Abstract
AIM
To examine the relationship between elevated granulocyte-macrophage colony-stimulating factor (GM-CSF) auto-antibodies (Ab) level and time to surgical recurrence after initial surgery for Crohn’s disease (CD).
METHODS
We reviewed 412 charts from a clinical database at tertiary academic hospital. Patients included in the study had ileal or ileocolonic CD and surgical resection of small bowel or ileocecal region for management of disease. Serum samples were analyzed for serological assays including GM-CSF cytokine, GM-CSF Ab, ASCA IgG and IgA, and genetic markers including SNPs rs2066843, rs2066844, rs2066845, rs2076756 and rs2066847 in NOD2, rs2241880 in ATG16L1, and rs13361189 in IRGM. Cox proportional-hazards models were used to assess the predictors of surgical recurrence.
RESULTS
Ninety six percent of patients underwent initial ileocecal resection (ICR) or ileal resection (IR) and subsequently 40% of patients required a second ICR/IR for CD. GM-CSF Ab level was elevated at a median of 3.81 mcg/mL. Factors predicting faster time to a second surgery included elevated GM-CSF Ab [hazard ratio (HR) 3.52, 95%CI: 1.45-8.53, P = 0.005] and elevated GM-CSF cytokine (HR = 2.48, 95%CI: 1.31-4.70, P = 0.005). Factors predicting longer duration between first and second surgery included use of Immunomodulators (HR = 0.49, 95%CI: 0.31-0.77, P = 0.002), the interaction effect of low GM-CSF Ab levels and smoking (HR = 0.60, 95%CI: 0.45-0.81, P = 0.001) and the interaction effect of low GM-CSF cytokine levels and ATG16L1 (HR = 0.65, 95%CI: 0.49-0.88, P = 0.006).
CONCLUSION
GM-CSF bioavailability plays a critical role in maintaining intestinal homeostasis. Decreased bioavailability coupled with the genetic risk markers and/or smoking results in aggressive CD behavior.
Keywords: Inflammatory bowel disease, Granulocyte-macrophage colony-stimulating factor antibody, Crohn’s disease, Surgery
Core tip: This retrospective study assesses the risk of surgery for management of ileal Crohn’s disease (CD) among patients with elevated granulocyte-macrophage colony-stimulating factor (GM-CSF) auto-antibodies (Ab). In this cohort, 396 subjects underwent initial ileocecal resection or ileal resection for management of disease. Subsequently 165 patients (41.7%) required a second ICR or IR. Factors predicting faster time to a second surgery were elevated GM-CSF Ab and elevated GM-CSF cytokine. Patients with low GM-CSF cytokine levels and the protective allele for ATG16L1 had longer intervals between a first and second surgery. To improve long term outcomes for patients with Ileal CD we need to optimize therapeutic options for patients with low GM-CSF bioavailability and genetic risk markers for ATG16L1.
INTRODUCTION
The incidence and prevalence of Crohn’s disease (CD) continues to rise with the highest rates reported to be 20.2 per 100000 person-years and 319 per 100000 persons in North America[1]. This chronic inflammatory disease is characterized by a transmural inflammation and can involve any region of the gastrointestinal tract. The transmural involvement particularly involving the ileum, often leads to fibrosis, luminal narrowing, and fistulas. In one population based cohort study of adult patients with CD, the cumulative probability of major abdominal surgery was 38%, 48%, and 58% at 5, 10, and 20 years after diagnosis, respectively[2]. Endoscopic recurrence after the initial ileocolic resection for CD may occur in up to 73% of patients within the first year after surgery[3]. Further, disease recurrence resulting in a second surgery for CD may occur in 31% to 50% of patients within 10 years of the initial surgery[4,5].
A growing body of literature supports the use of serological and genetic markers to determine the prognosis for patients with increased risk for surgical recurrence. Granulocyte macrophage colony-stimulating factor (GM-CSF) is a cytokine that promotes myeloid cell development and maturation. In mice, deficiency of this important hematopoietic growth factor can contribute to mucosal inflammation and immunodeficiency[6,7]. Clinical trials of recombinant GM-CSF in CD have demonstrated that it may benefit a subset of CD patients[8-10]. Conversely, as we and others have previously shown, neutralizing GM-CSF auto-antibodies (Ab) are associated with a reduced bioactivity of GM-CSF, impaired neutrophil bacterial killing, and increased rates of intestinal resection for ileal CD[7,11,12].
In the present study, we examined the predictive capacity of GM-CSF Ab in surgical recurrence rates after initial ileocolic resection for ileal CD. We also evaluated other clinical, serologic and genetic prognostic factors that might define the subset of patients with shorter time to surgical recurrence.
MATERIALS AND METHODS
Study population
After obtaining institutional review board approval, patients with CD enrolled between January 2005 and October 2015 at Washington University Medical Center in St. Louis, MO, were identified from a prospectively maintained database of Inflammatory Bowel Disease patients at the Digestive Diseases Research Core Centers (DDRCC). A retrospective chart review was performed on all patients. Patients were excluded if the pathology report was not consistent with a diagnosis of CD. The retrospective chart review included surgical and gastroenterology office notes, surgical and endoscopic operative reports, hospital admission and discharge notes, pathology reports, and radiology studies.
Study design
Patients included in the study had ileal or ileocolonic CD and surgical resection of small bowel or ileocecal region for management of disease. The diagnosis of CD was based on established clinical, radiological, endoscopic and histopathological criteria. The characteristics recorded at baseline included, age, gender, race/ethnicity, duration of disease, location and behavior according to the Montreal classification[13], smoking history, number and type of surgery, and exposure to IBD therapies. We also extracted available data for infection with Clostridium difficile (C. diff).
DNA samples were obtained from peripheral whole blood samples collected at enrollment in the registry. Samples were genotyped using the Illumina Golden Gate custom Immunochip array. Genetic analysis focused on SNPs rs2066843, rs2066844, rs2066845, rs2076756 and rs2066847 in NOD2, rs2241880 in ATG16L1, and rs13361189 in IRGM. These polymorphisms have a role in bacteria sensing and autophagy.
Serum concentrations of GM-CSF cytokine and Ab were quantified by enzyme-linked immunosorbent assay (ELISA) as previously described[7]. Based upon prior studies elevated serum GM-CSF Ab was defined as ≥ 1.6 mcg/mL.
ELISA for detection of anti-Saccharomyces cerevisiae antibodies
QUANTA Lite (INOVA Diagnostics, Inc., San Diego, CA, United States) ASCA IgG and IgA enzyme-linked immunosorbent assays (ELISA) were used for determination of serum anti-Saccharomyces cerevisiae antibodies (ASCAs) levels as described by the manufacturer’s instructions. Briefly, 100 microliters of patient’s serum at a dilution of 1:100 were added to 96-well polystyrene microwell plates adhered with partially purified and disrupted Saccharomyces cerevisiae antigen. Bound ASCAs were detected by incubation with horseradish peroxidase IgG or IgA conjugate (goat anti-human). The absorbance (optical density, OD) was read at 450 nm using a SpectraMax MiniMax Imaging Cytometer. On each plate a high and a low positive, as well as a negative control were included. ASCA reactivity was determined by the formula: sample OD⁄low positive OD × 25. The positive cut off values for both ASCA IgA and IgG as set by the manufacturer were 25 Units (U)/mL.
Statistical analysis
The statistical methods of this study were reviewed by Wei Zhu, PhD at Stony Brook University. Descriptive statistics of demographic variables were generated using Graph Pad Prism 5.04 for Windows, GraphPad Software, San Diego, CA, United States.
In order to detect risk factors associated with early surgical recurrence, a Cox proportional hazard model[14] was fitted with main effects and first-order interactions of clinical and genotype variables. A stepwise variable selection based on Bayesian information criteria (BIC) was performed to select the relevant subset of variables. Within the model, p value was adjusted as previously described by Li et al[15], with the cutoff at FDR < 0.05. Model fitting and selection were generated using R 3.1.1 (http://cran.r-project.org).
RESULTS
Patient demographic and clinical characteristics
The study group included 412 adult patients with CD and a prior history of surgery for management of disease (Table 1). The mean age of CD patients at the time of this study was 49.9 ± 14.5 years. The mean disease duration was 22.5 ± 12.5 years. The majority of patients (50.26 %) were smokers.
Table 1.
Demographic characteristics of the subjects
| Patient characteristics (n = 412) | n (%) |
| Gender, Female | 242 (58.74) |
| Race, White | 368 (89.3) |
| Age, mean ± SD | 49.88 ± 14.49 |
| Smoking | |
| Current smoker | 180 (43.69) |
| Ex-smoker | 27 (6.57) |
| Never smoker | 203 (49.27) |
| Surgery for CD1 | |
| First Ileocolic resection | 370 (89.80) |
| First Ileal resection | 26 (6.31) |
| Second Ileocolic resection | 152 (36.89) |
| Second Ileal resection | 13 (3.15) |
| Exposure to medication | |
| Immunomodulators pre-T1 | 105 (25.48) |
| Anti-TNF agent pre-T1 | 80 (19.41) |
| Immunomodulators pre-T2 | 187 (45.39) |
| Anti-TNF agent pre-T2 | 120 (29.13) |
3.2% of patients underwent other intestinal surgeries as noted in the results section of the text. CD: Crohn’s disease.
The first surgical procedure for IBD management occurred at a median of 3 years post diagnosis. Most patients underwent intestinal resection with ileocolonic anastomosis. Ileocecal resection (ICR) was performed in 370⁄412 (90%) patients and ileal resection (IR) was performed in 26⁄412 patients (6.3%). The remaining 3.2% of patients underwent other intestinal surgeries including total colectomy, ileostomy creation, upper small bowel surgery, partial colectomy, proctectomy, diverting colostomy, and enterofistula repair. Two hundred and fifty eight patients (62.62%) underwent the first surgery for management of CD prior to enrollment in the registry in 2005. Table 2 outlines the patient characteristics by Montreal Classification[13]. The mean age at diagnosis of CD patients was 27.8 ± 11.7 years. Chart review revealed that 105 patients (25.48%) had exposure to Immunomodulator therapy (Azathioprine, 6-mercaptopurine or Methotrexate) prior to the first surgery. In addition, 80 patients (19.41%) had exposure to Infliximab or Adalimumab prior to the first surgery.
Table 2.
Phenotypes by Montreal classification
| n (%) | |
| Age at diagnosis | |
| A1 (≤ 16) | 56 (13.63) |
| A2 (17-40) | 296 (72.57) |
| A3 (> 40) | 60 (14.6) |
| Disease location | |
| L1 ileal | 279 (67.71) |
| L2 colonic | 2 (0.485) |
| L3 ileocolonic | 131 (31.87) |
| L4 upper GI disease | 50 (12.17) |
| p perianal disease | 125 (30.41) |
| Disease behavior | |
| B1 non-stricturing, non-penetrating | 35 (08.49) |
| B2 stricturing | 167 (40.63) |
| B3 penetrating | 197 (47.93) |
Prevalence of GM-CSF Ab and ASCA
Serum samples were drawn from 254 and 150 patients before and after the first ICR/IR surgery for management of CD, respectively. Similarly serum samples were drawn from 98 and 67 patients before and after the second ICR/IR surgery, respectively. The serum GM-CSF Ab level was elevated at a median (interquartile range, IQR) of 3.81 (0.93-11.01) mcg/mL and consistent with previous studies. There were 273 (66.4%) CD cases with GM-CSF Ab level ≥ 1.6 mcg/mL. When we applied the positive cut off values for both ASCA IgA and IgG as set by the manufacturer, 25 U/mL, 313 ⁄ 412 (76%) of patients were positive for ASCA IgA and 334 ⁄ 412 (81%) were positive for ASCA IgG. Two hundred and eighty seven (70%) of patients were concordantly positive, 35 (8%) patients were concordantly negative, whereas 16 (4%) of patients were positive only for ASCA IgA and 28 (7%) only for ASCA IgG. The mean, median and range values of all four serological assays, ASCA IgA, IgG, GM-CSF cytokine and GM-CSF Ab are shown in Table 3.
Table 3.
Values for serological assays
| Serological assays | Mean ± SD | Median (IQRs) | Range |
| GM-CSF cytokine, pg/mL | 3.18 ± 8.61 | 1.28 (0.43-3.05) | 0.0052-136.5 |
| GM-CSF Ab, mcg/mL | 10.64 ± 19.76 | 3.81 (0.93-11.01) | 0.08-241.2 |
| ASCA IgA, U/mL | 95.21 ± 77.38 | 67.51 (27.86-174.4) | 1.92-242.8 |
| ASCA IgG, U/mL | 69.79 ± 41.09 | 66.86 (31.76-104.2) | 3.17-158.1 |
GM-CSF Ab: Granulocyte-macrophage colony-stimulating factor auto-antibodies; ASCA: Anti-Saccharomyces cerevisiae antibodies; CD: Crohn’s disease.
Surgical recurrence after initial ICR or small bowel resection and associated risk or protective factors
A total of 224 patients (54.5%) required two or more IBD related surgical procedures. One hundred and twenty one patients (29.37%) underwent the second surgery for management of CD prior to enrollment in the registry in 2005. Chart review revealed that 187 patients (45.39%) had exposure to Immunomodulator therapy (Azathioprine, 6-mercaptopurine or Methotrexate) prior to a second surgery. In addition, 80 patients (29.13%) had exposure to Infliximab or Adalimumab prior to a second surgery. Time to the second surgery was significantly shorter in patients with structuring (B2) or penetrating (B3) disease behavior as shown in Figure 1.
Figure 1.
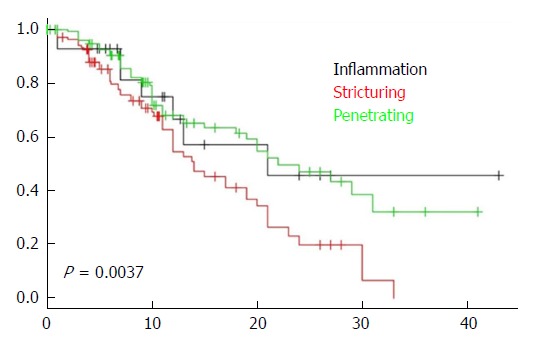
Comparison of survival Kaplan-Meier curves of patients according to disease behavior. Log-rank test, P = 0.0037. CD: Crohn’s disease.
Factors predicting a faster time to a second surgery were assessed by survival analysis using the Cox proportional hazard model to test for association with gender, disease phenotype, smoking status, serological assays, IBD polymorphisms and use of medications (Immunomodulators or anti-TNF) between the first and second surgery (Table 4). All serological assays (GM-CSF cytokine, GM-CSF Ab, ASCA IgA and ASCA IgG) were examined as continuous, log transformed variables while the remaining variables were included as categorical variables.
Table 4.
Risk factors at or after the initial Crohn’s disease surgery associated with time to the second Crohn’s disease surgery
| Main effects | HR | 95%CI | P value | FDR |
| Immunomodulators1 | 0.486 | 0.306-0.773 | 0.002 | 0.026 |
| GM-CSF Ab level | 3.493 | 1.430-8.538 | 0.005 | 0.030 |
| GM-CSF cytokine level | 2.489 | 1.292-4.796 | 0.005 | 0.030 |
| B2 disease behavior2 | 1.415 | 0.517-3.874 | 0.491 | 0.624 |
| B3 disease behavior3 | 0.415 | 0.146-1.176 | 0.091 | 0.145 |
| Smoking4 | 1.286 | 0.277-5.981 | 0.743 | 0.800 |
| ASCA IgG, U/mL | 1.318 | 0.799-2.174 | 0.270 | 0.378 |
| Age at diagnosis | 0.983 | 0.963-1.003 | 0.094 | 0.145 |
| Clostridium difficile infection | 0.035 | 0.001-1.092 | 0.051 | 0.113 |
| antiTNF-α5 | 1.272 | 0.662-2.447 | 0.461 | 0.615 |
| ATG16L1 polymorphism | 1.793 | 0.982-3.273 | 0.052 | 0.113 |
| IRGM polymorphism | 0.092 | 0.004-2.262 | 0.136 | 0.201 |
| ASCA IgA, U/mL | 0.976 | 0.755-1.262 | 0.852 | 0.852 |
| NOD2 polymorphisms | 1.123 | 0.741-1.702 | 0.577 | 0.673 |
| Interactions | ||||
| GM-CSF Ab level and smoking | 0.612 | 0.454-0.825 | 0.001 | 0.026 |
| GM-CSF cytokine level and ATG16L1 | 0.652 | 0.480-0.885 | 0.005 | 0.030 |
| GM-CSF Ab level and ASCA IgG, U/mL | 0.808 | 0.674-0.968 | 0.018 | 0.081 |
| GM-CSF Ab level and ATG16L1 | 0.719 | 0.538-0.962 | 0.023 | 0.081 |
| B2 disease behavior and Clostridium difficile | 21.57 | 1.456-319.271 | 0.023 | 0.081 |
| B2 disease behavior and smoking | 1.601 | 0.318-8.059 | 0.560 | 0.673 |
| B3 disease behavior and smoking | 5.117 | 0.966-27.141 | 0.050 | 0.113 |
| GM-CSF Ab level and Clostridium difficile | 2.865 | 1.065-7.715 | 0.033 | 0.104 |
| GM-CSF cytokine level and Clostridium difficile | 0.902 | 0.325-2.501 | 0.840 | 0.852 |
| Smoking and IRGM | 0.406 | 0.145-1.134 | 0.079 | 0.145 |
| ASCA IgG, U/mL and IRGM | 1.867 | 0.908-3.835 | 0.083 | 0.145 |
| GMCSF cytokine level and antiTNF-α | 0.765 | 0.562-1.041 | 0.082 | 0.145 |
| GM-CSF Ab level and antiTNF-α | 0.941 | 0.703-1.260 | 0.676 | 0.757 |
| GM-CSF cytokine level and ASCA IgA, U/mL | 0.865 | 0.750-0.997 | 0.041 | 0.113 |
Use of Immunomodulators between first and second CD surgery including: Azathioprine, 6-Mercaptopurine, Methotrexate;
Stricturing behavior (B2) compared to Inflammatory (B1) disease behavior;
Penetrating behavior (B3) compared to Inflammatory (B1) disease behavior;
Active smoker before the first CD surgery;
Use of Anti-tumor Necrosis Factor-alpha therapy between first and second CD surgery including: Infliximab, Adalimumab. Cox proportional hazard model; A Cox proportional hazard model was fitted with main effects and first-order interactions of clinical and genotype variables. A stepwise variable selection was performed to select the relevant subset of variables. Within the model, P value was adjusted with the cutoff at FDR < 0.05. Model fitting and selection were generated using R 3.1.1 (http://cran.r-project.org). Table 4 displays the P-values for all fitted terms in the final model. GM-CSF Ab: Granulocyte-macrophage colony-stimulating factor auto-antibodies; ASCA: Anti-Saccharomyces cerevisiae antibodies; CD: Crohn’s disease.
A total of 333 subjects were included in this analysis after removal of subjects who did not have ICR/IR and those with missing data. Patients with high GM-CSF cytokine or Ab levels had a significantly shorter time to a second surgery compared to those with low levels. Patients with exposure to immunomodulators between first and second surgery had significantly longer intervals to the second surgery.
When examining the interaction of GM-CSF with all the other variables patients with protective alleles for ATG16L1 and low GM-CSF cytokine levels had significantly longer intervals to the second surgery. Patients with the protective allele for ATG16L1 and low GM-CSF Ab levels also experienced a longer interval to the second surgery but this was not statistically significant. The other polymorphisms for the genes NOD2 and IRGM did not have significant correlation with time to the second surgery.
A positive smoking history did not correlate with a faster time to second surgery. The median GM-CSF Ab levels (Figure 2) was significantly lower in smokers when compared to non-smokers (P < 0.0001) and significantly lower when compared to ex-smokers (P < 0.01). In addition smokers who expressed low GM-CSF Ab has significantly longer intervals to the second surgery.
Figure 2.
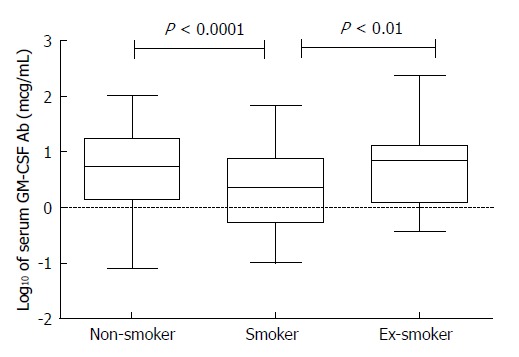
Serum granulocyte-macrophage colony-stimulating factor auto-antibodies levels in patients with Crohn’s disease stratified by smoking status. The log10 transformation of serum GM-CSF Ab (in micrograms per milliliter) is shown. The middle line represents the median, and the lower edge and the upper edge of the box represent the 25% and 75% quartiles, respectively. The bottom and top lines represent the minimum and maximum values, respectively. There were 201 non-smokers, 180 smokers and 27 ex-smokers with CD. Kruskal-Wallis test with Dunn’s post-test revealed significant differences between non-smokers and smokers and between smokers and ex-smokers. GM-CSF Ab: Granulocyte-macrophage colony-stimulating factor auto-antibodies; CD: Crohn’s disease.
DISCUSSION
In this cohort of 412 patients with ileal CD, 396 subjects underwent at least one ileocolic resection or ileal resection for management of their disease. Subsequently 165 of them (41.7%) required a second ICR or IR. Within the cohort of 412 patients, 410 had isolated Ileal or ileocolonic disease, 2 patients had colonic disease but evolved to ileocolonic location by the conclusion of the study. Ileal disease location is associated with stricturing and penetrating disease behavior (B2/B3) and increased risk of surgery. In fact, we demonstrated a faster time to the second surgery in those with B2 and B3 disease behavior compared to non-stricturing, non-penetrating disease (B1). In addition, patients with structuring disease had significantly decreased rates of survival (Figure 1) to the second surgery (P = 0.0037). However, we sought to determine additional variables that are potentially modifiable in this large cohort of CD patients with aggressive ileal CD.
We and others have reported previously, that elevated GM-CSF Ab is associated with increased rates of stricturing behavior and surgery in adult and pediatric CD[7,11,12]. Further, Däbritz et al[16], showed that longitudinal measurements of serum GM-CSF Ab levels in adult and pediatric patients with CD or ulcerative colitis reliably predicted disease recurrence 2 to 7 mo before a relapse as defined by clinical assessment using the Crohn’s or ulcerative colitis disease activity indices (CDAI, PCDAI, UCDAI, PUCAI) in Adults and Pediatric patients with CD or ulcerative colitis. In our cohort of ileal CD patients, the time to a second surgery was significantly accelerated in those with elevated GM-CSF Ab level even after accounting for all other variables in our analysis. This study confirms the prior association of elevated GM-CSF and aggressive CD and here we report the link between elevated GM-CSF Ab and a shorter time to surgical recurrence.
GM-CSF cytokine has a central role in intestinal homeostasis by enhancing innate immune responses to microbial pathogens[17]. Within the gastrointestinal tract, expression of GM-CSF cytokine is found on Paneth cells of the intestine and the GM-CSF receptor beta chain is present along epithelial cells of the small intestine[18]. While the underlying pathogenesis that drives over-expression of GM-CSF Ab is not fully defined, in vitro studies demonstrate a neutralizing effect on GM-CSF cytokine activity by reducing the bioactivity of free GM-CSF[7]. GM-CSF autoantibodies are produced by lamina propria mononuclear cells isolated from resection specimen from patients with stricturing CD[19]. In addition patients with elevated GM-CSF have reduced neutrophil bacterial killing[19]. The addition of GM-CSF cytokine increases neutrophil bacterial killing in CD only on washed neutrophils in which GM-CSF antibodies are depleted[19]. We examined GM-CSF cytokine level in this study and found that patients with elevated levels had significantly shorter intervals to the second surgery. We also evaluated the interaction between GM-CSF cytokine and its neutralizing antibody and found no interaction effect. In healthy individuals, GM-CSF in the circulation is tightly regulated at low or even undetectable levels but can rise to high levels in response to immune stimuli such as lipopolysaccharide. Chronic overexpression leads to pathological changes that can result in severe damage to affected organs[20]. This may be explained by the pleiotropic effects that result from GM-CSF cytokine function[21].
We confirmed that use of imunomodulators is associated with a longer duration between the first and second surgical resection for CD. This finding first reported by Unkart et al[22] highlights the importance of step up therapy in a subset of patients with aggressive CD behavior. It is reported that early use of TNF antagonists is associated with reduced risk of developing strictures in adults[23] and in children early monotherapy with TNF antagonists results in better overall clinical and growth outcomes at 1 year[24]. In this study, 70.2% of patients exposed to TNF antagonists after the initial ICR/IR did not require a second surgery. In addition, only 27.8% of the 165 patients that required a second ICR/IR had exposure to TNF antagonists prior to the second surgery. Nonetheless we did not find that exposure to TNF antagonists was associated with a longer duration between the first and second surgery. It is worthwhile mentioning that the earliest use of TNF antagonists for this cohort was the year 1994 but 31 subjects (18.8%) required 2 or more ICR/IR surgical events prior to this year. In addition 144 subjects (87.2%) who required a second surgery had long standing disease of 3 or more years prior to the second surgery.
In this study a large subset of patients were current or ex-smokers (50.1%) and practically all subjects except one had ileal disease location. In a prior study we found that smokers with Ileal CD had lower GM-CSF Ab levels[12]. Similarly, in the present cohort the median GM-CSF Ab level was 2.5 in smokers and 5.4 in non-smokers (Figure 2). This resulted in a complex interaction effect of smoking such that smokers who expressed low GM-CSF Ab level had a longer interval between the first and second surgery.
A limitation of the study was the retrospective nature of the clinical data collection. In addition we were not able to clearly delineate precise duration of exposure to immunomodulators or biologics, the response and tolerance of these medications. The serum collection was cross sectional and so future studies should examine serial measurements that correlate with time of diagnosis and surgical events.
The strengths of our study are that we were able to distinguish between modifiable and non-modifiable factors that impact disease behavior in CD. Modifiable factors like preventing exposure to smoking and early use of immunomodulators and TNF antagonists are well recognized but it is important to highlight their role in prevention of aggressive disease behavior. The non-modifiable factors like GM-CSF cytokine bioavailability and carriage of risk alleles for ATG16 L1 can be used to profile patients that may benefit from top-down therapy or alternatively newer more targeted therapy.
ARTICLE HIGHLIGHTS
Research background
Crohn’s disease (CD) is a chronic inflammatory disease is characterized by a transmural inflammation that targets any region of the gastrointestinal tract. The transmural involvement particularly involving the ileum, often leads to fibrosis, luminal narrowing, and fistulas. In one population based cohort study of adult patients with CD, the cumulative probability of major abdominal surgery was 38% to 58% within 5 to 20 years after diagnosis. Further, disease recurrence resulting in a second surgery for management of CD may occur in 31% to 50% of patients within 10 years of the initial surgery. There remains a need for serological and genetic markers that can reliably identify patients with increased risk for surgical recurrence.
Research motivation
Granulocyte macrophage colony-stimulating factor (GM-CSF) is a cytokine that promotes myeloid cell development and maturation. Deficiency of this important hematopoietic growth factor can contribute to mucosal inflammation and immunodeficiency. We and others have previously shown, neutralizing GM-CSF auto-antibodies (Ab) are associated with a reduced bioactivity of GM-CSF, impaired neutrophil bacterial killing, and increased rates of intestinal resection for ileal CD. In the present study, we examined the predictive capacity of GM-CSF Ab in surgical recurrence rates after initial ileocolic resection for ileal CD. We also evaluated other clinical, serologic and genetic prognostic factors that might define the subset of patients with shorter time to surgical recurrence.
Research objectives
The development of biomarkers for diagnosis and prognosis of CD is evolving and serological assays like ASCA, ANCA, Anti-glycan antibodies and GM-CSF Ab are important discoveries. Some assays are reliable predictors for diagnosis and for staging severity. Here we demonstrate that GM-CSF Ab is a reliable biomarker for characterizing severity and reliably predicts patients at greater risk for aggressive disease behavior and the need for surgery.
Research methods
We reviewed 412 patients which included in the study had ileal or ileocolonic CD and surgical resection of small bowel or ileocecal region for management of disease from a clinical database at tertiary academic hospital. Serum samples were analyzed for serological assays, which included GM-CSF cytokine, GM-CSF Ab, ASCA IgG and IgA, and genetic markers included SNPs rs2066843, rs2066844, rs2066845, rs2076756 and rs2066847 in NOD2, rs2241880 in ATG16L1, and rs13361189 in IRGM. The predictors of surgical recurrence were assessed by the Cox proportional-hazards models.
Research results
Of 96% patients underwent initial Ileocecal resection (ICR) or Ileal resection (IR) and subsequently 40% of patients required a second ICR/IR for CD. GM-CSF Ab level was elevated at a median of 3.81 mcg/mL. Factors predicting faster time to a second surgery included elevated GM-CSF Ab and elevated GM-CSF cytokine. Factors predicting longer duration between first and second surgery included use of Immunomodulators, the interaction effect of low GM-CSF Ab levels and smoking, and the interaction effect of low GM-CSF cytokine levels and ATG16L1.
Research conclusions
In this study patients with elevated GM-CSF Ab had shorter intervals between the first and second surgery for management of CD. This is the first study to describe this. Therefore routine surveillance of this serological assay may facilitate the identification of patients at risk for disease complications and allow clinicians to optimize medical therapy.
Research perspectives
Elevated GM-CSF Ab is a reliable assay for defining patients who are at a greater risk for surgery. A clinical assay is needed.
ACKNOWLEDGMENTS
We thank the Stony Brook Research Foundation for the institutional support and we gratefully acknowledge the staff at Washington University Medical Center in St. Louis, MO, United States.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Supported by (in part) the National Institutes of Health, No. R01 DK098231, R01 DK078683 and No. P30DK052574.
Institutional review board statement: This study was reviewed and approved by the Institutional Review Board at Washington University Hospital.
Informed consent statement: Adult patients are recruited in a consecutive fashion by the Washington University Digestive Diseases Research Tissue Procurement Facility and provide verbal and written consent for chart abstraction, blood, stool, tissue biopsies and/or surgical waste collection with analysis for research purposes and for their information to be stored in the hospital database. The IRB at Washington University approved this consent procedure.
Conflict-of-interest statement: The authors have declared that no potential conflicts (financial, professional, or personal) exist that are relevant to the manuscript.
Data sharing statement: No additional data are available.
Peer-review started: November 16, 2017
First decision: November 30, 2017
Article in press: December 12, 2017
P- Reviewer: Chiba T, Goral V S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Grace Gathungu, Department of Pediatrics, Division of Pediatric Gastroenterology, Stony Brook University, Stony Brook, NY 11794, United States. grace.gathungu@stonybrookmedicine.edu.
Yuanhao Zhang, Department of Medicine, Division of Gastroenterology, Stony Brook University, Stony Brook, NY 11794, United States.
Xinyu Tian, Department of Medicine, Division of Gastroenterology, Stony Brook University, Stony Brook, NY 11794, United States.
Erin Bonkowski, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229-3026, United States.
Leahana Rowehl, Department of Medicine, Division of Gastroenterology, Stony Brook University, Stony Brook, NY 11794, United States.
Julia Krumsiek, Department of Pediatrics, Division of Pediatric Gastroenterology, Stony Brook University, Stony Brook, NY 11794, United States.
Billy Nix, Department of Medicine, Washington University St. Louis, St. Louis, MO 63110, United States.
Claudia Chalk, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229-3026, United States.
Bruce Trapnell, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229-3026, United States.
Wei Zhu, Applied Mathematics and Statistics, Stony Brook University, Stony Brook, NY 11794, United states.
Rodney Newberry, Department of Medicine, Washington University St. Louis, St. Louis, MO 63110, United States.
Lee Denson, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229-3026, United States.
Ellen Li, Department of Medicine, Division of Gastroenterology, Stony Brook University, Stony Brook, NY 11794, United States.
References
- 1.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV Jr. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970-2004) Am J Gastroenterol. 2012;107:1693–1701. doi: 10.1038/ajg.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlando A, Mocciaro F, Renna S, Scimeca D, Rispo A, Lia Scribano M, Testa A, Aratari A, Bossa F, Tambasco R, et al. Early post-operative endoscopic recurrence in Crohn’s disease patients: data from an Italian Group for the study of inflammatory bowel disease (IG-IBD) study on a large prospective multicenter cohort. J Crohns Colitis. 2014;8:1217–1221. doi: 10.1016/j.crohns.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Penner RM, Madsen KL, Fedorak RN. Postoperative Crohn’s disease. Inflamm Bowel Dis. 2005;11:765–777. doi: 10.1097/01.mib.0000171273.09757.f2. [DOI] [PubMed] [Google Scholar]
- 5.Wolters FL, Russel MG, Sijbrandij J, Ambergen T, Odes S, Riis L, Langholz E, Politi P, Qasim A, Koutroubakis I, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut. 2006;55:1124–1130. doi: 10.1136/gut.2005.084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirata Y, Egea L, Dann SM, Eckmann L, Kagnoff MF. GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell Host Microbe. 2010;7:151–163. doi: 10.1016/j.chom.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Uchida K, Jurickova I, Koch D, Willson T, Samson C, Bonkowski E, Trauernicht A, Kim MO, Tomer G, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn’s disease. Gastroenterology. 2009;136:1261–1271, e1-e3. doi: 10.1053/j.gastro.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelsen JR, Rosh J, Heyman M, Winter HS, Ferry G, Cohen S, Mamula P, Baldassano RN. Phase I trial of sargramostim in pediatric Crohn’s disease. Inflamm Bowel Dis. 2010;16:1203–1208. doi: 10.1002/ibd.21204. [DOI] [PubMed] [Google Scholar]
- 9.Korzenik JR, Dieckgraefe BK, Valentine JF, Hausman DF, Gilbert MJ; Sargramostim in Crohn’s Disease Study Group. Sargramostim for active Crohn’s disease. N Engl J Med. 2005;352:2193–2201. doi: 10.1056/NEJMoa041109. [DOI] [PubMed] [Google Scholar]
- 10.Valentine JF, Fedorak RN, Feagan B, Fredlund P, Schmitt R, Ni P, Humphries TJ. Steroid-sparing properties of sargramostim in patients with corticosteroid-dependent Crohn’s disease: a randomised, double-blind, placebo-controlled, phase 2 study. Gut. 2009;58:1354–1362. doi: 10.1136/gut.2008.165738. [DOI] [PubMed] [Google Scholar]
- 11.Nylund CM, D’Mello S, Kim MO, Bonkowski E, Däbritz J, Foell D, Meddings J, Trapnell BC, Denson LA. Granulocyte macrophage-colony-stimulating factor autoantibodies and increased intestinal permeability in Crohn disease. J Pediatr Gastroenterol Nutr. 2011;52:542–548. doi: 10.1097/MPG.0b013e3181fe2d93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gathungu G, Kim MO, Ferguson JP, Sharma Y, Zhang W, Ng SM, Bonkowski E, Ning K, Simms LA, Croft AR, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies: a marker of aggressive Crohn’s disease. Inflamm Bowel Dis. 2013;19:1671–1680. doi: 10.1097/MIB.0b013e318281f506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox DR, Oakes D. Analysis of survival data. London; New York: Chapman and Hall; 1984. [Google Scholar]
- 15.Li E, Hamm CM, Gulati AS, Sartor RB, Chen H, Wu X, Zhang T, Rohlf FJ, Zhu W, Gu C, et al. Inflammatory bowel diseases phenotype, C. difficile and NOD2 genotype are associated with shifts in human ileum associated microbial composition. PLoS One. 2012;7:e26284. doi: 10.1371/journal.pone.0026284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Däbritz J, Bonkowski E, Chalk C, Trapnell BC, Langhorst J, Denson LA, Foell D. Granulocyte macrophage colony-stimulating factor auto-antibodies and disease relapse in inflammatory bowel disease. Am J Gastroenterol. 2013;108:1901–1910. doi: 10.1038/ajg.2013.360. [DOI] [PubMed] [Google Scholar]
- 17.Sainathan SK, Hanna EM, Gong Q, Bishnupuri KS, Luo Q, Colonna M, White FV, Croze E, Houchen C, Anant S, et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2008;14:88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuzawa H, Sawada M, Kayahara T, Morita-Fujisawa Y, Suzuki K, Seno H, Takaishi S, Chiba T. Identification of GM-CSF in Paneth cells using single-cell RT-PCR. Biochem Biophys Res Commun. 2003;312:897–902. doi: 10.1016/j.bbrc.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Jurickova I, Collins MH, Chalk C, Seese A, Bezold R, Lake K, von Allmen D, Frischer JS, Falcone RA, Trapnell BC, et al. Paediatric Crohn disease patients with stricturing behaviour exhibit ileal granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibody production and reduced neutrophil bacterial killing and GM-CSF bioactivity. Clin Exp Immunol. 2013;172:455–465. doi: 10.1111/cei.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Zhang L, Yuan ZR, Tan HS, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 22.Unkart JT, Anderson L, Li E, Miller C, Yan Y, Gu CC, Chen J, Stone CD, Hunt S, Dietz DW. Risk factors for surgical recurrence after ileocolic resection of Crohn’s disease. Dis Colon Rectum. 2008;51:1211–1216. doi: 10.1007/s10350-008-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safroneeva E, Vavricka SR, Fournier N, Pittet V, Peyrin-Biroulet L, Straumann A, Rogler G, Schoepfer AM; Swiss IBD Cohort Study Group. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Aliment Pharmacol Ther. 2015;42:977–989. doi: 10.1111/apt.13363. [DOI] [PubMed] [Google Scholar]
- 24.Walters TD, Kim MO, Denson LA, Griffiths AM, Dubinsky M, Markowitz J, Baldassano R, Crandall W, Rosh J, Pfefferkorn M, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn’s disease. Gastroenterology. 2014;146:383–391. doi: 10.1053/j.gastro.2013.10.027. [DOI] [PubMed] [Google Scholar]


