Abstract
One decade ago, our laboratory provided the first direct evidence linking orexin/hypocretin signaling with drug seeking by showing that activation of these neurons promotes conditioned morphine-seeking behavior. In the years since, contributions from many investigators have revealed roles for orexins in addiction for all drugs of abuse tested, but only under select circumstances. We recently proposed that orexins play a fundamentally unified role in coordinating “motivational activation” under numerous behavioral conditions, and here we unpack this hypothesis as it applies to drug addiction. We describe evidence collected over the past 10 years that elaborates the role of orexin in drug seeking under circumstances where high levels of effort are required to obtain the drug, or when motivation for drug reward is augmented by the presence of external stimuli like drug-associated cues/contexts or stressors. Evidence from studies using traditional self-administration and reinstatement models, as well as behavioral economic analyses of drug demand elasticity, clearly delineates a role for orexin in modulating motivational, rather than the primary reinforcing aspects of drug reward. We also discuss the anatomical interconnectedness of the orexin system with wider motivation and reward circuits, with a particular focus on how orexin modulates prefrontal and other glutamatergic inputs onto ventral tegmental area dopamine neurons. Last, we look ahead to the next decade of the research in this area, highlighting the recent FDA approval of the dual orexin receptor antagonist suvorexant (Belsomra®) for the treatment of insomnia as a promising sign of the potential clinical utility of orexin-based therapies for the treatment of addiction.
Keywords: Addiction, Alcohol, Behavioral economics, Cocaine, Dopamine, Drugs of abuse, Glutamate, Heroin, Hypocretin, Motivation, Orexin, Reward, VTA
1 Introduction
Orexin peptides A and B (also called hypocretin 1 and 2) are produced by a limited number of neurons within the hypothalamus, ranging mediolaterally from the dorsomedial hypothalamic nucleus (DMH) through the perifornical area (PFA) into the lateral hypothalamus (LH) proper [1, 2]. Orexins signal via two G-protein-coupled receptors (orexin 1 and 2 receptors; Ox1R and Ox2R) that are distributed throughout the central nervous system [1, 2]. Orexin A has equal affinity for Ox1R and Ox2R, whereas orexin-B preferentially binds Ox2R [2]. Consistent with their anatomical position within the lateral hypothalamus, orexin neurons were originally identified as playing a key role in feeding behavior, with exogenous application of orexins shown to increase food intake [2, 3], and this effect being blocked by antagonism of orexin receptors [4]. This role for orexins in appetitive behavior has since been extended to a range of reward-related processes, including drug abuse and addiction [5–12].
Indirect evidence for a role for orexins in addiction came from a series of interesting clinical observations of patients suffering from narcolepsy with cataplexy, a neurological disorder associated with frequent sleep/wake transitions resulting from an almost complete loss of neurons producing orexin [13, 14]. A number of clinical case studies reported that narcoleptic patients rarely develop stimulant abuse, despite long-term treatment with stimulant medications including amphetamines [15, 16]. Consistent with this, Georgescu et al. [17] reported that orexin cells are activated (as seen with the immediate early gene product, Fos) after chronic morphine and withdrawal, and orexin knockout mice develop attenuated physical signs of morphine dependence compared to wild type controls.
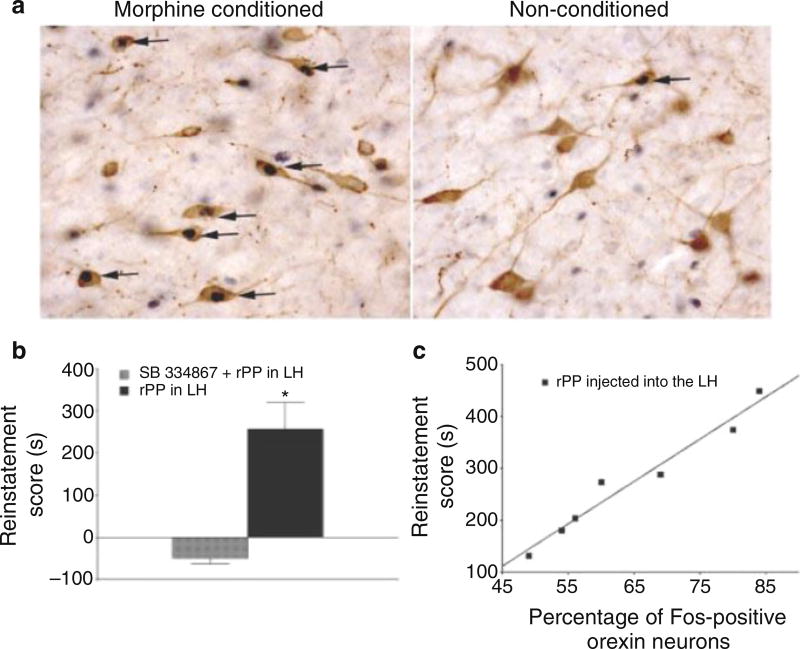
In 2005, our laboratory directly implicated orexin signaling in drug-seeking behavior by showing that activation of LH orexin neurons is strongly correlated with conditioned preference for environmental contexts associated with cocaine or morphine [18] (Fig. 1). This study also showed that local chemical stimulation of LH orexin neurons reinstates extinguished drug seeking, and that this effect is blocked by systemic administration of an Ox1R antagonist (Fig. 1). Soon after, Boutrel and colleagues reported that central infusions of orexin peptide reinstate extinguished cocaine seeking and systemic injections of the Ox1R antagonist block cocaine seeking elicited by stress [19]. Together, these papers prompted a surge in preclinical studies examining the involvement of the orexins in various animal models of addiction, with >200 journal articles on orexin/hypocretin and addiction now published. As this number indicates, significant progress has been made in the last decade in understanding the specific roles for orexins in drug seeking and how they interact with the brain reward circuitry to drive these processes.
Fig. 1.
First direct evidence that orexin signaling is involved in drug seeking behavior. (a) Using a two-chamber, non-biased, conditioned-place preference paradigm, animals were trained to associate one chamber with drug (morphine or cocaine) reward, whereas the other chamber was associated with no reward. Conditioned animals displayed reward-seeking by spending significantly more time in the reward-paired chamber. To determine if orexin neurons were stimulated during the expression of this preference, tissue was dual-labeled for both orexin and the immediate early gene protein Fos (a marker of neuronal stimulation). Only conditioned animals that exhibited a preference for the reward-paired chamber showed increased Fos expression in LH orexin cells. Similar percentages or orexin neurons in LH were activated following preference testing for morphine or cocaine. (b) Additional experiments sought to demonstrate that activation of orexin neurons could reinstate extinguished drug seeking. Animals were submitted to morphine conditioned place preference conditioning and then this behavior was extinguished by repeatedly exposing the rats to the chambers without morphine administration. To activate orexin neurons, animals received intra-LH infusions of the Y4 receptor agonist rPP (rat pancreatic polypeptide), as orexin neurons express the Y4 receptor and activation of this receptor by rPP induces Fos in these neurons. Infusions of rPP into LH robustly reinstated an extinguished morphine place preference, and this effect was blocked by pretreatment with the selective orexin 1 receptor antagonist SB-334867. (c) rPP-induced Fos expression in orexin neurons was strongly correlated with reinstatement score. Figure adapted from Harris et al. [18]
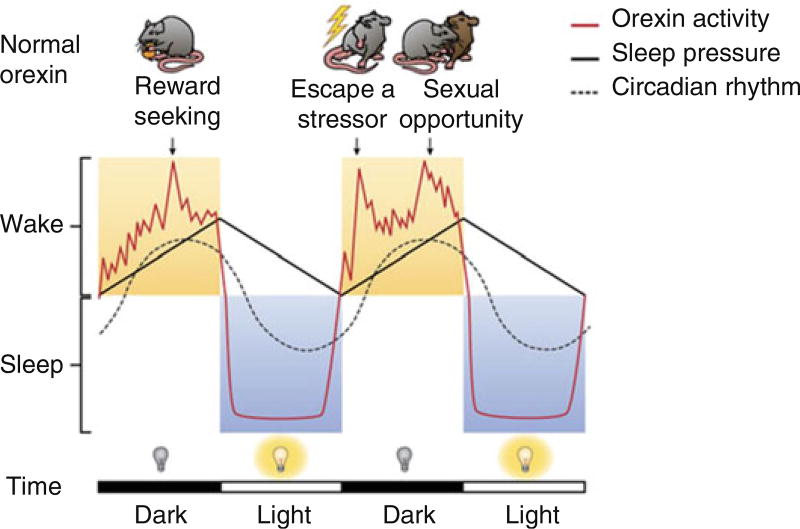
We recently articulated a hypothesis that the orexin system is preferentially engaged by situations of high motivational relevance, such as during physiological need states, exposure to threats, or reward opportunities [11]. We suggest that orexin neurons translate this “motivational activation” into organized suites of psychological and physiological processes that support adaptive behaviors (Fig. 2). With respect to addiction behaviors, we and others have argued that orexin neurons are not involved in modulating the primary reinforcing effects of drugs of abuse per se, but are critical to regulating motivated responding for these drugs [7, 11, 20]. Key to this is the observation that motivation for drug reward can be enhanced by various external stimuli, including drug-associated stimuli and stress, and that this occurs in an orexin-dependent manner [21].
Fig. 2.
“Motivational activation”: a unifying hypothesis of orexin/hypocretin function. We propose that orexin neurons have a fundamentally unified role that spans a variety of processes, including arousal, reward seeking, homeostatic regulation, and stress, which we term “motivational activation.” Orexin neuron activity (red line) has a circadian-like pattern but also exhibits phasic bursts during waking as a function of motivational state and adaptive behavior, such as reward seeking. We hypothesize that both suprachiasmatic nucleus-dependent tonic excitation and phasic orexin neuron burst firing help to produce and maintain wakefulness – the latter as a consequence of increased motivation to engage adaptive challenges or opportunities. Adapted from Mahler et al. [11]
Here, we seek to synthesize the findings of the past 10 years that support the “motivational activation” hypothesis as it relates to drug addiction. We present evidence from studies using a range of behavioral models of addiction that highlight a motivational role for orexin in drug seeking. Next, we provide an overview of how orexin neurons interact with motivational and reward circuits to drive these processes, with a particular emphasis on how orexin neurons modulate the efficacy of glutamatergic inputs onto VTA neurons. We also discuss heterogeneity in orexin cell subpopulations, and the anatomical and functional characteristics that may contribute to the diversity of orexin neuron function. Finally, we seek to identify key gaps in our knowledge regarding orexin function in addiction that will be important to address in the coming decade of research in this field. Throughout this article, we focus primarily on psychostimulant drugs, as the role of orexin in alcohol use and abuse is covered elsewhere in this volume [22].
2 Orexin Signaling and Self-Administration Behavior
2.1 Traditional Self-Administration Models
Studies investigating the role of orexin in drug reward have frequently utilized the self-administration model, which allows investigators to examine responding for drug under schedules of reinforcement that differ in terms of the amount of effort required to obtain the drug. Under low fixed-ratio (FR) schedules of reinforcement (e.g., FR1, FR3), animals can easily maintain a preferred blood level of drug [23–25] and responding under these schedules allows insight into the primary reinforcing properties of the drug. Systemic administration of the selective Ox1R antagonist SB-334867 has no effect on cocaine or amphetamine self-administration under FR1 or FR3 schedules of reinforcement [19, 26–28], suggesting limited involvement of the orexin system in modulating the hedonic properties of psychostimulants.
Instead, orexin signaling is required for psychostimulant self-administration when higher levels of effort are required to obtain the drug. Systemic administration of SB-334867, as well as genetic deletion of Ox1R receptors, greatly reduces responding for cocaine on an FR5 schedule of reinforcement [29]. Similarly, under a progressive ratio (PR) schedule of reinforcement, where animals must expend exponentially increasing amounts of effort to obtain each subsequent drug infusion, systemic or intra-VTA injections of SB-334867 reduce the maximal effort the animal is willing the exert (as indicated by reduced breakpoints) [20]. Further, when motivation for cocaine is increased by intermittently restricting access to the drug, intra-VTA infusions of the orexin-A peptide increase, whereas intra-VTA infusions SB-334867 decrease, cocaine self-administration [26, 30]. These findings are consistent with our recent results using behavioral economic procedures (described in Section 4) [21]. Importantly, these effects appear to be mediated primarily by the Ox1R, as selective Ox2R antagonists have no effect on highly motivated responding for cocaine [31].
2.2 Intracranial Self-Stimulation Models
Findings from self-administration studies are closely mirrored in studies that utilize the intracranial self-stimulation (ICSS) paradigm. In this paradigm, instead of responding for a drug reward, animals are required to make an operant response (generally turning a wheel) to deliver a rewarding electrical current into LH. When animals are trained to respond for LH stimulation under a continuous reinforcement schedule that requires low levels of effort (FR1) to obtain rewarding stimulation, SB-334867 has no effect on stimulation reward threshold, nor does it affect the ability of cocaine to reduce the minimum level of electrical current necessary to support self-stimulation [32]. In contrast however, the effects of cocaine on ICSS threshold are blocked by systemic SB-334867 when animals are trained to respond for ICSS under a discrete trial current-threshold procedure, which requires greater degrees of effort and motivation to obtain ICSS [29].
2.3 Conclusions
In summary, data from self-administration and ICSS studies point to a unique role for orexin in driving responding for psychostimulant reward under conditions of enhanced motivation. Orexin is required for psychostimulant self-administration behavior or ICSS responding only under schedules that require high amounts of effort to earn drug reward (FR5, PR). This is further supported by studies investigating orexin signaling in economic demand for psychostimulants (discussed in Section 4). In contrast, orexin is not necessary for the primary reinforcing effects of cocaine or amphetamine, or ICSS. Consistent with a more global role for orexin in modulating motivated behavior, SB-334867 also attenuates high-effort (FR5, PR) responding for other drugs of abuse, including alcohol [33–35], nicotine [36], and heroin [37], as well as high fat chocolate food (but not regular food or sucrose; [20, 33]. Unlike cocaine however, SB-334867 also affects low-effort (FR1 and FR3) responding for alcohol, nicotine, and heroin, indicating that there may be a role for orexin in mediating hedonic properties of some drugs [33, 36–38].
3 Orexin and Drug Seeking Elicited by External Stimuli
Environmental cues that are associated with drug use evoke motivational states that drive drug-seeking behavior, even in the absence of the drug. These cues engage the orexin system, which in turn acts to translate cue-induced motivation into appetitive actions. Studies investigating these processes utilize a variety of behavioral models, including conditioned place preference (CPP), operant reinstatement, and conditioned reinforcement paradigms. Exposure to stress is another potent stimulus known to drive drug seeking. Below, we discuss evidence from each of these paradigms that point to a central role for the orexin system in driving externally motivated drug-seeking behavior.
3.1 Orexin and Conditioned Place Preference
Harris et al. showed that activation of lateral (but not medial) hypothalamic orexin neurons is strongly correlated with CPP for cocaine and morphine [18] (Fig. 1). This study also reported that local chemical stimulation of LH orexin neurons reinstates extinguished CPP behavior, and that this effect was blocked by systemic administration of the Ox1R antagonist, SB-334867. Subsequent studies showed that systemic SB-334867 blocks the expression of CPP for amphetamine [27], and the dual OxR1 + OxR2 antagonist almorexant prevents the expression of cocaine CPP [39]. Expression of cocaine CPP depends on inputs to orexin neurons from the rostral lateral septum (LSr): Fos expression in LH-projecting LSr neurons is positively correlated with CPP, and inhibiting LSr cells prevents expression of CPP, and of Fos in LH orexin neurons [40]. Together, these findings are consistent with the notion that orexin neurons are engaged by stimuli (or environments) of high motivational relevance, and that this motivational activation is critical for the expression of reward-seeking behavior [11, 41].
3.2 Reinstatement Behavior
The orexin system is critically involved in the process of translating cue-induced motivation into appetitive actions. These processes are often probed experimentally using the reinstatement model of drug seeking whereby environmental cues paired with drug during self-administration are used to reinstate drug seeking following a period of abstinence or extinction training [12, 42]. Drug-associated cues are typically classified as either: (1) discrete; such as light or tone cues that are paired with cocaine infusions; (2) discriminative; whereby drug delivery is response-contingent but stimuli (lights, tones) predict drug availability (i.e., are not-response contingent); or (3) contextual; where animals are trained to self-administer and extinguish in two separate contexts with distinguishable compound cues (light, sound, olfactory or tactile cues), and reinstatement behavior is assessed by returning the animal to the original context where they learned to self-administer.
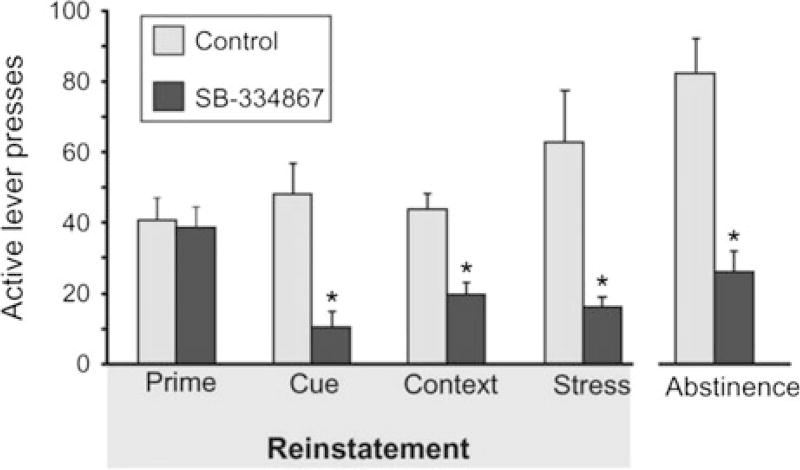
Systemic administration of SB-334867 dose-dependently blocks reinstatement of extinguished cocaine seeking elicited by discrete [21, 28, 43], discriminative [44], or contextual cues in rats [45] (Fig. 3). Similarly, systemic SB-334867 attenuates reinstatement of cocaine seeking when animals are returned to the self-administration chamber after either 1 day or 2 weeks of abstinence [45] (Fig. 3). These effects are driven by signaling at Ox1R, as pretreatment with the Ox2R antagonist 4PT had no effect on cue-induced cocaine seeking [27]. SB-334867 is also effective at blocking reinstatement of alcohol [34, 35, 46, 47], nicotine [48], and remifentanil [49] seeking, and there is evidence that Ox2R antagonists block reinstatement of nicotine seeking [50], but not cue-induced alcohol seeking [51].
Fig. 3.
Effects of SB-334867 treatment on reinstatement of cocaine seeking behavior. Systemic treatment with the orexin 1 receptor antagonist SB-334867 (30 mg/kg, i.p.) blocks reinstatement of cocaine seeking elicited by discrete cues, contextual cues, and stress. In contrast, SB-334867 has no effect on cocaine seeking elicited by a priming injection of cocaine. Figure adapted from Mahler et al. [12]
3.3 Conditioned Reinforcement
A model related to cue-induced drug seeking is the conditioned reinforcement (CR) paradigm, whereby animals learn to perform a novel response (e.g., nose poke) to receive a cue that was previously associated with drug delivery via another response (e.g., lever press) [52, 53]. Rats that were treated systemically with SB-334867 over 5 consecutive days of FR1 cocaine self-administration training showed attenuated responding for the cocaine-associated cue in a subsequent CR test [27]. This finding points to a role for orexin in ascribing motivational significance to discrete cues during self-administration training. In contrast however, Smith et al. [28] reported that SB-334867 administered systemically prior to a single cocaine-cue conditioning session had no effect on the ability of those cues to elicit reinstatement of cocaine seeking following extinction training. The reasons for the discrepancy in these two sets of findings are unclear, but may be related to differences in motivational significance acquired by the cue over a single versus repeated conditioning trials, or to different roles for orexin in operant vs Pavlovian conditioning. Importantly, treatment with SB-334867 prior to the CR test also attenuates responding [27], which is consistent with findings outlined above relating to cue-induced reinstatement, and supports a role for orexin in cue-driven motivated responding.
3.4 Stress
Orexin neurons are well positioned to modulate the behavioral and physiological responses to stress via bidirectional connections with various stress-relevant regions including bed nucleus of the stria terminalis (BNST), central amygdala (CeA), paraventricular nucleus of the hypothalamus (PVN), and medial prefrontal cortex (mPFC; for an overview, see James et al. [54]). Indeed, orexin neurons are activated by various stressors [8, 55–58] and optogenetic stimulation of orexin neurons reduces time spent in the interaction zone in a social interaction test and increases freezing in an open field test [59, 60]. Further, orexin receptor antagonists reduce anxiety behavior, and attenuate neuroendocrine responses to stress [48, 55, 61, 62]. These findings are consistent with a global role for orexin involvement in situations of high motivational relevance and translating this into adaptive behavior [7, 11, 20].
Drug-seeking behavior is strongly influenced by stress. Extinguished drug seeking can be reinstated by exposure to a range of stressors, including brief (15 min) intermittent, unpredictable electrical footshock [63, 64], food deprivation [65], or a range of pharmacological stressors, including the anxiogenic drug yohimbine [66, 67]. Systemic administration of SB-334867 reduces reinstatement of cocaine seeking elicited by yohimbine in both male and female rats [43]. Further, reinstatement of cocaine seeking elicited by intracerebroventricular (ICV) infusions of orexin-A is blocked by systemic administration of a non-selective CRF receptor antagonist [19]. The BNST appears to be an important brain region for these processes, as SB-334867 blocks yohimbine-induced changes in excitatory transmission in dorsal anterolateral BNST that are associated with the reinstatement of cocaine seeking, and these changes are absent in prepro-orexin knockout mice [68]. Orexin may also act at the nucleus accumbens shell (NAcSh) to mediate stress-induced drug seeking, as local infusions of either SB-334867 or TCS-OX2-29 block footshock-induced reinstatement of morphine seeking [69]. In contrast, the VTA and dentate gyrus of the hippocampus are unlikely sites of action, as local infusions of SB-334867 into these sites do not affect stress-induced seeking of cocaine or morphine, respectively [70, 71].
3.5 Conclusions
In summary, orexin signaling is necessary for motivated psychostimulant seeking elicited by external triggers like discrete drug-associated cues, contexts associated with prior drug use, or stress. Such stimuli are known to elicit craving and relapse behavior in human addicts [72–77]. This may reflect a role for orexin in conditioned responding for highly salient natural rewards, including certain foods and sex [20, 78, 79]. The orexin system may therefore offer a potential target for pharmacotherapies designed to reduce the risk of relapse during abstinence [10, 80, 81]. We propose that by decreasing OX1R signaling, and thereby attenuating responses of VTA dopamine neurons to cues associated with these rewards (discussed in Section 5.2.1), it may be possible to preferentially attenuate exaggerated craving responses without greatly affecting baseline reward processing. Indeed, many preclinical studies now suggest that natural reward seeking is unaffected following treatment with orexin receptor antagonists at doses that block drug-seeking behavior [9, 33, 44, 46].
4 Orexin and Behavioral Economics
4.1 Behavioral Economics Models
Behavioral-economic (BE) analyses of demand elasticity for drug and other rewards offer a sophisticated means by which to examine separately both reinforcing properties of a drug, and motivation to obtain and consume it. In a typical BE experiment, consumption of drug is plotted as a function of drug price, and both humans and animals are similarly responsive to increased drug price (in humans, monetary cost; in rats, lever presses). In rats, we employ a within-session BE paradigm [82, 83], and apply an exponential demand equation to generate estimates of preferred cocaine intake at zero cost (Q0, quantified by where computed demand curves intercept the ordinate) [84]. As such, Q0 can be considered a measure of hedonic set point for a drug, or the consumption of a drug under ’free’ (no cost) conditions. In addition, the slope of the demand curve conveys demand elasticity, or the sensitivity of drug seeking to increases in price. This elasticity parameter, termed alpha, is therefore an index of the animal’s motivation to obtain drug, or to defend preferred blood levels of drug. Importantly, Q0 and alpha are entirely orthogonal in cocaine demand tests, meaning that each parameter can be examined concurrently and independently [82, 85].
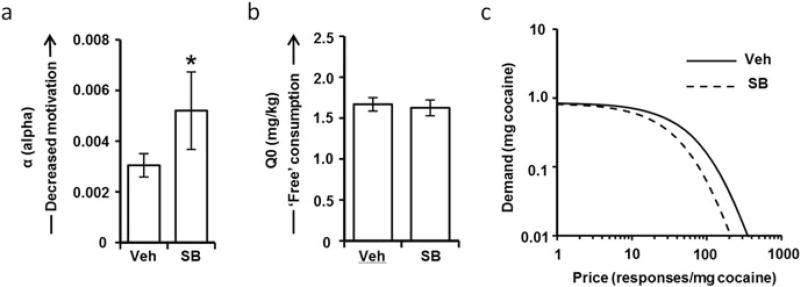
As described above, orexin is specifically involved in modulating motivated responding, as well as drug seeking elicited by external stimuli. A recent study in our laboratory used BE demand curve analysis to investigate these processes directly [21]. Different groups of rats were trained to self-administer cocaine with and without drug-paired discrete stimuli (light + tone), and demand curves for both groups of rats were calculated after pretreatment with SB-334867 or vehicle. Motivation (alpha) for cocaine was significantly higher when cocaine-associated cues were present and systemic administration of SB-334867 decreased motivation (increased alpha values) only in the presence of these cocaine-associated cues (Fig. 4). Further, SB-334867 had no effect on the hedonic set point (Q0) for cocaine, regardless of whether cocaine-associated cues were present. These findings are highly consistent with a previous study using a similar BE thresholding procedure to demonstrate that SB-334867 does not affect cocaine consumption, but reduces the maximal number of responses that rats were willing to make to maintain self-administration (PMax) [26].
Fig. 4.
Effects of SB-334867 treatment on economic demand for cocaine. (a) Pretreatment with SB-334867 increased cocaine demand elasticity (α), reflecting a decrease in motivation for cocaine. (b) SB-334867 had no effect on free cocaine consumption (Q0), consistent with previous studies showing that the orexin system is not involved in mediating the hedonic properties of cocaine. (c) Demand curves representing increased sensitivity of demand to price following SB-334867 treatment. Figure adapted from Bentzley et al. [21]
The observation that cocaine-paired discrete stimuli increased motivation for cocaine reward is consistent with previous findings that drug-paired stimuli take on motivational properties that drive responding independently of the reinforcing effects of the cocaine itself [86]. Interestingly, this cue-driven enhancement of motivation for cocaine was blocked by systemic SB-334867, indicating that signaling at Ox1Rs increases the reinforcing efficacy of cocaine-associated cues. These findings complement those outlined above, showing an important role for orexin signaling in stimulus-driven drug seeking. In contrast, blockade of orexin signaling had no effect on “free” consumption of cocaine (Q0). Together, these findings are strongly consistent with the “motivational activation” hypothesis of orexin function [11].
5 Interaction Between Orexin Neurons and Brain Reward Circuitry
Orexin signaling is crucial for highly motivated and conditioned drug seeking, but how do orexin neurons interact with wider reward circuits in the brain to modulate motivation? Examination of the afferent and efferent projection patterns of orexin neurons reveals that these cells are highly interconnected with key motivational and reward regions. The topography of the orexin system is described in detail elsewhere [87, 88], as well as in other chapters of this volume. Here, we summarize studies that have provided anatomical or functional evidence of orexin-related circuits in psychostimulant-seeking behavior. In particular, we focus on orexin projections to the midbrain dopamine (DA) system, and how orexin modulates glutamatergic inputs to VTA, including those that arise from the prefrontal cortex.
5.1 Orexin Afferents
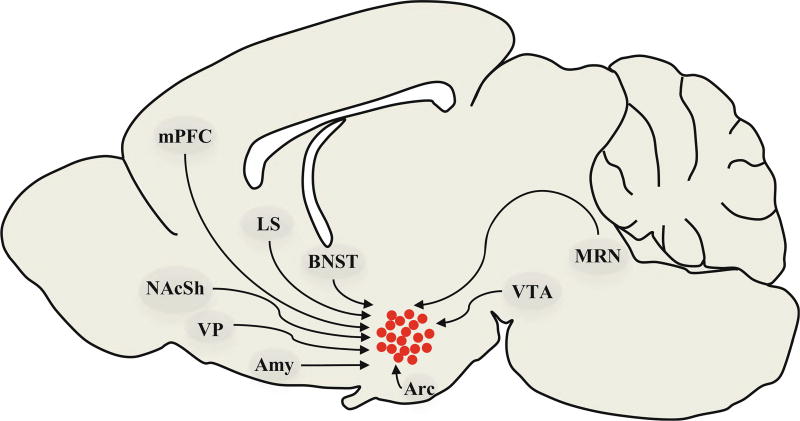
The first comprehensive study of inputs to orexin neurons was carried out by Sakurai and colleagues [89], using the retrograde tracer, tetanus toxin, that was genetically encoded to specifically reveal inputs to orexin neurons. Using this approach, the authors identified strong projections from various reward-related regions, including medial prefrontal cortex (mPFC), BNST, basal forebrain, basolateral and medial amygdala, arcuate nucleus, PVN, and median raphe nucleus (MRN). A subsequent study by Yoshida et al. [90] used the retrograde tracer cholera toxin B subunit (CTb) to identify afferents to LH and DMH orexin fields, and confirmed retrogradely labeled inputs using anterograde tracers and identification of appositions with orexin neurons. Using this approach, these authors confirmed many of the inputs identified by Sakurai et al., as well as strong projections from NAc shell, dorsolateral septum, ventral pallidum, central amygdala, VTA, and ventromedial and anterior hypothalamus. For a summary of afferent projections to orexin neurons from reward-related structures, see Fig. 5.
Fig. 5.
Afferent projections to orexin neurons from reward-related structures. A mid-sagittal section of a rat brain illustrating loci involved in drug seeking that provide input onto orexin neurons. Amy amygdala (central, medial, and basolateral amygdala provide input to orexin neurons), Arc arcuate nucleus of the hypothalamus, BNST bed nucleus of the stria terminalis, LS lateral septum, mPFC medial prefrontal cortex (both prelimbic and infralimbic structures), MRN medial raphe nucleus, NAcSh nucleus accumbens shell, VP ventral pallidum, VTA ventral tegmental area
To identify inputs to orexin neurons that are important for reward seeking, our laboratory examined Fos expression in various forebrain regions during the expression of cocaine CPP in animals that had previously received injections of the retrograde tracer CTb into the lateral or medial orexin fields [40]. This approach revealed that neurons in the rostral lateral septum and ventral BNST that project to the lateral orexin field are uniquely activated during cocaine CPP, and the magnitude of this activation was correlated with the degree of CPP expression. In a follow-up experiment, bilateral disconnection of the septum-LH orexin pathway attenuated the expression of cocaine CPP, confirming a functional role for this input in the expression of reward seeking [40].
Very few studies have otherwise been carried out examining the functional role of orexin afferents in reward seeking. One region of potential interest is the NAc shell, as this region projects strongly to the LH orexin area [90–92] and inactivation of this region induces Fos expression in the LH orexin field and promotes alcoholic beer seeking [93] and feeding behavior [94]. Another study, while not identifying the source of inputs, showed that excitatory drive from glutamatergic neurons onto orexin neurons is increased following cocaine exposure [95]. Given the apparent role for the orexin system in driving motivational behavior, future studies may benefit from focusing on inputs from regions involved in motivational processes, including prefrontal cortex and striatopallidal structures.
5.2 Orexin Efferents
5.2.1 Ventral Tegmental Area
Neurons in the VTA, along with the neighboring substantia nigra pars compacta, are major sources of DA in the brain [96]. VTA DA neurons mediate the effects of drugs of abuse, including psychostimulants, and play a key role in modulating motivational processes of drug-seeking behavior [6, 97–99]. VTA neurons express both Ox1R and Ox2R, although there are a limited number of orexin-containing synapses in this region, suggesting that the majority of orexin input to VTA may be nonsynaptic en passant fibers or non-synapsing terminals [100]. Regardless, orexin-A directly depolarizes VTA DA neurons [101] and there is considerable evidence that orexin facilitates glutamatergic inputs to modulate VTA activity and reward seeking.
A growing literature points to the VTA as a key site of orexin signaling in motivated reward seeking. Local infusions of orexin A into VTA increase cocaine self-administration under a PR, but not an FR1, schedule of reinforcement [30]. Similarly, local SB-334867 injections reduce PR breakpoints and reduce effort expended in a BE task, but have no effect on FR1 responding for cocaine [26]. Intra-VTA infusions of orexin-A also elicit reinstatement of extinguished cocaine seeking and morphine CPP [18, 71], whereas VTA injection of SB-334867 blocks both discrete and discriminative cue-induced reinstatement of cocaine seeking [102, 103]. Similar findings have been reported for cue-induced reinstatement of ethanol seeking [104]. Orexin signaling in VTA increases DA release in forebrain structures like NAc, which is known to be important for reward seeking behavior [105–112]. Infusions of orexin-A into VTA increase DA release in prefrontal cortex and ventral striatum [30, 113], whereas infusions of SB-334867 reduce cocaine-induced DA in NAc [71], showing clear modulation of VTA DA neurons by orexin inputs.
VTA DA neuron firing is regulated by glutamate inputs [114–117], and orexin plays an important role in modulating these. Borgland et al. first demonstrated that orexin modulates glutamatergic signaling in VTA DA neurons [118]. Bath application of orexin-A facilitates glutamatergic transmission in VTA DA neurons, initially via facilitation of NMDA signaling, and later, via facilitation of AMPA signaling – both via postsynaptic mechanisms. VTA Ox1R antagonism also blocks cocaine-induced LTP of excitatory DA afferents in vitro, and blocks locomotor sensitization to cocaine in vivo. Orexin-B also enhances glutamatergic inputs to VTA DA neurons, but does so via presynaptic as well as postsynaptic mechanisms [119].
We followed up these findings by demonstrating a functional interaction between glutamate and orexin in VTA mediation of conditioned cocaine seeking [103]. First, we showed that intra-VTA SB-334867 blocked cue-induced reinstatement of cocaine seeking, as did intra-VTA blockade of AMPA receptors with CNQX. In addition, blockade of VTA orexin in one cerebral hemisphere, with simultaneous blockade of AMPA signaling in the contralateral hemisphere, similarly blocked cue reinstatement, indicating necessity of concurrent orexin and AMPA signaling for cues to elicit cocaine seeking. Moreover, we showed that reductions in cocaine seeking by intra-VTA SB-334867 were reversed when we concurrently administered the AMPA allosteric modulator PEPA into VTA, to potentiate AMPA signaling. Together, these results show that by facilitating AMPA signaling in DA neurons, orexin in VTA potentiates both DA neuron activity and motivated behavioral responses to cocaine cues. Although orexin potentiates NMDA signaling in VTA neurons as well [118, 119], we did not see reductions in cue-induced cocaine seeking following intra-VTA blockade of NMDA receptors with AP-5 microinjections. VTA NMDA transmission is most important for acquisition of reward learning (presumably via induction of LTP; [117]), whereas AMPA may instead mediate expression of cocaine seeking elicited by previously learned cocaine cues [12, 103]. Therefore, orexin is likely capable of facilitating both reward learning, and expression of motivation elicited by prior learning, via facilitation of glutamate transmission in VTA.
The sources of glutamate inputs to VTA DA neurons that might be facilitated by orexin include hypothalamic, mid- and hind-brain nuclei, and/or the large projection from prelimbic mPFC [120, 121]. Although mPFC electrical stimulation usually elicits only weak enhancements of DA neuron firing in anesthetized animals [122, 123], we asked whether orexin application to VTA would increase the efficacy of mPFC stimulation-induced DA neuron activation [124]. Indeed, application of a low concentration of orexin-A that does not itself affect DA neuron firing robustly facilitated DA neuron firing elicited by prelimbic stimulation. This demonstrates that mPFC inputs to VTA DA neurons are facilitated by orexin-A in vivo. This said, we note that we did not see activation (as measured with Fos) in VTA afferent neurons from either mPFC or hypothalamic orexin neurons themselves during cue-induced cocaine seeking (compared to various control behaviors; [125]). Intriguingly, non-orexin-A expressing-, VTA-projecting neurons in the LH orexin field were instead activated during cued cocaine seeking.
5.2.2 Other Efferent Regions
Beyond the VTA, only a handful of regions have been investigated as potential targets of orexin signaling in drug seeking, and the large majority of these studies have been carried out in alcohol-seeking models. Perhaps the next most comprehensively studied orexin efferent region is the paraventricular thalamus (PVT), a midline thalamic structure that is more densely innervated by orexin-positive fibers than any other forebrain structure [88]. The PVT expresses both Ox1R and Ox2R in high abundance [87], and bath application of orexin peptide potently depolarizes PVT neurons [126]. Recent evidence has implicated the PVT as a key structure in brain reward circuitry, and a clear role has been shown for this structure in a range of drug-seeking paradigms [9, 81, 127–133]. Dayas and colleagues provided the first evidence of an LH orexin-PVT circuit in drug seeking, by showing that PVT neurons that are activated by drug-associated cues are closely apposed by orexin-positive fibers [129]. More direct evidence has come from studies showing that infusions of orexin-A and -B increase ethanol drinking and reinstate extinguished cocaine-seeking behavior. These effects appear to be mediated primarily via Ox2Rs, as Ox2R (but not Ox1R) levels in PVT are increased following ethanol drinking, and local infusions of the Ox2R antagonist TCS-OX2-29, but not the OxR1 antagonist SB-334867, reduces alcohol drinking [128]. A recent study suggests that signaling at the OxR2 in the PVT is also important for psychostimulant seeking, as infusions of orexin A into the PVT reinstated cocaine seeking and this was blocked by co-administration of TCS-OX2-29 but not SB-334867 [134]. These findings are consistent with an earlier demonstration that infusions of SB-334867 into the PVT have no effect on reinsatement of cocaine seeking elicited by discriminative cues [102].
The NAc is another potentially important site of orexin action in drug seeking, as this region expresses moderate to high levels of Ox2R [87, 135] and both orexin-A and B alter activity of NAc neurons [136, 137]. The effects of intra-NAc infusions of orexin receptor agonists or antagonists have not been examined for psychostimulants, but local infusions of SB-334867 or TCS-29-029 in NAcSh prevent stress-induced reinstatement of morphine conditioned place preference [69], and injections of TCS-OX2-029 in NAcC reduced responding for ethanol [51]. Also, injections of orexin-A into the rostral half of medial NAcSh increase the hedonic “liking” for sucrose taste [138], as do orexin-A injections into the posterior half of the ventral pallidum [139].
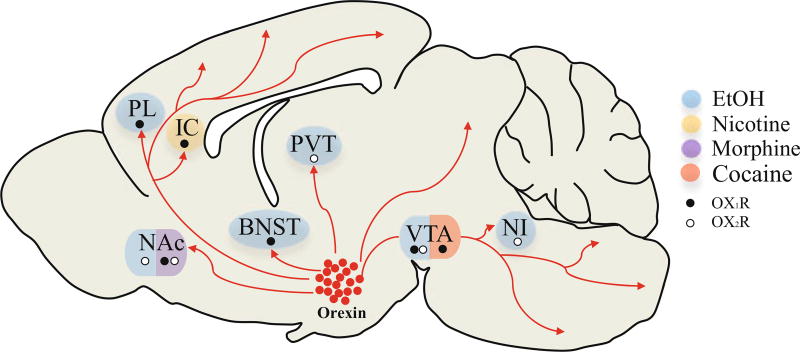
The BNST may be another target for orexin signaling in drug reward, as infusions of orexin-A into this region enhance alcohol seeking [140]. Also, as described above, OxR1 signaling in BNST appears to play an important role in stress-induced drug seeking. More recently, stress-induced reinstatement of alcohol seeking was shown to be dependent on signaling at the OX2R in the nucleus incertus, a small pontine region [141]. In the cortex, infusions of SB-334867 into the prelimbic cortex attenuate cue-induced reinstatement of ethanol seeking [104], and local infusions of SB-334867 in the insular cortex reduce nicotine self-administration behavior [36]. These findings highlight potential targets for orexin signaling beyond the mesocorticolimbic DA system that may be important in modulating psychostimulant-seeking behavior. For a summary of regions shown to be important sites of orexin signaling in drug seeking, see Fig. 6.
Fig. 6.
Sites of orexin signaling in drug-seeking behavior. Orexin neurons in the lateral hypothalamus project to a number of reward structures involved in drug-seeking behavior. The ventral tegmental area (VTA) has been identified as an important site of orexin signaling in cocaine seeking behavior (red). Intra-VTA infusions of the orexin receptor 1 antagonist SB-334867 attenuate reinstatement of cocaine seeking elicited by discriminative stimuli, discrete stimuli, and stress [71, 102, 103]. Similarly, intra-VTA infusions of SB-334867 block cue-induced reinstatement of ethanol seeking [104], and infusions of the dual orexin receptor antagonist almorexant into the VTA reduce alcohol drinking [142]. Ethanol-seeking behaviors have been shown to be dependent on orexin signaling at other loci, including the prelimbic cortex (PL; [104]), nucleus accumbens (NAc; [51]), the anterior paraventricular thalamus (PVT; [128]), the bed nucleus of the stria terminalis [140], and the nucleus incertus (NI; [141]). Signaling at both the OX1R and OX2 in the NAc is important for stress-induced reinstatement of morphine conditioned place preference [69], and infusions of SB-334867 into the insular cortex (IC) reduce nicotine seeking behavior [36]. (PNAS)
6 Functional Heterogeneity of Orexin Neurons: A Medial/Lateral Dichotomy in Orexin Cell Function?
Orexin neurons participate in a wide variety of functions including, but not limited to arousal, stress, and appetitive motivation [11, 143, 144]. A question arises as to how the orexin system participates in such diverse, and even apparently contradictory functions (e.g., stress and reward). One possibility is that there are heterogeneous subpopulations of orexin neurons that selectively participate in specific behavioral or physiological functions. There are multiple potential sources of heterogeneity in the orexin system. One of the more prominent factors appears to be differences in circuit functions of orexin neurons located in different hypothalamic subregions. Our laboratory proposed that orexin neurons in LH preferentially encode reward motivation whereas orexin neurons in DMH and PFA process arousal and stress [8]. This proposal was initially based on our demonstration that LH orexin neurons were Fos-activated by cues associated with food, morphine, and cocaine but not footshock, whereas PFA-DMH orexin neurons were preferentially activated by arousal/anxiety-inducing footshock stimulation [8]. Furthermore, chemical activation of LH, but not PFA-DMH, orexin neurons reinstated preference for morphine [18]. We also noted studies in which activation of LH, and not DMH, orexin neurons was associated with antipsychotic-induced weight gain [145] as well as increased feeding associated with NAcSh inhibition [94]. Conversely, PFA-DMH orexin neuron Fos activation was preferentially associated with wakefulness and arousal [145–147] as well as footshock, restraint, and cold stress [148, 149].
Since the publication of Harris and Aston-Jones [8], a number of studies have been performed investigating the activation of orexin neurons in a variety of appetitive and aversive contexts. In many cases, the distinction between lateral neurons encoding appetitive motivated behaviors and medial neurons encoding arousal or aversive motivated behaviors has been replicated. Work from our lab has shown preferential encoding of drug preference in LH orexin neurons [40, 150–152] and preferential signaling of arousal by PFA/DMH orexin neurons [153]. A number of other groups have also found preferential encoding of reward seeking by lateral orexin neurons. Context-induced reinstatement (ABA renewal) of beer seeking induced Fos activation of PFA and LH, but not DMH, orexin neurons, and the activity of LH orexin neurons (and not PFA/DMH) was correlated with strength of renewal [154]. Increased Fos activation of DMH and PFA, but not LH orexin neurons was observed following ABA renewal of cocaine seeking, though this increase was also seen in ABB controls in which animals were tested in their extinguished environment (B) instead of the conditioned environment (A) [91]. This is similar to the increased Fos observed in PFA and DMH (but not LH) orexin neurons during extinction from sucrose self-administration [78].
The increased reinstatement for cocaine seeking following ICV infusions of neuropeptide S depended primarily on LH orexin neurons [155]. Alcohol seeking induced by baclofen/muscimol infusions in the NAc induced Fos activation of PFA and LH, but not DMH, orexin neurons [93]. Morphine CPP preferentially activated LH, and not PFH or DMH, orexin neurons [156]. In a recent report, LH orexin neurons exhibited stronger firing in response to reward- vs. punishment-predicting cues [157], though the authors noted that LH orexin neurons also exhibited activity correlations with arousal in their previous studies [158].
Also supporting lateral vs. medial differences in orexin neuron function is the finding that medial orexin neurons preferentially encode arousing or aversive stimuli, events, and outcomes. Unpredictable chronic mild stress, a rodent model of depression, increased Fos expression in PFA/DMH orexin neurons, but not LH [159]. Naloxone-induced morphine withdrawal activates DMH and PFA, but not LH orexin neurons [160]. Anxiogenic doses of acute nicotine increased Fos in PFA/DMH but not LH, whereas cue-induced reinstatement of nicotine seeking increased Fos in PFA and LH but not DMH orexin neurons [48, 161]. DMH and PFA, but not LH neurons are activated by panic induced by sodium lactate, CO2, caffeine, or the anxiogenic drug FG-7142 [62, 162–164]. Inflammation using formalin injections into the hindpaw increased Fos in DMH and PFA, but not LH orexin neurons, and this activation was correlated with the extent of licking and grooming responses [165].
However, a medial/lateral dichotomy in orexin neuron function has not been observed in every study (see Tables 1 and 2). Stressful situations such as exposure to a brightly lit novel environment induced Fos activation of medial and lateral orexin neurons [172], as did a range of behaviors in mice, including progressive ratio responding for food reward [173]. In one study, restraint stress resulted in increased Fos in LH but not PFA/DMH orexin neurons [166]. Discriminative stimulus-driven reinstatement of alcohol seeking increased Fos activity in DMH, PFA, and LH orexin neurons [129]. Similarly, in a conditioned sucrose-seeking model, orexin neuron activation was observed across all orexin cell fields in food-restricted rats [78]. Cue-evoked feeding in sated animals produced preferential activation of PFA orexin neurons [174, 175], as did expectation of chocolate [176], while feeding induced by DAMGO infusions in the medial prefrontal cortex [177] or NAc [178] activated medial but not lateral orexin neurons. Finally, in a recent study from our laboratory, we found that the strength of context-induced reinstatement of ethanol seeking was correlated with the strength of Fos activation of LH and DMH (but not PFA) orexin neurons [151]. Together, these results indicate that the lateral vs. medial dichotomy in orexin neuron function may not always be as simple as encoding appetitive vs. aversive behaviors, respectively. The role of the PFA orexin neurons, which in some cases appears to associate more with appetitive and in other cases with aversive behaviors, needs to be investigated further.
Table 1.
Summary of studies that have reported patterns of orexin neuron activation under conditions of stress or arousal
| Stimuli/paradigm | DMH orexin | PFA orexin | LH orexin | Reference | |
|---|---|---|---|---|---|
| Stress | Acute amphetamine injection (1.5 mg/kg; i.p.) | ↑ Fosa | ↑ Fosa | Fadel et al. [145] | |
| Acute amphetamine injection (0.5 mg/kg; i.p.) | b | ↑ Fos (correlated with wakefulness) | b | Estabrooke et al. [146] | |
| Acute caffeine injection (10, 30, 75 mg/kg; i.p.) | ↑ Fos | ↑ Fos | ↑ Fos (10, 30 mg/kg dose) | Murphy et al. [147] | |
| Acute caffeine injection (50 mg/kg; i.p.) | ↑ Fosa | ↑ Fosa | Johnson et al. [163] | ||
| Acute nicotine injection (0.8 mg/kg; s.c.) | ↑ Fosa | ↑ Fosa | Plaza-Zabala et al. [48] | ||
| Footshock | ↑ Fos | ↑ Fos | Harris and Aston-Jones [8] | ||
| Footshock | b | ↑ Fos | b | Winsky-Sommerer et al. [149] | |
| Immobilization stress (30 min) | b | ↑ Fos | b | Winsky-Sommerer et al. [149] | |
| Immobilization stress (20 min) | c | ↑ Fosc | c | Sakamoto et al. [148] | |
| Immobilization stress (30 min; used to reinstate cocaine CPP) | a | a | ↑ Fos | Tung et al. [166] | |
| Cold water stress | b | ↑ Fosb | b | Sakamoto et al. [148] | |
| Carbon-dioxide stress | ↑ Fosa | ↑ Fosa | Sunanaga et al. [164] and Johnson et al. [167] | ||
| Sodium lactate injections | ↑ Fos | ↑ Fos | Johnson et al. [62] | ||
| FG-7142 injections (7.5 mg/kg) | ↑ Fosa | ↑ Fosa | Johnson et al. [163] | ||
| Hindpaw formalin injections | ↑ Fos (correlated with pain response) | ↑ Fos (correlated with pain response) | Campbell et al. [165] | ||
| Naloxone-induced morphine withdrawal | ↑ Fos | ↑ Fos | Sharf et al. [160] | ||
| Unpredictable chronic mild stress | ↑ Fosa | ↑ Fosa | Nollet et al. [159] | ||
| Arousal | Sleep deprivation | b | ↑ Fos (correlated with wakefulness) | b | Estabrooke et al. [146] |
| Active vs rest period | ↑ Fos in active period | ↑ Fos in active period (correlated with wakefulness) | Estabrooke et al. [146] | ||
| Active vs rest period | ↑ Fos in active period | ↑ Fos in active period | Gompf and Aston-Jones [153] | ||
| Running wheel confinement-induced resetting of circadian clock | ↑ Fos | ↑ Fos | ↑ Fos | Webb et al. [168] | |
| Wakefulness induced by inhibition of basal forebrain | ↑ Fos | ↑ Fos | ↑ Fos | Satoh et al. [111] | |
| Acute modafinil injection (150 mg/kg; i.p.) | b | ↑ Fos | b | Chemelli et al. [169] |
In cases where cell is blank, no effect of the experimental manipulation was observed in this region
Indicates that PFA and DMH regions were not quantified separately
Indicates that this region was not examined
Indicates that in this paper, subregions were not quantified separately in the original paper – subregional effects are estimated here based on original figure depicting Orx+/Fos+ cells across entire orexin field
Table 2.
Summary of studies that have reported patterns of orexin neuron activation in Pavlovian vs. instrumental reward seeking paradigms
| Stimuli/paradigm | DMH orexin |
PFA orexin | LH orexin | Reference | ||
|---|---|---|---|---|---|---|
| Pavlovian | Opiates | Morphine CPP | ↑ Fos (Fos correlated with CPP) | Harris and Aston-Jones [18], Harris et al. [150], and Lasheras et al. [156] | ||
| Morphine CPP | ↑ Fos in VTA-projecting neurons (Fos correlated with CPP) | Richardson and Aston-Jones [152] | ||||
| Cocaine | Cocaine CPP | ↑ Fos (Fos correlated with CPP) | Harris and Aston-Jones [18] | |||
| Cocaine CPP | a | a | ↑ Fos in LS-projecting neurons (Fos in LH-projecting LS neurons correlated with CPP) | Sartor and Aston-Jones [40] | ||
| Instrumental | Cocaine | ABA renewal | ↑ Fos (also seen in ABB) | ↑ Fos (also been in ABB) | Hamlin et al. [91] | |
| Cued-reinstatement (with NPS infusions) | ↑ Fos | ↑ Fos | Kallupi et al. [155] | |||
| Alcohol | Context-induced reinstatement | Fos correlated with seeking | Fos correlated with seeking | Moorman et al. [151] | ||
| ABA renewal (beer) | ↑ Fos | ↑ Fos (Fos correlated with seeking) | Hamlin et al. [154] | |||
| DS+ reinstatemet | ↑ Fos | ↑ Fos | ↑ Fos | Dayas et al. [129] | ||
| EtOH-seeking elicited by inhibition of NAcSh | ↑ Fos | ↑ Fos | Millan et al. [93] | |||
| Homecage EtOH seeking | For correlated with seeking | Fos correlated with seeking | Moorman et al. [151] | |||
| Nicotine | Cue-induced reinstatement | ↑ Fos | ↑ Fos | Plaza-Zabala [170] | ||
| Methamphetamine | Methamphetamine self-administration | ↑ Fos (not seen in meth-seeking rats) | ↑ Fos (not seen in meth-seeking rats) | Cornish et al. [171] |
In case where cell is blank, no effect of the experimental manipulation was observed in this region
Indicates that PFA and DMH regions were not quantified separately
As shown in Table 2, there may be a dichotomy whereby Pavlovian reward-associated behaviors (such as conditioned place preference) primarily engage LH and not PFA orexin neurons. In contrast, instrumental reward seeking, often performed in a reinstatement context in which the primary reinforcer is unavailable, recruits both LH and PFA neurons. One possible explanation for this observation is that instrumental reinstatement in the absence of a reward produces both motivation to acquire reward along with frustration in not receiving reward, thus engaging both LH and PFA (and sometimes DMH) orexin neurons. Another possible explanation may arise from the fact that instrumental, and not Pavlovian, reward-related behaviors require increased arousal and/or motor activity, in addition to motivation for reward. The simultaneous increase in arousal/motor output as well as reward motivation thus activates both LH and PFA orexin neurons to facilitate an active reward-seeking behavior, hypotheses presented in previous reports [8, 173]. These proposals should be directly tested in behavioral paradigms designed to isolate subcomponents of reward seeking, arousal, motor behavior, etc., in order to identify specific relationships to orexin neuron activity.
In addition, it will be important to consider different behavioral categories beyond simply appetitive vs. aversive, which may further dissect selective contributions of orexin neuron populations. Future work will also benefit from characterizing real-time activation of orexin neurons (as shown, for example, in [157, 158, 179]), direct modulation of orexin neuron populations [59, 180–183], and correlating strength of activation with specific behaviors [18, 151, 154].
Differential anatomical connectivity of neurons within orexin subfields may provide a basis for functional differences between lateral vs medial orexin cell groups. In support of this possibility, a number of groups reported that different brain areas innervate lateral vs. medial orexin fields [90, 184]. Although somewhat speculative, an overview of the regions that preferentially project to the lateral vs. medial orexin fields reflects regions that could be broadly defined as appetitive/motivational vs. arousal/anxiety categories, respectively. For example, projections from dorsal mPFC in rats (prelimbic and cingulate), which are frequently associated with expression of conditioned fear [185], target medial orexin fields, whereas ventral medial prefrontal cortex (infralimbic) and orbitofrontal cortex, which are commonly associated with extinction of fear and reward seeking [185], preferentially target lateral regions of the orexin field [184]. As discussed above, studies in mice using genetic tools for identifying neuronal populations specifically projecting to orexin neurons revealed a range of brain areas including those related to sleep/wake (e.g., basal forebrain, median raphe, and ventrolateral preoptic area) as well as motivation and emotion (e.g., infralimbic medial prefrontal cortex, amygdala, NAc, BNST, and lateral septum) [89]. In these studies, however, lateral vs. medial differences were not considered indicating that the interaction between orexin neuron anatomical location and afferents may be a useful research path.
There is also evidence for differential physiological properties of orexin neurons based on projection targets; this provides another possible basis for functional heterogeneity among orexin cell groups. As outlined above, orexin neurons signal at a number of target structures to regulate drug seeking, including VTA [20, 30, 71, 102, 103], PVT [128, 131, 132], and other reward and motivation-related targets [12, 186]. Early work revealed that lateral (LH/PFA) orexin neurons preferentially projected to VTA in rats [187], although orexin neuron projections to VTA in mice appear to originate from both medial and lateral subdivisions [188]. Only a small number of studies to date have explored the function of orexin neurons based on their projection targets [150, 189]. Work from our lab demonstrated that VTA-projecting, but not locus coeruleus-projecting, orexin neurons were activated during morphine conditioned place preference [152]. Interestingly, the same study demonstrated that VTA-projecting LH, but not PFA/DMH orexin neurons exhibited Fos activation correlated with CPP scores. These and other results indicate that selective projections of specific orexin neuron populations may serve as a mechanism for differentiating functional ensembles.
Orexin neurons co-express other neurotransmitter molecules, and this provides another possible basis for functional differences in orexin neuron populations [11]. Such co-expressed signaling molecules include classical neurotransmitters (eg. glutamate [190–192]) and neuropeptides (eg. dynorphin [193, 194]).
Finally, differences in physiological properties may also contribute to functional heterogeneity of orexin neuron subpopulations. Burdakov and colleagues have characterized two distinct populations of orexin neurons based on a combination of physiological (transient vs. persistent inhibitory responses to glucose administration in vitro) and anatomical (medial vs. lateral) location [195]. Additional studies may further delineate subpopulations of orexin neurons by endogenous physiological properties.
6.1 Summary
Different populations of orexin neurons may preferentially participate in specific functions within the greater context of motivational activation. Whether the primary differentiating factor is anatomical location within the medial/lateral axis of the hypothalamus, as our group proposed 10 years ago [8], has so far not been conclusively proven or dismissed. A likely future outcome will be the revelation that a confluence of factors – anatomical, hodological, physiological, and molecular – will differentiate orexin neurons into specific functional populations. Future integrative studies, using well-designed behavioral paradigms to isolate specific physiological or behavioral factors, will ultimately assist in understanding how the range of functionally distinct subpopulations of orexin neurons, and how these populations are segregated and how they interact, both with each other and with other hypothalamic neuronal populations.
7 Conclusions and Future Directions
The past decade has seen significant advancement of our understanding of the orexin system in reward and addiction. In drug-seeking paradigms, orexin neurons are preferentially engaged under circumstances where high levels of effort are required to obtain drug, or when motivation for drug is augmented by external stimuli such as drug-related cues or stress. These findings are consistent with what we believe constitutes a broader, fundamentally unified role for the orexin system in facilitating motivational states – both appetitive and aversive – and translating these states into behaviors that the organism uses to exploit a reward opportunity or respond to pressing threats – a process we termed “motivational activation” [11]. We suggest that this diverse, integrative role must be the result of heterogeneous functional connectivity with wider brain circuits that allow orexin neurons to modulate a range of behavioral and physiological outputs. Indeed, a priority of future studies must be to characterize this heterogeneity. As we discuss above, we believe that an important first step will be examining how different orexin subpopulations (for example, medial versus lateral) differ in terms of their precise afferent and efferent topography. Recent advances in cell-specific targeting approaches that allow for the selective regulation of orexin neurons and their afferents or efferents, using either optogenetics or chemogenetics (e.g., DREADDs) will allow these questions to be addressed at both an anatomical and functional level.
We note that the majority of studies of orexin in the context of drug abuse have been carried out in short-access models that may not fully recapitulate the behavioral features of addiction [196]. It is imperative that future studies examine how the orexin system is involved in the compulsive behavioral phenotypes that are precipitated by alternative models such long [196, 197] or intermittent [198] access models. Indeed, rats that are exposed to these paradigms exhibit behavioral characteristics reflective of increased motivational drive for drug, including escalated cocaine intake and compulsive drug use despite punishment. Currently, our laboratory is investigating how the orexin system may be involved in this motivational shift and whether this system can be targeted to reduce addiction-like behaviors.
In August 2014, the dual orexin receptor antagonist Suvorexant (Belsomra®) was approved by the US FDA for the treatment of insomnia. Clinical trials suggest that this drug is relatively well tolerated with few side effects reported at doses required to achieve sleep-promoting effects [199]. In addition, a number of other orexin receptor antagonist compounds are also currently at various stages of preclinical and clinical testing [10]. As such, there is significant interest in the potential use of orexin receptor antagonists for the treatment of other disorders associated with orexin dysfunction, including substance use disorders. Although we agree that these compounds may offer an exciting new approach to treating psychostimulant addiction – for which there are currently no effective pharmacotherapies – we also caution that our understanding of the orexin system in addiction is far from complete. This said, the rapid growth in our understanding of orexin in the last 10 years, including the introduction of the first orexin receptor antagonist for clinical use, bodes well for the next 10 years of research into this intriguing neuropeptide system.
Acknowledgments
This work is supported by an NHMRC CJ Martin Fellowship (1072706) to M.H.J., and PHS grants R00 DA035251 to SVM, and R01 DA006214 to GAJ. Support for DEM: R21 DA041674.
Contributor Information
Morgan H. James, Brain Health Institute, Rutgers University/Rutgers Biomedical and Health Sciences, Piscataway, NJ 08854, USA Florey Institute of Neuroscience and Mental Health, University of Melbourne, Parkville, VIC 2337, Australia.
Stephen V. Mahler, Department of Neurobiology and Behavior, University of California, Irvine, Irvine, CA 92967, USA
David E. Moorman, Department of Psychological and Brain Sciences & Neuroscience and Behavior Graduate Program, University of Massachusetts Amherst, Amherst, MA 01003, USA
Gary Aston-Jones, Brain Health Institute, Rutgers University/Rutgers Biomedical and Health Sciences, Piscataway, NJ 08854, USA.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821(2):535–538. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- 4.Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96(1–2):45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 5.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol. 2014;172:334–348. doi: 10.1111/bph.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010;1314:103–111. doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29(10):571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 9.James MH, Yeoh JW, Graham BA, Dayas CV. Insights for developing pharmacological treatments for psychostimulant relapse targeting hypothalamic peptide systems. J Addict Res Ther. 2012;S4:008. [Google Scholar]
- 10.Khoo SY, Brown RM. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs. 2014;28(8):713–730. doi: 10.1007/s40263-014-0179-x. [DOI] [PubMed] [Google Scholar]
- 11.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17(10):1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355(9197):39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 14.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 15.Akimoto H, Honda Y, Takahashi Y. Pharmacotherapy in narcolepsy. Dis Nerv Syst. 1960;21:704–706. [PubMed] [Google Scholar]
- 16.Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52(1):27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 17.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23(8):3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 19.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102(52):19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41(9):1149–1156. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker LC, Lawrence AJ. The role of orexins/hypocretins in alcohol use and abuse. Curr Top Behav Neurosci. 2016 doi: 10.1007/7854_2016_55. [DOI] [PubMed] [Google Scholar]
- 23.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 24.Wise RA. Intravenous drug self-administration: a special case of positive reinforcement. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer; New York: 1987. pp. 117–141. [Google Scholar]
- 25.Wise RA. Drug self-administration viewed as ingestive behaviour. Appetite. 1997;28(1):1–5. doi: 10.1006/appe.1996.0059. [DOI] [PubMed] [Google Scholar]
- 26.Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31(2):336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol. 2011;22(2):173–181. doi: 10.1097/FBP.0b013e328343d761. [DOI] [PubMed] [Google Scholar]
- 28.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30(3):493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 2011;214(2):415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Nerosci. 2015;6(1):138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riday TT, Fish EW, Robinson JE, Jarrett TM, McGuigan MM, Malanga CJ. Orexin-1 receptor antagonism does not reduce the rewarding potency of cocaine in Swiss–Webster mice. Brain Res. 2012;1431:53–61. doi: 10.1016/j.brainres.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 2011a;1391:54–59. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 34.Jupp B, Krstew E, Dezsi G, Lawrence AJ. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. Br J Pharmacol. 2011b;162(4):880–889. doi: 10.1111/j.1476-5381.2010.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148(6):752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105(49):19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith RJ, Aston-Jones G. Orexin/hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. 2012;35(5):798–804. doi: 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 2010;209(2):203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steiner MA, Lecourt H, Jenck F. The dual orexin receptor antagonist almorexant, alone and in combination with morphine, cocaine and amphetamine, on conditioned place preference and locomotor sensitization in the rat. Int J Neuropsychopharmacol. 2013;16(2):417–432. doi: 10.1017/S1461145712000193. [DOI] [PubMed] [Google Scholar]
- 40.Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci. 2012;32(13):4623–4631. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao Y, Mineur YS, Gan G, Wang AH, Liu ZW, Wu X, Suyama S, de Lecea L, Horvath TL, Picciotto MR, Gao XB. Repeated in vivo exposure of cocaine induces long-lasting synaptic plasticity in hypocretin/orexin-producing neurons in the lateral hypothalamus in mice. J Physiol. 2013;591(Pt 7):1951–1966. doi: 10.1113/jphysiol.2012.246983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23(4):675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther. 2012;340(3):801–809. doi: 10.1124/jpet.111.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin-Fardon R, Weiss F. Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: comparison with natural reward seeking. Neuroreport. 2014a;25(7):485–488. doi: 10.1097/WNR.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58(1):179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin-Fardon R, Weiss F. N-(2-methyl-6-benzoxazolyl)-N′-1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: comparison with natural reward seeking. Addict Biol. 2014b;19(2):233–236. doi: 10.1111/j.1369-1600.2012.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moorman DE, James MH, Kilroy EA, Aston-Jones G. Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res. 2016;1654:34–42. doi: 10.1016/j.brainres.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30(6):2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porter-Stransky KA, Bentzley BS, Aston-Jones G. Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol. 2015 doi: 10.1111/adb.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uslaner JM, Winrow CJ, Gotter AL, Roecker AJ, Coleman PJ, Hutson PH, Le AD, Renger JJ. Selective orexin 2 receptor antagonism blocks cue-induced reinstatement, but not nicotine self-administration or nicotine-induced reinstatement. Behav Brain Res. 2014;269:61–65. doi: 10.1016/j.bbr.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Brown RM, Khoo SY-S, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol. 2013;16(9):2067–2079. doi: 10.1017/S1461145713000333. [DOI] [PubMed] [Google Scholar]
- 52.Di Ciano P, Everitt BJ. Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. Eur J Pharmacol. 2005;526(1–3):186–198. doi: 10.1016/j.ejphar.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 53.Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl) 2002;163(3–4):327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- 54.James MH, Campbell EJ, Dayas CV. Role of the orexin/hypocretin system in stress-related psychiatric disorders. Curr Top Behav Neurosci. 2016 doi: 10.1007/7854_2016_56. [DOI] [PubMed] [Google Scholar]
- 55.Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, Vrontakis ME, Kirouac GJ. Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct Funct. 2013;219:2103–2118. doi: 10.1007/s00429-013-0626-3. [DOI] [PubMed] [Google Scholar]
- 56.Furlong TM, Vianna DM, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 2009;30(8):1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- 57.James MH, Campbell EJ, Walker FR, Smith DW, Richardson HN, Hodgson DM, Dayas CV. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front Behav Neurosci. 2014;8:244. doi: 10.3389/fnbeh.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martins PJ, D’Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept. 2004;117(3):155–158. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Bonnavion P, Jackson AC, Carter ME, de Lecea L. Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat Commun. 2015;6:6266. doi: 10.1038/ncomms7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heydendael W, Sengupta A, Beck S, Bhatnagar S. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol Behav. 2013;130:182–190. doi: 10.1016/j.physbeh.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang H, Saito T, Ohiwa N, Tateoka M, Deocaris CC, Fujikawa T, Soya H. Inhibitory effects of an orexin-2 receptor antagonist on orexin A- and stress-induced ACTH responses in conscious rats. Neurosci Res. 2007;57(3):462–466. doi: 10.1016/j.neures.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med. 2010;16(1):111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33(1):13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 64.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 65.Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2003;168(1–2):170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- 66.Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179(2):366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- 67.Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29(4):686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- 68.Conrad KL, Davis AR, Silberman Y, Sheffler DJ, Shields AD, Saleh SA, Sen N, Matthies HJG, Javitch JA, Lindsley CW, Winder DG. Yohimbine depresses excitatory transmission in BNST and impairs extinction of cocaine place preference through orexin-dependent, norepinephrine-independent processes. Neuropsychopharmacology. 2012;37(10):2253–2266. doi: 10.1038/npp.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi K, Wei C, Li Y, Sui N. Orexin receptors within the nucleus accumbens shell mediate the stress but not drug priming-induced reinstatement of morphine conditioned place preference. Front Behav Neurosci. 2013;7:144. doi: 10.3389/fnbeh.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ebrahimian F, Naghavi FS, Yazdi F, Sadeghzadeh F, Taslimi Z, Haghparast A. Differential roles of orexin receptors within the dentate gyrus in stress- and drug priming-induced reinstatement of conditioned place preference in rats. Behav Neurosci. 2016;130(1):91–102. doi: 10.1037/bne0000112. [DOI] [PubMed] [Google Scholar]
- 71.Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65(10):857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107(4):523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 73.Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 74.O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 75.Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68(9):942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 77.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39(3):1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 2013;226(1):155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27(11):2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cruz HG, Hoever P, Chakraborty B, Schoedel K, Sellers EM, Dingemanse J. Assessment of the abuse liability of a dual orexin receptor antagonist: a crossover study of almorexant and zolpidem in recreational drug users. CNS Drugs. 2014;28(4):361–372. doi: 10.1007/s40263-014-0150-x. [DOI] [PubMed] [Google Scholar]
- 81.Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci. 2014a;8:36. doi: 10.3389/fnins.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 2013;226(1):113–125. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oleson EB, Roberts DCS. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2008;34(3):796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115(1):186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- 85.Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A. 2014;111(32):11822–11827. doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76(Pt B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 88.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494(5):845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151(3):659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 92.Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29(5):1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30(13):4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19(2):376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- 95.Yeoh JW, James MH, Jobling P, Bains JS, Graham BA, Dayas CV. Cocaine potentiates excitatory drive in the perifornical/lateral hypothalamus. J Physiol. 2012;590(Pt 16):3677–3689. doi: 10.1113/jphysiol.2012.230268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1–6):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 97.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 98.Ranaldi R, Wise RA. Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci. 2001;21(15):5841–5846. doi: 10.1523/JNEUROSCI.21-15-05841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14(2–3):169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503(5):668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- 101.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011b;14(5):684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- 103.Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2013;226(4):687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown RM, Kim AK, Khoo SY, Kim JH, Jupp B, Lawrence AJ. Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in iP rats. Addict Biol. 2016;21(3):603–612. doi: 10.1111/adb.12251. [DOI] [PubMed] [Google Scholar]
- 105.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 106.Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54(2):237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 107.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 108.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 109.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 110.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 111.Satoh T, Nakai S, Sato T, Kimura M. Correlated coding of motivation and outcome of decision by dopamine neurons. J Neurosci. 2003;23(30):9913–9923. doi: 10.1523/JNEUROSCI.23-30-09913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30(5):853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- 113.Vittoz NM, Schmeichel B, Berridge CW. Hypocretin/orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28(8):1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci. 1993;5(2):137–144. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 115.Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28(1):73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- 116.Wang T, O’Connor WT, Ungerstedt U, French ED. N-methyl-d-aspartic acid biphasically regulates the biochemical and electrophysiological response of A10 dopamine neurons in the ventral tegmental area: in vivo microdialysis and in vitro electrophysiological studies. Brain Res. 1994;666(2):255–262. doi: 10.1016/0006-8993(94)90780-3. [DOI] [PubMed] [Google Scholar]
- 117.Zellner MR, Ranaldi R. How conditioned stimuli acquire the ability to activate VTA dopamine cells: a proposed neurobiological component of reward-related learning. Neurosci Biobehav Rev. 2010;34(5):769–780. doi: 10.1016/j.neubiorev.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 118.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 119.Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28(8):1545–1556. doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- 120.Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, Meredith GE, Zahm DS. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33(11):2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2015;1628(Pt A):130–146. doi: 10.1016/j.brainres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gariano RF, Groves PM. Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res. 1988;462(1):194–198. doi: 10.1016/0006-8993(88)90606-3. [DOI] [PubMed] [Google Scholar]
- 123.Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996;22(3):195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 124.Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci. 2010;30(46):15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32(38):13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ishibashi M, Takano S, Yanagida H, Takatsuna M, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides. 2005;26(3):471–481. doi: 10.1016/j.peptides.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 127.James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV. Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011a;199:235–242. doi: 10.1016/j.neuroscience.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 128.Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2015;20(3):469–481. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63(2):152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 130.James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Dayas CV. Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One. 2010;5(9):e12980. doi: 10.1371/journal.pone.0012980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.James MH, Dayas CV. What about me…? The PVT: a role for the paraventricular thalamus (PVT) in drug-seeking behavior. Front Behav Neurosci. 2013;7:18. doi: 10.3389/fnbeh.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martin-Fardon R, Boutrel B. Orexin/hypocretin (Orx/Hcrt) transmission and drug-seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Front Behav Neurosci. 2012;6:75. doi: 10.3389/fnbeh.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yeoh JW, James MH, Graham BA, Dayas CV. Electrophysiological characteristics of paraventricular thalamic (PVT) neurons in response to cocaine and cocaine- and amphetamine-regulated transcript (CART) Front Behav Neurosci. 2014b;8:280. doi: 10.3389/fnbeh.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matzeu A, Kerr TM, Weiss F, Martin-Fardon R. Orexin-A/hypocretin-1 mediates cocaine-seeking behavior in the posterior paraventricular nucleus of the thalamus via orexin/hypocretin receptor-2. J Pharmacol Exp Ther. 2016;359:273–279. doi: 10.1124/jpet.116.235945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438(1–2):71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 136.Mori K, Kim J, Sasaki K. Electrophysiological effects of orexin-B and dopamine on rat nucleus accumbens shell neurons in vitro. Peptides. 2011;32(2):246–252. doi: 10.1016/j.peptides.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 137.Mukai K, Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Electrophysiological effects of orexin/hypocretin on nucleus accumbens shell neurons in rats: an in vitro study. Peptides. 2009;30(8):1487–1496. doi: 10.1016/j.peptides.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 138.Castro DC, Terry RA, Berridge KC. Orexin in rostral hotspot of nucleus accumbens enhances sucrose ‘liking’ and intake but scopolamine in caudal shell shifts ‘liking’ toward ‘disgust’ and ‘fear’. Neuropsychopharmacology. 2016;41:2101–2111. doi: 10.1038/npp.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ho CY, Berridge KC. An orexin hotspot in ventral pallidum amplifies hedonic ’liking’ for sweetness. Neuropsychopharmacology. 2013;38(9):1655–1664. doi: 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ubaldi M, Giordano A, Severi I, Li H, Kallupi M, de Guglielmo G, Ruggeri B, Stopponi S, Ciccocioppo R, Cannella N. Activation of hypocretin-1/orexin-A neurons projecting to the bed nucleus of the stria terminalis and paraventricular nucleus is critical for reinstatement of alcohol seeking by neuropeptide S. Biol Psychiatry. 2016;79(6):452–462. doi: 10.1016/j.biopsych.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 141.Kastman HE, Blasiak A, Walker L, Siwiec M, Krstew EV, Gundlach AL, Lawrence AJ. Nucleus incertus Orexin2 receptors mediate alcohol seeking in rats. Neuropharmacology. 2016;110(Part A):82–91. doi: 10.1016/j.neuropharm.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 142.Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, Hopf FW, Bonci A, Bartlett SE. The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS One. 2012;7(9):e44726. doi: 10.1371/journal.pone.0044726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol. 2014;171(2):332–350. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15(11):719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 145.Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22(15):6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21(5):1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murphy JA, Deurveilher S, Semba K. Stimulant doses of caffeine induce c-FOS activation in orexin/hypocretin-containing neurons in rat. Neuroscience. 2003;121(2):269–275. doi: 10.1016/s0306-4522(03)00461-5. [DOI] [PubMed] [Google Scholar]
- 148.Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 2004;118(3):183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 149.Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24(50):11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183(1):43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moorman DE, James MH, Kilroy EA, Aston-Jones G. Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci. 2016;43:710–720. doi: 10.1111/ejn.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Richardson KA, Aston-Jones G. Lateral hypothalamic orexin/hypocretin neurons that project to ventral tegmental area are differentially activated with morphine preference. J Neurosci. 2012;32(11):3809–3817. doi: 10.1523/JNEUROSCI.3917-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gompf HS, Aston-Jones G. Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity. Brain Res. 2008;1224:43–52. doi: 10.1016/j.brainres.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146(2):525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 155.Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, de Lecea L, Ciccocioppo R. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc Natl Acad Sci U S A. 2010;107(45):19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lasheras MC, Laorden ML, Milanes MV, Nunez C. Corticotropin-releasing factor 1 receptor mediates the activity of the reward system evoked by morphine-induced conditioned place preference. Neuropharmacology. 2015;95:168–180. doi: 10.1016/j.neuropharm.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 157.Hassani OK, Krause MR, Mainville L, Cordova CA, Jones BE. Orexin neurons respond differentially to auditory cues associated with appetitive versus aversive outcomes. J Neurosci. 2016;36(5):1747–1757. doi: 10.1523/JNEUROSCI.3903-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25(28):6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology. 2011;61(1–2):336–346. doi: 10.1016/j.neuropharm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 160.Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64(3):175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Plaza-Zabala A, Flores A, Maldonado R, Berrendero F. Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol Psychiatry. 2012;71(3):214–223. doi: 10.1016/j.biopsych.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 162.Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012a;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, Truitt W, Lowry CA, Shekhar A. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012b;107(5):733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166(3):184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 165.Campbell EJ, Watters SM, Zouikr I, Hodgson DM, Dayas CV. Recruitment of hypothalamic orexin neurons after formalin injections in adult male rats exposed to a neonatal immune challenge. Front Neurosci. 2015;9:65. doi: 10.3389/fnins.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tung LW, Lu GL, Lee YH, Yu L, Lee HJ, Leishman E, Bradshaw H, Hwang LL, Hung MS, Mackie K, Zimmer A, Chiou LC. Orexins contribute to restraint stress-induced cocaine relapse by endocannabinoid-mediated disinhibition of dopaminergic neurons. NatCommun. 2016;7:12199. doi: 10.1038/ncomms12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Johnson PL, et al. Induction of c-Fos in ‘panic/defence’-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol. 2011;25:26–36. doi: 10.1177/0269881109353464. [DOI] [PubMed] [Google Scholar]
- 168.Webb IC, Patton DF, Hamson DK, Mistlberger RE. Neural correlates of arousal-induced circadian clock resetting: hypocretin/orexin and the intergeniculate leaflet. Eur J Neurosci. 2008;27:828–835. doi: 10.1111/j.1460-9568.2008.06074. [DOI] [PubMed] [Google Scholar]
- 169.Chemelli RM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;20:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 170.Plaza-Zabala A, et al. A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior. Neuropsychopharmacology. 2013;38:1724–1736. doi: 10.1038/npp.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cornish JL, et al. Regional c-Fos and FosB/ΔFosB expression associated with chronic methamphetamine self-administration and methamphetamine-seeking behavior in rat. Neuroscience. 2012;206:100–114. doi: 10.1016/j.neuroscience.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 172.Espana RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121(1):201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- 173.McGregor R, Wu MF, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J Neurosci. 2011;31(43):15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cole S, Hobin MP, Petrovich GD. Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience. 2015;286:187–202. doi: 10.1016/j.neuroscience.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Petrovich GD, Hobin MP, Reppucci CJ. Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience. 2012;224:70–80. doi: 10.1016/j.neuroscience.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167(1):11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 177.Mena JD, Selleck RA, Baldo BA. Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci. 2013;33(47):18540–18552. doi: 10.1523/JNEUROSCI.3323-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27(41):11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46(5):787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schone C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci. 2012;32(36):12437–12443. doi: 10.1523/JNEUROSCI.0706-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tsunematsu T, Kilduff TS, Boyden ES, Takahashi S, Tominaga M, Yamanaka A. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci. 2011;31(29):10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tsunematsu T, Tabuchi S, Tanaka KF, Boyden ES, Tominaga M, Yamanaka A. Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behav Brain Res. 2013;255:64–74. doi: 10.1016/j.bbr.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 184.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432(3):307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- 185.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16(5):279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Giardino WJ, de Lecea L. Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol. 2014;29:103–108. doi: 10.1016/j.conb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 188.Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Convergent inputs from electrically and topographically distinct orexin cells to locus coeruleus and ventral tegmental area. Eur J Neurosci. 2012;35(9):1426–1432. doi: 10.1111/j.1460-9568.2012.08057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26(2):398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schone C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7(3):697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schone C, Burdakov D. Glutamate and GABA as rapid effectors of hypothalamic "peptidergic" neurons. Front Behav Neurosci. 2012;6:81. doi: 10.3389/fnbeh.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience. 2003;119(4):1033–1044. doi: 10.1016/s0306-4522(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 193.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21(19):RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA., Jr Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111(16):E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105(33):11975–11980. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 197.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22(4):413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 198.Zimmer BA, Oleson EB, Roberts DCS. The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology. 2012;37(8):1901–1910. doi: 10.1038/npp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Michelson D, Snyder E, Paradis E, Chengan-Liu M, Snavely DB, Hutzelmann J, Walsh JK, Krystal AD, Benca RM, Cohn M, Lines C, Roth T, Herring WJ. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461–471. doi: 10.1016/S1474-4422(14)70053-5. [DOI] [PubMed] [Google Scholar]