Abstract
Background
Despite the implications for tumor growth and cancer drug resistance, the mechanisms underlying differences in energy metabolism among cells remain unclear.
Methods
To analyze differences between cell types, cell viability, ATP and α-ketoglutaric acid levels, the oxygen consumption rate and extracellular acidification rate, and the expression of key enzymes involved in α-KG metabolism and transfer were examined. Additionally, UPLC-MS/MS was used to determine the doxorubicin (DOX) content in SMMC-7721 and SMMC-7721/DOX cells.
Results
We found that energy metabolism in SMMC-7721 cells is mainly dependent on the glycolysis pathway, whereas SMMC-7721/DOX cells depend more heavily on the oxidative phosphorylation pathway. Cell viability and intracellular ATP levels in SMMC-7721/DOX cells were significantly reduced by rotenone and oligomycin, inhibitors of oxidative phosphorylation. However, SMMC-7721 cell properties were more strongly influenced by an inhibitor of glycolysis, 2-deoxy-d-glucose. Furthermore, the suppressive effect of α-KG on ATP synthase plays an important role in the low levels of oxidative phosphorylation in SMMC-7721 cells; this effect could be strengthened by the metabolic poison methotrexate and reversed by l-(−)-malic acid, an accelerator of the malate-aspartate cycle.
Conclusions
The inhibitory effect of α-KG on ATP synthase was uncoupled with the tricarboxylic acid cycle and oxidative phosphorylation in SMMC-7721 cells; accordingly, energy metabolism was mainly determined by glycolysis. In drug-resistant cells, a remarkable reduction in the inhibitory effects of α-KG on ATP synthase resulted in better coordination among the TCA cycle, oxidative phosphorylation, and glycolysis, providing novel potential strategies for clinical treatment of liver cancer resistance.
Keywords: Drug resistance, Hepatocellular carcinoma, Mechanism, α-ketoglutaric acid, Energy metabolism
Background
The reprogramming of energy metabolism, including aerobic glycolysis and the inhibition of mitochondrial energy metabolism, promotes tumor growth [1–8]. Additionally, it is closely related to multidrug resistance (MDR) in chemotherapy [9, 10]. The effect of mitochondrial energy metabolism on MDR is complex and research progress in this area is slow [11].
The inhibition of mitochondrial energy metabolism in hepatocellular carcinoma cells shows significant plasticity. The process is only partially and reversibly inhibited in normal conditions and is reactivated under stress conditions, e.g., under a lack of oxygen and chemical therapy, and is involved in MDR. Further studies of its proximal mechanisms can provide new targets for reversing MDR [12–17]. According to previous studies, tricarboxylic acid cycle (TCA) enzyme activity in AS-30D hepatoma cells is higher than typical activity levels in non-cancer cells and there is no change in mitochondrial electron transport chain function, indicating that the TCA cycle is not significantly suppressed in these cells [14]. However, it is not clear how glycolysis affects the TCA cycle and oxidative phosphorylation (OXPHOS) or how adenosine triphosphate (ATP) production is reduced in cases where the TCA cycle is constant or even enhanced. Therefore, it is necessary to study characteristic changes in glycolysis and mitochondrial energy metabolism to determine the mechanisms underlying the reactivation of mitochondrial energy metabolism.
Oxaloacetic acid metabolized by lactic acid in hepatocyte mitochondria can enter the TCA cycle, and can be metabolized into aspartic acid by ammonification to enter the gluconeogenic pathway to generate glucose. When the gluconeogenic pathway is blocked, oxaloacetic acid enters the TCA cycle to maintain a normal mitochondrial membrane potential difference and morphology [18]. Studies have shown that there are no significant differences in the function of the complexes (I, II, III, and IV) that maintain mitochondrial membrane potential [19, 20]. However, complex V (ATP synthase) often exhibits abnormal expression or function, and this is considered an important factor in mitochondrial energy metabolism dysfunction [13]. However, the specific mechanisms, particularly the mechanism underlying the reactivation of mitochondrial energy metabolism after chemotherapy, have not been clarified.
A complete loss of mitochondrial function is bad for normal cells, but partial inhibition of the electron transport chain can prolong the life of cells, and this is commonly observed in low-grade and mammalian cells [21]. α-Ketoglutaric acid (α-KG), an intermediate in the TCA cycle, can reduce ATP production by inhibiting the activity of ATP synthase, without affecting electron flow through the electron transport chain (mediated by complexes I, II, III, and IV); it has an antiapoptotic role by increasing mitochondrial autophagy [22–24].
Recent research has shown that α-KG is not just a regulator of many energy metabolism enzymes, but is also a precursor of a variety of amino acids involved in cell metabolism. However, it is not clear whether the inhibitory effects of α-KG on ATP synthase also occur in hepatoma carcinoma cells. In this study, SMMC-7721 and doxorubicin (DOX)-resistant SMMC-7721/DOX cells were used to investigate resistance mechanisms from the perspective of energy metabolism.
Methods
Establishment of a drug-resistant cell model and cell culture
Continuous induction by applying low-dose DOX with increasing concentrations to SMMC-7721 cells was used to generate drug-resistant cells. The cells were grown in medium with 0.43 μM DOX until resistance was acquired, and resistant cells are referred to as SMMC-7721/DOX. They were cultured under standard cell culture conditions with 10% fetal bovine serum with 1% penicillin-streptomycin at 37 °C in a humidified incubator with 5% CO2.
In vitro cytotoxicity test
Cells (2 × 104) were seeded in 96-well plates, and the growth curve was recorded by the IncuCyte real-time video imaging system (Essen Instruments, Ann Arbor, MI, USA). In the cytotoxicity test, cells were seeded in 96-well plates, allowed to attach overnight, and treated with DOX (0.1–40 μM) for 24 h. Cell viability was measured by the MTT method and the IC50 of DOX was calculated using Graphpad prism. The resistance index (RI) was calculated as follows: RI = IC50 (SMMC-7721/DOX)/IC50 (SMMC-7721).
Determination of ATP and protein levels
SMMC-7721 and SMMC-7721/DOX cells were administrated DOX and other drugs for 24 h. Endogenous expression of ATP in lysates of treated cells was detected using an Enhanced ATP Assay Kit following the manufacturer’s instructions (093016161116; Shanghai Biyuntian Biological Co., Ltd., Shanghai, China).
Mitochondrial stress test and glycolysis stress test
Cells (2 × 104) were seeded in 96-well plates, cultured for 24 h to achieve 80% coverage, and treated with drugs at various time points. The oxygen consumption rate (OCR) in the mitochondrial stress test and extracellular acidification rate (ECAR) in the glycolysis stress test were determined using the Seahorse-XF 96 (Seahorse Bioscience, North Billerica, MA, USA), and data were analyzed using Seahorse Wave.
Determination of α-KG
The α-KG content was determined using commercially available kits according to the manufacturer’s protocols (#3C09K06770; Sigma, St. Louis, MO, USA). Briefly, 2 × 106 cells were homogenized in 100 μL of ice-cold α-KG buffer. Samples were centrifuged at 13,000×g for 10 min to remove insoluble material, adjusted to a final volume of 50 μL with α-KG assay buffer, and deproteinized with 10-kDa MWCO (Millipore, Billerica, MA, USA) spin filter before addition to the reaction to prevent interference from enzymes in the samples. Reactions consisted of 44 μL of the sample or standard, 2 μL of α-KG converting enzyme, 2 μL of α-KG development enzyme mix, and 2 μL of fluorescent peroxidase substrate; they were incubated at 37 °C for 30 min. The absorbance of each reaction system was measured at 570 nm (A570) on a microplate reader.
Immunofluorescence analyses
Cells (5 × 103) were cultured in 6-well Merck Millicell EZ slides (Merck Millipore, Darmstadt, Germany), allowed to attach overnight, and treated with DOX for 24 h. Afterwards, the slides were washed twice in PBS, fixed in 4% formaldehyde in PBS (10 min, room temperature) and incubated with 0.2% Triton X-100 in PBS (10 min, room temperature). The fixed cells were incubated overnight at 4 °C with specific antibodies. Protein expression was detected using mouse monoclonal Ab against P-gp. The primary antibodies were detected after 1 h of incubation with anti-rabbit HRP-conjugated antibodies at a dilution 1:2000 in antibody diluent. Finally, the slides were washed 3 times in PBS and Pro Long Gold Mounting Medium with DNA intercalating dye 4,6-diamidino-2-phenylindole (DAPI) was added to visualize the cell nucleus. The analysis was conducted under fluorescence microscope.
Western blot analysis
Cell extracts were acquired from treated SMMC-7721 and SMMC-7721/DOX with RIPA buffer plus proteinase inhibitors. Proteins were resolved by electrophoresis on SDS-polyacrylamide gels and transferred to a polyvinylidene fluoride membrane (Millipore). Proteins of interest were detected using specific primary antibodies, followed by specific secondary antibodies. The expression of proteins of interest was analyzed using ImageJ (NIH, Bethesda, MD, USA). Changes in the density of bands are expressed as fold changes compared to the control in the blot after normalization to β-actin.
Determination of intracellular DOX by UPLC-MS/MS
RIPA buffer (100 μL) was added to cells after treatment for 24 h. The protein in cell lysates was precipitated by methanol, and the supernatant after high-speed centrifugation (12,000 g, 10 min, 4 °C) was dried with nitrogen and re-dissolved in methanol. The supernatant after high-speed centrifugation was directly injected into the UPLC-MS/MS system. This system was a Shimadzu UPLC system equipped with a LC-30 AD binary pump, an on-line degasser (DGU-20A5R), an auto-sampler (Model SIL-30SD), a column temperature controller compartment (CTO-30A), and a 5500 Triple Quad Tandem Mass Spectrometer (AB Sciex, Concord, Ontario, Canada) with an electrospray ionization (ESI) source. Analytes were separated using an Extend C18 column (2.1 mm × 100 mm, 1.8 μm; Agilent, Santa Clara, CA, USA). The mobile phase was composed of a mixture of 1% formic acid water (A) and acetonitrile (B) and a gradient elution program was used (0–2.5 min, 15% B to 40% B, 2.5–4.0 min, 40% B, 4.0–4.1 min, 40% B to 95% B, 4.1–5.0 min, 95% B, 5.0–5.1 min, 95% B to 15% B, 5.1–6.6 min, 15% B). The flow rate was set at 0.3 mL/min, the column temperature was 40 °C, and the injection volume was 2 μL. The ESI source was operated in positive ionization mode. The mass spectrometer was operated in multiple reactions monitoring (MRM) mode. The MS parameters of DOX are presented in Table 1. The optimized parameters were as follows: ion source temperature, 550 °C; curtain gas, 35 psi; ion source gas 1, 55 psi; ion source gas 2, 55 psi; ion spray voltage, 5500 V.
Table 1.
Optimized multiple reaction monitoring (MRM) parameters for DOX
| Compounds | Q1 | Q3 | CE/V | DP/V | EP/V |
|---|---|---|---|---|---|
| DOX | 544.3 | 397.1 | 15.03 | 57.05 | 10.00 |
Statistical analysis
Data are presented as means ± standard deviation (SD). One-way analysis of variance (ANOVA) and t-tests were used for comparisons between groups. All statistical analyses were implemented in SPSS 15.0 with a significance threshold of p < 0.05.
Results
Low-dose DOX-induced drug resistance in hepatoma SMMC-7721 cells
Treatment of SMMC-7721 cells with low-dose DOX at increasing concentrations was successfully used to establish drug-resistant cells. Compared with the sensitive cells, SMMC-7721/DOX cells showed increased slightly volume, relatively irregular shape, unequal size, unclear border, and more intracellular granules (Fig. 1a). The 48-h growth curve showed slightly delayed proliferation of drug-resistant cells (Fig. 1b). In the cytotoxicity test, SMMC-7721 and SMMC-7721/DOX cells exhibited significant differences in viability to DOX at doses ranging from 0.1 to 40 μM (Fig. 1c). The IC50 values of DOX in SMMC-7721 and SMMC-7721/DOX cells were 23.27 μM and 185.00 μM, respectively, and the RI for SMMC-7721/DOX was 7.95.
Fig. 1.
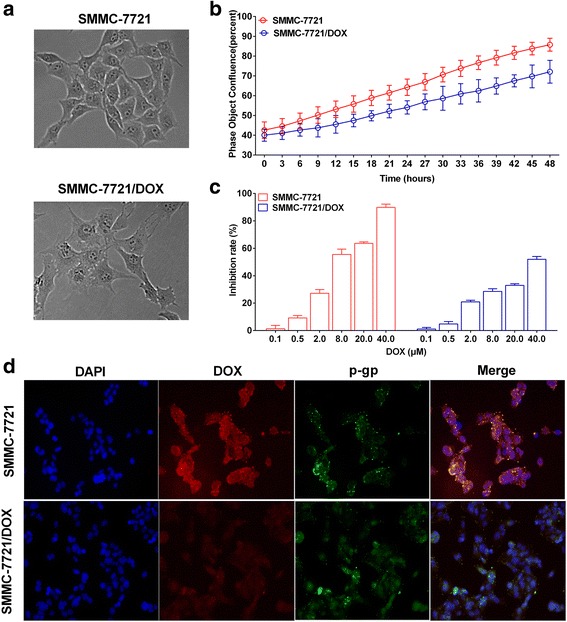
Low-dose DOX-induced drug resistance in hepatoma SMMC-7721 cells. a SMMC-7721 and SMMC-7721/DOX cell configuration. b Growth curves of SMMC-7721 and SMMC-7721/DOX cells c Change in cell viability induced by different concentrations of DOX. d Immunofluorescence staining for P-gp with polyclonal mouse anti-P-gp minigene (green). Nuclear DNA was stained with DAPI (blue). DOX showed red fluorescence. Merge, maximum projection
DOX is a type of anthracycline antitumor drug and exhibits red fluorescence. The fluorescence intensity and relative dose of DOX in cells showed a linear relationship; thus, we analyzed the red fluorescence in SMMC-7721 and SMMC-7721/DOX cells by fluorescence microscopy. After 24-h incubation, the amount of DOX in SMMC-7721/DOX cells was more than that in SMMC-7721 cells. However, the expression of P-gp did not show any significant difference between sensitive and resistant cells (Fig. 1d). These results suggest that the expression of P-gp was not the main mechanism of the decreasing in DOX content in SMMC-7721/DOX cells.
Difference in energy metabolism mode between SMMC-7721 and SMMC-7721/DOX cells
Intracellular ATP levels and energy metabolism mechanisms were examined in SMMC-7721 and SMMC-7721/DOX cells. Cellular ATP levels in SMMC-7721/DOX were significantly greater than those in SMMC-7721 (Fig. 2a). In a mitochondrial stress test, the basal OCR in SMMC-7721/DOX cells was greater than that in SMMC-7721 cells. In response to oligomycin (Oli), an inhibitor of ATP synthase, the OCR was reduced in both cell types, and this reduction was greater for the resistant cells, indicating that SMMC-7721/DOX cells had higher ATP synthase activity and thereby had increased mitochondrial ATP production. In response to carbonyl carbonylcyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP), the OCR increased for both cell types, and the maximal respiration in the resistant cells was much greater than that in SMMC-7721 cells, indicating that SMMC-7721/DOX had a strong spare respiratory capacity in OXPHOS (Fig. 2c and d). In a glycolysis stress test, the ECAR was increased in the culture media of the two kinds of cells, providing evidence for glycolysis. In response to Oli, the increase in ECAR was significantly greater for SMMC-7721/DOX than SMMC-7721 cells, suggesting that SMMC-7721 cells had a greater glycolytic capacity when OXPHOS was inhibited. In response to 2-deoxy-d-glucose (2-DG), as expected, glycolysis was significantly inhibited in the two cell types (Fig. 2e and f). These results suggested that glycolysis was dominant in SMMC-7721 cells, and mitochondrial energy metabolism and glycolysis were both significantly increased in SMMC-7721/DOX cells (Fig. 2b). Glycolysis was dominant in SMMC-7721 cells, and mitochondrial energy metabolism and glycolysis were both significantly increased in SMMC-7721/DOX cells (Fig. 2b).
Fig. 2.
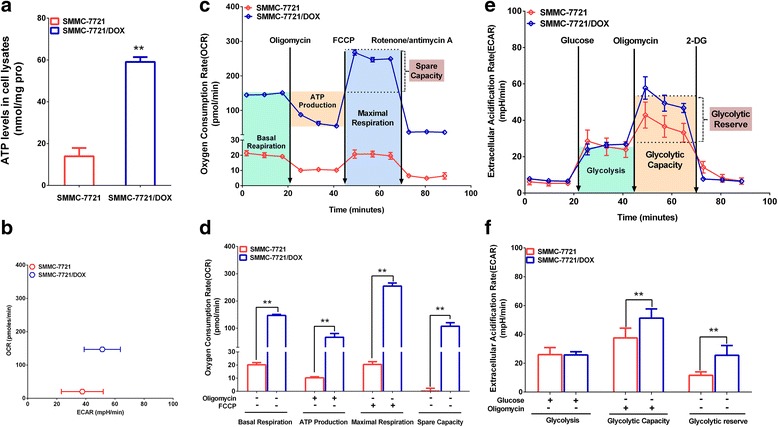
Differences in ATP levels and energy metabolism mode between SMMC-7721 and SMMC-7721DOX cells. a Intracellular ATP levels in SMMC-7721 and SMMC-7721/DOX cells. b Cell respiration (OCR) against glycolysis (ECAR) in SMMC-7721 and SMMC-7721DOX. c OCR based on a mitochondrial stress test. d Effects of various drugs on the OCR in a mitochondrial stress test. e ECAR of the culture media in a glycolysis stress test. f Effects of various drugs on the ECAR in a glycolysis stress test. Significance: **p < 0.01 vs. SMMC-7721
Effects of blocking cell energy metabolism on cell viability and intracellular ATP levels
To explore the effects of glycolysis and OXPHOS on drug resistance, cell viability was assessed after treatment with 2-DG, rotenone (Rot, an inhibitor of respiratory chain complex I), and Oli at various concentrations for 24 h. 2-DG inhibited cellular activity of both cell types, and the IC50 of 2-DG was lower for SMMC-7721 than SMMC-7721/DOX, suggesting that SMMC-7721 cells depend on glycolysis (Fig. 3a, Table 2). In contrast, drug-resistant cells were sensitive to Rot and Oli (Fig. 3b and c, Table 2). Intracellular ATP levels was measured after treatment with 2-DG (5 μM), Rot (0.1 μM), and Oli (0.5 μM) for 24 h. 2-DG reduced the intracellular ATP level in SMMC-7721 cells, and Rot and Oli reduced the intracellular ATP levels in SMMC-7721/DOX cells (Fig. 3d). These findings indicated that the activation of OXPHOS is an essential characteristic of drug-resistant cells.
Fig. 3.
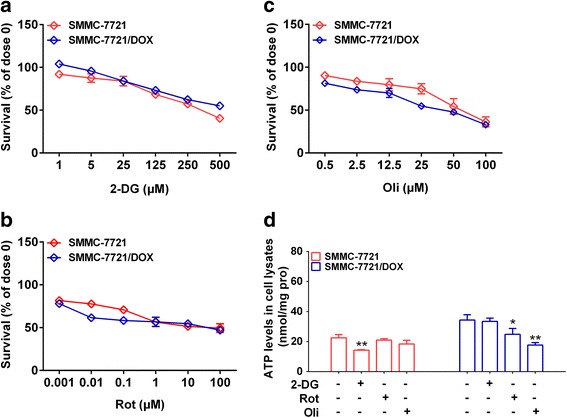
Effects of blocking cell energy metabolism on cell viability. a Effects of the inhibitor 2-DG on cell viability. b Effects of the inhibitor Rot on cell viability. c Effects of the inhibitor Oli on cell viability. d Effects of 2-DG, Rot, and Oli on intracellular ATP levels in SMMC-7721 and SMMC-7721/DOX cells. Significance: *p < 0.05, **p < 0.01 vs. Control
Table 2.
IC50 of glycolysis inhibitors and OXPHOS inhibitors in hepatoma carcinoma cells
| IC50 (μM) | SMMC-7721 | SMMC-7721/DOX |
|---|---|---|
| 2-DG | 358.5 | 643.6 |
| Rot | 28.47 | 22.55 |
| Oli | 63.53 | 36.16 |
Differences in α-KG content and the expression of α-KG-metabolizing enzymes between SMMC-7721 and SMMC-7721DOX cells
α-KG is an intermediate in the TCA cycle, but it is also a biologically active substance involved in OXPHOS and glycolysis via the regulation of ATP synthase and hypoxia inducible factor-1 (HIF-1). We investigated the relationship between α-KG and the activation of oxidative phosphorylation in drug-resistant cells. The α-KG content was significantly higher in drug-resistant cells than in SMMC-7721 cells (Fig. 4a). We examined the expression of ketoglutarate dehydrogenase (OGDH), isocitrate dehydrogenase 2 (IDH2), malic dehydrogenase (MDH1, MDH2), glutamate dehydrogenase 1 (GLUD1), and ATP synthase (ATP5), which are involved in α-KG metabolism. Compared with expression levels in SMMC-7721 cells (Fig. 4b, c, d, and e), OGDH, MDH1, MDH2, and ATP5 activity levels were higher, GLUD1 levels were not different, and IDH2 levels were lower in the drug-resistant cells. Consequently, the inhibition and activation of the malate-aspartate shuttle might be an important factor underlying the phenotypic differences in energy metabolism between SMMC-7721 cells and drug-resistant cells.
Fig. 4.
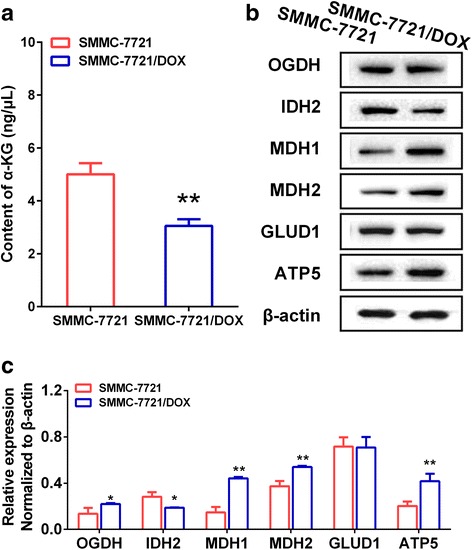
Differences in α-KG content and expression of α-KG-metabolizing enzymes between SMMC-7721 and SMMC-7721DOX. a α-KG content in SMMC-7721 and SMMC-7721/DOX cells. b Blots of OGDH, IDH, MDH1, MDH2, GLUD1, and ATP5 in SMMC-7721 and SMMC-7721/DOX cells. c Expression of OGDH, IDH, MDH1, MDH2, GLUD1, and ATP5 in SMMC-7721 and SMMC-7721/DOX cells. Significance: *p < 0.05, **p < 0.01 vs. Control
Influence of α-KG on cell viability and intracellular ATP levels
To explore the effect of α-KG on the drug resistance mechanism, cell viability was assessed after treatment for 24 h with methotrexate (Met), which inhibits OGDH and influences α-KG levels in the mitochondria, sodium oxamate (Sod), an inhibitor of the malate-aspartate shuttle affecting the α-KG distribution in the mitochondria via NADH metabolism, and l-(−)-malic acid (Mal), which can change the α-KG distribution in the mitochondria by activating the malate-aspartate shuttle. Met inhibited cellular activity in both cell types, and the IC50 of Met was lower for SMMC-7721 than SMMC-7721/DOX, suggesting a stronger inhibitory effect on SMMC-7721 (Fig. 5a, Table 2). Sod had a stronger inhibitory effect on SMMC-7721/DOX than on SMMC-7721 when the concentration was greater than 1 μM (Fig. 5b, Table 3). Mal had no significant effect on viability in either cell type in the range of 0.001 to 10 mM (Fig. 5c, Table 3). In general, the suppression of α-KG metabolism and transfer in the mitochondria could reduce the viability of hepatocellular carcinoma cells, and had a stronger inhibitory effect on resistant cells than non- resistant cells. Intracellular ATP levels were measured after treatment with Met (0.1 μM), Sod (1 μM), and Mal (1 μM) for 24 h. Met and Sod, which increase the accumulation of α-KG in mitochondria, significantly reduced ATP levels in the resistant cells, but had no effect on SMMC-7721 cells. Mal increased ATP levels in both cell types (Fig. 5d). These findings indicated that the accumulation of α-KG in mitochondria by the inhibition of metabolism or reduction in transfer can greatly reduce intracellular ATP levels in the drug-resistant cells.
Fig. 5.
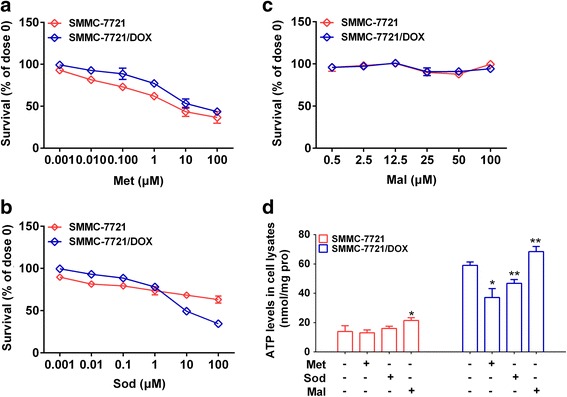
Effects of blocking cell energy metabolism on cell viability. a Effects of Met inhibition on cell viability. b Effects of Sod inhibition on cell viability. c Effects of Mal inhibition on cell viability. d Effects of Met, Sod, and Mal on intracellular ATP levels in SMMC-7721 and SMMC-7721/DOX cells. Significance: *p < 0.05, **p < 0.01 vs. Control
Table 3.
IC50 of regulators of α-KG metabolism in hepatoma carcinoma cells
| IC50 (μM) | SMMC-7721 | SMMC-7721/DOX |
|---|---|---|
| Met | 6.23 | 29.82 |
| Sod | 61,526 | 149.7 |
| Mal | – | – |
Influence of met on the energy metabolism mode
The basal oxygen consumption, ATP production, maximal respiration, and spare capacity in OXPHOS were significantly lower in SMMC-7721/DOX than in SMMC-7721, and only maximal respiration was reduced in SMMC-7721 cells when Met was used to inhibit OGDH activity and thereby reduce α-KG metabolism (Fig. 6a and b).
Fig. 6.
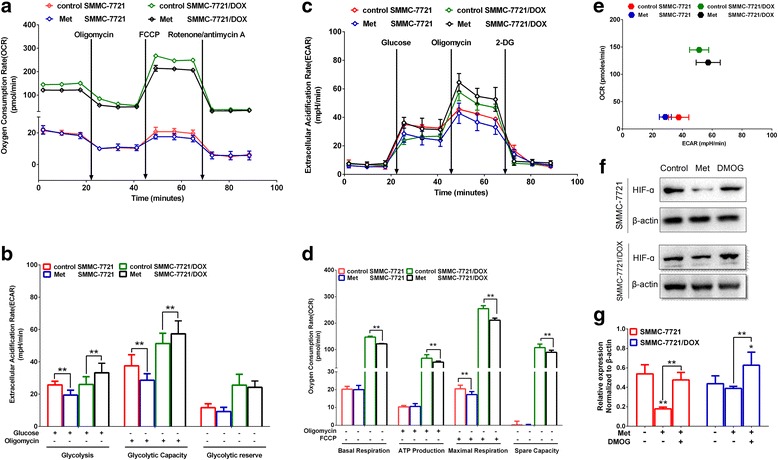
Influence of Met on the energy metabolism mode. a OCR based on a mitochondrial stress test. b Effects of Met on OCR in a mitochondrial stress test. c ECAR of the culture media in a glycolysis stress test. d Effects of Met on ECAR in a glycolysis stress test. e Cell respiration (OCR) against glycolysis (ECAR) in SMMC-7721 and SMMC-7721/DOX cells. f, g Expression of HIF-α in SMMC-7721 and SMMC-7721/DOX cells Significance: **p < 0.01 vs. SMMC-7721 or SMMC-7721/DOX
Glycolysis and the glycolytic capacity of SMMC-7721 were significantly decreased and glycolysis and glycolytic capacity of SMMC-7721/DOX were increased (Fig. 6c and d). It is possible that α-KG that entered the cytoplasm activated the proly-4-hydroxylase domain (PHD) and degraded hypoxia-inducible factor (HIF). In drug-resistant cells, the inhibition of α-KG metabolism can reduce the OXPHOS level and increase the glycolysis level, demonstrating that mitochondrial energy metabolism and glycolysis are coordinated, and the inhibition of mitochondrial energy metabolism can activate glycolysis (Fig. 6d).
This HIF-1α stabilization phenotype is similar to that after treatment of cells with desferrioxamine (DFO), an iron chelator, or dimethyloxalyglycine (DMOG), an established PHD inhibitor, but was not recapitulated with other α-KG analogues, such as Octyl-2KG, MPTOM001 and MPTOM002. Our study is the first example of an α-KG precursor which increases HIF-1αabundance and activity. We propose that DKG acts as a potent HIF-1α activator, highlighting the potential use of DKG to investigate the contribution of the PHD2-HIF-1α pathway to tumor biology.
Influence of sod on the energy metabolism mode
When Sod was used to suppress the malate-aspartate shuttle to reduce the α-KG transfer, the basal oxygen consumption and ATP production of SMMC-7721 and SMMC-7721/DOX decreased significantly (Fig. 7a and b), and glycolysis and the glycolytic capacity increased significantly (Fig. 7c and d), which may be explained by reduced inhibitory effects on PHD and HIF. However, Sod significantly increased the maximal respiration, spare capacity in OXPHOS, and glycolytic reserve for SMMC-7721/DOX cells, indicating that the accumulation of α-KG had inhibitory effects on ATP synthase, and mitochondrial energy metabolism and glycolysis can be activated by suppressing the malate-aspartate shuttle (Fig. 7e).
Fig. 7.
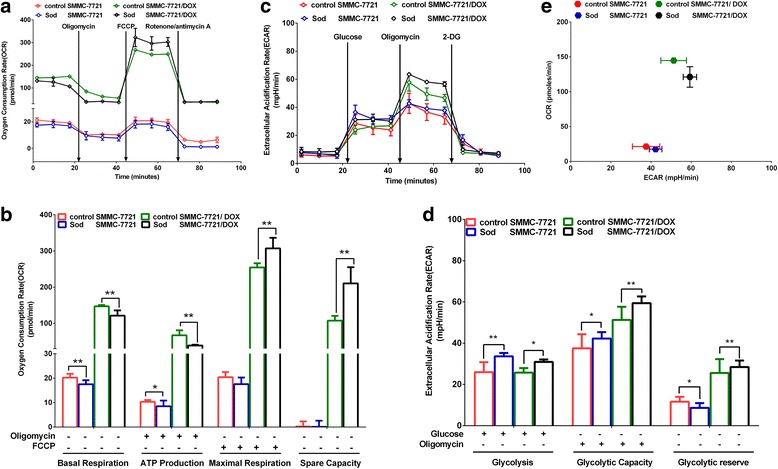
Influence of Sod on the energy metabolism mode. a OCR in a mitochondrial stress test. b Effects of Sod on OCR in a mitochondrial stress test. c ECAR of the culture media in a glycolysis stress test. d Effects of Sod on ECAR in a glycolysis stress test. e Cell respiration (OCR) against glycolysis (ECAR) in SMMC-7721 and SMMC-7721/DOX cells. Significance: *p < 0.05, **p < 0.01 vs. SMMC-7721 or SMMC-7721/DOX
Influence of mal on the energy metabolism mode
Basal oxygen consumption, ATP production, maximal respiration, and spare capacity in OXPHOS increased in SMMC-7721 and SMMC-7721/DOX cells (Fig. 8a and b), and glycolysis, glycolytic capacity, and glycolytic reserve increased (Fig. 8c and d) when Mal was used to activate the malate-aspartate shuttle to increase the α-KG transfer. Mal could promote mitochondrial energy metabolism and glycolysis (Fig. 8e).
Fig. 8.
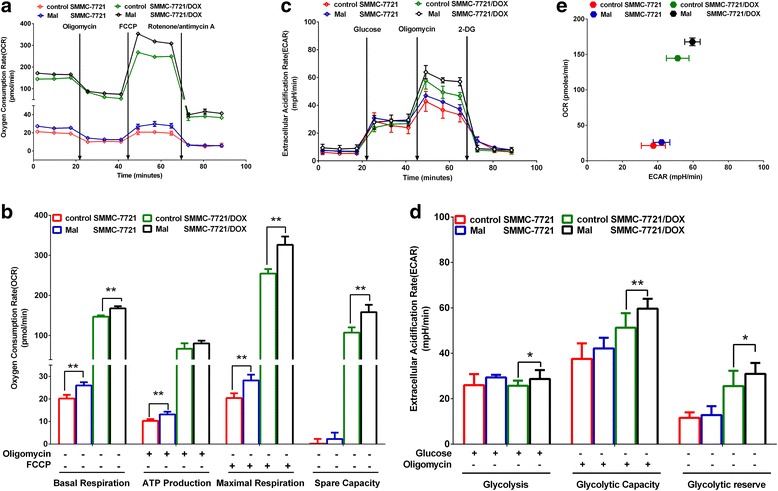
Influence of Mal on the energy metabolism mode. a OCR in a mitochondrial stress test. b Effects of Mal on OCR in a mitochondrial stress test. c ECAR of the culture media in a glycolysis stress test. d Effects of Mal on ECAR in a glycolysis stress test. e Cell respiration (OCR) against glycolysis (ECAR) in SMMC-7721 and SMMC-7721/DOX cells. Significance: *p < 0.05, **p < 0.01 vs. SMMC-7721 or SMMC-7721/DOX
Influence of met, sod, and mal on the α-KG content and expression of key enzymes involved in α-KG metabolism and transfer
The balance and transformation between mitochondrial energy metabolism and glycolysis could be affected by α-KG metabolism and transfer, but the role of key enzymes involved in α-KG metabolism and transfer is unclear. We first examined the α-KG content and found that Met and Sod increased the α-KG content in SMMC-7721/DOX cells (Fig. 9a). We further examined the expression of key enzymes, including OGDH, IDH2, MDH1, MDH2, GLUD1, and ATP5. Met increased the expression of MDH1, MDH2, and ATP5 in SMMC-7721 cells (Fig. 9b and c), and had no significant influence on the expression of these enzymes in SMMC-7721/DOX cells (Fig. 9d and e). Sod significantly reduced the expression of GLUD1 in SMMC-7721 cells (Fig. 9b and c). Mal increased the expression of MDH1, MDH2, and ATP5 in SMMC-7721 cells (Fig. 9b and c), but had little influence on the expression of these enzymes in SMMC-7721/DOX cells (Fig. 9d and e). These results suggested that the inhibition of α-KG metabolism or regulation of α-KG transfer in SMMC-7721 cells could not only regulate mitochondrial energy metabolism and glycolysis, but could also trigger feedback in metabolic enzyme expression. However, in SMMC-7721/DOX cells, α-KG-metabolizing enzyme expression was not affected.
Fig. 9.
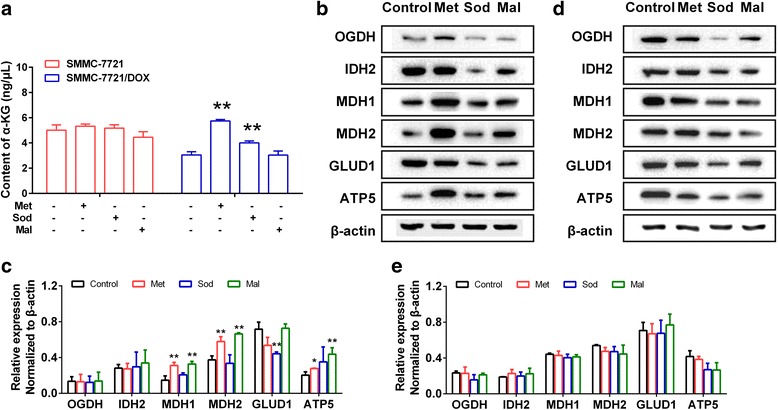
Influence of Met, Sod, and Mal on the α-KG content and expression of key enzymes involved in α-KG metabolism and transfer. a α-KG content in SMMC-7721 and SMMC-7721/DOX. b, c Blots of OGDH, IDH, MDH1, MDH2, GLUD1, and ATP5 in SMMC-7721. d, e. Expression of OGDH, IDH, MDH1, MDH2, GLUD1, and ATP5 in SMMC-7721/DOX. Significance: *p < 0.05, **p < 0.01 vs. Control
Influence of met, sod, and mal on the content of DOX and resistance index
We developed a novel and simple UPLC-MS/MS method to evaluate DOX (Supplementary Material). The results indicated that there was no significant effect of Met, Sod, and Mal on the DOX content in SMMC-7721 cells. However, in SMMC-7721/DOX cells, the inhibition of α-KG metabolism or the malate-aspartate shuttle could significantly increase intracellular DOX, which increases the sensitivity to DOX and reduces the resistance index (Fig. 10a-c, Table 4). The activation of the malate-aspartate shuttle had no significant effect on intracellular DOX levels, reduced the sensitivity to DOX, and improved the resistance index (Fig. 10a and d, Table 4).
Fig. 10.
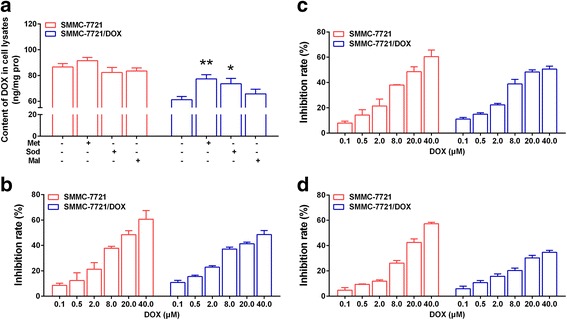
Influence of Met, Sod, and Mal on the DOX content and resistance index. a Effects of Met, Sod, and Mal on the DOX content in SMMC-7721 and SMMC-7721/DOX cells. b Effects of Met on the resistance index. c Effects of Sod on the resistance index. d Effects of Mal on the resistance index. Significance: *p < 0.05, **p < 0.01 vs. Control
Table 4.
IC50 and RI of DOX after treatment with Met, Sod, and Mal
| IC50 (μM) | SMMC-7721 | SMMC-7721/DOX | RI |
|---|---|---|---|
| DOX | 23.27 | 185.00 | 7.95 |
| Methotrexate | 20.37 | 46.81 | 2.30 |
| Sodium oxamate | 20.33 | 29.87 | 1.47 |
| l-(−)-Malic acid | 29.55 | 240.90 | 8.15 |
Discussion
We examined the mechanisms of drug resistance from the perspective of energy metabolism in liver cancer cells, with a focus on the role of α-KG. It is well known that energy metabolism reprogramming is an important characteristic of tumor cells and exhibits substantial heterogeneity and variability. According to the results of this study, mitochondrial energy metabolism and the spare capacity in OXPHOS were weak in SMMC-7721 cells. When OXPHOS was inhibited, viability and intracellular ATP levels were not affected in SMMC-7721 cells. In contrast, when glycolysis was inhibited, viability and intracellular ATP levels were significantly reduced in SMMC-7721 cells. When liver cancer cells acquired drug resistance, mitochondrial energy metabolism and glycolysis increased significantly. In drug-resistant cells, viability and intracellular ATP levels could be significantly reduced by inhibiting OXPHOS.
α-KG is an important intermediate in the TCA cycle and participates in mitochondrial energy metabolism by regulating ATP synthase, PHD, and HIF. Owing to its role in the transition between energy metabolism modes, α-KG is involved in aging, tumorigenesis, and tumor drug resistance.
The regulatory effect of α-KG in mitochondrial energy metabolism has been attributed to its impact on ATP synthase, and the regulatory effect of α-KG on glycolysis has been ascribed to its impact on the activity of PHD and HIF in the cytoplasm. How do α-KG metabolism and distribution affect energy metabolism in drug-resistant cells? In SMMC-7721, the inhibition of α-KG metabolism in mitochondria enhanced the function of the malate-aspartate shuttle, which did not influence the level of OXPHOS, but significantly inhibited glycolysis. The inhibition of α-KG in the cytoplasm further inhibited OXPHOS and enhanced glycolysis. In Smmc-7721/DOX cells, the inhibition of α-KG metabolism or its distribution to the cytoplasm could reduce the function of OXPHOS and strengthen glycolysis. A decrease in intracellular ATP levels suggested a coordinated transformation of energy metabolism and the metabolic phenotype. Conversely, the activation of the malate-aspartate shuttle could increase intracellular ATP levels. In particular, OXPHOS and glycolysis levels were significantly higher in the drug-resistant cells than in non-resistant cells.
Conclusions
In conclusion, the results of this study suggested that the inhibitory effect of α-KG on ATP synthase was uncoupled with the TCA cycle and OXPHOS in SMMC-7721 cells, for which glycolysis was the main energy source (Fig. 11). In drug-resistant cells, a remarkable reduction of the inhibitory effects of α-KG on ATP synthase improved the coordination among TCA, OXPHOS, and glycolysis, and this may be associated with strong malate-aspartate shuttling, promoting the timely metabolism of intermediate products (Fig. 12).
Fig. 11.
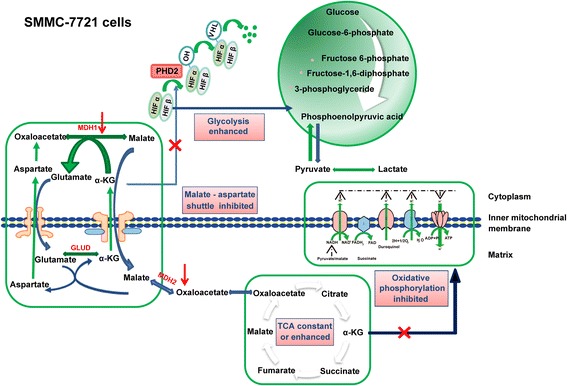
Schematic diagram of the energy metabolism mechanism in SMMC-7721 cells
Fig. 12.
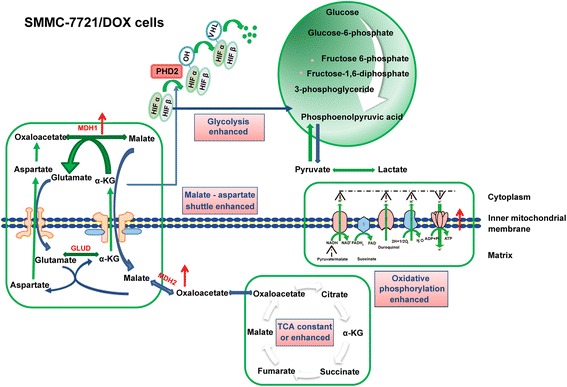
Schematic diagram of the energy metabolism mechanism in SMMC-7721/DOX cells
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation of China (81503308, 81673600, and 81473147), the Project of Administration of Traditional Chinese Medicine of Jiangsu Province (YB2015007), the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015A070), the Postgraduate Research Innovation Project of Jiangsu Higher Education Institutions (KYLX16_1160), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP), and the Open Project Program of Jiangsu Key Laboratory for Pharmacology and Safety Evaluation of Chinese Materia Medica, Nanjing University of Chinese Medicine (JKLPSE201608).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- 2-DG
2-deoxy-d-glucose
- ATP
Adenosine triphosphate
- ATP5
ATP synthase
- DOX
Doxorubicin
- ECAR
Extracellular acidification rate
- FCCP
Carbonyl carbonylcyanide - 4 - (trifluoromethoxy) phenylhydrazone
- GLUD1
Glutamate dehydrogenase 1
- HIF-1
Hypoxia inducible factor-1
- IDH2
Isocitrate dehydrogenase 2
- Mal
l-(−)-malic acid
- MDH
Malic dehydrogenase
- MDR
Multidrug resistance
- Met
methotrexate
- MRM
Multiple reactions monitoring
- OCR
Oxygen consumption rate
- OGDH
Ketoglutarate dehydrogenase
- Oli
Oligomycin
- OXPHOS
Oxidative phosphorylation
- PHD
Proly-4-hydroxylase domain
- RI
Resistance index
- Rot
Rotenone
- Sod
Sodium oxamate
- TCA
Tricarboxylic acid cycle
- α-KG
α-Ketoglutaric acid
Authors’ contributions
Conception and design: JZ, XL and LW. Analysis and interpretation of the data: All authors. Drafting of the article or critical revision of the article for important intellectual content: JZ, KC, XL, HC and LW. Final approval of the article: All authors. Funding procurement: LW, HC, ZC, and JZ. Data collection and assembly: All authors. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Li Wu, Phone: 13913813645, Email: wuli@njucm.edu.cn.
Zhipeng Chen, Phone: 13913877964, Email: czpcpu2000@hotmail.com.
References
- 1.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 2.Ohta S. Contribution of somatic mutations in the mitochondrial genome to the development of cancer and tolerance against anticancer drugs. Oncogene. 2006;25:4768–4776. doi: 10.1038/sj.onc.1209602. [DOI] [PubMed] [Google Scholar]
- 3.Warburg O. The chemical constitution of respiration ferment. Science. 1928;68:437–443. doi: 10.1126/science.68.1767.437. [DOI] [PubMed] [Google Scholar]
- 4.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:1519–1530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Qian Y, Wu S. The Warburg effect: evolving interpretations of an established concept. Free Radic Biol Med. 2015;79:253–263. doi: 10.1016/j.freeradbiomed.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Modica-Napolitano JS, Singh KK. Mitochondrial dysfunction in cancer. Mitochondrion. 2004;4:755–762. doi: 10.1016/j.mito.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Li L, Li W. Gene co-expression analysis identifies common modules related to prognosis and drug resistance in cancer cell lines. Int J Cancer. 2014;135:2795–2803. doi: 10.1002/ijc.28935. [DOI] [PubMed] [Google Scholar]
- 11.Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S, Encalada R, Moreno-Sanchez R, Saavedra E. Modeling cancer glycolysis. Biochim Biophys Acta. 1807;2011:755–767. doi: 10.1016/j.bbabio.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Loiseau D, Morvan D, Chevrollier A, Demidem A, Douay O, Reynier P, Stepien G. Mitochondrial bioenergetic background confers a survival advantage to HepG2 cells in response to chemotherapy. Mol Carcinog. 2009;48:733–741. doi: 10.1002/mc.20539. [DOI] [PubMed] [Google Scholar]
- 13.Domenis R, Bisetto E, Rossi D, Comelli M, Mavelli I. Glucose-modulated mitochondria adaptation in tumor cells: a focus on ATP synthase and inhibitor factor 1. Int J Mol Sci. 2012;13:1933. doi: 10.3390/ijms13021933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietzen DJ, Davis EJ. Oxidation of pyruvate, malate, citrate, and cytosolic reducing equivalents by AS-30D hepatoma mitochondria. Arch Biochem Biophys. 1993;305:91–102. doi: 10.1006/abbi.1993.1397. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;27:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 16.Moreno-Sánchez R, Rodríguez-Enríquez S, Saavedra E, Marín-Hernández A, Gallardo-Pérez JC. The bioenergetics of cancer: is glycolysis the main ATP supplier in all tumor cells? Biofactors. 2009;35:209–225. doi: 10.1002/biof.31. [DOI] [PubMed] [Google Scholar]
- 17.Marín-Hernández A, Gallardo-Pérez JC, López-Ramírez SY, García-García JD, Rodríguez-Zavala JS, Ruiz-Ramírez L, Gracia-Mora I, Zentella-Dehesa A, Sosa-Garrocho M, Macías-Silva M, Moreno-Sánchez R, Rodríguez-Enríquez S. Casiopeina II-gly and bromo-pyruvate inhibition of tumor hexokinase, glycolysis, and oxidative phosphorylation. Arch Toxicol. 2012;86:753–766. doi: 10.1007/s00204-012-0809-3. [DOI] [PubMed] [Google Scholar]
- 18.Ma R, Zhang W, Tang K, Zhang H, Li D, Li Y, Xu P, Luo S, Cai W, Ji T, Katirai F, Ye D, Huang B. Switch of glycolysis to gluconeogenesis by dexamethasone for treatment of hepatocarcinoma. Nat Commun. 2013;4:2508. doi: 10.1038/ncomms3508. [DOI] [PubMed] [Google Scholar]
- 19.Domenis R, Comelli M, Bisetto E, Mavelli I. Mitochondrial bioenergetic profile and responses to metabolic inhibition in human hepatocarcinoma cell lines with distinct differentiation characteristics. J Bioenerg Biomembr. 2011;43:493–505. doi: 10.1007/s10863-011-9380-5. [DOI] [PubMed] [Google Scholar]
- 20.Lu JJ, Yang Z, Lu DZ, Wo XD, Shi JJ, Lin TQ, Wang MM, Li Y, Tang LH. Dihydroartemisinin-induced inhibition of proliferation in BEL-7402 cells: an analysis of the mitochondrial proteome. Mol Med Rep. 2012;6:429. doi: 10.3892/mmr.2012.906. [DOI] [PubMed] [Google Scholar]
- 21.Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, Hu E, Whelan SA, Wang JX, Jung G, Solis GM, Fazlollahi F, Kaweeteerawat C, Quach A, Nili M, Krall AS, Godwin HA, Chang HR, Faull KF, Guo F, Jiang M, Trauger SA, Saghatelian A, Braas D, Christofk HR, Clarke CF, Teitell MA, Petrascheck M, Reue K, Jung ME, Frand AR, Huang J. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson CR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan M, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, Dematteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


