Abstract
We previously demonstrated that pancreatic stellate cells within pancreatic ductal adenocarcinoma (PDAC) stroma secrete lumican and its presence is associated with prolonged survival of patients with localized PDAC. Here, we observed that extracellular lumican decreases PDAC tumour cell growth in xenograft and syngeneic orthotopic animal models, and induces growth inhibition of low-passage human PDAC cells in a species-specific manner. PDAC cells grown in variant culture conditions and exposed to extracellular lumican display typical characterizations of cancer cell in a quiescent state, such as growth inhibition, apoptosis, G0/G1 arrest and chemoresistance. Importantly, extracellular lumican is associated with diminished ERK1/2 phosphorylation and increased p38 phosphorylation within PDAC cells. We further demonstrated that extracellular lumican physically binds with EGFR to trigger EGFR internalization and downregulation of EGFR and its downstream signal molecule ERK. Lumican enhances casitas B-lineage lymphoma expression, which stabilized the TGFβ Type II receptor sensitizing PDAC cells to TGFβ-mediated activation of p38 and SMAD signals. These provide a mechanism for the shift in signalling and phenotypic changes we observed after prolonged exposure to lumican. Together, our findings demonstrate that stromal lumican restrains PDAC cell growth through mediating cell entry into a quiescent state.
INTRODUCTION
It is increasingly evident that the genetic events necessary for development and expansion of a pancreatic ductal adenocarcinoma (PDAC) primary tumour occur over more than a decade before most patients are clinically diagnosed.1 Identifying the role of these genetic molecules in tumour progression could be beneficial for PDAC treatment and prevention.
During tumour progression, PDAC cells come in contact with the rich surrounding stroma triggering a stromal reaction, leading to formation of a tumour-specific microenvironment, which may play either a restrictive role or supportive role in the growth and progression of the tumour.2–4 Lumican, a small leucine-rich proteoglycan of the extracellular matrix, has been shown to regulate tissue repair and tumour cell behaviour.5,6 Because of the complexity and diversity of its proteoglycan structure, lumican is capable of influencing cell function through a variety of mechanisms in different types of tumours.7–9 Within PDAC tumours, lumican transcript and protein can be identified in cancer cells and stromal tissues surrounding the tumour.10 The role of lumican in pancreatic cancer is still controversial, and is dependent on the growth stage of the primary tumour. Ishiwata et al7 evaluated tissues from 53 patients with invasive PDAC (81% stage III or IV and 19% stage I or II), and found that patients with lumican-positive stromal cells have a shorter survival than the patients with lumican-negative stromal cells, indicating that lumican fosters PDAC aggressiveness. However, we previously reported that patients (stage I – II, smaller tumours) with stromal lumican were less likely to experience metastasis after surgery and experience a threefold longer survival than the patients without stromal lumican,10 suggesting that stromal lumican may play a restrictive role in regulating PDAC cell growth in early stage.
Here we report results from studies using syngeneic mouse PDAC models in lumican knock-out mice, patient-derived xenografts (PDX) and low-passage primary human PDAC cell lines and designed to further explore the function of extracellular lumican in PDAC. Through these experimental models we determined that extracellular lumican directly interacts with PDAC cells to inhibit replication and restrain PDAC tumour expansion.
RESULTS AND DISCUSSION
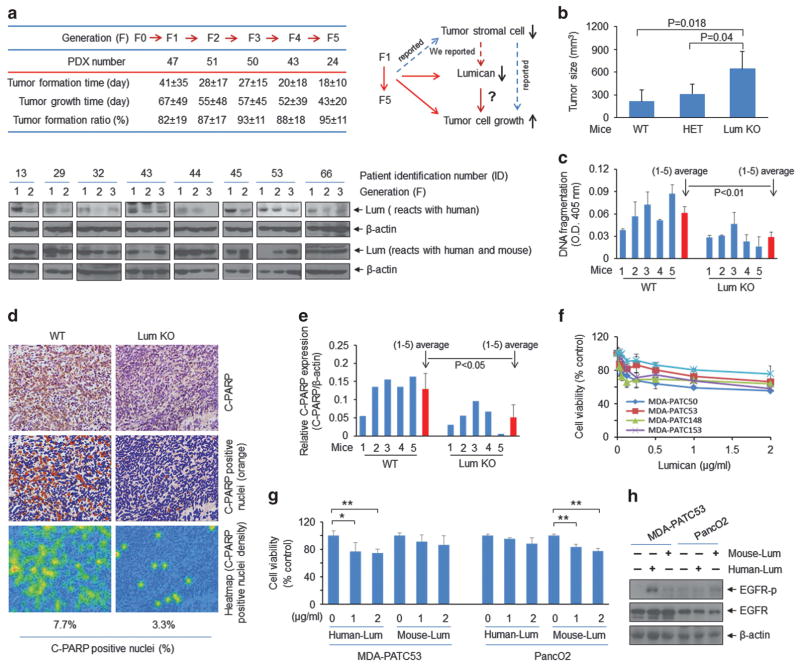
We first observed that the time to tumour identification and tumour harvest decreased as PDX tumours passed from the F1 to the F5 generation; we observed that tumour lumican expression within PDX diminished concurrently (Figure 1a, lower-human lumican antibody). The inverse association between lumican and tumour progression suggested that lumican and other human stromal elements that restrain PDAC cell growth decrease as PDX tumours pass through mice. This finding is consistent with our previous studies of PDX grown in fluorescent nude mice and other recent publications demonstrating that human tumour-derived stromal fibroblasts are lost and concomitantly replaced by mouse-derived stromal fibroblasts during each PDX tumour passage.11,12 Furthermore, numerous publications support the concept that stromal fibroblasts and fibrosis can prevent cancer from spreading.2,4 Our recent work demonstrated that activated pancreatic stellate cells within the tumour are a principle source of stromal lumican and the presence of lumican within the stroma of primary PDAC tumours is associated with decreased metastasis and prolonged survival.10,13 Based on these results, we investigated whether the absence of host lumican would influence PDAC growth (Figure 1a, upper-right). Using a syngeneic orthotopic pancreatic tumour model, we compared growth of PanO2 cells within the pancreas of wild-type C57B6 mice versus those with loss of one or both copies of the LUM gene. PDAC tumours were larger and more expansive in lumican knockout mice (Figure 1b) and contained fewer apoptotic cells with less DNA fragmentation (Figure 1c), and lower cleaved poly (ADP-ribose) polymerase (PARP) expression (Figures 1d and e). Direct evidence of the inhibitory effect of extracellular lumican on PDAC cells was further demonstrated by the MTT assay observed after low-passage human PDAC were exposed to recombinant human lumican (Figure 1f). These results indicate that extracellular lumican inhibits tumour growth and induces tumour cell apoptosis.
Figure 1.
Stromal lumican inhibits tumour growth. (a) Upper-left: patient tumour tissues (F0) were implanted into immunodeficient mice to obtain direct xenograft tumours (PDX: F1, F2, F3, F4, and F5). PDX number: statistical number of PDX from different patients. Tumour formation time: implantation to the tumour reached 0.25 cm in diameter; tumour growth time: tumour grows from 0.25 cm to 1.5 cm. Tumour formation ratio (%): tumour formation number/implanted number. Female NOD/SCID mice (4–8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). All animal studies were approved by the MD Anderson Cancer Center Institutional Animal Care and Use Committee (IRB: Lab00-396. ACUF: 00001089-RN00). A blind analysis was performed in animal data statistical analysis. The protocols of engraftment and expansion of PDX tumours were performed as previously described.23,24 Briefly, excised patient tumour tissue (F0) was mechanically minced into fragments (about 2 mm), and five tumour fragments were individually placed in a formed tissue pocket. Once tumours reached 1.5 cm in greatest diameter, mice were killed, and the tumours were dissected (F1). F1 tumour tissue fragments were placed into NOD/SCID mice to generate the F2 to F5 generations. Lower: western blot analysis showed the lumican expression in tumour tissues from different PDX generations. Antibodies used were lumican (ab70191, Abcam, Cambridge, MA, USA) and β-actin (sc-69879, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Upper-right: schematic drawing depicts the conclusions from the chart (left), our previous findings, reported results and our hypothesis for the current study. (b) Tumour size was measured in orthotopic syngeneic mouse PDAC models. WT: wild-type mice; HET: heterozygous mice; Lum KO: lumican knockout mice. Freshly cultured PancO2 cells (purchased from ATCC) were re-suspended in sterile 1X PBS at 1 ×106 cells per 100 μl, and this volume of the suspension was directly injected into the pancreas of wild-type (WT) and lumican knockout (KO) C57BL/6 mice aged approximately 10 weeks, and were randomly placed into different groups ( six mice per condition). Lumican WT and KO mice were purchased from Jackson Laboratory. All mice were killed 5 weeks after PancO2 cells injection. (c) Tumour tissue lysates were subjected to a cell death detection ELISA kit (11774425001, Roche, Mannheim, Germany). (d) Immunohistochemistry staining of tumour tissues from lumican WT and KO mice with anti-cleaved PARP antibody (ab32064, Abcam). The C-PARP labelled pancreatic tumour tissues were scanned using Olympus BX51. Within DAB-stained slides, six random fields in tumours were scanned at× 10. The obtained images were analysed using the ‘Nucleus detection, Nucleus Morphology and Filter, and Nucleus classification’ algorithm in Tissue Studio 2.5 (Definiens, Cambridge, MA, USA). The C-PARP positive nuclei were quantified. (e) Tumour tissue lysates were subjected to western blot analysis with cleaved PARP (C-PARP) antibody (#9542, Cell Signaling Technology, Beverly, MA, USA). Quantification of C-PARP protein level was normalized to β-actin. The Student’s t-test was used (in (c) and (e)) to detect the significance of differences between groups. The data are presented as means ± s.d. All experiments were repeated at least once with reproducible findings. (f) Cell viability was measured by methylthiazolyldiphenyl-tetrazolium bromide (MTT) (M2128, Sigma-Aldrich, St Louis, MO, USA) assay after lumican (recombinant human lumican protein CF, R&D Systems, 2846-LU-050, Minneapolis, MN, USA) treatment for 3 days. Primary PDAC cell lines MDA-PATC50, MDA-PATC53, MDA-PATC148 and MDA-PATC153 were isolated from PDX tumours in our laboratory.10 All cell lines were verified by DNA fingerprinting at the Characterized Cell Line Core Facility of the MD Anderson Cancer Center. The OD value of each treatment group was expressed as a percentage of the OD value of the untreated control cells. (g) Human MDA-PATC53 cells and mouse PancO2 cells were treated with human recombinant lumican protein and mouse recombinant lumican protein (recombinant mouse lumican protein CF, R&D Systems, 2745-LU-050) with indicated doses for 3 days, respectively. Cell viability was measured by MTT assay. *P<0.05 and **P<0.01. (h) MDA-PATC53 and PancO2 cells were treated with 2 μg/ml human lumican or mouse lumican for 10 min, respectively. Cell lysates were subjected to western blot analysis with anti-phospho-EGF receptor (#2234, Cell Signaling Technology), anti-EGFR (sc-373746, Santa Cruz Biotechnology) and anti-β-actin.
Another important finding from Figure 1 (lower) is that total lumican (human and mouse) levels within each PDX tumour do not change during tumour passage, whereas levels of human lumican decrease. This suggests that mouse-derived stromal lumican does not influence human PDAC cell growth within the PDX. To further confirm this species-specific role of lumican in growth of cancer cells, we measured cell viability (Figure 1g) and EGFR activity (Figure 1h) in human (MDA-PATC53) or mouse (PancO2) PDAC cells after exposure to human or mouse lumican. We previously reported that EGFR could be rapidly activated in MDA-PATC53 cells after brief exposure to human recombinant lumican. 10 Our results showed that human cancer cells are sensitive only to human lumican and mouse cancer cells are sensitive solely to mouse lumican, indicating that human and mouse PDAC cells respond to lumican in a species-specific manner.
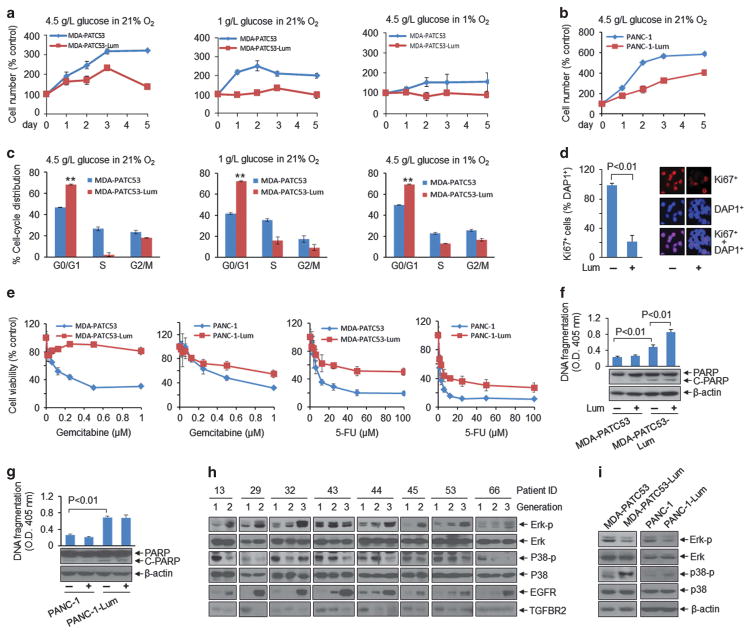
Since PDAC has a long period of genetic evolution before clinical diagnosis, we investigated whether prolonged exposure to extracellular lumican induces PDAC cells to switch to a quiescent state leading to growth inhibition. Compared with untreated controls, MDA-PATC53 and PANC-1 cells exposed to extracellular lumican demonstrated lower cell proliferation in various culture conditions modelling the tumour microenvironment (Figure 2a and b). We also found more G0/G1 arrest (Figure 2c), fewer Ki67-positive cells (Figure 2d), more resistance to chemotherapy (Figure 2e) and increased apoptotic cells death in MDA-PATC53-Lum (Figure 2f) and PANC-1-Lum cells (Figure 2g) than their parallel control cells. Interestingly, we have recently reported that short term (≤ 5d) lumican treatment augments cytotoxicity of chemotherapy.14 These results from long-term lumican exposure suggest that prolonged exposure to lumican induces some molecular changes in PDAC that protect against damaging events.
Figure 2.
Extracellular lumican induces phenotypic and signalling changes in PDAC cells. (a, b) MDA-PATC53 and PANC-1 (purchased from ATCC) cells were cultured with or without lumican (Lum; 2 μg/ml) in different culture conditions and cell number was counted. (c) Cell-cycle distribution was quantified by flow cytometry in MDA-PATC53 and MDA-PATC53-Lum cells in various culture conditions. **P<0.01. Parental MDA-PATC53 or PANC-1 cells were split to two parts, one was continuously exposed to 0.2 μg/ml lumican in culture medium around 30–90 days, named as MDA-PATC53-Lum or PANC-1-Lum cells; another was regularly cultured without lumican within the same period, serves as a control of MDA-PATC53-Lum or PANC-1-Lum cells. Cell DNA fingerprinting test at the Characterized Cell Line Core Facility of the MD Anderson Cancer Center (case number: CCLCF-YK-1421) verified the parental cells and lumican treated cells have the same DNA pattern and exactly match to primary cell line ID in our cell line database, suggesting they come from the same source and have the same basic genetic makeup. (d) Immunofluorescent staining showed Ki67-positive MDA-PATC53 cells after treatment with lumican for 3 days. Anti-Ki67 (sc-15402) was purchased from Santa Cruz Biotechnology. (e) MDA-PATC53, MDA-PATC53-Lum, PANC-1 and PANC-1-Lum cells were treated with gemcitabine or 5-fluorouracil (5-FU) for 72 h, and cell viability was measured by MTT assay. (f, g) Indicated cells were treated with lumican (2 μg/ml) for 24 h, cell lysates were subjected to a cell death detection ELISA kit (top) and western blot analysis (bottom). (h, i) Western blot results showed the expression of indicated molecules in PDX tumour tissues from different generations (h), and in MDA-PATC53, MDA-PATC53-Lum, PANC-1 and PANC-1-Lum cells (i). Anti- phospho-p44/42 MAPK (ERK1/2) (#9101), anti-p44/42 MAPK (ERK1/2) (#9102), anti-phospho-p38 MAPK (#9211) and anti-p38 MAPK (#9212) were purchased from Cell Signaling Technology. Anti-TGFBR2 (ab61213) was from Abcam.
The observed phenotypic changes accompanying growth suppression are typical of those observed when tumour cells are in a quiescent state. Recent reports have shown that changes in mitogenic signalling from the ERK pathway to the p38 pathway accompany a switch from proliferation to dormancy.15,16 To determine whether this process was involved in PDAC quiescence after prolonged lumican exposure, we examined the effect of lumican on the ERK and p38 pathways. We first checked them in PDX from serial passages (Figure 2h) and found that ERK1/2 phosphorylation increased and p38 phosphorylation decreased coincident with decreased lumican levels during passage from generation F1 to F3 (shown in Figure 1a). Due to the nature of PDX tumours and antibody sensitivity, it is not possible to attribute the signalling changes to cancer cells alone. For this reason, we further analysed human cancer cells MDA-PATC53 and PANC-1 after exposure to extracellular lumican for alterations in Erk and P38 signalling pathways. Compared with control cells, MDA-PATC53-Lum and PANC-1-Lum cells demonstrated lower ERK1/2 phosphorylation but higher p38 phosphorylation (Figure 2g). Taken together, these results suggest that extracellular lumican induces cell inhibitory signalling in PDAC cells.
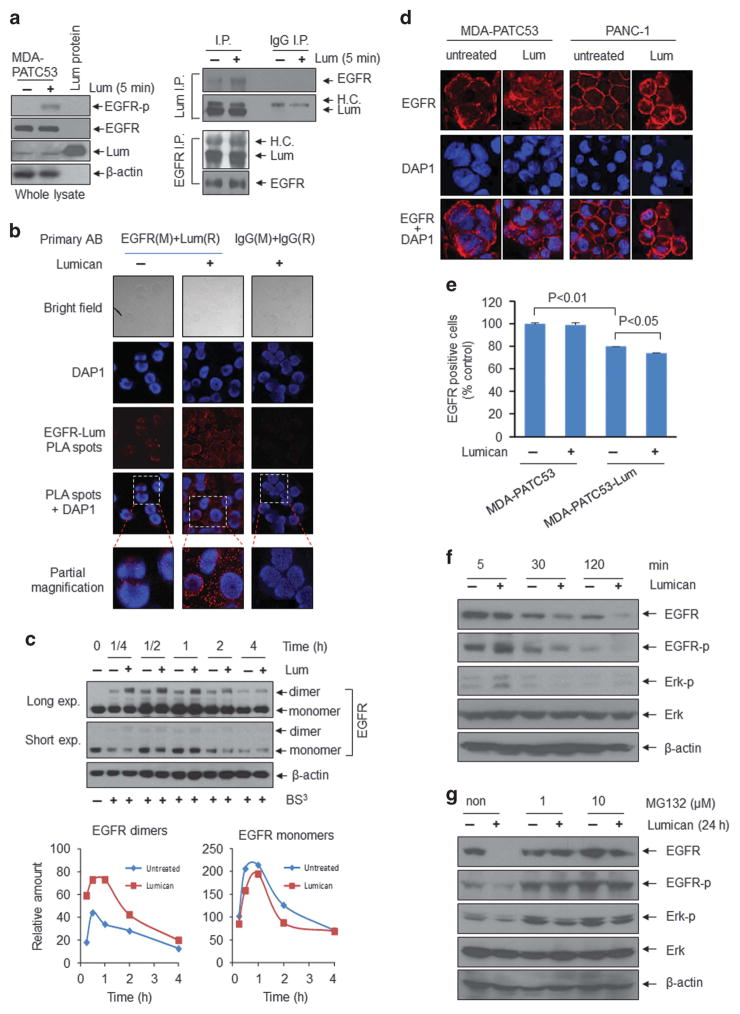
Our previous work indicated that extracellular lumican decreases EGFR signals, resulting in glycolytic metabolism inhibition and apoptotic cell death.10 Figure 2h also determined that EGFR expression is increased following lumican levels decreased from F1 to F3 generations, and consistent with ERK1/2 phosphorylation level. Here, we further investigated how lumican regulates EGFR and its downstream signal ERK, a key regulator of cell growth inhibition. We found that lumican stimulated EGFR phosphorylation (Figure 3a, left), and that both intracellular lumican (cell basal level) and extracellular lumican were bound with EGFR (Figure 3a, right). Importantly, although cell EGFR and lumican levels were the same with or without lumican treatment (Figure 3a, left), the association between extracellular lumican and EGFR was stronger than that between intracellular lumican and EGFR, suggesting that extracellular lumican binds with EGFR. To further confirm these results, we performed proximity-ligation-based assays on MDA-PATC53 cells. Cells were first incubated with both a mouse antibody against EGFR and a rabbit antibody against lumican, and then incubated with antibodies against mouse and rabbit antibodies that have a unique short DNA strand attached to each antibody. If the two epitopes on EGFR and lumican are sufficiently close, the attached oligonucleotides on the respective antibodies hybridize or become ligated, producing a template for a rolling circle DNA amplification, which can be probed efficiently with fluorescent oligonucleotide probes. The appearance of discrete fluorescent spots in the immunofluorescent images indicates that EGFR and lumican are present in close proximity. We found the fluorescent spots existed both on the membrane and in the cytoplasm of cells, and the number was significantly increased after 10-min treatment with lumican (Figure 3b). These observations further support the notion that there is a specific interaction between lumican and EGFR. Next, we verified that extracellular lumican induces EGFR dimerization (Figure 3c) and subsequent internalization from the cell membrane into the cytoplasm in both MDA-PATC53 and PANC-1 cells (Figure 3d). Flow cytometry also provided evidence that the EGFR level of cell surface is reduced following prolonged exposure to lumican (Figure 3e). We confirmed that lumican-induced EGFR internalization led to downregulation of EGFR and ERK activities (Figure 3f). When cells were pretreated with MG132, a proteasome inhibitor, and were subsequently exposed to lumican, EGFR degradation was prevented as was resultant ERK phosphorylation (Figure 3g). Taken together, these results verify that extracellular lumican binds with EGFR, induces EGFR dimerization and subsequent internalization, resulting in inhibition of EGFR and ERK activities.
Figure 3.
Extracellular lumican binds with EGFR and induces EGFR internalization, thereby downregulating EGFR/ERK signalling activity. (a) MDA-PATC53 cells were stimulated with lumican (2 μg/ml) for 5 min. Cell pellets were lysed in RIPA buffer for use in all procedures. Whole cell lysates (left) were subjected to western blot analysis with anti-phospho-EGF receptor, anti-EGFR, anti-lumican and anti-β actin. For immunoprecipitation studies, cell lysates were incubated with primary antibodies, Lumican, EGFR and lgG (immunoglobulin G) (sc-2027, Santa Cruz Biotechnology), then the resulting immune complexes were precipitated with protein A/G plus-Agarose (sc-2003, Santa Cruz Biotechnology). Lumican, EGFR and lgG immunoprecipitates (right) were separated by SDS-PAGE, and subjected to immune blotting analysis with EGFR and lumican antibodies. H.C.: heavy chain. (b) The binding between EGFR and lumican was detected by proximity ligation assay in MDA-PATC53 cells with or without lumican treatment for 10 min. The proximity ligation assay was performed using Duolink proximity ligation assay in Situ Red Starter Kit (Mouse and Rabbit, Sigma-Aldrich, DU092101) as per the manufacturer’s instruction. The primary antibodies were mouse anti-EGFR antibody (1:150) and rabbit anti-Lumican antibody (1:100). Images were acquired using a FluoView 1000 IX2 confocal microscope (Olympus, Center Valley, PA, USA). (c) MDA-PATC53 cells were treated with lumican (2 μg/ml) for the indicated time periods, washed twice with cold PBS to remove serum proteins, and then incubated with 3-mM crosslinker bis(sulfosuccinimidyl) suberate (BS3) (PI21580, Thermo Scientific Pierce, Rockford, IL, USA) in PBS for 30 min at RT with gentle shaking. The reaction was quenched at RT with 90 mM glycine, 9 mM Tris-HCl (final concentrations), pH 8.5, for 15 min. Cell lysates were subjected to western immunoblotting for EGFR and β-actin. EGFR dimers and monomers were quantified. exp.: exposure. (d) Immunofluorescent staining of MDA-PATC53 and PANC1 cells with anti-EGFR antibody and DAP1 (D9542, Sigma-Aldrich) after treatment with lumican for 30 min. (e) MDA-PATC53 and MDA-PATC53-Lum cells were treated with lumican (2 μg/ml) for 24 h, and EGFR-positive cells were quantified by flow cytometry after staining for EGFR antibody. (f, g) MDA-PATC53 cells were treated with lumican (f) or were treated with lumican and/or MG132 (M7449, Sigma-Aldrich) for the indicated time periods (g). Cell lysates were subjected to western blot analysis.
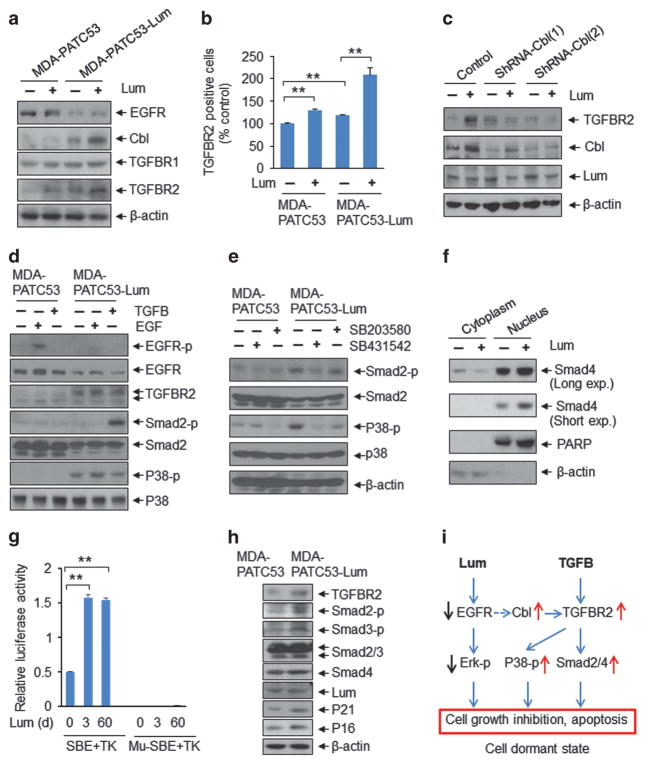
Interestingly, we found that transforming growth factor beta receptor 2 (TGFBR2) expression was reduced in PDX from F1 to F3 generations along with a decrease in lumican and an increase in EGFR (Figure 2h). TGFβ signals have been reported to induce cell inhibition through downstream activation of SMAD effectors as well as p38 signalling pathways.17,18 Thus, we investigated TGFβ receptor expression after short-term lumican exposure in MDA-PATC53 and MDA-PATC53-Lum cells. Lumican-treated cells had higher TGFBR2 levels than control cells (Figure 4a), which was confirmed by flow cytometry analysis (Figure 4b). Several studies have reported that casitas B-lineage lymphoma (CBL), an ubiquitin E3 ligase and proto-oncogene involved in the EGFR internalization/degradation pathway, promotes TGFβ signalling by stabilizing TGFBR2.19–21 We treated MDA-PATC53 cells with CBL shRNA to block CBL signalling before lumican treatment. The shRNA-CBL-treated cells had significantly lower lumican-enhanced TGFBR2 levels than control cells (Figure 4c), indicating that lumican-regulated TGFBR2 is CBL-dependent. Along with decreased EGFR expression, MDA-PATC53-Lum cells had no response to EGF stimulation but had enhanced sensitization to TGFβ treatment showing the activation of the downstream signals SMAD2 and p38 (Figure 4d). Furthermore, the TGFBR2 inhibitor SB431542 significantly reduced lumican-increased phosphorylation of p38 and Smad2 (Figure 4e). These data suggest that TGFβ stimulates SMAD2 and p38 signalling through lumican-increased TGFBR2. Importantly, Yamanaka et al have reported that lumican directly binds ALK5 (TGFBRI) and activates TGFB and its downstream signals. 22 We believe that lumican/CBL-increased TGFBR2 can also trigger TGFB signalling through lumican/ALK5. Lumican also induced SMAD4 translocation from the cytoplasm to the nucleus (Figure 4f), followed by activation of the SMAD4-binding site (Figure 4g) and enhancement of downstream SMAD4 targets and cell-cycle inhibitors, p21 and p16 (Figure 4h). Together, these results indicate that lumican activates SMAD and p38 signals by enhancing the CBL-TGFBR2 signalling pathway.
Figure 4.
Extracellular lumican increases SMAD and p38 signalling activities by enhancing TGFBR2 expression through EGFR/CBL signalling. (a, b) MDA-PATC53 and MDA-PATC53-Lum cells were treated with lumican (2 μg/ml) for 24 h, and cell lysates were subjected to western blot analysis with anti-EGFR, anti-Cbl (sc-170, Santa Cruz Biotechnology), anti-TGFBR1 (ab31013, Abcam), anti-TGFBR2 and anti-β-actin (a). TGFBR2-positive cells were quantified by flow cytometry after staining for TGFBR2 antibody (b). (c) MDA-PATC53 cells were treated with CBL-shRNA (Sigma-Aldrich, SHCLNG-NM_005188) for 48 h and then were cultured with lumican for another 24 h. Cell lysates were subjected to western blot analysis. (d, e) MDA-PATC53 and MDA-PATC53-Lum cells were stimulated with EGF (10 nmol/l) (E9644, Sigma-Aldrich) or TGFβ (10 ng/ml) for 10 min (d) or were treated with p38 inhibitor SB203580 (10 μM) (#5633, Cell Signaling Technology) or TGFBR2 inhibitor SB431542 (10 μM) (S4317, Sigma-Aldrich) for 24 h (e). Cell lysates were subjected to western blot analysis by anti-phospho-EGF receptor, anti-EGFR, anti-TGFBR2, anti- phosphor-SMAD2 (#3101, Cell Signaling Technology), anti-SMAD2/3 (#5678, Cell Signaling Technology), anti-phospho-p38 MAPK, anti-p38 MAPK and anti-β-actin. (f) Expression levels of SMAD4 in the nucleus and cytosol of MDA-PATC53 cells treated with lumican (2 μg/ml; 24 h). PARP and β-actin were used as the nucleus and cytosol loading control, respectively. SMAD4 (#9515) were purchased from Cell Signaling Technology. (g) TGFβ/SMAD4 responsive promoter activity was measured by Dual-Luciferase Reporter Assay. MDA-PATC53 and lumican-treated MDA-PATC53 cells were co-transfected with pRL-TK and 6XSBE luciferase or pRL-TK and Mu-SBE (mutated-SBE) luciferase constructs for 24 h. The activities of Firefly and Renilla luciferase were determined using a dual luciferase reporter assay system (E1910, Promega, San Luis Obispo, CA, USA) and compared to the activity obtained for the mutated vector. All results were normalized against the activity of the co-transfected Renilla luciferase vector pRL-TK. Data are presented as the means of three independent experiments. (h) Cell lysates were subjected to western blot analysis with indicated antibodies. Anti-phosphor-SMAD3 (#9502) was purchased from Cell Signaling Technology. Anti-p21 (ab109199) and anti-p16 (ab51243) were purchased from Abcam. (i) Schematic model depicts how lumican induces phenotypic and signalling changes in PDAC cells inhibition.
In this study, we report a novel mechanism by which stromal lumican inhibits PDAC growth by inducing cell into a quiescent state. Our results indicate that lumican binds with ‘lumican receptor’ EGFR and downregulates EGFR and its downstream signal ERK. Lumican-enhanced CBL stabilizes TGFBR2, which in turn sensitizes cells to TGFB stimulation, leading to activation of p38 and SMAD signals. A working model summarizing these results is shown in Figure 4i. These signalling changes mediate PDAC cell entry into a quiescent state, resulting in cell growth inhibition. Our findings indicate that extracellular lumican represents a naturally occurring inhibitor of PDAC tumour cell growth whose properties and actions may offer strategies for therapeutic intervention against pancreatic cancer.
Acknowledgments
This work was supported by grants from the Skip Viragh Family Foundation (to JF), the W. Smith Foundation (to JF), National Institutes of Health (NIH) grant T32CA009599 (to DR and MRP), U54 CA210181-01 ‘Center for Immunotherapeutic Transport Oncophysics (CITO)’ grant (to EK), and CABI GE in-kind grant (to EK). This research was conducted at the MD Anderson Cancer Center for Advanced Biomedical Imaging in-part with equipment support from General Electric Healthcare.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Bui AT, Laurent F, Havard M, Dautry F, Tchenio T. SMAD signaling and redox imbalance cooperate to induce prostate cancer cell dormancy. Cell Cycle. 2015;14:1218–1231. doi: 10.1080/15384101.2015.1014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proia DA, Kuperwasser C. Stroma: tumor agonist or antagonist. Cell Cycle. 2005;4:1022–1025. doi: 10.4161/cc.4.8.1903. [DOI] [PubMed] [Google Scholar]
- 4.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba H, Ishiwata T, Takashi E, Xu G, Asano G. Expression and localization of lumican in the ischemic and reperfused rat heart. Jpn Circ J. 2001;65:445–450. doi: 10.1253/jcj.65.445. [DOI] [PubMed] [Google Scholar]
- 6.Lu YP, Ishiwata T, Kawahara K, Watanabe M, Naito Z, Moriyama Y, et al. Expression of lumican in human colorectal cancer cells. Pathol Int. 2002;52:519–526. doi: 10.1046/j.1440-1827.2002.01384.x. [DOI] [PubMed] [Google Scholar]
- 7.Ishiwata T, Cho K, Kawahara K, Yamamoto T, Fujiwara Y, Uchida E, et al. Role of lumican in cancer cells and adjacent stromal tissues in human pancreatic cancer. Oncol Rep. 2007;18:537–543. [PubMed] [Google Scholar]
- 8.Williams KE, Fulford LA, Albig AR. Lumican reduces tumor growth via induction of fas-mediated endothelial cell apoptosis. Cancer Microenviron. 2010;4:115–126. doi: 10.1007/s12307-010-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto T, Matsuda Y, Kawahara K, Ishiwata T, Naito Z. Secreted 70 kDa lumican stimulates growth and inhibits invasion of human pancreatic cancer. Cancer Lett. 2012;320:31–39. doi: 10.1016/j.canlet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Truty MA, Kang Y, Chopin-Laly X, Zhang R, Roife D, et al. Extracellular lumican inhibits pancreatic cancer cell growth and is associated with prolonged survival after surgery. Clin Cancer Res. 2014;20:6529–6540. doi: 10.1158/1078-0432.CCR-14-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maykel J, Liu JH, Li H, Shultz LD, Greiner DL, Houghton J. NOD-scidIl2rg (tm1Wjl) and NOD-Rag1 (null) Il2rg (tm1Wjl): a model for stromal cell-tumor cell interaction for human colon cancer. Dig Dis Sci. 2014;59:1169–1179. doi: 10.1007/s10620-014-3168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Moriwaki H, et al. Multi-color palette of fluorescent proteins for imaging the tumor microenvironment of orthotopic tumorgraft mouse models of clinical pancreatic cancer specimens. J Cell Biochem. 2012;113:2290–2295. doi: 10.1002/jcb.24099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang Y, Roife D, Lee Y, Lv H, Suzuki R, Ling J, et al. Transforming growth factor-beta limits secretion of lumican by activated stellate cells within primary pancreatic adenocarcinoma tumors. Clin Cancer Res. 2016;22:4934–4946. doi: 10.1158/1078-0432.CCR-15-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Roife D, Kang Y, Dai B, Pratt M, Fleming JB. Extracellular lumican augments cytotoxicity of chemotherapy in pancreatic ductal adenocarcinoma cells via autophagy inhibition. Oncogene. 2016;35:4881–4890. doi: 10.1038/onc.2016.20. [DOI] [PubMed] [Google Scholar]
- 15.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK (MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63:1684–1695. [PubMed] [Google Scholar]
- 16.Ranganathan AC, Adam AP, Aguirre-Ghiso JA. Opposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancy. Cell Cycle. 2006;5:1799–1807. doi: 10.4161/cc.5.16.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salm SN, Burger PE, Coetzee S, Goto K, Moscatelli D, Wilson EL. TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol. 2005;170:81–90. doi: 10.1083/jcb.200412015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber T, Hinterleitner R, Hermann-Kleiter N, Meisel M, Kleiter I, Wang CM, et al. Cbl-b mediates TGFbeta sensitivity by downregulating inhibitory SMAD7 in primary T cells. J Mol Cell Biol. 2013;5:358–368. doi: 10.1093/jmcb/mjt017. [DOI] [PubMed] [Google Scholar]
- 20.Kang JM, Park S, Kim SJ, Hong HY, Jeong J, Kim HS. CBL enhances breast tumor formation by inhibiting tumor suppressive activity of TGF-beta signaling. Oncogene. 2012;31:5123–5131. doi: 10.1038/onc.2012.18. [DOI] [PubMed] [Google Scholar]
- 21.Zuo W, Huang F, Chiang YJ, Li M, Du J, Ding Y, et al. c-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-beta type II receptor. Mol Cell. 2013;49:499–510. doi: 10.1016/j.molcel.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka O, Yuan Y, Coulson-Thomas VJ, Gesteira TF, Call MK, Zhang Y, et al. Lumican binds ALK5 to promote epithelium wound healing. PloS One. 2013;8:e82730. doi: 10.1371/journal.pone.0082730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MP, Truty MJ, Choi W, Kang Y, Chopin-Lally X, Gallick GE, et al. Molecular profiling of direct xenograft tumors established from human pancreatic adenocarcinoma after neoadjuvant therapy. Ann Surg Oncol. 2012;19(Suppl 3):S395–S403. doi: 10.1245/s10434-011-1839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]