Abstract
Objective:
To determine the efficacy of the stair-step protocol (SSP) using clomiphene citrate (CC) in patients with polycystic ovary syndrome (PCOS) and compare it with traditional regimen.
Design:
This was randomized control trial.
Setting:
Infertility Clinic.
Patient(s):
Sixty infertile PCOS women.
Intervention(s):
Patients were randomized into the study (SSP – 30 patients) and control group (traditional protocol – 30 patients). In the SSP, patients were treated with CC 50 mg/day for 5 days and in nonresponsive patients, the dosage was increased to 100 mg/day for 5 days in the same cycle. Maximum dose of 150 mg was given until the dominant follicle was generated. In control group, the dose increment in nonovulatory cases was done in subsequent cycle. Ultrasonography follow-up was done to detect ovulation.
Main Outcome Measure(s):
Ovulation rate and duration of treatment.
Results:
Ovulation (66.7% vs. 50% respectively) and pregnancy rates (26.7% vs. 15.7%) were similar between the stair step and the control group. The duration of treatment was significantly shorter in stair step compared to traditional protocol (17.23 vs. 53 days). CC 100 mg was the most effective dose for ovulation in either group. There were no significant differences in the systemic side effect.
Conclusions:
By using SSP, effective treatment is provided in significantly shorter time period without any detrimental effect on the ovulation and pregnancy rates.
KEYWORDS: Clomiphene citrate, ovulation induction, polycystic ovary, stair-step protocol
INTRODUCTION
According to American Society for Reproductive Medicine/ European Society of Human Reproduction and Embryology, clomiphene citrate (CC) is the recommended first-line treatment for polycystic ovary syndrome (PCOS) because it is readily available, cheap, well tolerated, with a good safety and efficacy profile.[1,2,3] A commonly used CC protocol for ovulation induction involves a starting dose of 50 mg/day for 5 days during the follicular phase. If ovulation does not occur, the dose is increased by 50 mg in the next cycle after a progesterone-induced withdrawal bleeding. A new protocol is the SSP in which the CC dose is escalated and administered without intervening menses between the dosages. It is shown that the patient with SSP takes shorter time to ovulate, and the potential advantage of SSP is the lack of waiting period until the next menstruation.[4] The cumulative effect of the dose due to repetitive use and delayed excretion may actually augment the response in ovulation. The purpose of this study was to establish if SSP is an effective way for ovulation induction in women who fails ovulating with 50 mg CC.
MATERIAL AND METHODS
Infertile PCOS women booked in the infertility clinic of the Department of Obstetrics and Gynaecology, in whom cause of infertility has been PCOS were screened. The exclusion criteria were women with other confounding factors for infertility such as tubal pathology, endocrinological disorders, previous gynecological operation, and age ≥39 years or male infertility. The study was approved by the Ethics Board of the hospital. Informed consent was obtained from all the cases enrolled in the study.
Randomization was done with envelop method and was enrolled in either study or control group. The initial dose of 50 mg of CC was given for 5 days. In stair-step protocol (SSP), patients were called on Day 11 of menstrual cycle for follicular monitoring by ultrasonography (USG). When the diameter of the largest follicle size was below 10 mm, it was considered as failure of ovulation and a higher dose of 100 mg was given from the same day for the next 5 days. An alternate day, USG was done if the size was between 11 and 16 mm. The increment was carried till maximum 150 mg of dose when no dominant follicles were seen. USG after the dose increment was done 2 days after the last dose. Injection human chorionic gonadotropin 5000 IU was given if anytime the follicles were of size >16 mm and women were called after 2 days to see USG feature for ovulation in the form of presence of free fluid and collapsed dominant follicle. Luteal phase support was given if ovulation was suggestive. When no follicular response was observed with dose till 150 mg, the patient was considered resistant to CC.
In the control group, the doses were increased in subsequent cycles after inducing progesterone withdrawal if no dominant follicle was seen. Ovulation was detected in a similar way as in the study group. The maximum dose of 150 mg was given and response noted. For each individual enrolled in the study, receiving either SSP or traditional protocol of CC (Group A and B, respectively), the time to achieve ovulation were calculated from the day 1 of menstruation cycle when she was enrolled to receive 50 mg CC (defined as cycle day 1 of the first treatment cycle) to the date of successful ovulation. Any systemic side effects were recorded, and number of women conceiving following this therapy was recorded and analyzed.
RESULTS
A total of 60 patients took ovulation induction treatment as per the methodology [Figure 1]. The demographic characteristics between the study group and the control groups were comparable [Table 1]. Among these 60 patients, 35 (58.3%) had ovulation. Twenty (66.7%) patients ovulated under SSP and 15 (50%) ovulated under traditional regimen. A mean of 13.65 days was taken to ovulate with stair-step regimen compared to 32.80 days in the traditional regimen as shown in Table 2. Totally of 18 patients ovulated with 100 mg dose equally distributed in each group. In stair-step group, among 16 patients who received 150 mg of CC, 6 (37.5%) ovulated whereas in control group of 17 women received 150 mg of CC only 2 (11.74%) ovulated. The most effective dose was 100 mg and the result with this specific dose in each group was statistically significant compared to 50 mg and 150 mg [Table 3]. This difference in time period to ovulate with study group compared to the control group was statistically significant with P < 0.003. Few systemic side effects such as nausea, gastritis, and flushing were seen in both the groups, but no additional side effects were seen on using the drug in a cumulative fashion in the SSP. Further, follow-up showed pregnancy in 8 (26.7%) women of study group and 5 (16.7%) women in control group. There was a trend for a higher ovulation rate and pregnancy rate in the SSP group compared to the traditional protocol group. Although these outcomes could not achieve statistical significance, similar results were achieved in a shorter period with no adverse effects recorded.
Figure 1.
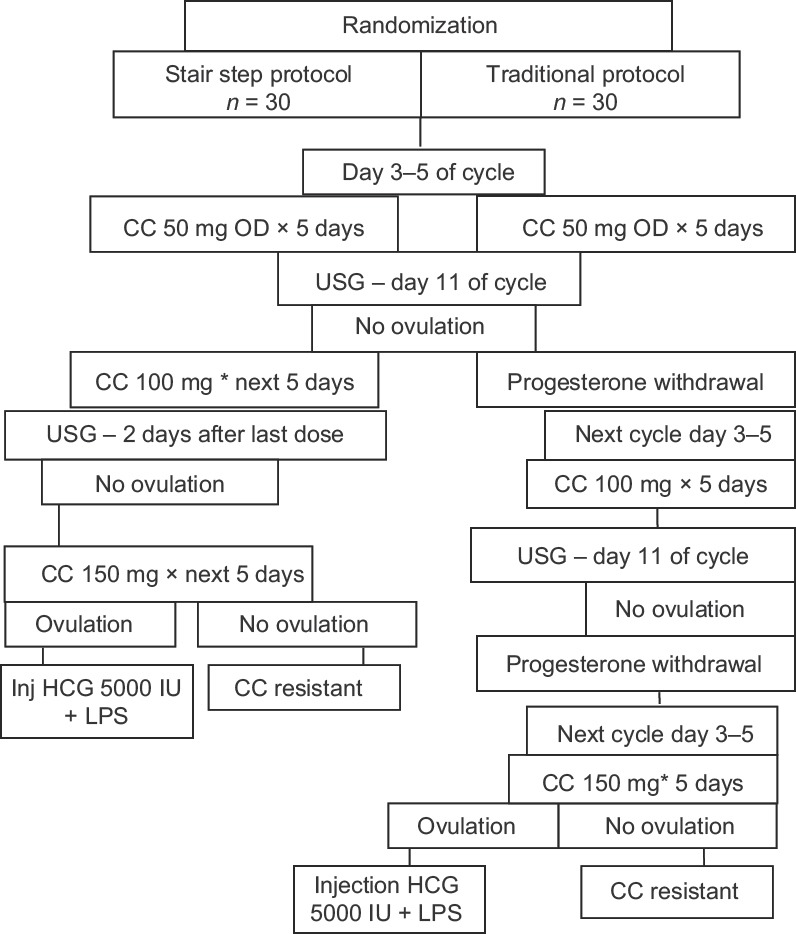
Consort diagram of treatment of the patients
Table 1.
The demographic characteristics of the women
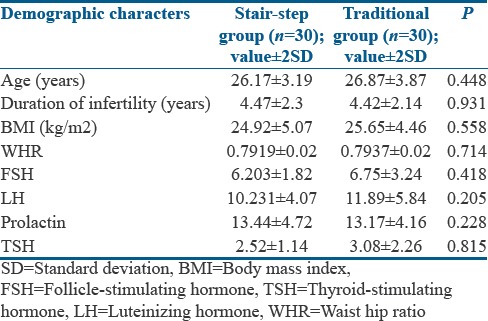
Table 2.
Comparison of the primary results
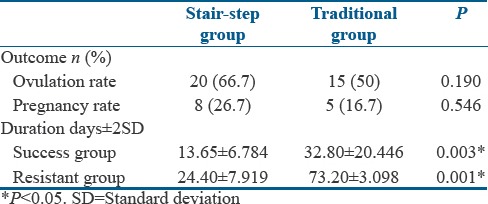
Table 3.
Ovulation number at different doses

DISCUSSION
This prospective study demonstrated that CC given in stair-step regimen shortens the period of treatment. Ovulation rate and pregnancy rate remain comparable in both the groups without any detrimental side effects in any patients. Thus, the duration of follicular phase can be prolonged without any detrimental effect on the endometrium if ovulation begins after a longer time interval with a higher dose of clomiphene. Hurst et al. in 2009[4] published pioneer work in stair-step approach of CC, where he reported a significantly higher ovulation rate of 64% (95% confidence interval, 45–81) with the SSP at 100 mg dose compared with ovulation rate of 22% with traditional regimen. Successful ovulation was achieved much earlier with SSP (23–35 days) compared with a traditional progestin-withdrawal regimen (55–88 days).
In the present study, the ovulation rate in Group A was 20 (66.7%) women out of 30 while in Group B, it was 15 (50%), successful outcome for ovulation did not attain significance when compared. Similarly, Deveci et al.[5] after performing similar study reported his results with an ovulation rate of 43.3% in the study group and 33.3% in the control group.
Discussing the pregnancy rate, 8 (26.66%) women became pregnant in the SSP group whereas in the traditional protocol group, 5 (16.66%) women had positive pregnancy test. Pregnancy rate in both the groups was comparable though this result was not statistically significant with a P = 0.546.
Hurst et al.[4] study resulted in a clinical pregnancy rate of 13% with the SSP and 15% with the traditional protocol. Study done by Deveci et al.[5] reflected similar pregnancy outcome where pregnancy rate in the SSP was 16.7% and that in the traditional protocol was 10%.
In this study, the mean duration of treatment taken for success in SSP group was 13.65 ± 6.78 days. In traditional group, this time duration for ovulation was 32.80 ± 20.44 days. The difference in the period required to attain ovulation was statistically significance (P = 0.003). Hurst et al. in his study concluded with similar results where ovulation time was shorter with the SSP (23–25 days) compared with the traditional regimen (55–88 days). The shortened time required to achieve ovulation or determine failure was a clear advantage over traditional regimen.
In present study, the duration of treatment for the women to consider as resistant to clomiphene therapy in this study was 24.40 ± 7.919 in the SSP group whereas this duration was 73.20 ± 3.098 in the traditional protocol group. This difference in time duration required to label the women as being resistant to the therapy was statistically significant (P = 0.001). These findings were also observed by the study recently published by Deveci et al. where after comparing similar study; the duration of treatment for ovulation was significantly shorter in stair step compared to traditional protocol (20.5 ± 2.0 vs. 48.6 ± 2.4 days, respectively) in his study. In conclusion, the clomiphene SSP significantly decreased the time to ovulate and for occurrence of pregnancy.
In clomiphene salt mixture, enclomiphene is more potent isomer and is responsible for its ovulation inducing actions.[6] Half-life of this potent isomer is relatively shorter and thus excreted quickly, whereas zuclomiphene persists for week duration with no clinical effects. It may be that zuclomiphene is responsible for the cumulative effect of the SSP, but its clinical significance has no proven evidence. Despite a significant increase in ovulation rate, overall pregnancy rates with CC remain low. Many reports have explained the low pregnancy rates with antiestrogenic effect of clomiphene and its metabolites on cervical mucus, endometrial, and oocytes. Further, decrease in uterine vascularity in periovulatory period and endometrial thinning may disturb implantation and cause increase in pregnancy loss.[7] Roy Homburg [8] reported that negative effect of suppressing endometrial proliferation using CC was unrelated to dose or duration of treatment but apparently sporadic. In the present study, endometrial thickness was recorded on USG during ovulation evaluation posttherapy. The mean endometrial thickness was between 7 and 9 mm in either group of patients who ovulated. Pregnancies were also noted at an endometrium thickness of 7–9 mm at 100 mg dose.
In a retrospective study by Budinetz in 2015,[9] ovulation rate and cycle characteristics were studied in patients who had previously been ovulatory after a stair-step ovulation induction. He summarized that women ovulating with stair-step regimen will again ovulate after taking the previously ovulatory CC dose in a subsequent cycle. Those who do not ovulate are likely to ovulate with a further increase in CC dose. Many patients desire a more active and aggressive management and get very distressed by having to await menses or have their menses induced. They are at higher risk for depressive illnesses, anxiety symptoms, and social phobias.[10,11] For many patients, the SSP might be a good option, since women are more likely to drop out of therapy if they are anovulatory with treatment as their frustration increases.[12]
CONCLUSIONS
The results from this study reflected the effectiveness of clomiphene when being used as stair-step regimen. This regimen improves the ovulation rate and pregnancy rate without any detrimental side effects compared to traditional regimen. It helps to know the sensitivity and resistance of an individual to CC much earlier and helps to plan ahead with alternative treatment for desired outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181. [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 3.Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89:505–22. doi: 10.1016/j.fertnstert.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Hurst BS, Hickman JM, Matthews ML, Usadi RS, Marshburn PB. Novel clomiphene “stair-step” protocol reduces time to ovulation in women with polycystic ovarian syndrome. 2009;200:510. Am J Obstet Gynecol. 2009;200:510.e1–4. doi: 10.1016/j.ajog.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Deveci CD, Demir B, Sengul O, Dilbaz B, Goktolga U. Clomiphene citrate 'stair-step' protocol vs. Traditional protocol in patients with polycystic ovary syndrome: A randomized controlled trial. Arch Gynecol Obstet. 2015;291:179–84. doi: 10.1007/s00404-014-3398-y. [DOI] [PubMed] [Google Scholar]
- 6.Dickey RP, Holtkamp DE. Development, pharmacology and clinical experience with clomiphene citrate. Hum Reprod Update. 1996;2:483–506. doi: 10.1093/humupd/2.6.483. [DOI] [PubMed] [Google Scholar]
- 7.Nakai A, Yokota A, Koshino T, Araki T. Assessment of endometrial perfusion with Doppler ultrasound in spontaneous and stimulated menstrual cycles. J Nippon Med Sch. 2002;69:328–32. doi: 10.1272/jnms.69.328. [DOI] [PubMed] [Google Scholar]
- 8.Homburg R. Clomiphene citrate – End of an era? A mini-review. Hum Reprod. 2005;20:2043–51. doi: 10.1093/humrep/dei042. [DOI] [PubMed] [Google Scholar]
- 9.Budinetz TH, Benadiva CA, Griffin DW, Engmann LL, Nulsen JC, DiLuigi AJ, et al. Ovulation rate and cycle characteristics in a subsequent clomiphene citrate cycle after stair-step protocol. Fertil Steril. 2015;103:675–9. doi: 10.1016/j.fertnstert.2014.12.088. [DOI] [PubMed] [Google Scholar]
- 10.Hollinrake E, Abreu A, Maifeld M, Van Voorhis BJ, Dokras A. Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil Steril. 2007;87:1369–76. doi: 10.1016/j.fertnstert.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: Systematic review and meta-analysis. Fertil Steril. 2012;97:225–3000. doi: 10.1016/j.fertnstert.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Verhaak CM, Smeenk JM, Evers AW, van Minnen A, Kremer JA, Kraaimaat FW, et al. Predicting emotional response to unsuccessful fertility treatment: A prospective study. J Behav Med. 2005;28:181–90. doi: 10.1007/s10865-005-3667-0. [DOI] [PubMed] [Google Scholar]


