Abstract
Background
Grasslands are one of the most representative vegetation types accounting for about 20% of the global land area and thus the response of grasslands to climate change plays a pivotal role in terrestrial carbon balance. However, many current climate change models, based on earlier results of the doubling-CO2 experiments, may overestimate the CO2 fertilization effect, and as a result underestimate the potentially effects of future climate change on global grasslands when the atmospheric CO2 concentration goes beyond the optimal level. Here, we examined the optimal atmospheric CO2 concentration effect on CO2 fertilization and further on the growth of three perennial grasses in growth chambers with the CO2 concentration at 400, 600, 800, 1000, and 1200 ppm, respectively.
Results
All three perennial grasses featured an apparent optimal CO2 concentration for growth. Initial increases in atmospheric CO2 concentration substantially enhanced the plant biomass of the three perennial grasses through the CO2 fertilization effect, but this CO2 fertilization effect was dramatically compromised with further rising atmospheric CO2 concentration beyond the optimum. The optimal CO2 concentration for the growth of tall fescue was lower than those of perennial ryegrass and Kentucky bluegrass, and thus the CO2 fertilization effect on tall fescue disappeared earlier than the other two species. By contrast, the weaker CO2 fertilization effect on the growth of perennial ryegrass and Kentucky bluegrass was sustained for a longer period due to their higher optimal CO2 concentrations than tall fescue. The limiting effects of excessively high CO2 concentrations may not only associate with changes in the biochemical and photochemical processes of photosynthesis, but also attribute to the declines in stomatal conductance and nitrogen availability.
Conclusions
In this study, we found apparent differences in the optimal CO2 concentrations for the growth of three grasses. These results suggest that the growth of different types of grasses may respond differently to future elevated CO2 concentrations through the CO2 fertilization effect, and thus potentially alter the community composition and structure of grasslands. Meanwhile, our results may also be helpful for improving current process-based ecological models to more accurately predict the structure and function of grassland ecosystems under future rising atmospheric CO2 concentration and climate change scenarios.
Background
It is widely evident that global atmospheric carbon dioxide (CO2) concentration has dramatically increased since the nineteenth century industrial revolution, elevating by about 1.6 ppm/yr. during the past five decades [1, 2]. According to the most recent report released by the Inter-Governmental Panel on Climate Change (IPCC, 2013), global atmospheric CO2 levels have increased from the pre-industrial level of 280 ppm to the present level of nearly 410 ppm and the growth rate of CO2 concentration is projected to be accelerated with an unprecedented pace of ∼1.0 ppm/yr. [2–4]. Moreover, the global atmospheric CO2 concentration may even reach 1000 ppm by the end of this century and nearly 2000 ppm by the end of the next century if no effective control measures are implemented [4]. This elevated global atmospheric CO2 concentration may not only cause climate warming, but also cause profound impacts on the net primary productivity of agricultural and natural ecosystems [5–9].
It is well known that CO2 is not only one of the most important greenhouse gases, but also a critical reactant for the biochemical processes of plant photosynthesis, and thus future elevated CO2 concentrations may affect plant growth by altering metabolic rates [10–13]. Many studies have reported that most plants may benefit from enriched atmospheric CO2 concentrations through the “CO2 fertilization effect”. Plant growth can be boosted by absorbing more CO2 molecules for photosynthesis under elevated CO2 concentrations [10, 14–17]. For example, Wand [18] reviewed the responses of wild grasses to elevated atmospheric CO2 concentrations and found that elevated CO2 increased the total biomass of C3 grass species by about 50%. However, other studies have shown that the CO2 fertilization effect on plant growth might decline or vanish beyond certain CO2 concentrations [7, 19, 20], and even CO2 enrichment induced adverse effects on some plants when the ambient CO2 level was above 1000 ppm [21]. In addition, many previous studies also found that the CO2 fertilization effect on plants had a large variation among different species. For example, Wang [18] reported a substantial increase of the biomass of young birch tree by 59% when CO2 concentration was doubled from about 350 ppm to 700 ppm. By contrast, Körner et al. [22] showed that the growth and biomass of five tree species in a mature deciduous forest were barely affected by increasing CO2 concentration to 530 ppm based on a four-year FACE experiment. These results indicate that different plant species may have different optimal CO2 concentrations, and that plants with higher optimal CO2 concentrations are likely to benefit the most from the CO2 fertilization effect, and at the same time, suffer less negative impacts from future climate change, mainly due to higher nitrogen and water use efficiency [23, 24].
The CO2 fertilization effect on plant growth was fundamentally mediated by leaf photosynthesis [19, 25, 26], which is highly correlated with plant carbon balance [27] and biochemical composition [28, 29]. Previous studies have demonstrated that elevated CO2 could dramatically affect net photosynthetic rates through various processes including up-regulation or down-regulation when the growth CO2 below or above the optimal CO2 for plants. Elevated CO2 levels generally stimulate net photosynthetic rate through directly enhancing carboxylation rates [13, 30] while competitively reducing photorespiration and dark respiration [19, 22, 31–33]. Nevertheless, the decline of net photosynthetic rate under high CO2 levels may be related to changes in leaf biochemical composition associated with reductions in the amount and/or activity of Rubisco [22, 26], and increases in total non-structural carbohydrates [7, 34]. Moreover, the down-regulation of net photosynthetic rate is also associated with the availability of nutrients such as nitrogen (N), which exerts an important control over the response of plants and ecosystems in rising atmospheric CO2 conditions [28, 35–37]. Previous studies showed that down-regulation of photosynthesis occurred in plants grown in elevated CO2 and limited N indicated decreased leaf N concentration [38, 39] High N availability could alleviate the down-regulation of photosynthesis in plants under elevated CO2 environments [19, 26, 29].
Grasslands are an important part of terrestrial ecosystems, and account for about 20% of the earth’s land area [6, 40]. Perennial grasses are the dominant species in temperate grasslands and pastures [40], and are utilized as fine turf grass, which serves many important environmental functions including erosion control, surface water detoxification and control of allergens and diseases [41, 42]. A majority of the research investigating plant response to elevated CO2 have been focused on crops [43–45] or trees [26, 29, 34, 46–48] and few studies have examined the effects of elevated CO2 on perennial grasses [17, 19, 40]. In addition, most previous studies regarding the CO2 fertilization effect have focused primarily on “doubling-CO2 experiments” with twofold higher CO2 concentration of about 700 or 800 ppm than the current global CO2 concentration [40, 42, 45, 48]. Nevertheless, the CO2 fertilization effect may sustain up to about 1000 ppm for leaf photosynthesis [46, 49] and 1800 ppm for grain yield of crops [50]. For example, Xu [23] examined the optimal atmospheric CO2 concentration of the CO2 fertilization effect on the growth of winter wheat and found that the optimal atmospheric CO2 concentration was 894 and 968 ppm for total biomass and leaf photosynthesis. So far, few experimental studies have been conducted to examine the optimal CO2 concentration for maximizing the CO2 fertilization effect on perennial grasses, which are the most important grass species in both natural grasslands and managed turf grass. Moreover, most of the modeling projections are based on strong CO2 fertilization according to the conclusions from earlier “doubling-CO2 experiments” [29, 34]. However, it should be noted that in the future, continuously rising atmospheric CO2 concentrations may substantially lower the CO2 fertilization effect when the atmospheric CO2 concentration rises beyond the optimal CO2 level [23]. As a result, many current climate change models based on earlier results of the doubling-CO2 experiments may overestimate the CO2 fertilization effect and underestimate the potential risks that climate change poses on global grasslands when the atmospheric CO2 concentration goes beyond the optimal CO2 level. Therefore, identifying optimal CO2 concentrations and understanding the mechanisms that determine these optima are not only critical to accurately estimating the impacts of climate change on global grassland production, but also have important significance for policy implementations under future climate change scenarios. Therefore, this study was conducted based on the following objectives: (1) investigate the effects of elevated CO2 concentrations on the growth of three perennial grass species, (2) examine the optimal CO2 concentration for maximizing the CO2 fertilization effect of these grasses, and (3) explore potential mechanisms that determine the optimal CO2 concentrations for the growth of perennial grasses.
Methods
Plant materials and growing conditions
Three grass species, tall fescue (Festuca arundinacea Schreb.), perennial ryegrass (Lolium perenne L.), and Kentucky bluegrass (Poa pratensis L.), were collected using a golf-hole cutter (10 cm diameter × 20 cm long) to ensure the same aboveground and belowground biomass of each species from field plots in the research farm at Rutgers University (Adelphia, NJ, USA). These grasses were irrigated with groundwater once a week in the field research farm to maintain a 10-cm soil surface moisture of about 40% (% volume) during the growing season. Then the collected plants were transplanted into pots (10 cm diameter × 40 cm long) filled with fritted clay and maintained in a greenhouse with an average temperature of 21/16 °C (day/night) and about 800 μmol photon m− 2 s− 1 Photosynthetic Active Radiation (PAR) in natural sun light, and 65% relative humidity for 70 d (May–June 2012) to establish canopy and root system. During the establishment period, grasses were irrigated daily to water-holding capacity and fertilized twice per week with half-strength Hoagland’s solution [51]. We trimmed grasses once a week to maintain a canopy height of 5 cm during the canopy development and root establishment period. Then the plants were trimmed to a 2-cm canopy height and moved to growth chambers (Environmental Growth Chamber) with temperatures set at 21/18 °C (day/night), 60–70% Relative Humidity (RH), light level at grass canopy of 1000 μmol m− 2 s− 1 PAR, and a 12-h photoperiod for 2 weeks prior to the CO2 treatment. During the eight weeks of the CO2 treatment, these grasses were maintained under the same environmental factors as before the start of CO2 treatment, such as chamber temperature of 21/18 °C (day/night), relative humidity of 60–70%, light level at the grass canopy of 1000 μmol m− 2 s− 1 PAR, and 12-h photoperiod (6:00–18:00). In addition, the grasses were also well-watered with daily irrigation and fertilized with half-strength Hoagland’s solution twice a week.
Treatments and experimental design
We exposed grasses to five CO2 treatments: ambient concentration (400 ± 10 ppm) or elevated concentrations (600, 800, 1000, and 1200 ± 10 ppm). In order to minimize confounding effects of environmental variation between different chambers, we randomly changed the CO2 concentration of each growth chamber every three days, and then relocated the CO2 treated grasses to the growth chambers with corresponding CO2 concentrations. The experiment was arranged in a randomized complete block design with four replicates (pots) per treatment. The ambient and elevated CO2 concentrations within the chambers were maintained through an automatic CO2 control system connected to a CO2 source-tank containing 100% research-grade CO2 (Airgas, Inc.). The CO2 concentrations inside the chambers were continuously monitored through an infrared gas analyzer (LI-820; LICOR, Inc., Lincoln, NB, USA) connected to a computer logger maintaining the CO2 concentration within 10 ppm of the ambient and elevated target levels.
Plant biomass measurements
We trimmed the plants to a 2-cm canopy height again at 14, 28, 42, and 56 days after the CO2 treatments. The trimmed leaves were collected and oven dried at 80 °C for 7 days, and the dry weights were subsequently measured. The dry weights of leaves collected at 14, 28, 42, and 56 days of CO2 treatment were put together for calculating shoot biomass during the CO2 treatment period. At the end of the treatment period (56 days), all plant samples were destructively removed for an analysis of root biomass accumulation. The roots were severed from the shoots at the soil line and washed to make free of fritted clay medium. All of the washed roots were then oven dried at 80 °C for 3 days, and the dry weights were subsequently measured.
Leaf gas exchange measurements
Leaf gas exchange measurements were performed at the end of the CO2 treatment period (56 days). Five fully expanded leaves were randomly selected and arranged in a 2 × 3 cm2 cuvette chamber attached to a portable photosynthetic system (LI-6400; LICOR, Inc.). Before each measurement, leaves were equilibrated in the cuvette at saturating PPFD (1000 μmol photon m− 2 s− 1), the growth CO2 level, the target temperature and Vapor Pressure Deficit (VPD). CO2 concentrations in the cuvette were controlled using an injector system (LI-6400, LI-COR Inc.), which utilizes a CO2 mixer and compressed CO2 cartridges sealed with plasticene to prevent leakage. Then, the photosynthesis vs intercellular CO2 (An-Ci) curves were measured at cuvette chamber CO2 of 50, 100, 150, 200, 300, 400, 600, 800, 1000, 1200, and 1400 ppm. Data from An-Ci curves were used to compare treatment effects on the light-saturated net photosynthetic rates at ambient or elevated CO2 (An), the maximum carboxylation rate of Rubisco (Vcmax), and the maximum capacity of electron transport mediated ribulose bisphosphate (RuBP) regeneration (Jmax). An estimation method was used to obtain Vcmax and Jmax for each observed An-Ci curve [52]. Meanwhile, stomatal conductance (gs), and transpiration rate (Tr) were also determined with the portable photosynthesis system (LI-6400; LICOR, Inc.). Water Use Efficiency (WUE) was determined by the values of the net photosynthetic rate (An) and transpiration rate (Tr) according to the formula WUE = An / Tr.
Biochemical analysis
After the CO2 treatment period (56 days), the leaves and roots for analyzing Total Non-structural Carbohydrates (TNC) were sampled at midday, immediately frozen in liquid nitrogen and stored at − 80 °C until freeze-drying. Freeze-dried tissues were then ground to fine powder with a ball mill (MM2, Fa. Retsch, Haan, Germany), applied desiccant and stored at 20 °C. Total carbon (C) and nitrogen (N) contents in leaves and roots were determined using an elemental analyzer (Vario Max CN, Elemnetar Corp., Germany). Glucose, fructose, sucrose and starch concentrations were determined spectrophometrically (UV-1750, Shimadzu Corp., Tokyo, Japan), using a glucose kit (GAHK-20, Sigma, St Louis, MO, USA). Phospho-glucose isomerase (P5381–1 KU, Sigma) and invertase (I-4504, Sigma) were used to convert fructose to glucose and sucrose to glucose respectively. Biochemical analyses were repeated five times and expressed on a percentage dry matter basis for each.
Data analysis
The raw data from the leaf photosynthesis measurements was cleaned and processed in Excel spreadsheets where the non-linear An-Ci curve fitting was performed as in Sharkey et al. (2007) [52]. The net assimilation rate (An) versus intercellular CO2 concentration (An-Ci curve), were fitted to estimate the maximum carboxylation rate (Vcmax), maximum electron transport rate (Jmax) based on the measurements of An-Ci curves. In addition, linear and non-linear (quadratic equations) regressions were employed to examine relationships between CO2 concentration and other variables.
Results
Elevated CO2 effects on plant biomass
We found very strong CO2 fertilization effects on the aboveground and total biomass of the three species. The optimal CO2 levels for the aboveground biomass were 945, 915, and 1151 ppm, and for the total biomass were 915, 1178, and 1386 ppm for tall fescue, perennial ryegrass, and Kentucky bluegrass, respectively (Fig. 1). However, an optimal CO2 of 895 ppm for the belowground was found only for the tall fescue, while no obviously optimal CO2 of the belowground biomass for the other two species was detected. Beyond the optimum, further elevating the ambient CO2 concentration significantly reduced the growth of perennial grasses, indicating the adverse impacts of high CO2 concentration on the grass species. Quadratic models can be used to adequately quantify the CO2 fertilization effect on the biomass of the three grasses (Fig. 1).
Fig. 1.
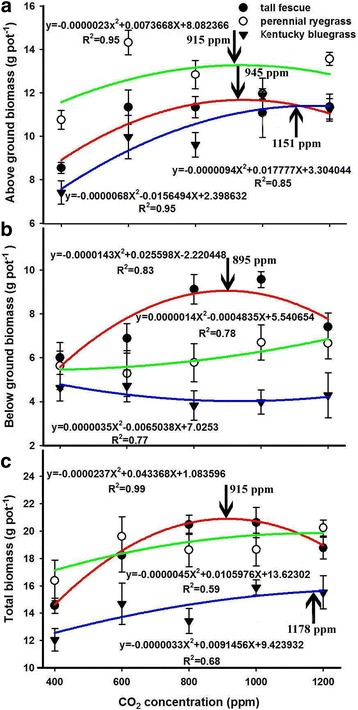
Effects of elevated CO2 concentrations on above ground biomass (a), below ground biomass (b), and total biomass (c) of the three grass species. Values given are mean ± standard deviation for n = 4 pots
Elevated CO2 effects on leaf gas exchange
As with plant growth, the CO2 fertilization effect was also evident in the leaf net photosynthetic rate (An) of Kentucky bluegrass, stimulating An by 75% when the CO2 increased from 400 ppm to 1000 ppm. The CO2 stimulation effect on An reached a maximum at 959 ppm, at which point further increase in CO2 resulted in a decline of An (Fig. 2). However, the response of An to elevated CO2 also varied with grass species. The leaf net photosynthetic rates of the other two species (tall fescue and perennial ryegrass) consistently increased with increasing CO2, which can also be described by quadratic relationships with optimal CO2 beyond the maximum CO2 treatment of this study. In contrast to An, the stomatal conductance (gs) and transpiration rates (Tr) of the three grasses decreased non-linearly with the increase of CO2 and the relationships of CO2-gs and CO2-Tr also typically followed quadratic equations with maximum gs and Tr occurring around 400 ppm, which was much lower than the optimal CO2 for plant growth and leaf photosynthesis.
Fig. 2.
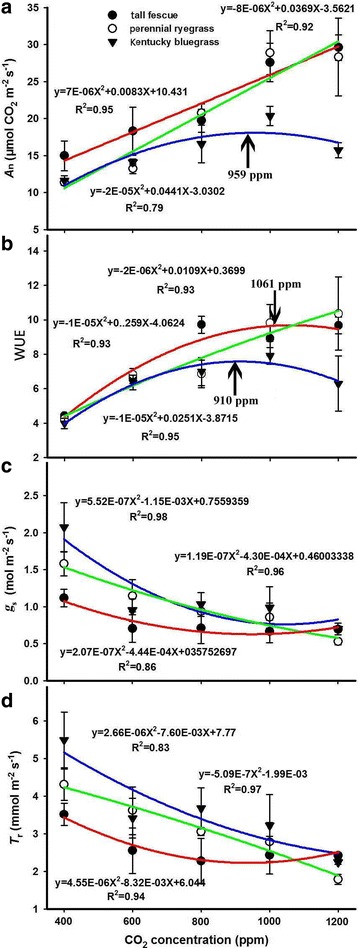
Effects of elevated CO2 concentrations on (a) leaf net photosynthesis rate (An), (b) water use efficiency (WUE), (c) stomatal conductance (gs), and (d) transpiration rate (Tr) of the three grass species. Values given are mean ± standard deviation for n = 4 pots
As a result, the WUE of tall fescue and Kentucky bluegrass also featured bell-shaped curves in relation to CO2 concentration, with the maximum CO2 fertilization effect occurring at approximately 1062 ppm and 910 ppm, respectively. However, the maximum WUE of perennial ryegrass was beyond the highest CO2 concentration treatment of 1200 ppm. Thus, we quantified the relationship between CO2 and WUE of perennial ryegrass through quadratic models and found that the optimal CO2 for WUE would occur at about 2700 ppm, which was much higher than those of the other two species (Fig. 2).
The maximum carboxylation rate (Vcmax) of the three grasses demonstrated bell-shaped curves in relation to CO2 concentration, peaking at 906 ppm, 863 ppm, and 743 ppm for tall fescue, perennial ryegrass, and Kentucky bluegrass, respectively (Fig. 3a). Similar to the Vcmax, the maximum electron transport rate (Jmax) in response to increasing CO2 concentrations also shared bell-shaped curves for all three grasses. The optimal CO2 concentration of Jmax was 877 ppm, 941 ppm, and 665 ppm for tall fescue, perennial ryegrass, and Kentucky bluegrass, respectively (Fig. 3b).
Fig. 3.
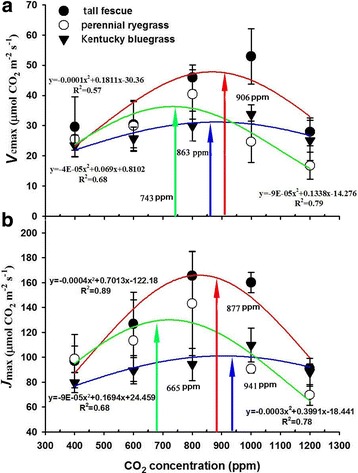
Effects of elevated CO2 concentrations on (a) the maximum carboxylation rate (Vcmax) and (b) the maximum capacity of electron transport RuBP regeneration (Jmax) of the three grasses. Values given are mean ± standard deviation for n = 4 pots
Elevated CO2 effects on leaf dark respiration and non-structural carbohydrates
Our results showed that leaf dark respiration (Rd) of the three species substantially declined with increasing CO2 (Fig. 4). The relationships between Rd and CO2 of the three species were quantified through quadratic models with R2 values of 0.99, 0.99 and 0.94 for tall fescue, perennial ryegrass and Kentucky bluegrass respectively (Fig. 4a). Similar to the Rd, the leaf total non-structural carbohydrate (TNC) of the three grasses also quadratically decreased with elevated CO2 (Fig. 4b). Meanwhile, we estimated the relationships between Rd and TNC (Fig. 5) and found that Rd was increased linearly by the enhancement of TNC, with R2 values such as 0.73, 0.78 and 0.95 for the tall fescue, perennial ryegrass, and Kentucky bluegrass, respectively.
Fig. 4.
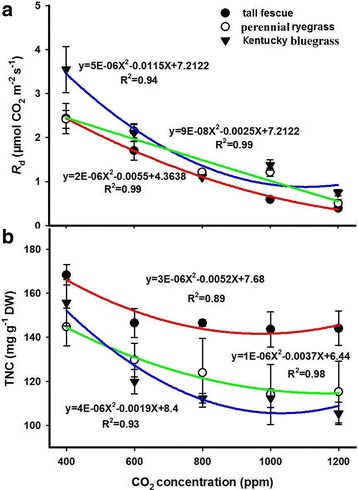
Effects of elevated CO2 concentrations on (a) the leaf dark respiration rates (Rd) and (b) total non-structural carbohydrates (TNC) of the three grass species
Fig. 5.
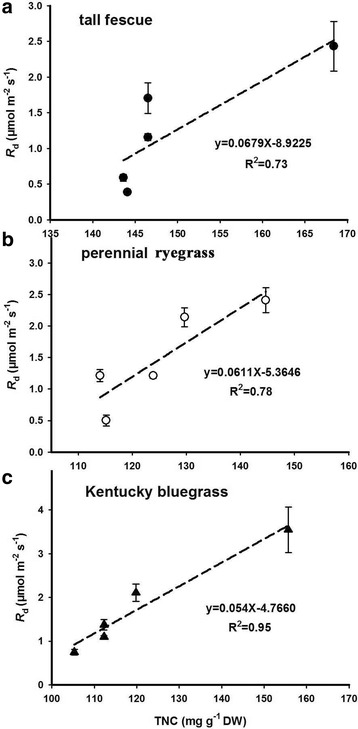
Effects of elevated CO2 concentrations on the linear relationship between TNC and Rd for tall fescue (a), perennial ryegrass (b), and Kentucky bluegrass (c)
Elevated CO2 effects on tissue carbon (C) and nitrogen (N) contents and the relationships between leaf N and Vcmax or leaf N and Jmax
We found optimal CO2 concentrations in both the leaf and root of tall fescue and perennial ryegrass. The relationships between leaf carbon and CO2 featured bell-shaped curves with maximum values occurring at approximately 1388 and 1600 ppm for tall fescue and perennial ryegrass with R2 values 0.96 and 0.99 respectively (Fig. 6a). Interestingly, root carbon in response to elevated CO2 was also characterized by similar curves with R2 values 0.71 and 0.78 and optimal CO2 levels of 1011 and 1200 ppm for tall fescue and perennial ryegrass, respectively. However, we obtained very weak relationships between CO2 and tissue carbon with R2 values 0.23 for leaf and 0.19 for root of Kentucky bluegrass (Fig. 6a). In contrast to tissue carbon, both the leaf and root nitrogen of the tall fescue and Kentucky bluegrass quadratically decreased with elevated CO2 (Fig. 6b). By using the quadratic functions, we analyzed the relationships of leaf and root nitrogen with CO2 and found the R2 values to be 0.79 and 0.71, and 0.31 and 0.44 for the tall fescue and Kentucky bluegrass respectively (Fig. 6d). Our results also showed that elevated CO2 barely affected the tissue nitrogen of perennial ryegrass, evidenced by the weak quadratic relationships between CO2 and nitrogen with R2 values 0.03 and 0.04 for leaf and root respectively (Fig. 6c-d).
Fig. 6.
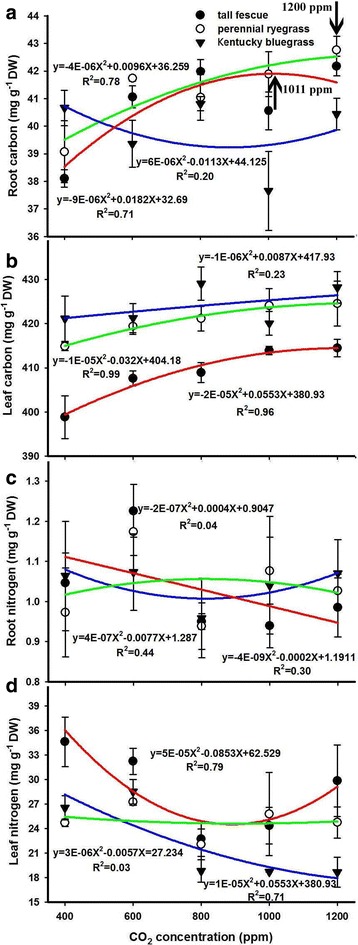
Effects of elevated CO2 concentrations on the carbon content of root (a) and leaf (b) as well as the nitrogen content of root (c) and leaf (d) of the three grass species. Values given are mean ± standard deviation for n = 4 pots
We also evaluated the relationships between leaf N and Vcmax as well as leaf N and Jmax of the three grass species (Fig. 7). Our results showed that the Vcmax values were linearly enhanced with the increases of leaf N for tall fescue (R2 = 0.70), perennial ryegrass (R2 = 0.70), and Kentucky bluegrass (R2 = 0.65, Fig. 7a-c). Similarly, we also found linearly positive relationships between leaf N and Jmax with R2 values of 0.57, 0.55, and 0.62 for tall fescue, perennial ryegrass, and Kentucky bluegrass, respectively (Fig. 7d-f).
Fig. 7.
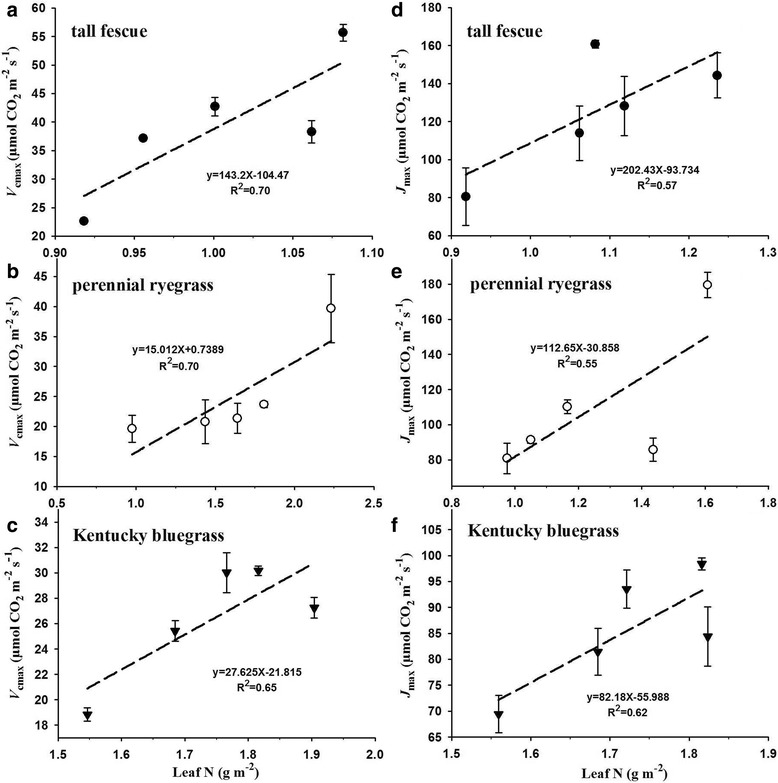
Effects of elevated CO2 concentrations on the relationships between Vcmax and leaf N (a-c) or Jmax and leaf N (d-f) for the three grass species. Values given are mean ± standard deviation for n = 4 pots
Discussion
Different optimal CO2 fertilization concentrations for the growth of perennial grasses
Most plants generally benefit from elevated atmospheric CO2 concentration through the “CO2 fertilization effect”, which boosts growth and yield [19, 23, 46, 52]. However, this positive CO2 fertilization effect strongly depends on the plant functional groups and species [7, 22, 53–56]. Even within the same species of winter wheat, the results from previous studies are inconsistent [22, 50, 57–60]. These contradictory results suggest that different plants and/or species may have different optimal CO2 concentrations for their growth. Our results showed that the optimal CO2 concentrations occurred at 945, 915, and 1151 ppm for the aboveground biomass and at 915, 1178, and 1386 ppm for the total biomass of tall fescue, perennial ryegrass, and Kentucky bluegrass (Fig. 1), suggesting that a strong CO2 fertilization effect occurred at different optimal CO2 concentrations for these three perennial grasses. This result also indicated that Kentucky bluegrass has the highest optimal CO2 concentration among the three grasses, and thus may suffer less from future climate change than the other two grasses. In addition, by enhancing the atmospheric CO2 concentration from 400 ppm to the optimum for each grass species, the maximum CO2 fertilization effect substantially increased the total biomass of the by 60%, 15%, and 30% for tall fescue, perennial ryegrass, and Kentucky bluegrass respectively. Interestingly, biomass enhancements of 15% and 30% for perennial ryegrass and Kentucky bluegrass are very similar with the average of approximately 20% for C3 plants as estimated in meta-analysis of Free-Air CO2 Enrichment (FACE) studies [61, 62], and 32% of Open Top Chamber (OTC) and greenhouse experiments [63]. However, the increased rate of tall fescue (60%) is much higher than those of the other two species, indicating this specie will benefit the most from the positive fertilization effect among these three perennial grasses under future high CO2 environmental conditions. It is noted that we found no obviously optimal CO2 for the belowground biomass of two species (Kentucky bluegrass and perennial ryegrass), as evidenced by the upward quadratic relationships between belowground biomass and CO2 concentrations. These results suggest that the carbon allocation between aboveground and belowground of the three grasses characterize different strategies, and tall fescue might select a more effective strategy to balance the carbon investment between aboveground and belowground than the other two species under high CO2 concentrations.
The positive CO2 fertilization effect on the growth of perennial grasses
Previous studies have well demonstrated that plant growth is highly correlated with biochemical and photochemical processes [64, 65] such as photosynthesis and respiration, through which the CO2 fertilization effect is developed and regulated [22]. In the current study, the photosynthesis-CO2 relationship followed a similar bell-shaped curve like the biomass-CO2 relationship (Figs. 1 and 2), suggesting that the positive CO2 fertilization effect might be attributed to the up-regulation of An, as evidenced by the increased leaf net photosynthetic rates (An), with the maximum CO2 fertilization effect occurring at 959 ppm for Kentucky bluegrass, and 1200 ppm for both tall fescue and perennial ryegrass (Fig. 2a). Further analysis showed that leaf biochemical and photochemical processes played a key role in determining the positive CO2 fertilization effect through directly increasing both carboxylation rates and electron transport rates of perennial grasses. Our results showed that both the maximum carboxylation rate of Rubisco (Vcmax) and the maximum capacity of electron transport RuBP regeneration (Jmax) of the three grasses were dramatically stimulated by elevated CO2 concentrations before reaching their optimums (Fig. 3), suggesting that the initial increase in CO2 concentration may favor both the light and dark reactions of photosynthesis through boosting the Rubisco carboxylation and the RuBP regeneration processes. Also, a recent study has reported that the Vcmax of winter wheat was dramatically increased by elevating ambient CO2 concentrations from 400 ppm to about 800 ppm [23].
In addition to leaf photosynthesis, the positive CO2 fertilization effect on the growth of perennial grasses may also closely associate with the changes in leaf respiration and total non-structural carbohydrates (TNC) under high CO2 concentrations. Our results showed that the leaf dark respiratory rates (Rd) and leaf TNC of the three grasses consistently decreased with elevated CO2 concentrations. Meanwhile, we found a linear relationship between leaf Rd and TNC, suggesting that Rd reduction may partially attribute to decrease in leaf TNC, which is the most important substrate for leaf respiration [14, 66]. Overall, the up-regulation of An and the decline of Rd may both play pivotal roles in explaining the positive CO2 fertilization effects on the growth of perennial grasses in the current study.
The diminishing returns of CO2 fertilization effect on perennial grasses
Previous studies have found that beyond certain thresholds, high CO2 concentration cause diminishing returns of CO2 fertilization effect on plants [13, 22, 23]. Several studies found that the stimulation of An induced by elevated CO2 decreased or even diminished if exposed for a longer time period, because plants acclimate to elevated CO2 concentrations through a process known as down-regulation [19, 32]. We also found bell-shaped curves for biomass-CO2 relationships for the three grasses similar to the An-CO2, indicating a reduction in biomass due to a decline in the photosynthetic rate at high CO2 concentrations. It is well demonstrated that the down-regulation of An is possibly attributed to the changes in carbohydrates [31], under high CO2 environments. In the current study, elevated CO2 concentrations beyond the optima of the three grasses consistently reduced leaf TNC, suggesting that the imbalance of carbohydrate concentration in the source and sink was not a limiting factor for the down-regulation of An. In addition, it is important to noted that hexokinase is a key functional enzyme for mediating sugar sensing [67] and may also decrease Rubisco content through inhibiting the expression of photosynthetic genes [68]. Previous studies have well demonstrated that the Rubisco content and activity of higher plants were dramatically decreased under high CO2 concentrations [68, 69], because leaf N was prior to enzymes relating to the metabolic processes of starch and sucrose than invested in Rubisco when plants was subjected to high CO2 concentrations [70]. Consequently, the changes in hexokinase with CO2 concentrations may contribute to the bell-shaped relationship between An and CO2 concentration, especially for the down-regulation of An under high CO2 concentrations.
It is well documented that stomatal conductance (gs) declines when exposed to elevated atmospheric CO2 concentration, and a doubling of CO2 from the present ambient concentration generally results in a reduction in gs of 10–70% depending on species or functional groups [58]. In the current study, we also found that the gs of all three grasses were dramatically decreased with elevated CO2 concentrations, which may be partly due to the down-regulation of An caused by CO2. Moreover, the reduced gs under high CO2 concentrations might result in a decline in leaf transpiration and thus reduced nutrient availability, as observed in many previous studies [22]. Previous studies have claimed that elevated CO2 concentration increased plant C/N ratios mainly due to a decrease in N content [12, 26]. Similarly, we also found that the nitrogen contents of both tall fescue and Kentucky bluegrass were markedly decreased with increasing CO2 concentrations, which may also be caused by the CO2 effects on An, since nitrogen content is associated with photosynthetic enzymes such as Rubisco [35–37]. In addition, the linearly positive relationships between leaf N and Vcmax for the three grasses (Fig. 7) were directly supporting the above conclusion that the down-regulation of An was partly attributed to the decline of leaf N under high CO2 concentrations.
It should be noted that the CO2 fertilization effect on plant growth may be confounded by future climate change such as global warming, nitrogen deposition, and drought, which may reduce or cancel out the CO2 fertilization effect [39]. For example, the global surface temperature may continue to increase and cause global precipitation to become unevenly distributed both temporally and spatially [2]. As a result, drought stress caused by the increased global surface temperature and the declined precipitation may also be a critical factor affecting leaf photosynthesis and respiration [17] and thus plant growth and biomass accumulation [49], and in turn the structure and function of ecosystems such as grasslands and pastures [37, 40]. Therefore, the fates of the three grasses cannot only be determined by elevated CO2 concentrations because warming and drought may have interactive effects with CO2 enhancement on the growth, physiological, and biological processes of the three grasses under future climate change [20]. Therefore, more controlled experiments with multiple factors such as temperature, drought, nutrition availability and CO2 concentration are needed for predicting the fates of grass species and thus the community dynamics of grasslands under future global climate change [31]. However, it is important to note that this study was carried out under controlled conditions with sufficient nutrients and water for plants during the experiment, which is obviously different from actual field conditions. Therefore, many similar experiments should be carried out in natural conditions without fertilization and watering for predicting the fates of the three cool-season C3 grasses in future climate change scenarios.
Conclusions
We found that the optimal CO2 concentrations occurred at 945, 915, and 1151 ppm for the aboveground biomass of tall fescue, perennial ryegrass, and Kentucky bluegrass, respectively. Higher CO2 concentrations had diminishing returns of CO2 fertilization effect on plant growth, causing limiting effects on stomatal conductance, nitrogen availability and changes in the biochemical and photochemical processes of photosynthesis. Our results suggest that the continuously increasing atmospheric CO2 concentration in the future may dramatically lower the CO2 fertilization effect, and thus many current climate change models based on earlier results of “doubling–CO2” experiments may overestimate the CO2 fertilization effect on grasslands beyond the optimum CO2 concentration. According to recent IPCC reports, if global CO2 emissions are not effectively mitigated, the atmospheric CO2 concentration might be over 900 ppm in the second half of this Century. Nevertheless, the optimal CO2 concentrations found in this study can be used as an indicator in predicting the fates of the cool-season C3 grasses under future rising atmospheric CO2 concentration and climate change, because grasses with high optimal CO2 concentrations may take full advantage of the CO2 fertilization effect.
Acknowledgments
We gratefully thank two anonymous reviewers for their constructive comments and suggestions on revising the manuscript. We also acknowledge Dr. Patrick Burgess and Mr. Steven Xu (Rutgers University, USA) for managing the growth chamber and editing the English of the manuscript.
Funding
This research was partially supported by the Natural Science Foundation of China (31400418), Natural Science Foundation of Hebei Province (C2016402088), Young Outstanding Innovative Talents of Hebei Province (BJ2016012), The Hebei Province Foundation for Returnees (CN201702), the Forestry Department of Sichuan Province (Forest Carbon Monitoring and Accounting Project, 2009–204), the Project on Promoting New Rural Construction with Science and Technology in Qinghai Province (2013-N-556), and the China Postdoctoral Science Foundation funded projects (2014 M561044 and 2016 T90128). The funders have no role in the study design, data analysis and interpretation, and manuscript writing, but just provide the financial support.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- An
net photosynthetic rates
- gs
stomatal conductance
- Rd
dark respiration
- TNC
total nonstructural carbohydrates
- Tr
transpiration rates
- VPD
vapor pressure deficit
- WUE
water use efficiency
Authors’ contributions
YPZ, FL, MX and BH designed the experiments. FL, LHH, LLG and CM performed the experiments and analyzed the data. YPZ, MX, AAS and BH analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bingru Huang, Email: huang@aesop.rutgers.edu.
Ming Xu, Email: mingxu@crssa.rutgers.edu.
References
- 1.Pearson PN, Palmer MR. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature. 2000;406:695–699. doi: 10.1038/35021000. [DOI] [PubMed] [Google Scholar]
- 2.IPCC: Summary for Policy makers: Climate Change 2013: The Physical Science Basis . In: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Church J, Clark P, Cazenave A, Gregory J, Jevrejeva S, Levermann A, Merrifield M, Milne G, Nerem SR, Nunn P, Payne A, Pfeffer WT, Stammer D, Alakkat U, editors. UK: Cambridge University Press; 2013. [Google Scholar]
- 3.NOAA: The history of atmospheric carbon dioxide on earth 2013. Available at: http://www. Planet for life. Com/CO2 history/.
- 4.NASA: Global climate change: Vital signs of the planet 2014. Available at: http:// climate. nasa.
- 5.Bazzaz FA. The response of natural ecosystems to the leaf photosynthesis and water use of big bluestem under elevated carbon dioxide. Crop Sci. 1990;31:1589–1594. [Google Scholar]
- 6.Steffen WL, Canadell JG. Carbon dioxide fertilization and climate change policy. Department of Environment and Heritage. AGO: Australian Greenhouse Office; 2005. [Google Scholar]
- 7.Wand SJE, Midgley GF, Jones MH, Curtis PS. Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Glob Change Biol. 1999;5:723–741. doi: 10.1046/j.1365-2486.1999.00265.x. [DOI] [Google Scholar]
- 8.Arnone JA, Zaller JG, Spehn EM, Niklaus PA, Wells CE, Korner C. Dynamics of root systems in native grassland: effects of elevated atmospheric CO2. New Phytol. 2000;147:73–85. doi: 10.1046/j.1469-8137.2000.00685.x. [DOI] [Google Scholar]
- 9.Kirkham MB. elevated carbon dioxide, impacts on soil and plant water relations. Boca Raton, FL: CRC Press. 2011;978:4398–5504. [Google Scholar]
- 10.Ainsworth EA. Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob Change Biol. 2008;14:1642–1650. doi: 10.1111/j.1365-2486.2008.01594.x. [DOI] [Google Scholar]
- 11.Lloyd J, Farquhar GD. The CO2 dependence of photosynthesis, plant growth response to elevated atmospheric CO2 concentrations and their interaction with soil nutrient status. I. General principles and forest ecosystems. Funct Ecol. 1996;10:4–32. doi: 10.2307/2390258. [DOI] [Google Scholar]
- 12.LeCain DR, Morgan JA. Growth, gas exchange, leaf nitrogen and carbohydrate concentrations in NAD-ME and NADP-ME C4 grasses grown in elevated CO2. Physiol Plantarum. 1998;102:2927–2306. doi: 10.1034/j.1399-3054.1998.1020218.x. [DOI] [Google Scholar]
- 13.Reddy AR, Rasineni GK, Raghavendra AS. the impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr Sci. 2010;99:46–57. [Google Scholar]
- 14.Amthor JS. Effects of atmospheric CO2 concentration on wheat yield: review of results from experiments using various approaches to control CO2 concentration. Field Crops Res. 2001;73:1–34. doi: 10.1016/S0378-4290(01)00179-4. [DOI] [Google Scholar]
- 15.Jablonski LM, Wang X, Curtis PS. Plant reproduction under elevated CO2conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol. 2002;156:9–26. doi: 10.1046/j.1469-8137.2002.00494.x. [DOI] [Google Scholar]
- 16.Drewry DT, kumar P, Long S, Bernacchi C, Liang XZ, Sivapalan M. Ecohydrological responses of dense canopies to environmental variability: 2. Role of acclimation under elevated CO2. J Geophys Res. 2010;155:2005–2012. [Google Scholar]
- 17.Yu J, Chen L, Xu M, Huang B. Effects of elevated CO2 on physiological responses of tall fescue to elevated temperature, drought stress, and the combined stress. Crop Sci. 2012;52:1848–1858. doi: 10.2135/cropsci2012.01.0030. [DOI] [Google Scholar]
- 18.Wang X, Taub DR. Interactive effects of elevated carbon dioxide and environmental stress on root mass fraction in plants: a meta-analytical synthesis using Pairwise Techniques. Oecologia. 2010;163:1–11. doi: 10.1007/s00442-010-1572-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee TD, Tjoelker MG, Ellsworth DS, Reich PB. Leaf gas exchange responses of 13 prairie grassland species to elevated CO2 and increased nitrogen supply. New Phytol. 2001;150:405–418. doi: 10.1046/j.1469-8137.2001.00095.x. [DOI] [Google Scholar]
- 20.Poorter H, Navas M. Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol. 2003;157:175–198. doi: 10.1046/j.1469-8137.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Du H, Xu M. Metabolic responses to heat stress under elevated atmospheric CO2 concentration in a cool-season grass species. J Am Soc Hortic Sci. 2012;137:221–228. [Google Scholar]
- 22.Körner C, Asshoff R, Bignucolo O, Hättenschwiler S, Keel SG, Peláez-Riedl S, Pepin S, Siegwolf RTW, Zotz G. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science. 2005;309:1360–1362. doi: 10.1126/science.1113977. [DOI] [PubMed] [Google Scholar]
- 23.Xu M. The optimal atmospheric CO2 concentration for the growth of winter wheat (Triticum aestivum) J Plant Physiol. 2015;184:89–97. doi: 10.1016/j.jplph.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- 25.Morgan JA, Milchunas DG, LeCain DR. Carbon dioxide enrichment alters plant community structure and accelerates shrub growth in the shortgrass steppe. Proc Natl Acad Sci U S A. 2007;104:14724–14729. doi: 10.1073/pnas.0703427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot. 2009;60:2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- 27.Arp WJ. Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 1991;14:869–875. doi: 10.1111/j.1365-3040.1991.tb01450.x. [DOI] [Google Scholar]
- 28.Lewis JD, Lucash M, Olszyk DM, Tingey DT. Relationships between needle nitrogen concentration and photosynthetic responses of Douglas-fir seedlings to elevated CO2 and temperature. New Phytol. 2004;162:355–364. doi: 10.1111/j.1469-8137.2004.01036.x. [DOI] [Google Scholar]
- 29.Borjigidai A, Hikosak K, Hirose T. Carbon balance in a monospecific stand of an annual herb Chenopodium album at an elevated CO2 concentration. Plant Ecol. 2009;203:33–44. doi: 10.1007/s11258-008-9502-2. [DOI] [Google Scholar]
- 30.Jin VL, Evans RD. Elevated CO2 increases plant uptake of organic and inorganic N in the desert shrub Larrea tridentate. Oecologia. 2010;163:257–266. doi: 10.1007/s00442-010-1562-z. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Yang Y, Zhan X, Zhan XY, Zhang CJ, Zhou SX, Wu DX. responses of a dominant temperate grass plant (Leymus chinensis) to elevated carbon dioxide and nitrogen addition in China. J Environ Qual. 2010;39:251–259. doi: 10.2134/jeq2009.0109. [DOI] [PubMed] [Google Scholar]
- 32.Leakey ADB, Uribelarrea M, Ainsworth EA, Naidu SL, Rogers A, Ort DR, Long SP. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006;140:779–790. doi: 10.1104/pp.105.073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunderson CA, Wullschleger SD. photosynthetic acclimation in trees to rising CO2: a broader perspective. Photosynth Res. 1994;39:369–388. doi: 10.1007/BF00014592. [DOI] [PubMed] [Google Scholar]
- 34.Rey A, Jarvis PG. Long-term photosynthetic acclimation to increased atmospheric CO2 concentration in young birch (Betula pendula) trees. Tree Physiol. 1998;18:441–450. doi: 10.1093/treephys/18.7.441. [DOI] [PubMed] [Google Scholar]
- 35.Cure JD, Acock B. Crop responses to carbon dioxide doubling: a literature survey. Agric For Meteorol. 1986;38:127–145. doi: 10.1016/0168-1923(86)90054-7. [DOI] [Google Scholar]
- 36.Hendrix MS, Dumitru TA, Graham SA. Late oligocene-early miocene unroofing in the Chinese tian Shan; an early effect of the India-Asia collision. Geology. 1994;22:487–490. doi: 10.1130/0091-7613(1994)022<0487:LOEMUI>2.3.CO;2. [DOI] [Google Scholar]
- 37.Luo Y, Hui D, Zhang D. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology. 2006;87:53–63. doi: 10.1890/04-1724. [DOI] [PubMed] [Google Scholar]
- 38.Taub DR, Wang XZ. Why are nitrogen concentrations in plant tissue lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol. 2008;50:1365–1374. doi: 10.1111/j.1744-7909.2008.00754.x. [DOI] [PubMed] [Google Scholar]
- 39.Arndal MF, Schmidt IK, Kongstad J, Beier C, Michelsen A. Root growth and N dynamics in response to multi-year experimental warming, summer drought and elevated CO2 in a mixed heathland-grass ecosystem. Funct Plant Biol. 2014;41:1–10. doi: 10.1071/FP13117. [DOI] [PubMed] [Google Scholar]
- 40.Coleman JS, McConnaughay KDM, Bazzaz FA. Elevated CO2 and plant nitrogen-use: is reduced tissue nitrogen concentration size-dependent? Oecologia. 1993;93:195–200. doi: 10.1007/BF00317671. [DOI] [PubMed] [Google Scholar]
- 41.Cotrufo MF, Ineson P, Scott A. Elevated CO2 reduces the nitrogen concentration of plant tissue. Glob Change Biol. 1998;4:43–54. doi: 10.1046/j.1365-2486.1998.00101.x. [DOI] [Google Scholar]
- 42.Suter D, Frehner M, Fischer BU, Nösberger J, Lüscher A. Elevated CO2 increases carbon allocation to the roots of Lolium perenne under free-air CO2 enhancement but not in a controlled environment. New Phytol. 2002;154:65–75. doi: 10.1046/j.1469-8137.2002.00368.x. [DOI] [Google Scholar]
- 43.Beard JB, Green RL. The rule of turfgrasses in environmental protection and their benefits to humans. J Environ Qual. 1994;23:452–460. doi: 10.2134/jeq1994.00472425002300030007x. [DOI] [Google Scholar]
- 44.Burgess P, Huang B. Growth and physiological responses of creeping bentgrass (Agrostis stolonifera) to elevated carbon dioxide concentrations. Hort Res. 2014;1:14021. doi: 10.1038/hortres.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo Y, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience. 2004;54:731–739. doi: 10.1641/0006-3568(2004)054[0731:PNLOER]2.0.CO;2. [DOI] [Google Scholar]
- 46.Li X, Zhang G, Sun B, Zhang S, Zhang Y, Liao Y, Zhou Y, Xia X, Shi K, Yu J. Stimulated leaf dark respiration in tomato in an elevated carbon dioxide atmosphere. Sci Rep. 2013;3:3433. doi: 10.1038/srep03433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan K, Zhou GS, Ren SX. Responses of leaf dark respiration of winter wheat to changes in CO2 concentration and temperature. Chin Sci Bull. 2013;58:1795–1800. doi: 10.1007/s11434-012-5605-1. [DOI] [Google Scholar]
- 48.Ziska LH, Hogan KP, Smith AP, Drake BG. Growth and photosynthetic response of nine tropical species with long-term exposure to elevated carbon dioxide. Oecologia. 1991;86:383–389. doi: 10.1007/BF00317605. [DOI] [PubMed] [Google Scholar]
- 49.Jump A, Hunt JM, Peñuelas J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob Change Biol. 2006;12:2163–2174. doi: 10.1111/j.1365-2486.2006.01250.x. [DOI] [Google Scholar]
- 50.Crous KY, Zaragoz-Castells J, Löw M, Ellsworth D, Tissue DT, Tjoelker M, CVM B, Gimeno T, Atkin OK. Seasonal acclimation of leaf respiration in Eucalyptus saligna trees: impacts of elevated atmospheric CO2 and summer drought. Glob Change Biol. 2011;17:1560–1576. doi: 10.1111/j.1365-2486.2010.02325.x. [DOI] [Google Scholar]
- 51.Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif. Agr Exp Stat. 1950;347:1–32. [Google Scholar]
- 52.Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Envion. 2007;30:1035–1040. doi: 10.1111/j.1365-3040.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- 53.Reuveni J, Bugbee B. Very high CO2 reduces photosynthesis dark respiration and yield in wheat. Ann Bot. 1997;80:539–546. doi: 10.1006/anbo.1997.0489. [DOI] [PubMed] [Google Scholar]
- 54.Graaff MA, Van Groenigen KJ, Six J, Hungate B, Van Kessel C. Elevated CO2 increases nitrogen rhizodeposition and microbial immobilization of root-derived nitrogen. New Phytol. 2006, 173:778–86. [DOI] [PubMed]
- 55.Zhang L, Wu D, Shi H, Zhang CJ, Zhan XY, Zhou SX. Effects of elevated CO2 and N addition on growth and N2 fixation of a legume subshrub (Caragana microphylla lam.) in temperate grassland in China. PLoS One. 2011;6:e26842. doi: 10.1371/journal.pone.0026842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu LG, Kimble HJ, Hail JL Wu HF. Generation of squeezed states by parametric down conversion. Phys Rev Lett. 1986;57:2520–2523. doi: 10.1103/PhysRevLett.57.2520. [DOI] [PubMed] [Google Scholar]
- 57.Pooter H. Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio. 1993;104:77–79. doi: 10.1007/BF00048146. [DOI] [Google Scholar]
- 58.Drennan PM, Nobel PS. Responses of CAM species to increasing atmospheric CO2 concentrations. Plant Cell Environ. 2000;23:767–781. doi: 10.1046/j.1365-3040.2000.00588.x. [DOI] [Google Scholar]
- 59.Kerstiens G. Meta-analysis of the interaction between shade-tolerancw, light environment and growth response of woody species to elevated CO2. Acta Oecol. 2001;22:61–69. doi: 10.1016/S1146-609X(00)01096-1. [DOI] [Google Scholar]
- 60.Rawson HM. Yield responses of two wheat genotypes to carbon dioxide and temperature in field studies using temperature gradient tunnels. Aust J Plant Physiol. 1995;22:23–32. doi: 10.1071/PP9950023. [DOI] [Google Scholar]
- 61.Grotenhuis TP, Bugbee B. Super optimal CO2 reduces seed yield but not vegetative growth in wheat. Crop Sci. 1997;37:1215–1222. doi: 10.2135/cropsci1997.0011183X003700040031x. [DOI] [PubMed] [Google Scholar]
- 62.Pleijel H, Gelang J, Sild E, Danielsson H, Younis S, Karlsson PE, Wllin G, Skärby L, Selldén G. Effects of elevated carbon dioxide, ozone and water availability on spring wheat growth and yield. Physiol Plant. 2000;108:61–70. doi: 10.1034/j.1399-3054.2000.108001061.x. [DOI] [Google Scholar]
- 63.Hogy P, Brunnbauer M, Koehler P, Schwadorf K, Breuer J, Franzaring J, Zhunusbayeva D, Fangmeier A. Grain quality characteristics of springwheat (Triticum aestivum) as affected by free-air CO2 enrichment. Environ Exp Bot. 2013;88:11–18. doi: 10.1016/j.envexpbot.2011.12.007. [DOI] [Google Scholar]
- 64.Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- 65.Wang L, Feng ZZ, Schjoerring JK. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): a meta-analytic test of current hypotheses. Agric Ecosyst Environ. 2013;178:57–63. doi: 10.1016/j.agee.2013.06.013. [DOI] [Google Scholar]
- 66.Curtis PS, Wang X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia. 1998;113:299–313. doi: 10.1007/s004420050381. [DOI] [PubMed] [Google Scholar]
- 67.Kelly G, Sade N, Attia Z, Secchi F, Zwieniecki M, Holbrook NM, Asher L, Alchanatis V, Moshelion M, Granot D. Relationship between hexokinase and the aquaporin PIP1 in the regulation of photosynthesis and plant growth. PLoS One. 9(2):e87888. doi:10.1371/journal.pone.0087888. [DOI] [PMC free article] [PubMed]
- 68.Salvucci ME, Crafts-Brandner SJ. Inhibition of photosynthesis by heatstress: the activation state of rubisco as a limiting factor in photosynthesis. Physiol Plant. 2004;120:179–186. doi: 10.1111/j.0031-9317.2004.0173.x. [DOI] [PubMed] [Google Scholar]
- 69.Sicher RC, Bunce JA. Relationship of photosynthetic acclimation to changes of rubisco activity in field-grown winter wheat and barley during growth in elevated carbon dioxide. Photosynth Res. 1997;52:27–38. doi: 10.1023/A:1005874932233. [DOI] [Google Scholar]
- 70.Sharwood RE, Crous KY, WhitneySM EDS, Ghannoum O. Linking photosynthesis and leaf N allocation under future elevated CO2 and climate warming in Eucalyptus globulus. J Exp Bot. 2017;68:1157–1167. doi: 10.1093/jxb/erw484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


