Summary
OBJECTIVES
To assess the efficacy of amodiaquine-artesunate in an area with high chloroquine resistance in western Kenya.
METHODS
Twenty-eight day in-vivo efficacy trial of amodiaquine-artesunate in 103 children aged 6–59 months in western Kenya with smear-confirmed uncomplicated Plasmodium falciparum malaria.
RESULTS
The 28-day uncorrected adequate clinical and parasitological response (ACPR) was 69.0%, with 15.5% Late Clinical Failure and 15.5% Late Parasitologic Failure rates. The PCR-corrected 28-day ACPR was 90.2%. Clinical risk factors for recurrent infection (recrudescences and reinfections) were lower axillary temperature at enrolment and low weight-for-age Z-score. The presence of single nucleotide polymorphisms pfcrt 76T and pfmdr1 86Y at baseline was associated with increased risk of recurrent infections, both reinfections and recrudescences.
CONCLUSION
Although artemether-lumefantrine (Coartem®) is the first line ACT in Kenya, amodiaquine-artesunate is registered as an option for treatment of uncomplicated P. falciparum and remains an effective alternative to Coartem® in western Kenya. Continued amodiaquine monotherapy in the private sector may jeopardise the future use of amodiaquine-artesunate as an alternative artemisinin-based combination therapy.
Keywords: artemether-lumefantrine, amodiaquine-artesunate, malaria, resistance, chloroquine, clinical trial, Kenya
Introduction
Artemisinin-based combination therapy has been adopted as first-line treatment for uncomplicated Plasmodium falciparum malaria in most of sub-Saharan Africa, with artemether-lumefantrine (AL) and amodiaquine-artesunate (AQ-AS) being the most common currently adopted regimens. Monitoring the development of resistance to these drug combinations is paramount to preserving artemisinin efficacy. Amodiaquine, a 4-aminoquinolone, raises particular concern given its similarity to chloroquine and the common resistance mechanisms.
In recent years, single nucleotide polymorphisms (SNPs) have been implicated in resistance to antimalarials, and several have been identified that are involved in resistance to 4-aminoquinolones. Both chloroquine and amodiaquine monotherapy are associated with SNPs in pfcrt and pfmdr1. The association of pfcrt 76T and pfmdr1 86Y with chloroquine resistance was first shown in Mali (Djimdé et al. 2001). Associations between resistance to amodiaquine and SNPs pfcrt 76T and pfmdr1 86Y, 184Y, 1246Y, and the pfmdr1 haplotype (a.a 86, 184, 1246) YYY have also been noted after amodiaquine monotherapy (Happi et al. 2006; Holmgren et al. 2006, 2007; Humphreys et al. 2007; Tinto et al. 2008).
Addition of artesunate to amodiaquine results in dramatically improved efficacy over amodiaquine alone in most areas (Adjuik et al. 2002; Abacassamo et al. 2004; Rwagacondo et al. 2004; Mutabingwa et al. 2005; Sowunmi et al. 2007); addition of artesunate to amodiaquine also reduces gametocytemia compared to amodiaquine monotherapy, thereby theoretically reducing transmission (Abacassamo et al. 2004; Mutabingwa et al. 2005; Sowunmi et al. 2007). Trials of AQ-AS in east Africa have found 28-day PCR-corrected cure rates of 89% or greater in Rwanda, Uganda, and Tanzania (Rwagacondo et al. 2004; Mårtensson et al. 2005; Mutabingwa et al. 2005; Dorsey et al. 2007; Holmgren et al. 2007; Kabanywanyi et al. 2007). Molecular studies of AQ-AS combinations have found differing patterns of SNP selection; in one study AQ-AS therapy selected for pfmdr1 1246Y and for the pfmdr1 haplotype (a.a 86, 184, 1246) YYY, but not for pfcrt 76T or pfmdr1 86Y independently (Holmgren et al. 2007). In another study, treatment with amodiaquine selected for pfmdr1 86Y, 184Y (wild-type), and 1246Y; pfcrt 76T frequency was too high at baseline to see a difference (Nsobya et al. 2007). In both studies, codons 1034 and 1042 remained wild-type in all samples.
We conducted a single arm in-vivo efficacy trial of AQ-AS in western Kenya. The trial had originally been designed to obtain in-vivo efficacy data for regimens being tested in a concurrent intermittent preventive treatment in infants (IPTi) trial comparing IPTi with SP-AS, AQ-AS, and chlorproguanil–dapsone. Due to recent concerns about resistance to SP, even in combination with artesunate, and chlorproguanil–dapsone in this population in which routine use is likely not feasible, we limited this trial to evaluation of AQ-AS.
Methods
Enrolment
Enrolment and follow-up took place at the Maternal Child Health clinic at Bondo District Hospital from 14th May to 24th August 2007, during the rainy season. A standard WHO 28-day in-vivo protocol for measuring antimalarial drug efficacy in an area of intense malaria transmission was used. Children aged 6–59 months with an axillary temperature ≥37.5 °C or a history of fever in the past 24 h, whose caregivers reported easy access to Bondo District Hospital and ability make all study visits, were screened for infection with P. falciparum. Exclusion criteria were: signs of severe disease, use of antimalarials in the previous 14 days, malnutrition, hypersensitivity to a study medication, enrolment in the main IPTi trial, chronic illness, and co-trimoxazole prophylaxis. Children who met screening criteria and whose guardians provided informed consent underwent laboratory evaluation. Laboratory inclusion criteria were: smear positive for P. falciparum, and parasitemia 2000–200 000 parasites/µl; exclusion criteria were mixed infection or severe anaemia (Hgb ≤7 g/dl). Children with haemoglobin <7 g/dl and ≥5 g/dl were not enrolled, but were treated with oral iron. If the haemoglobin was <5 g/dl, the child was hospitalised for treatment and was not enrolled.
The study was approved by the Kenya Medical Research Institute (KEMRI) National Ethics Review Committee and the Centers for Disease Control and Prevention Institutional Review Board.
Clinical procedures
For each child, finger prick blood was taken for blood smears, filter paper, and haemoglobin measurement. All participating children underwent anamnesis and physical examination by study clinicians at enrolment. The children were given oral artesunate (Cosmos Pharmaceuticals Limited, Nairobi, Kenya, expiration April 2008) and amodiaquine (Pfizer Dakar, Senegal, expiration November 2008) dosed using a dosing chart that approximately 25 mg/kg total of amodiaquine and 4 mg/kg/day of artesunate on days 0, 1 and 2. The children were monitored for 30 min. Any child who vomited was given a repeat dose of the same treatment and observed for a further 30 min. If the child vomited again, he or she was admitted for parenteral quinine administration and withdrawn from the trial. Enrolled children were provided treatment for fever and for any other conditions diagnosed. Children with a haemoglobin < 8 g/dl were treated with oral iron tablets according to national policy.
Follow-up
The children were asked to return on days 1, 2, 3, 7, 14, 21 and 28 and on any non-scheduled day if ill. Physical examination and axillary temperature were documented, and history was elicited with attention to illness, side effects, possible adverse events, and history of concomitant medication. On days 1 and 2, the study drugs were administered under observation. Any concomitant illnesses were treated by the study clinicians. Follow-up laboratory evaluation included finger prick blood for blood smears and filter paper dried blood spots on days 1, 2, 3, 7, 14, 21, 28 and on any other day the child presented with an axillary temperature ≥37.5 °C or reported fever in the previous 24 h. Haemoglobin was measured on days 7, 14, and 28, and when clinically indicated.
Any safety concerns and serious adverse events were reported to the KEMRI Ethics Review Committee and the Centers for Disease Control and Prevention Institutional Review Board.
Laboratory procedures
Haemoglobin was assessed with a Hemocue machine® (Hemocue AB, Angelholm, Sweden). The blood smears were read for quantitative baseline parasite counts and parasite density. Smears were read by two independent microscopists and, in case of discordances >50% in positivity or in species, read by a third microscopist. The final density was determined by taking the average of the two closest readings, and smear positivity and species by the reading of the majority. Smears were considered negative if no parasites were seen after examining 100 high power fields.
Outcomes
Study outcomes were evaluated by clinical and parasitological responses using standard WHO definitions (WHO 2005). Parasitological and clinical failures were treated with Coartem®, or if signs of severe illness were present, with intravenous quinine. Those who remained asymptomatic and parasite free on day 28, without previously meeting criteria of Early Treatment Failure (ETF), Late Clinical Failure (LCF), or Late Parasitological Failure (LPF), were classified as adequate clinical and parasitological response (ACPR).
Molecular analysis
DNA was extracted from bloodspots dried on filter papers by soaking overnight in 1 ml of 0.5% saponin-1× phosphate buffered saline (PBS). The segment was then washed twice in 1 ml of PBS and boiled for 8 min in 100 µl PCR quality water with 50 µl 20% chelex suspension (pH 9.5). Recrudescent and new infections were differentiated by typing the highly polymorphic repeat region of MSP2 (Snounou et al.1999). Pre- and post-treatment samples for each patient were compared by running the amplified MSP2 products in adjacent lanes of the gel. Recrudescent infections were characterised as having at least one identical allele (within 15 bp) present in both pre- and post-treatment samples. Samples where no alleles matched in the pre- and post-treatment were classified as new infections. If either pre- or post-sample failed to amplify, they were classified as undetermined.
Pfmdr1 and pfcrt genes were analysed in all individuals who failed treatment in baseline and failure samples. Pfcrt alleles were analysed at baseline in all patients who reached 28-day ACPR, and pfmdr 1 alleles were analysed at baseline in randomly selected ACPR individuals equal to the number of recurrent infections (32). Pfcrt and pfmdr1 genes were amplified by nested PCR using the primers and PCR protocol described by Humphreys et al. (2007). The pfcrt haplotype was analysed using a multiplex, real-time PCR (qPCR) assay as described by Sutherland et al. (2007). Samples were considered positive for a particular genotype if a CT-value of 35 cycles or fewer was obtained in at least two independent PCR experiments.
Statistical analysis
The sample size of 110 was based on an estimated ACPR for AQ-AS of 85%, and an ability to detect at the 5% significance level an error/precision rate of ±7.5%. Allowance was made for a 10% loss to follow-up at day 28 and a further 10% loss due to non-adherence. Data were collected and scanned in using Cardiff Teleforms (Cardiff Software Inc., Vista, CA, USA) and all data were rechecked after scanning. Data was analysed using sas version 9.1 (Statistical Application Software Institute, Cary, NC, USA). To compare 28-day PCR-corrected ACPR with recrudescences, Fisher’s exact test was used to compare categorical variables. Similarly, the Wilcoxon rank sum test was used to compare continuous variables. Logistic regression with backward selection was used to determine baseline factors associated with treatment failure.
Results
Study population
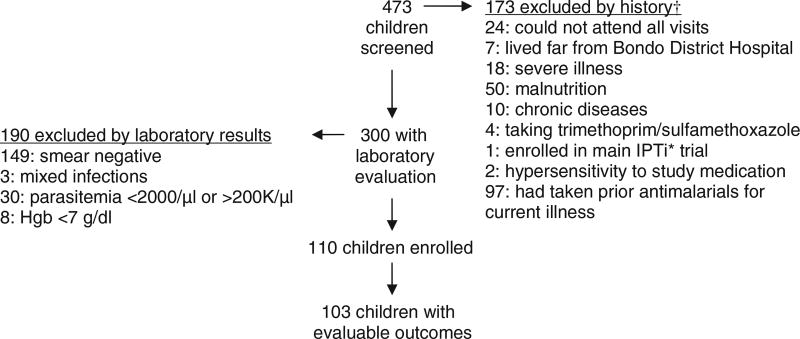
A total of 473 children presented to the Maternal Child Health Clinic at Bondo District Hospital with fever or a history of fever and were screened. Of these, 110 met study criteria and were enrolled in the study. There were no refusals. Reasons for exclusion are presented in Figure 1. Baseline characteristics of enrolled children are presented in Table 1. Of the 110 enrolled children, 103 (93.6%) had evaluable clinical and parasitological outcomes. Three children were lost to follow up, one missed a dose and was withdrawn, and one vomited persistently and was withdrawn. One patient was hospitalised for amebiasis after clearing parasitemia, was treated with quinine despite a negative blood smear and was withdrawn. Another patient died at home after clearing parasitemia (see Adverse Events).
Figure 1.
Reasons for exclusion. †Some children have multiple reasons for exclusion by history. *IPTi, Intermittent Preventive Treatment in Infancy.
Table 1.
Baseline characteristics of enrolled children
| Enrolled (n = 110) |
(95% CI) | |
|---|---|---|
| Mean height (cm) | 87.2 | (85.1–89.2) |
| Mean weight (kg) | 12.0 | (11.5–12.5) |
| Mean age (months) | 30.7 | (28.0–33.4) |
| Percent male | 47.3 | (37.9–56.6) |
| Mean temperature (°C) | 38.2 | (38.0–38.4) |
| Mean haemoglobin (g/dl) | 9.9 | (9.6–10.2) |
| Geometric Mean Parasite Density | 54915 | (46043–65497) |
Clinical and parasitological response
There were no ETF. Of the 103 children who reached a study endpoint, 30.0% had cleared parasitemia by day 1, 84.6% by day 2, and 98.2% by day 3. The mean parasitological clearance time was 1.9 days (95% CI 1.8–2.1). Although 54.5% of children were febrile at enrolment, only one was febrile at the following day’s visit. Of the 103 children who completed the trial, 16 (15.5%) were classified as LCF, 1 on day 19, 11 on day 21, 1 on day 26, and 3 on day 28. An additional 16 (15.5%) children experienced LPF, 1 on day 14, 13 on day 21, and 2 on day 28. A total of 32 children (31.1%) experienced treatment failure, and 71 children (68.9%) had an ACPR at day 28 (uncorrected). After parasite clearance, the median time to recurrent parasitemia was 21 days with 75% of recurrent infections documented on day 21, thus an interquartile range of 21–21. The actual dose achieved was 4.4 mg/kg/day of artesunate (95% CI 3.8–5.1) and 8.4 mg/kg/day of amodiaquine (95% CI 7.0–9.8). There was no difference between 28-day ACPRs and recurrent infections in artesunate dose per kg (P = 0.43) or amodiaquine dose per kg (P = 0.96).
Molecular analysis
Molecular analysis demonstrated that of the 32 children who experienced treatment failure, 21 had new infections, 10 had recrudescences, and one could not be classified and was therefore not included in the ACPR calculation; thus, the PCR-corrected 28-day ACPR was 90.2% (92/102). There was no statistically significant difference between children who achieved PCR-corrected 28-day ACPR and children who had recrudescence in terms of baseline height, weight, age, gender, haemoglobin, or parasitemia. Children who achieved 28-day PCR-corrected ACPR had a higher temperature at enrolment (38.3 °C vs. 37.4 °C, P-value 0.03) and higher weight-for-age Z-scores (−0.88 vs. −1.71, P-value 0.03) (Table 2), which remained significant in logistic regression analysis. Neither baseline gametocytemia nor failure to clear gametocytemia was significantly associated with recrudescence. There was no difference in time to recurrence between reinfection and recrudescences.
Table 2.
Baseline characteristics of patients with PCR-corrected 28-day adequate clinical and parasitological response (ACPR) vs. those with recrudescence
| ACPR (n = 92) |
Recrudescence (n = 10) |
P-value | |
|---|---|---|---|
| Mean height (cm) | 87.4 | 85.3 | 0.53 |
| Mean weight (kg) | 12.1 | 10.5 | 0.10 |
| Mean age (months) | 31.3 | 27.5 | 0.44 |
| Percent male | 47.8% | 40.0% | 0.75 |
| Mean temperature (°C) | 38.3 | 37.4 | 0.03 |
| Mean haemoglobin (g/dl) | 9.9 | 9.4 | 0.31 |
| Geometric mean parasite density | 52856 | 71096 | 0.31 |
| Z-score (weight for age) | −0.88 | −1.71 | 0.03 |
Markers of resistance
The pfcrt 76T mutation was high at baseline in our population (Table 3), but was present in 100% of analysed samples from recurrent infections (data available for 26/32). In pfcrt codons 72–76 at baseline, of analysed ACPR samples (n = 29), 72.4% had the CVIET haplotype, 24.1% had wild-type CVMNK, and 3.4% had both haplotypes, while of recurrent infections (n = 32), 71.9% had the CVIET haplotype, 6.3% had CVMNT, and 21.9% had both haplotypes. All recurrent infections had the CVIET haplotype on the day of failure.
Table 3.
Presence of single nucleotide polymorphisms (SNPs) at baseline among patients with 28 day adequate clinical and parasitological response (ACPR) (n = 71) compared with patients with recurrent infection (n = 32)
| SNP* | ACPRs | Recurrent infections |
Odds Ratio† (95% C.I.) P-value |
|---|---|---|---|
| pfcrt 76T | 49/65 (75%) | 29/31 (94%) | 4.74 (1.02–22.09) |
| 0.049 | |||
| pfmdr1 86Y | 11/28 (39%) | 19/27 (70%) | 3.67 (1.20–11.26) |
| 0.023 | |||
| pfmdr1 184Y | 22/28 (79%) | 25/28 (89%) | 2.27 (0.51–10.18) |
| 0.283 | |||
| pfmdr1 1246Y | 9/29 (31%) | 17/32 (53%) | 2.52 (0.88–7.19) |
| 0.085 | |||
| pfmdr1 YYY | 8/27 (30%) | 12/27 (44%) | 1.90 (0.62–5.83) |
| 0.262 |
For analysis of pfmdr1, a random sample from patients with ACPR was selected equal to the number of samples from patients with recurrent infections (n = 32). The denominator (n) for each SNP is number of samples for which that SNP was analysable.
Odds Ratio, odds of a recurrent infection in the presence of a specified SNP at baseline.
Pfmdr1 1034 and 1042 alleles were wild-type in all analysable samples, both at baseline and failure. Resistant pfmdr1 86Y, wild-type pfmdr1 184Y, and resistant pfmdr1 1246Y were all more frequent in baseline samples from recrudescences and reinfections than baseline samples from ACPRs (Table 3), but only pfcrt 76T and pfmdr1 86Y were significantly associated with recurrent infection. In recurrent infections at baseline and at day of failure, we found similar frequencies in every SNP at baseline compared to day of failure (Table 4).
Table 4.
Presence of single nucleotide polymorphisms (SNPs) at baseline and at day of failure, among patients with recurrent infections (n = 32)
| SNP* | Baseline | Day of failure |
|---|---|---|
| pfcrt 76T | 29/31 (94%) | 27/27 (100%) |
| pfmdr1 86Y | 19/27 (70%) | 22/32 (69%) |
| pfmdr1 184Y | 25/28 (89%) | 28/32 (88%) |
| pfmdr1 1246Y | 17/32 (53%) | 17/27 (63%) |
| pfmdr1 YYY | 12/27 (44%) | 16/27 (59%) |
The denominator (n) for each SNP is number of samples for which that SNP was analysable.
Haematological response
Mean haemoglobin for all children who completed the study increased, with mean haemoglobin increasing from 9.9 g/dl (95% CI 9.6–10.2) on day 0 to 11.1 g/dl (95% CI 10.8–11.4) on day 28.
Adverse events
We saw no study medication related severe adverse events during the study, although one child was hospitalised for an unrelated cause. Another child developed a rapidly enlarging lateral neck mass, recurrent fever, and vomiting after clearing parasitemia. Care was not sought, and the patient died at home, likely of untreated neck abscess and subsequent septicaemia. Fifteen children (13.6%) vomited the first dose, requiring re-administration, and tolerated the second dose. Only one child had vomiting of both doses requiring intravenous quinine.
Discussion
We found the combination of AQ-AS to be efficacious against acute, uncomplicated P. falciparum malaria in children in western Kenya, consistent with previously published studies of AQ-AS combination therapy in surrounding countries. There were no ETF, and most treatment failures occurred at 21 or more days after treatment. AQ-AS was well-tolerated and there was a very low rate of side effects requiring change of therapy.
The two clinical associations with risk of recurrent infection, lower temperature at enrolment and low Z-score, may be related. Malnutrition, especially protein malnutrition, can reduce the immune function and ability to mount a febrile response to a variety of infections (Phillips & Wharton 1968); a lower Z-score is associated with treatment failure (Hamel et al. 2005). Although we excluded children below the third percentile on the Kenyan growth charts to exclude severe malnourishment, children with relatively lower Z-scores may have had some degree of immune dysfunction and a compromised ability to mount a febrile response.
We found that the presence of pfcrt 76T and pfmdr1 86Y at baseline was associated with risk for recurrent infections, both reinfections and recrudescences, consistent with observations in previously published efficacy studies of amodiaquine-artesunate in the neighbouring countries of Tanzania and Uganda (Holmgren et al. 2007; Nsobya et al. 2007).
One limitation of this study is that we did not compare AQ-AS to AL; there was an AL efficacy trial being completed concurrently in the same area, and the selection of drugs for this trial was based on those included in the IPTi trial. The sample size, which was calculated to estimate treatment efficacy, also was too small to allow us to observe selection of some resistant polymorphisms.
Artemether-lumefantrine was adopted as first-line therapy for treatment of uncomplicated P. falciparum malaria in Kenya in April 2004, and was fully implemented in late 2006. AQ-AS is registered as an approved alternative. Other antimalarials are commonly used in the private sector, including amodiaquine as monotherapy. In a recent household survey in three districts of Kenya, 86% of antimalarials purchased for self-treatment were either sulfadoxine-pyrimethamine or amodiaquine, with each equally likely to be used; amodiaquine was likely to be underdosed (Abuya et al. 2007).
Amodiaquine-artesunate remains a safe and effective alternative to AL in children with uncomplicated P. falciparum in western Kenya, although continued amodiaquine monotherapy in the community may increase resistance and jeopardise the future of this combination. The results presented here provide a baseline for monitoring changes in the susceptibility of P. falciparum to AQ-AS in western Kenya.
Acknowledgments
We would first like to thank the children in Bondo District who participated in the study and their parents and caregivers. We are grateful to our excellent staff who worked tirelessly: Simon Omollo, Judith Otieno, Sylvia Odera, Caroline Nyatuoro, Winifred Otieno, Mary Owidhi, Eveline Delila, Irene Nyagwachi, Sophie Omondi, Charles Ojwang’, Japheth Adika, Philip Olang, Franklin Komino, Malaki Ogalo, Telesphorus Odawo, Thomas Otieno, Philip Onyona, Daniel K’ouko, and Kephas Otieno. We would also like to thank Roselyne Odera, Linnet Odula, and Everline Sikuku for their administrative and analytic support; Dr Billis, Bondo District Medical Officer of Health, and Dr Austin Ochwila, Bondo District Hospital Director, for allowing us to conduct the trial at Bondo District Hospital, allocating clinic space, and for all their support; Dr Kayla Laserson, Director KEMRI/CDC Research Station; and Venkatachalam Udhayakumar for assistance with interpretation of molecular marker data. Funding was provided by the Drug Resistance Working Group and IPTi Consortium.
References
- Abacassamo F, Enosse S, Aponte JJ, et al. Efficacy of chloroquine, amodiaquine, sulphadoxine-pyrimethamine and combination therapy with artesunate in Mozambican children with non-complicated malaria. Tropical Medicine and International Health. 2004;9:200–208. doi: 10.1046/j.1365-3156.2003.01182.x. [DOI] [PubMed] [Google Scholar]
- Abuya TO, Mutemi W, Karisa B, Ochola SA, Fegan G, Marsh V. Use of over-the-counter malaria medicines in children and adults in three districts in Kenya: implications for private medicine retailer interventions. Malaria Journal. 2007;6:e57. doi: 10.1186/1475-2875-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjuik M, Agnamey P, Babiker A, et al. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomised, multicentre trial. Lancet. 2002;359:1365–1372. doi: 10.1016/s0140-6736(02)08348-4. [DOI] [PubMed] [Google Scholar]
- Djimdé A, Doumbo OK, Cortese JF, et al. A molecular marker for chloroquine-resistant falciparum malaria. New England Journal of Medicine. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- Dorsey G, Staedke S, Clark TD, et al. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. The Journal of the American Medical Association. 2007;297:2210–2219. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- Hamel MJ, Holtz T, Mkandala C, et al. Efficacy of trimethoprim-sulfamethoxazole compared with sulfadoxine-pyrimethamine plus erythromycin for the treatment of uncomplicated malaria in children with integrated management of childhood illness dual classifications of malaria and pneumonia. American Journal of Tropical Medicine and Hygiene. 2005;73:609–615. [PubMed] [Google Scholar]
- Happi CT, Gbotosho GO, Folarin OA, et al. Association between mutations in Plasmodium falciparum chloroquine resistance transporter and P. falciparum multidrug resistance 1 genes and in vivo amodiaquine resistance in P. falciparum malaria-infected children in Nigeria. American Journal of Tropical Medicine and Hygiene. 2006;75:155–161. [PubMed] [Google Scholar]
- Holmgren G, Gil JP, Ferreira PM, Veiga MI, Obonyo CO, Björkman A. Amodiaquine resistant Plasmodium falciparum malaria in vivo is associated with selection of pfcrt 76T and pfmdr1 86Y. Infection Genetics Evolution. 2006;6:309–314. doi: 10.1016/j.meegid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Holmgren G, Hamrin J, Svård J, Mårtensson A, Pedro Gil JP, Björkman A. Selection of pfmdr1 mutations after amodiaquine monotherapy and amodiaquine plus artemisinin combination therapy in East Africa. Infection Genetics Evolution. 2007;7:562–569. doi: 10.1016/j.meegid.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Humphreys GS, Merinopoulos I, Ahmed J, et al. Amodiaquine and Artemether-Lumefantrine Select Distinct Alleles of the Plasmodium falciparum mdr1 Gene in Tanzanian Children Treated for Uncomplicated Malaria. Antimicrobial Agents and Chemotherapy. 2007;51:991–997. doi: 10.1128/AAC.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanywanyi AM, Mwita A, Sumari D, Mandike R, Mugittu K, Abdulla S. Efficacy and safety of artemisinin-based antimalarial in the treatment of uncomplicated malaria in children in southern Tanzania. Malaria Journal. 2007;6:e146. doi: 10.1186/1475-2875-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson A, Strömberg J, Sisowath C, et al. Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clinical Infectious Diseases. 2005;41:1079–1086. doi: 10.1086/444460. [DOI] [PubMed] [Google Scholar]
- Mutabingwa TK, Anthony D, Heller A, et al. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet. 2005;365:1474–1480. doi: 10.1016/S0140-6736(05)66417-3. [DOI] [PubMed] [Google Scholar]
- Nsobya SL, Dokomajila C, Joloba M, Dorsey G, Rosenthal PJ. Resistance-Mediating Plasmodium falciparum pfcrt and pfmdr1 Alleles after Treatment with artesunate-amodiaquine in Uganda. Antimicrobial Agents and Chemotherapy. 2007;51:3023–3025. doi: 10.1128/AAC.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I, Wharton B. Acute bacterial infection in kwashiorkor and marasmus. BMJ. 1968;i:407–409. doi: 10.1136/bmj.1.5589.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rwagacondo CE, Karema C, Mugisha V, et al. Is amodiaquine failing in Rwanda? Efficacy of amodiaquine alone and combined with artesunate in children with uncomplicated malaria. Tropical Medicine and International Health. 2004;9:1091–1098. doi: 10.1111/j.1365-3156.2004.01316.x. [DOI] [PubMed] [Google Scholar]
- Snounou G, Zhu X, Siripoon N, et al. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93:369–374. doi: 10.1016/s0035-9203(99)90120-7. [DOI] [PubMed] [Google Scholar]
- Sowunmi A, Balogun T, Gbotosho GO, Happi CT, Adedeji AA, Fehintola FA. Activities of amodiaquine, artesunate, and artesunate-amodiaquine against asexual- and sexual-stage parasites in falciparum malaria in children. Antimicrobial Agents and Chemotherapy. 2007;51:1694–1699. doi: 10.1128/AAC.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland CJ, Haustein T, Gadalla N, Armstrong M, Doherty J, Chiodini P. Chloroquine-resistant Plasmodium falciparum infections among UK travellers returning with malaria after chloroquine prophylaxis. Journal of Antimicrobial Chemotherapy. 2007;59:1197–1199. doi: 10.1093/jac/dkm104. [DOI] [PubMed] [Google Scholar]
- Tinto H, Guekoun L, Zongo I, Guiguemdé RT, D’Alessandro U, Ouédraogo JB. Chloroquine-resistance molecular markers (Pfcrt T76 and Pfmdr-1 Y86) and amodiaquine resistance in Burkina Faso. Tropical Medicine and International Health. 2008;13:238–240. doi: 10.1111/j.1365-3156.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- WHO. Classification of Treatment Outcomes. 2005 Available at: http://www.malariadrugresistance.net/warn/clinical/files/WHOClassificationofTreatmentOutcomes.pdf.