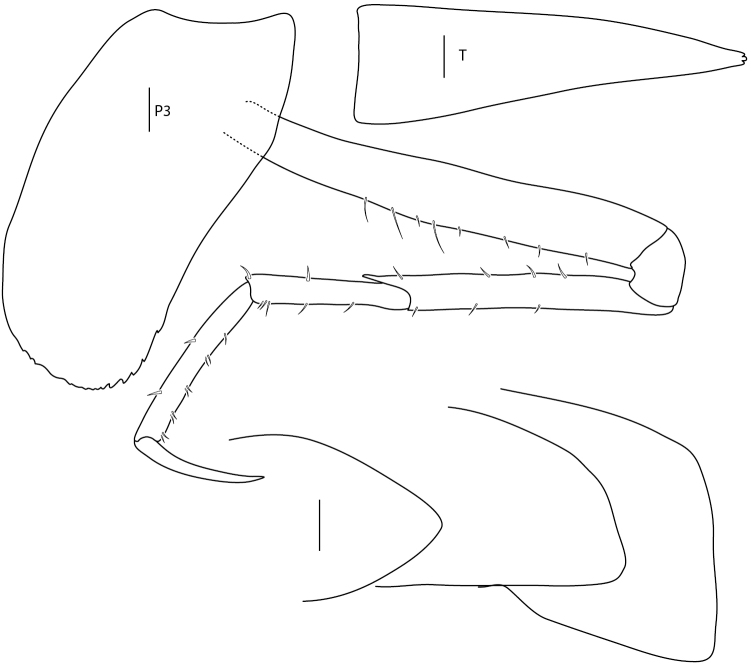
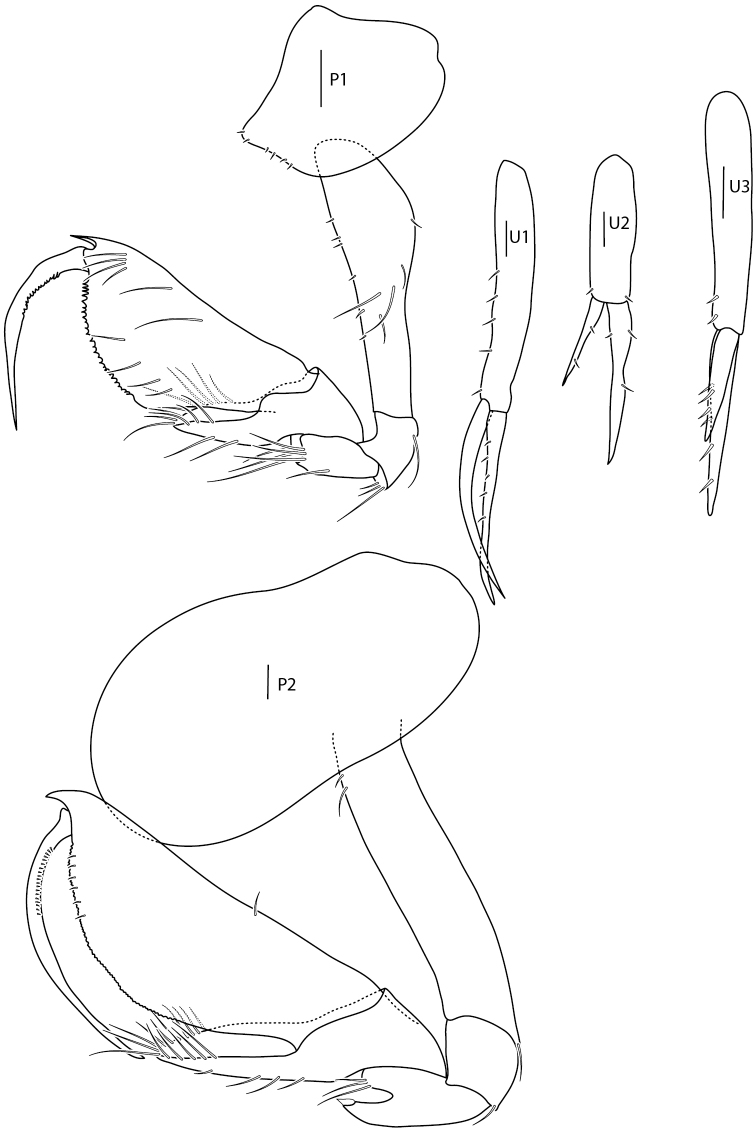
Amphilochus anoculus sp. n. Pereopods 5, 6 and 7, Epimeral plates, Uropods 1, 2 from ZMBN121952. Uropod 3 and telson from BioIce station 2367. Scale bars: 0.1 mm.
Figure 3.
Amphilochus anoculus sp. n. Pereopods 1, 2, 3 and 4. ZMBN121952. Pereopod 1 dactylus from ZMH K-47225. Scale bars: 0.1 mm.
Description.
Description is based on a composite of studied material. No observed sexual dimorphism.
Head. Rostrum subequal to peduncle article 1 of antenna 1, curved. Eyes absent. Cephalic lobes produced, broadly rounded, tips of mouthparts just visible under the edge of cephalon. Antenna 1 subequal to antenna 2; peduncle strong, longer than six-articulate flagellum; accessory flagellum absent. Setae on both peduncle and flagellum few and short. Antenna 2 peduncle longer than eight-articulate flagellum. Few and short setae distally on peduncle articles, all articles of the peduncle are longer than broad.
Labrum symmetrically bilobed. Mandible molar small but triturative, rounded cone-shaped, with setation on entire chewing area, which is ridged; incisor serrate; eleven accessory spines; palp slender, 3-articulate; article 1 is shorter than article 2, which is shorter than article 3; article 3 with setae; lacinia mobilis laterally expanded. Labium symmetrical, without inner lobes. Maxilla 1 palp biarticulate, with two apical setae; inner plate reduced, with one seta; outer plate with eight robust and six thinner setae. Maxilla 2 inner plate shorter than outer plate, nine setae on distal margin; outer plate long and thin with four distal setae. Maxilliped inner plate reaching end of merus, well separated, thin, two robust distal setae; outer plate reaches middle of carpus of palp, one robust seta and ridge of serrations; palp slim, heavily setulated on propodus.
Mesosome dorsally smooth; segment 3 is shorter than segment 4. Coxa 1 reduced and covered by coxa 2, which is longer than broad. Coxa 2 distal margin serrate and with setae. Coxa 3 and 4 distal margin not serrate, without setae. Coxa 5–7 concave.
Pereopod 1 basis longer than propodus, upper half distally widened, few and short setae; carpal lobe well developed, reaching 65% of posterior margin of propodus; propodus triangular; palm oblique, serrate with setae, no seta defining palmar corner, anterodistal tooth of medium size (half as long as the base of dactylus is broad); dactylus smooth with few, thin setae on inner margin. Pereopod 2 basis little longer than propodus, upper half not as widened distally as pereopod 1; carpal lobe covers all of posterior margin of propodus; propodus elongate, palm oblique, serrate with minute setae, no setae defining the palmar corner, anterodistal tooth well developed (same size as the breadth of the base of dactylus); dactylus inner margin weakly serrate on proximal half. Pereopod 3 missing in holotype. Pereopod 4 basis with four anterior setae, dactylus half-length of propodus. Pereopod 5 with posterior lobe on basis and merus. Pereopod 6 with posterior lobe on basis; posterior lobe on merus boat-shaped; carpus shorter than propodus; dactylus more than half length of propodus. Pereopod 7, posterior lobe on basis and merus, meral lobe covers 50% of carpus; dactylus more than half-length propodus.
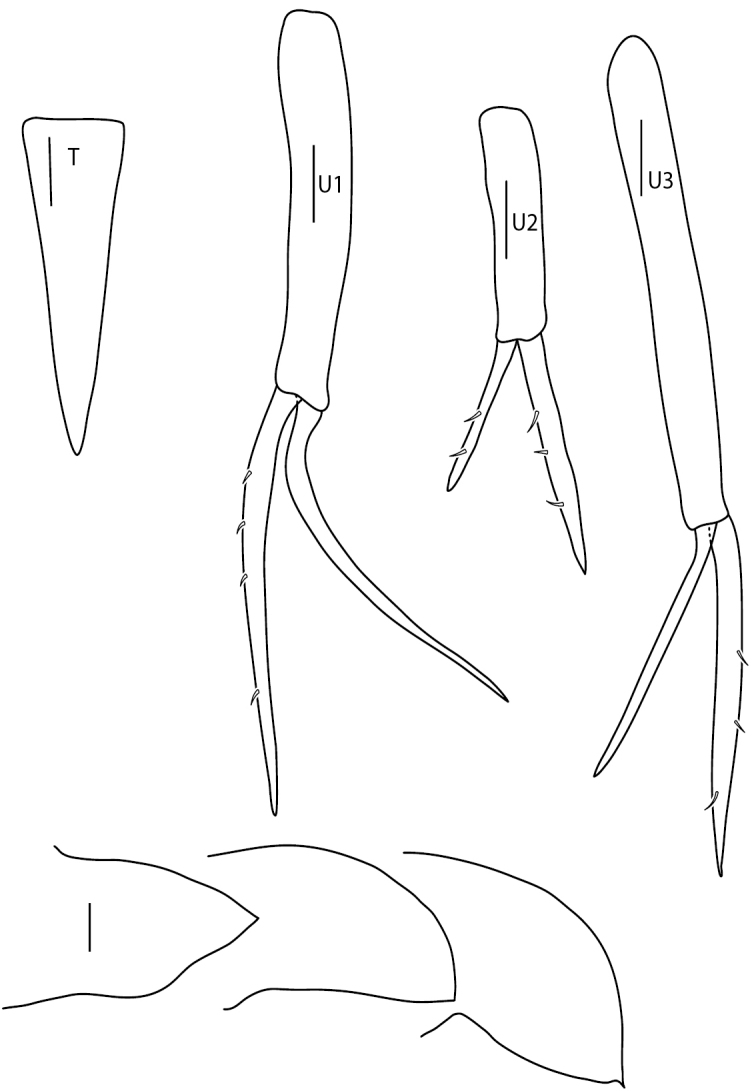
Metasome smooth. Epimeral plates 1 and 3 rounded; plate 2 right-angled. Urosome smooth; segment 1 long; segments 2 and 3 shorter. Uropod 1 peduncle longer than rami; outer ramus marginally longer than inner; three to four setae on outer margins. Uropod 2 peduncle longer than rami; outer ramus half-length of inner; setae on both rami. Uropod 3 peduncle with clear flange, smooth; outer ramus weakly shorter than inner ramus; uropod 3 longer than telson. Gills on segments 2 to 7; oostegites on segments 2 to 6. Telson elongate and boat-shaped; distal end entire, acute and with one seta.
Living colour. Semi-transparent, virtually colourless.
Distribution.
Iceland, Faroe Channel, Norwegian Sea, Polar basin. Has only been found in cold and deep water.
Remarks.
This species is easily recognized because it lacks eyes and has an anterodistal tooth on the propodi of pereopods 1 and 2. Amphilochus manudens and A. hamatus are the only other Amphilochidae having this tooth, but unlike Amphilochus anoculus sp. n., they both have eyes. The telson has a robust seta distally, a character not seen in any other Amphilochidae. The flange on the distal end of uropod 3 peduncle is also a good character-state to use when separating it from A. hamatus. A synoptic list of characters separating the three species is shown in Table 2.
Table 2.
Comparison of character states between Amphilochus anoculus sp. n., A. manudens, and A. hamatus.
| Character | Amphilochus anoculus sp. n. | Amphilochus manudens | Amphilochus hamatus |
| Cephalic lobes | rounded | acute | rounded |
| Labrum | symmetrically bilobed | asymmetrically bilobed | asymmetrically bilobed |
| Mandible | molar rounded | molar conical | molar conical |
| 1st Maxilla | palp 2-articulate | palp 2-articulate | palp 1-articulate |
| 2nd Maxilla | outer plate with 4 setae | outer plate with 3 setae | outer plate with 3 setae |
| Labium | tooth on inner edge of outer plate | no tooth on inner edge of outer plate | tooth on inner edge of outer plate |
| Eyes | absent | round, strongly coloured | ill defined, bean-shaped or oval |
| Gnathopod 2 | elongate | subtriangular | elongate |
| Oostegites on P6 | present | absent | present |
| Epimeral plate 1 | rounded | angular | angular |
| Epimeral plate 3 | rounded | with clear tooth | rounded |
| Uropod 3 | with flange on peduncle | no flange on peduncle | no flange on peduncle |
| Telson | tip with robust seta | tip smooth | tip tridentate – all lobes rounded. |
| Temperature (°C) | -0.6 to + 1.7 | +1.7 to + 7 | -0.85 to +7 |
| Depth (m) | 303m to 1055m | 81m to 350m (single specimens at 772m and 1390m) | 260m to 1407m |
Biology.
This species appears to be restricted to cold water (it is only found at a temperature range of -0.6 °C to +1.7 °C. Three stations from the Mareano-project have higher temperatures than this (stations R1180 RP86, R1200 RP90 and R1225 RP112). These are also the three of the shallowest stations where this species has been found, and constitute a statistical outlier in the dataset. They are all in the eastern Barents Sea, an area where winter-temperatures are much colder, and thus still might fall within the proposed ecological niche of the species. It has been found north and east of Iceland, south of the Faroe Islands, north in the Norwegian Sea and in the Polar basin, at depths ranging from 303 to 2100 meters. In contrast, the closely related Amphilochus manudens has, during BioIce, IceAGE, and several other collection efforts in the area been found mainly at depths from 81 to 360 meters, with single specimens found at 772 and 1390 meters (see Fig. 1 for specimens from BioIce and IceAGE). No Amphilochus manudens were found in the Faroe-samples from AkvaplanNiva.
Derivatio nominis.
The name anoculus (an = no, oculus = eye) refers to the absence of eyes. It is a noun in apposition.
Amphilochus manudens
Spence Bate, 1862
Amphilochus manudens Spence Bate, 1862:107, pl 17 fig 6; Sars 1890-95: 217, pl 74; Chevreux and Fage 1925: 114, fig 109; Lincoln 1979: 150, fig 65 e-f, fig 66 a-d; Krapp-Schickel 1982: 75, fig 51.
Remarks.
Although Amphilochus manudens is one of the best described species within the Amphilochidae (Sars 1890–95; Lincoln 1979; KrappSchickel 1982), we have included a redescription of material from Iceland, to facilitate direct comparison with the new species.
Material examined.
all drawings are made from specimens found during the BioIce program. For the complete set of drawings (Figs 5–8) we have used specimens IINH37889 (BioIce 2207), IINH37887 (BioIce 2215) and IINH37885 (BioIce 2237). Type material not examined. Additional material of Amphilochidae from a Statoil funded baseline survey of some Faroe waters has been examined, and only Amphilochus anoculus and Amphilochus tenuimanus were found. We have also examined all Amphilochidae from the BioFar program, and only Amphilochus manudens was found (no Amphilochus anoculus sp. n.). During a cruise in the Polar basin in 2005 both Amphilochus manudens and Amphilochus anoculus sp. n. were found, but at different stations (see discussion below). Material from several Norwegian surveys (summarised in the project NorAmph) and the IceAGE project included several Amphilochus manudens. For information about the specific sample-stations, see Table 1.
Figure 5.
Amphilochus manudens. Habitus and antennae. IINH37889. Scale bar habitus 0.5 mm, other scale bars 0.1 mm.
Figure 8.
Amphilochus manudens. Appendages from pleon and urosome. IINH37885. Scale bars: 0.1 mm.
Description.
Head. Rostrum curved, smaller than peduncle article 1 of antenna 1. Eyes round, no ommatidial framing, small, deep brown-red in colour. Cephalic lobe produced, distally acute. Antenna 1 subequal to antenna 2; peduncle article 1 is longer than article 2, which is longer than article 3; peduncle is longer than six-articulate flagellum; accessory flagellum absent. Antenna 2 peduncle longer than eight-articulate flagellum; peduncle articles have few short setae.
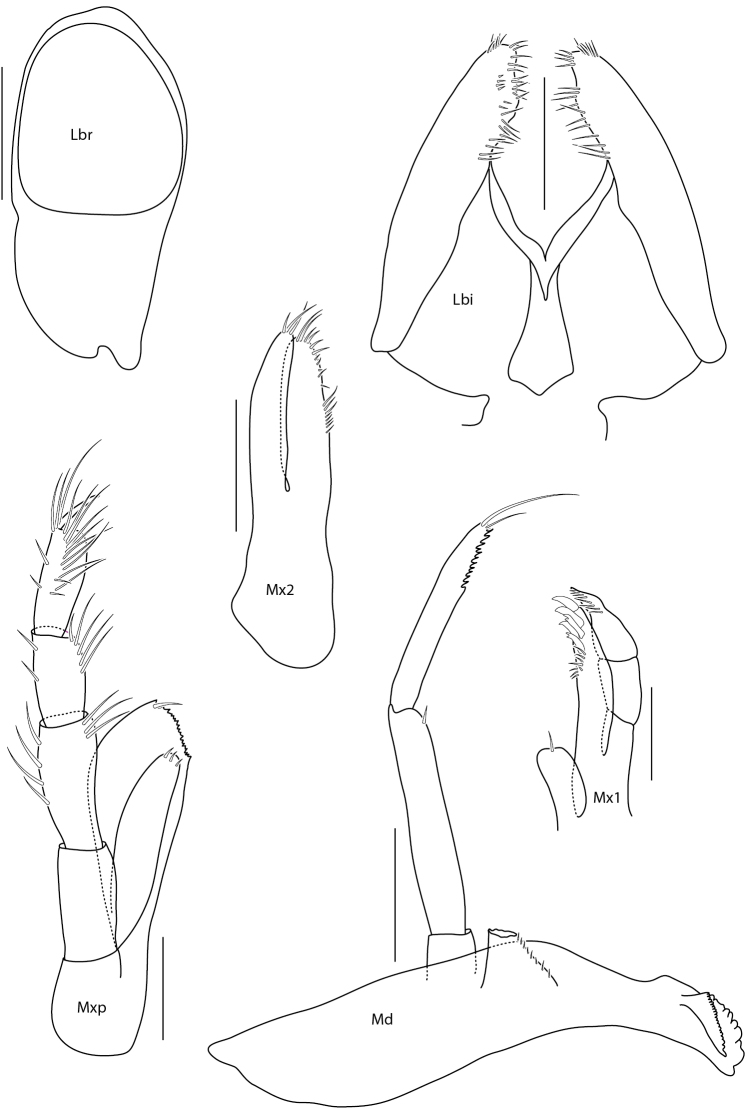
Labrum asymmetrically bilobed. Mandible molar small but triturative, cone-shaped, with a row of short setae around the ridged chewing area; incisor serrate; nine accessory spines; palp slender, 3-articulate; article 1 is shorter than article 2, which is longer than article 3; article 3 with two long setae distally and distal third of margin serrate; lacinia mobilis laterally expanded. Labium symmetric; inner lobes reduced. Maxilla 1 palp 2-articulate, with eight setae; inner plate reduced, with 1 seta; outer plate with six strong setae and two rows with four and three smaller setae. Maxilla 2 inner plate shorter than outer plate, six long setae distally and a row of five short, three long and five short setae; outer plate is long and thin with three distal setae. Maxilliped inner plate is long and thin, well separated, three short and strong setae distally; outer plate reaches just past merus of palp; palp slim, heavily setulated on carpus and propodus.
Mesosome dorsally smooth; length segment 3 is smaller than segment 4. Coxa 1 reduced and covered by coxa 2, which is longer than broad. Coxa 2 distal margin serrate, with setae. Coxa 3 concave; distal margin serrate, without setae. Coxa 4 distal margin serrate; without setae. Coxa 5–7 concave.
Pereopod 1 basis longer than propodus, upper half distally widened, few and short setae on anterior margin, longer setae on posterior margin; carpal lobe well developed, 50% of posterior margin of propodus; propodus subtriangular, proximal half of oblique palm serrate, distal half with short evenly spaced setae, no seta defining palm, anterodistal tooth strong; dactylus longer than palm, narrow and acute, apparently smooth. Pereopod 2 basis longer than propodus, linear, several short setae; one robust seta distally on ischium; merus with small distal ‘hook’; carpal lobe covers 100% of posterior margin of propodus, lined with setae posteriorly, small crown of setae distally; propodus elongate with a regularly convex serrate palm without seta, anterodistal tooth strong; dactylus longer than palm, narrow, apparently smooth. Pereopod 3 coxa elongate, pereopod 4 coxa posteriorly produced, both with basis to propodus anterior edge lined with short setae, dactylus more than half propodus. Pereopod 5 to 7 basis and merus with posterior lobes; carpus shorter than propodus; dactylus longer than half propodus.
Metasome smooth. Epimeral plate 1 with small, blunt posterodistal tooth, distal margin convex; plate 2 angular, distal margin convex; plate 3 with clear posterodistal tooth, distal margin weakly concave. Urosome smooth; segment 1 as long as segments 2 and 3 together. Uropod 1 peduncle and rami subequal; rami subequal; setae on outer ramus. Uropod 2 peduncle subequal to inner ramus; outer ramus about half-length of inner ramus; setation on both rami. Uropod 3 peduncle longer than rami; outer ramus is shorter than inner ramus; rami longer than telson; rami with setae.
Gills on segments 2 to 6. Oostegites on segments 2 to 5. Telson elongate; distal end entire and acute; no setae.
Figure 7.
Amphilochus manudens. Pereopods. IINH37885. Scale bars: 0.1 mm.
Distribution.
North East Atlantic and Arctic Ocean (Lincoln, 1979); Barents Sea and Murmansk area (Gurjanova 1951; Vader and Bryazgin 1998; Vader et al. 2001); Spitsbergen (Stephensen 1935; Vader et al. 2001); Mediterranean (Marseilles, Capri) (Krapp-Schickel 1982); amphi-Atlantic (Watling 1979); Gulf of St Lawrence (Brunel et al. 1998).
Amphilochus hamatus
(Stephensen, 1925) comb. n.
Amphilochopsis hamatus Stephensen, 1925: 173, figs 52–53; Gurjanova 1951: 402, fig. 246; Barnard and Karaman 1991: 95.
Material examined.
Drawings are made from IINH37894 (BioIce 2077), IINH37898 (BioIce 2236), IINH37900 (BioIce 2318) and IINH37903 (BioIce 2367). Material from IceAGE and NorAmph has been used for molecular sequencing and comparisons. For a list of stations for the material, see Table 1. Type material not examined. The drawings are shown on Figs 9–12.
Figure 9.
Amphilochus hamatus. Habitus. IINH37900. Scale bar: 0.5 mm.
Figure 12.
Amphilochus hamatus. Pereopod 3, epimeral plates, telson. IINH37898 and IINH37894. Scale bars: 0.1 mm.
Description.
Head. Rostrum curved, reaches tip of article 1, antenna 1. Eyes not evident, but an ill-defined eye-patch can be seen. Cephalic lobe produced distally and rounded. Antenna 1 shorter than antenna 2; second peduncle-article with a triangular production on the apex the size of third peduncle article; peduncle subequal to ten-articulate flagellum; no accessory flagellum. Antenna 2 peduncle longer than flagellum; short setae on peduncle, and a pair of long setae at tip of flagellum.
Labrum asymmetrically bilobed. Mandible molar small but triturative, cone-shaped; incisor serrate; ten accessory spines; palp slender, 3-articulate with series of short setae on article 3, one long seta at tip; lacinia mobilis laterally expanded. Labium symmetric, with inner lobe reduced; sharp tooth making tip of outer lobe look dentate. Maxilla 1 palp 1-articulate, with a crown of two robust setae and a serrate distal margin; inner plate reduced, 1 seta; outer plate with four and eight heavy and five smaller setae. Maxilla 2 long and thin; inner plate shorter than outer plate; three heavy setae and eight smaller setae on outer plate; inner plate with seven short setae distally and one to two thin setae medially. Maxilliped inner plate small and thin, well separated, just reaching past ischium; outer plate reaching mid-merus, two strong setae distally, serrations on inner margin; palp slim, heavily setulated on carpus and propodus.
Mesosome dorsally smooth; segment 3 shorter than segment 4. Coxa 1 reduced, subquadratic and covered by coxa 2, which is longer than broad. Coxa 2 distal margin smooth, no setae. Coxa 3 concave, smooth, with setae on distal margin. Coxa 4 distal margin smooth with setae. Coxa 5–7 concave.
Pereopod 1 basis subequal to propodus length, upper half distally widened, three long setae posteriorly; carpal lobe 65% of propodus posterior margin; propodus subtriangular, palm oblique, serrate, no setae defining palmer corner, anterodistal tooth of medium size; dactylus with inner margin partly serrate. Pereopod 2 basis weakly longer than propodus, linear; merus with a clearly defined “hook” on posterior side, close to carpus; carpal lobe 100% of propodus posterior margin, boat-shaped with a row of setae on margin; propodus subovate, palm oblique, defined mostly by its serration and upper third with small setae, no setae defining palmar corner, anterodistal tooth large (same size as breadth of the base of dactylus); dactylus inner margin with a row of small and strong setae, otherwise smooth. Pereopod 3 posterior margin of basis with an even row of slender setae; merus with small lobe. Pereopod 4 basis with very thin setae. Pereopods 5 and 6 basis and merus with posterior lobes; dactylus longer than half-length propodus. Pereopod 7, basis and merus with posterior lobes.
Metasome smooth. Epimeral plates rounded, no teeth. Urosome smooth; segment 1 long; segments 2 and 3 short. Uropod 1 peduncle subequal to rami; rami subequal; both peduncle and outer ramus with marginal setae. Uropod 2 peduncle subequal to inner ramus; outer ramus half-length of inner ramus; setation on peduncle and rami. Uropod 3 peduncle marginally longer than rami; outer ramus half-length of inner ramus; rami shorter than telson; setation on peduncle and rami. Gills on segments 2 to 6. Oostegites on segments 2 to 6. Telson elongate, longer than broad; distal end entire and tridentate, all lobes rounded; no setae.
Figure 10.
Amphilochus hamatus. Mouthparts. IINH37894. Scale bars: 0.1 mm.
Figure 11.
Amphilochus hamatus. Pereopods 1 and 2, uropods. IINH37898 and IINH37903. Scale bars: 0.1 mm.
Distribution.
This species appears to have a wide depth range based on our collections (206 to 1407 m), although Stephensen (1925) found it only in deep water (700 to 2702 m). The temperatures it has been found at range from -0.6 to +7.0 °C. It is also recorded from the deep Norwegian Sea (Dahl 1979), the Arctic basin (Gurjanova 1951), Greenland (Brandt et al. 1996) and the deep polar basin (Tzvetkova and Golikov 2001).
Discussion
Genetic delimitation of the species
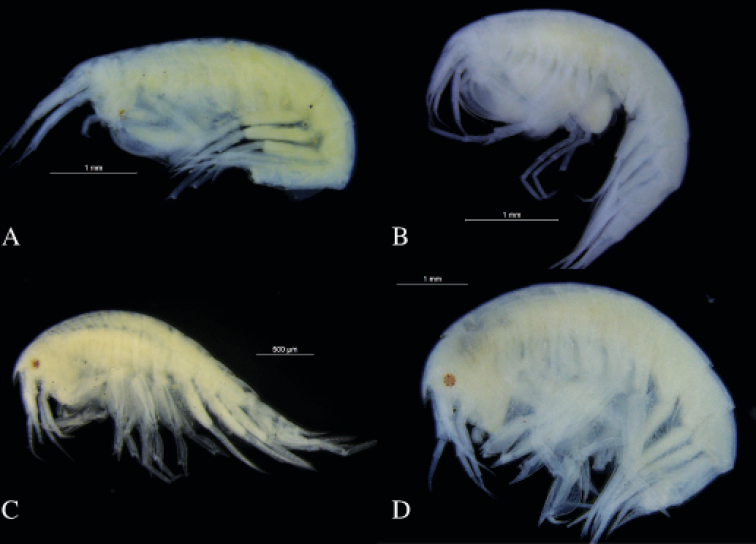
Examinations of the COI-gene (Folmer segment) of Amphilochus manudens, Amphilochus anoculus sp. n. and Amphilochopsis hamatus from both IceAGE (Icelandic waters) and NorBol (Norwegian waters) show a clear separation of the new species Amphilochus anoculus from other Amphilochidae tested. (Jazdzewska et al. 2018; NorAmph in Barcode of Life Project (BOLD) www.boldsystems.org). Using Barcode Identification Numbers (BIN) to make a quick check on species delimitation gives four different BINs for Amphilochus manudens from the two projects, as well as separate BINs for Amphilochus anoculus and Amphilochopsis hamatus. It has, however, been very difficult to get good sequences for A. anoculus; after thorough scrutiny we only found one non-ambiguous sequence. Many of our discarded sequences were removed from the analyses from being too short, but the parts we have are identical to the full COI-sequence we tested, and that thoroughly separates it from all clades of A. manudens and A. hamatus. Calculating the distance between groups using Mega7 (Kumar et al. 2015) shows this (Table 3), even though it must be noted that since A. manudens separated into several clades, the within-distance for this group was also very large (0.283). Clearly, a more thorough genetic analysis and possibly a larger sample-pool (especially a larger genetic sample pool) will reveal if we have further new species to be separated from Amphilochus manudens, but for this study it will suffice to note that Amphilochus manudens may constitute a species complex. Specimens of Amphilochus manudens assigned to two of the different BINs as well as Amphilochus anoculus sp. n. and Amphilochopsis hamatus are photographed (Fig. 13).
Table 3.
P-distances between groups (species) of Amphilochidae from NorAmph and IceAGE projects.
| Amphilochoides boecki | 0,426 | |||||
| Amphilochus anoculus | 0,343 | 0,359 | ||||
| Amphilochus hamatus | 0,331 | 0,302 | 0,196 | |||
| Amphilochus manudens | 0,388 | 0,400 | 0,335 | 0,279 | ||
| Amphilochus sp1 | 0,336 | 0,317 | 0,223 | 0,095 | 0,238 | |
| Amphilochus tenuimanus | 0,357 | 0,327 | 0,312 | 0,285 | 0,378 | 0,294 |
Figure 13.
Photographs of habitus A Amphilochus anoculus sp. n. ZMBN_104532 B Amphilochus hamatus ZMBN_104479 C Amphilochus manudens ZMBN_103989 D Amphilochus manudens UMBergen_NBamph_123.
The status of the genus Amphilochopsis Stephensen, 1925
The genus Amphilochopsis was erected by Stephensen (1925) for the species A. hamatus. Stephensen wrote: ‘The present genus is very closely allied to Amphilochus, but is characterised especially in having the molar of the maxillae (sic!) well developed (but not very large) and in having only one joint in the palp of maxilla 1’.The type species of the relatively large (Barnard and Karaman 1991) and probably not monophyletic (Hoover and Bousfield 2001) genus Amphilochus Spence Bate, 1862 is Amphilochus manudens Spence Bate, 1862; this species is usually described as having a non-triturative molar on the mandible, but in reality the molar, although much reduced in size and conical in form, has a small flat triturating surface on top (see Fig. 6). Amphilochus manudens has a 2-articulate palp on maxilla 1. Later authors have often allied the genus Amphilochopsis with the basic amphilochid genus, the also extremely variable Gitanopsis G.O. Sars. This is probably mainly because in keys to the genera the first dichotomy usually concerns the molar, and Amphilochopsis is deemed to have a well-developed molar, while Amphilochus is judged to have a feebly developed, non-triturative molar. Thus Barnard and Karaman (1991) write in their monograph for Amphilochopsis sub ‘Relationship’: ‘Differing from Gitanopsis in the 1-articulate palp of maxilla 1’, and also Hoover and Bousfield (2001), in their phenograms, ally Amphilochopsis closely to Gitanopsis.
Figure 6.
Amphilochus manudens. Mouthparts. IINH37887. Scale bars: 0.1 mm.
In reality the molar of Amphilochus manudens is only quantitatively different from that of Amphilochopsis hamatus, with the new species A. anoculus in an intermediate position between the two. These molars are completely different from the well-developed cylindrical molars of Gitanopsis and Gitana species, as well as from the almost completely reduced molars of many other species in Amphilochus s.l. A number of other species in Amphilochus s. l., e.g. the west Atlantic A. casahoya and A. delacaya, both described by McKinney (1978), and the Hawaiian species described by Barnard in 1970, have the same type of ‘intermediate’ molar as A. manudens.
Amphilochopsis hamatus has a clearly 1-articulate palp on mx 1, while all Amphilochus species that we have seen have a 2-articulate palp. This type of character-state has been used extensively elsewhere in the division of genera in the Amphilochidae (cf. the discussion in Barnard (1962)). We feel, however, that this difference alone is not sufficient to warrant a separate genus for A. hamatus, especially as the articulation of the palp in some Amphilochus species, i.e., A. anoculus, is not always easy to perceive and may even be incomplete.
As shown by Hoover and Bousfield (2001) who in their ‘partial revision’ split up Amphilochus s. l. and erected the genus Apolochus for some of its species, Amphilochus s. l. is definitely not a monophyletic genus, and is in great need of a complete revision. A preliminary phylogenetic analysis of amphilochid species, based on literature data (Tandberg 2000) came to the same conclusion: species of Amphilochus and Gitanopsis were scattered over the entire cladogram. The cladogram did, however, show a clear clade around Amphilochus manudens, the type species of Amphilochus, and thus Amphilochus s. str.: this clade included, besides A. manudens, the new species A. anoculus, A. opunake Barnard, 1972 from New Zealand, the Mediterranean A. planierensis Ledoyer, 1977, and Amphilochopsis hamatus.
An easily observed and spectacular character of A. hamatus is the characteristic hook on the merus of P2, from which its name is derived. However, this same hook occurs, albeit in greatly reduced form, in both A. manudens and A. planierensis. The new species described above, A. anoculus, also has a meral hook on P2; this is another character where the character state present in A. anoculus falls between the more extreme versions of the states in A. manudens and A. hamatus. We therefore do not think the meral hook on P2 to be of more than specific value.
For these reasons, we have decided to transfer Amphilochopsis hamatus to Amphilochus s. str. and to submerge the genus Amphilochopsis as a junior synonym of Amphilochus s. str.
Ecology of the species
In Icelandic waters, Amphilochus manudens and Amphilochus hamatus seem to be confined to shallower and warmer waters. The only parameter that seems to be limiting is temperature – they are only found in “warm” waters: + 1,7˚C to +7˚C. Amphilochus manudens is common, and from the literature known to be found mostly on gravel and silty sand, and on hydroids (Jones 1948; Schellenberg 1942).
Given the distribution-data on Amphilochus manudens from BioIce, BioFar, IceAGE and other studies in the Faroe channel and our surveys in the Norwegian Sea and Polar basin, it seems that Amphilochus anoculus sp. n. replaces Amphilochus manudens in cold waters. Temperatures for the stations in the Faroe channel and a few in the Norwegian Sea were not reported, but Westerberg (1990) has shown that the general benthic temperatures in this area are always lower than 0.5 °C and temperatures in the deep waters of the Norwegian Sea are lower than 1 °C, which supports our hypothesis that A. anoculus replaces A. manudens at temperatures below 1.7 °C.
Key to Amphilochidae in the North-East Atlantic
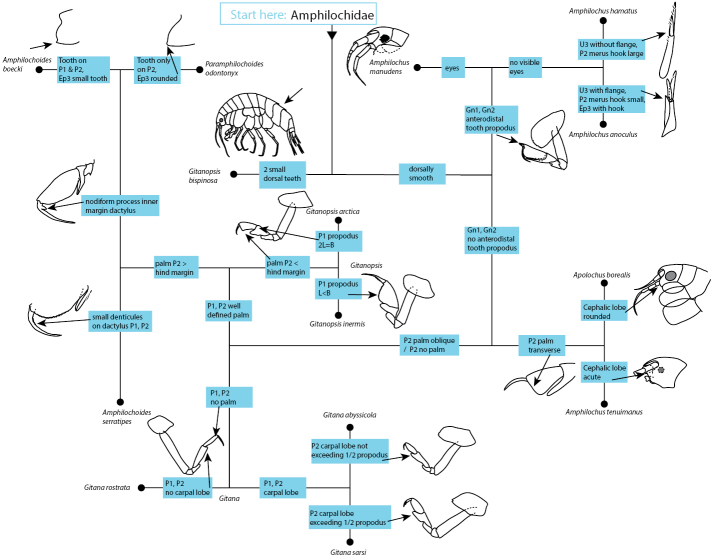
A pictorial key, loosely based on Stephensen (1935) with the new species added, is shown in Fig. 14.
Figure 14.
Pictorial key to Amphilochidae in the NE Atlantic.
Supplementary Material
Acknowledgements
The work on this new species has been financially supported by the Norwegian Biodiversity Information Centre and the Volkswagen Fund. The material has been submitted by the BioIce, IceAGE and Mareano programmes, in addition to collections at University Museum of Bergen, Tromsø University Museum, the University Centre at Svalbard and Akvaplan-niva – all of which have been kind and helpful with sharing their specimens. A. Fischer at the DZMB, Hamburg and J. Kongsrud at the University Museum of Bergen have helped with curatorial work. Dr A. Jazdzewska is thanked for her work with the barcodes of the IceAGE-material of the Amphilochidae, NorBOL has facilitated barcoding of Norwegian specimens, and N. Gatzemeier at the DZMB, Hamburg, has produced an invaluable extra set of sequences. Prof E. Willassen and Dr M. Stokkan helped with sequence-analyses. Dr J. Berge was a great help in the initial part of this work. We thank Dr K. White and Dr A. Myers for kind and thoughtful reviews and comments that helped improve this manuscript significantly.
Citation
Tandberg AHS, Vader W (2018) On a new species of Amphilochus from deep and cold Atlantic waters, with a note on the genus Amphilochopsis (Amphipoda, Gammaridea, Amphilochidae). In: Brix S, Lörz A-N, Stransky B, Svavarsson J (Eds) Amphipoda from the IceAGE-project (Icelandic marine Animals: Genetics and Ecology). ZooKeys 731: 103–134. https://doi.org/10.3897/zookeys.731.19899
Funding Statement
Norwegian Biodiversity Initiative
References
- Barnard JL. (1962) Benthic marine Amphipoda of southern California: families Amphilochidae, Leucothoidae, Stenothoidae, Argissidae, Hyalidae. Pacific Naturalist 3: 116–163. [Google Scholar]
- Barnard JL. (1970) Sublittoral Gammaridea (Amphipoda) of the Hawaiian Islands. Smithsonian Contributions to Zoology 34: 1–296. https://doi.org/10.5479/si.00810282.34 [Google Scholar]
- Barnard JL, Karaman GS. (1991) The families and genera of marine gammaridean Amphipoda (except marine gammaroids). Part 1. Records of the Australian Museum Supplement 13(1): 1–417. https://doi.org/10.3853/j.0812-7387.13.1991.91 [Google Scholar]
- Berge J, Vader W. (1997) Stegocephalid (Crustacea, Amphipoda) species collected in the BIOFAR and BIOICE programmes. Sarsia 82: 347–370. https://doi.org/10.1080/00364827.1997.10413662 [Google Scholar]
- Boeck A. (1871) Crustacea Amphipoda Borealia et Arctica. Videnskabs Selskapets forhandlinger, Kristiania 1870: 1–222. [Google Scholar]
- Boeck A. (1873) De skandinaviske og arktiske amfipoder. Christiania, 864 pp.
- Brandt A, Vassilenko S, Piepenburg D, Thurston M. (1996) The species composition of the Peracarid fauna (Crustacea, Malacostraca) of the Northeast Water Polynya (Greenland). Meddelelser om Grønland Bioscience 44: 1–30. [Google Scholar]
- Brix S, Meißner K, Stransky B, Halanych KM, Jennings RM, Kocot K, Svavarsson J. (2014) The IceAGE project – a follow up of BIOICE. Polish Polar Research 35(2): 141–150. [Google Scholar]
- Brix S, Lörz A-N, Jażdżewska AM, Hughes L, Tandberg AHS, Pabis K, Stransky B, Krapp-Schickel T, Sorbe JC, Hendrycks E, Vader W, Frutos I, Horton T, Jażdżewski K, Peart R, Beermann J, Coleman CO, Buhl-Mortensen L, Corbari L, Havermans C, Tato R, Campean AJ. (2018) Amphipod family distributions around Iceland. In: Brix S, Lörz A-N, Stransky B, Svavarsson J. (Eds) Icelandic marine Animals: Genetics and Ecology (IceAGE Amphipoda project). ZooKeys 731: 41–53. https://doi.org/10.3897/zookeys.731.19854 [DOI] [PMC free article] [PubMed]
- Brunel P, Bossé L, Lamarche G. (1998) Catalogue of the Marine Invertebrates of the Estuary and Gulf of Saint Lawrence. Canadian Special Publication of Fisheries Aquatic Sciences 126: 1–405. [Google Scholar]
- Buhl-Mortensen L, Hodnesdal H, Thorsnes T. (Eds) (2015) The Norwegian Sea Floor – New Knowledge from MAREANO for Ecosystem-Based Management. MAREANO, 192 pp.
- Chevreux ME, Fage L. (1925) Amphipodes. Faune de France 9: 1–487. [Google Scholar]
- Coleman CO. (2003) “Digital inking” How to make perfect line drawings on computers. Organisms Diversity & Evolution 3(4): 1–14. https://doi.org/10.1078/1439-6092-00081 [Google Scholar]
- Coleman CO. (2009) Drawing setae the digital way. Zoosystematics and Evolution 85(2): 305–310. https://doi.org/10.1002/zoos.200900008 [Google Scholar]
- Dahl E. (1979) Amphipoda Gammaridea from the deep Norwegian Sea. A preliminary report. Sarsia 64: 57–59. https://doi.org/10.1080/00364827.1979.10411363 [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3(5): 294–299. [PubMed] [Google Scholar]
- Gurjanova EF. (1951) [Amphipods of the USSR seas and adjacent waters (Amphipoda - Gammaridea). ] Izdadelstvo Akademii Nauk SSSR 41: 1–1031. [In Russian] [Google Scholar]
- Hoover PM, Bousfield EL. (2001) The amphipod superfamily Leucothoidea on the Pacific coast of North America: Family Amphilochidae: Systematics and distributional ecology. Amphipacifica 3(1): 3–28. [Google Scholar]
- Horton T, Lowry J, De Broyer C, Bellan-Santini D, Coleman CO, Daneliya M, Dauvin JC, Fišer C, Gasca R, Grabowski M, Guerra-García JM, Hendrycks E, Holsinger J, Hughes L, Jaume D, Jazdzewski K, Just J, Kamaltynov RM, Kim YH, King R, Krapp-Schickel T, LeCroy S, Lörz AN, Senna AR, Serejo C, Sket B, Tandberg AH, Thomas J, Thurston M, Vader W, Väinölä R, Vonk R, White K, Zeidler W. (2017) World Amphipoda Database. http://www.marinespecies.org/amphipoda [on 2017–07–20]
- Jażdżewska AM, Corbari L, Driskell A, Frutos I, Havermans C, Hendrycks E, Hughes L, Lörz A-N, Stransky B, Tandberg AHS, Vader W, Brix S. (2018) A genetic fingerprint of Amphipoda from Icelandic waters – the baseline for further biodiversity and biogeography studies. In: Brix S, Lörz A-N, Stransky B, Svavarsson J. (Eds) Icelandic marine Animals: Genetics and Ecology (IceAGE Amphipoda project). ZooKeys 731: 55–73. https://doi.org/10.3897/zookeys.731.19931 [DOI] [PMC free article] [PubMed]
- Jones NS. (1948) The ecology of the Amphipoda of the South of the Isle of Man. The Journal of the Marine Biological Association of the United Kingdom 27: 400–439. https://doi.org/10.1017/S0025315400025455 [Google Scholar]
- Krapp-Schickel G. (1982) Family Amphilochidae. In: Ruffo S. (Ed.) The Amphipoda of the Mediterranean. Family Amphilochidae. Mémoires de l’Institut Océanographique 13: 70–93.
- Kumar S, Stecher G, Tamura K. (2015) MEGA7: Molecular Evolutionary GEnetic Analysis version 7.0. Molecular Biology and Evolution. http://www.megasoftware.net [DOI] [PMC free article] [PubMed]
- Ledoyer M. (1977) Contribution à l´étude de l´écologie de la faune vagile profonde de la Méditerranée nord occidentale. I. Les Gammariens (Crustacea, Amphipoda). Bolletino del Museo Civico di Storia Naturale di Verona 4: 321–421. [Google Scholar]
- Lörz A-N, Tandberg AHS, Willassen E, Driskell A. (2018) Rhachotropis (Eusiroidea, Amphipoda) from the North East Atlantic. In: Brix S, Lörz A-N, Stransky B, Svavarsson J. (Eds) Icelandic marine Animals: Genetics and Ecology (IceAGE Amphipoda project). ZooKeys 731: 75–101. https://doi.org/10.3897/zookeys.731.19814 [DOI] [PMC free article] [PubMed]
- Lincoln RJ. (1979) British Marine Amphipoda: Gammaridea. British Museum (Natural History), London, 658 pp. [Google Scholar]
- McKinney LD. (1978) Amphilochidae (Crustacea: Amphipoda) from the western Gulf of Mexico and Caribbean Sea. Gulf Research Reports 6: 137–143. https://doi.org/10.18785/grr.0602.04 [Google Scholar]
- Mannvik HP, Pettersen A, Carrol JL. (2002) Environmental baseline survey of the Faroe offshore licence areas 001–004 in the Faroe-Shetland Channel, 2001. Akvaplan-niva reports, APN-411.2201.03.
- Nørrevang A, Brattegard T, Josefson AB, Sneli JA, Tendal OS. (1994) List of BioFar stations. Sarsia 79: 165–180. https://doi.org/10.1080/00364827.1994.10413557 [Google Scholar]
- Sars GO. (1890–95) Amphipoda. An Account of the Crustacea of Norway With Short Descriptions and Figures of All the Species, 1, Al. Cammermeyer, Christiania, 717 pp. [Google Scholar]
- Schellenberg A. (1942) Krebstiere oder Crustacea. IV. Flohkrebse oder Amphipoda. Die Tierweld Deutschlands 40: 1–252. [Google Scholar]
- Spence Bate C. (1862) Catalogue of the specimens of amphipodous Crustacea in the collection of the British Museum. British Museum of Natural History, London.
- Stebbing TRR. (1876) On some new and little-known amphipodous Crustacea Annals and Magazine of Natural History, series 4, 18: 443–449. https://doi.org/10.1080/00222937608682076
- Stebbing TRR. (1906) Das Tierreich. EIne Zusammenstellung und Kennzeichning der rezenten Tierformen. Amphipoda 1. Gammaridea, 1–854.
- Stephensen K. (1925) Crustacea Malacostraca. VI. (Amphipoda. II.). The Danish Ingolf-Expedition 3: 101–178. [Google Scholar]
- Stephensen K. (1935) The Amphipoda of N. Norway and Spitsbergen with adjacent waters. Tromsø Museums Skrifter 3: 1–140. [Google Scholar]
- Tandberg AHS. (2000) Studies on Amphilochidae (Amphipoda, Gammaridea), With a Phylogenetic Analysis of the Family, and a Description of a New Species. MSc Thesis, Univ of Tromsø: 1–82.
- Tzvetkova NL, Golikov AA. (2001) List of species of free-living invertebrates of Eurasian Arctic Seas and adjacent deep water. Amphipoda, (Explorations of the seas) 51: 79–94. [Google Scholar]
- Vader W, Brattegard T, Buhl-Mortensen L, Miskov Larsen K. (2001) Chapter “Amphipoda, suborder Gammaridea”. In: Brattegard T, Holthe T (2001) Distribution of marine benthic macroorganisms in Norway. A tabulated catalogue. Update of Direktoratet for Naturforvaltning Utredning 1997–1: 183–207.
- Vader W, Bryazgin VF. (1998) Karelian-Norwegian Cooperation on the Crustacea Amphipoda of the Barents Sea Area, With a Preliminary Checklist of Amphipoda Gammaridea. Proceedings of the Scientific Conference held in Karelian Research Centre RAS within the framework of the Days of Norway in Republic of Karelia, Petrozavodsk, 28–31 May, 1997.Russian Academy of Science Karelian Research Centre, 40–45.
- Watling L. (1979) Zoogeographic affinities of northeastern North American gammaridean Amphipoda. Bulletin of the Biological Society of Washington 3: 256–282. [Google Scholar]
- Westerberg H. (1990) Benthic Temperature in the Faroe Area. Report from the Department of Oceanography, University of Gothenburg 51: 1–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.