Abstract
Aims:
This study aims to measure the quality of life (QOL), treatment satisfaction, and tolerability of antidiabetic drugs in patients suffering from type 2 diabetes mellitus (DM).
Methods:
The prospective, observational study was conducted in consenting patients of type 2 DM attending the outpatient department of a tertiary care hospital in Western India. The QOL instrument for Indian diabetes (QOLID) patients questionnaire and the Diabetes Treatment Satisfaction Questionnaire were administered to all patients at baseline, 3 months, and 6 months of treatment. Tukey–Kramer comparison test was used to analyze the difference in QOLID scores in various domains at baseline, 3 months, and 6 months. WHO-UMC scale, Naranjo's probability scale, Hartwig and Siegel, and Schumock and Thornton modified criteria were used to analyze the adverse drug reactions.
Results:
A male preponderance was observed in 200 patients enrolled in the study. The mean duration of diabetes was 10.96 ± 5.99 years. The patients received metformin alone (40), metformin and glipizide (47), metformin, glipizide and other oral hypoglycemic agents (OHAs) (78), and OHAs and insulin (35). A significant improvement in fasting and postprandial blood sugar was observed at 6 months as compared to the baseline (P < 0.05). A total of 39 (19.5%) patients suffered from adverse effects to metformin and insulin. Physical health and physical endurance improved in patients receiving metformin alone or in combination with glipizide as compared to patients receiving other OHAs and/or insulin. Treatment satisfaction, highest in patients receiving metformin and least in those receiving insulin, was unaltered during the study period.
Conclusions:
While polypharmacy is evident, using lesser medicines offers better treatment satisfaction and QOL in DM. Periodic assessment of QOL and treatment satisfaction are recommended in DM.
Keywords: Diabetes mellitus, insulin, metformin, oral hypoglycemic agents, Quality of Life Instrument for Indian Diabetes, treatment satisfaction
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism resulting from defects in insulin secretion, insulin action, or both.[1] The worldwide prevalence of DM has risen dramatically from an estimated 30 million cases in 1985 to 177 million in 2000.[2] In India, an estimated 40 million people suffered from diabetes in 2007 and is expected to rise to 70 million by 2025.[3] The quality of life (QOL) as “an individual's perception of their position in life in context of culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns. Uncontrolled or poorly controlled diabetes affects organ functions badly, which ultimately affects the patient's QOL.” A patient's view of his/her QOL is important for evaluating and understanding treatment effects from the patient's perspective and for improving future care.[4] Measurement of QOL is considered vital for the care of diabetic patients. These measures have been used to guide and evaluate treatment interventions.[5] Treatment satisfaction may play an important role in adherence to the anti-diabetic treatment. Various factors such as route of drug administration, cost of therapy, and time spent on managing the illness affect treatment satisfaction in patients suffering from DM. This study aims to evaluate the impact of drug treatment on QOL of patients and measure the treatment satisfaction and tolerability of commonly used antidiabetic drugs in patients suffering from DM.
METHODS
This was a prospective, observational study conducted at the diabetes outpatient department (OPD) in Department of Medicine, Civil Hospital, Ahmedabad, a tertiary care hospital in India. Prior permission to conduct the study was obtained from Institutional Ethics Committee. The incidence and prevalence of DM in Indian population was considered for calculation of sample size. All patients diagnosed with type 2 DM (treatment naïve or otherwise) attending the OPD, who consented to participate, were included in the study. Illiterate patients and patients who had difficulty in comprehending the questionnaire were excluded from the study.
Patients were initially assessed by the clinician. During the first visit, a detailed history, demographic characteristics, and details of the disease such as presenting complaints, treatment history, and relevant personal and family history were recorded in a prevalidated case record form. Details of general and systemic examination were recorded. Relevant investigations such as blood sugar, glycosylated hemoglobin (HbA1c), complete blood count, blood pressure, heart rate, and respiratory rate were performed in each case at predetermined intervals. QOL was measured using QOL Instrument for Indian Diabetes (QOLID) patients questionnaire.[6] The treatment satisfaction of antidiabetic drugs was assessed using Diabetes Treatment Satisfaction questionnaire (DTSQ).[7] Both the questionnaires were translated by language experts, validated, and administered in vernacular language. Each patient was evaluated at 3rd and 6th month of enrollment. DTSQ and QOLID were administered in all patients at baseline, 3 months, and 6 months. The data were recorded in Microsoft Excel Worksheet version 2007. Tukey–Kramer comparison test using the GraphPad demo version software (GraphPad Software, Inc. (USA) was used to analyze the difference in QOLID scores in various domains at baseline, 3 months, and 6 months. A value of q > 3.436 (P < 0.05) was considered statistically significant. To compare the scores between two treatments or within treatment groups, unpaired or paired t-test was used as appropriate. P < 0.05 was considered statistically significant. Analysis of the suspected adverse drug reactions (ADRs) and their causality assessment was done by WHO-UMC and Naranjo's probability score.[8] The severity and preventability of ADRs were assessed by scales described by Hartwig and Siegel and modified Schumock and Thornton criteria, respectively.[9,10]
RESULTS
Two hundred patients were enrolled in the study, with a male preponderance. The mean duration of diabetes was 10.96 ± 5.99 years. The age of patients ranged from 34 to 65 years (mean age: 52.17 ± 8.23 years). The mean weight was 64.04 ± 6.17 kg and mean body mass index (BMI) was 23.75 ± 2.11 kg/m2. All patients were literate with the majority (81%) having completed their graduation. The majority of patients (75.5%) had a sedentary occupation and belonged to the lower socioeconomic group (73%) with an annual income of 40,000–80,000 INR.
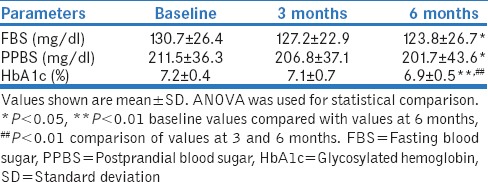
The majority of patients were asymptomatic (68%). Common presenting complaints were polyuria (29%), polydipsia (26.5%), insomnia (17.5%), polyphagia (12%), vision problems (9.5%), neuropathic pain (9%), and other complaints such as fatigue and lack of energy (6%). Diabetic complications were reported in 67 (33.5%) patients. A nonsignificant reduction in the number of patients presenting with these symptoms was observed at 3 and 6 months as compared to baseline (P > 0.05). A significant improvement in fasting blood sugar (FBS) and postprandial blood sugar (PPBS) was observed at 6-month follow-up as compared to the baseline (P < 0.05). The improvement in FBS and PPBS at 3 months was statistically nonsignificant compared to baseline. The HbA1c levels were significantly improved at 6 months as compared to baseline (P < 0.001) [Table 1].
Table 1.
Biochemical evaluation of patients suffering from type 2 diabetes mellitus (n=200)
Drug therapy
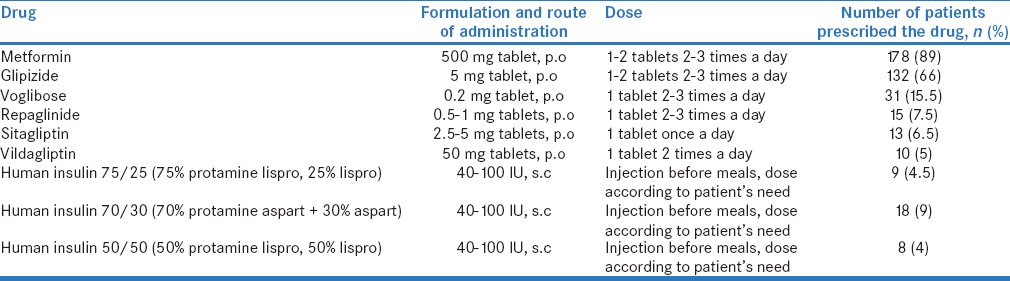
Of the 200 patients, 40 (20%) received metformin alone, 47 (23.5%) patients received metformin and glipizide, 78 (39%) patients received metformin, glipizide, and other oral hypoglycemic agents (OHAs) (sitagliptin, voglibose, etc.), and 35 (17.5%) patients received OHAs and insulin [Table 2]. A total of 414 drugs were prescribed, of which 58 (14%) were prescribed as fixed-dose combinations, and 199 (48%) were prescribed by brand names. Eighty-six (43%) patients received concomitant medicines for hypertension (HT), coronary artery disease, hyperlipidemia, and/or diabetic neuropathy. A total of 39 (19.5%) patients suffered from adverse effects such as gastritis (10), diarrhea (9), hypoglycemia (5), abdominal pain (3), nausea (3), flatulence (3), headache (2), fatigue (2), vomiting (1), and pharyngitis (1). The causal drugs were metformin (13), insulin (9), glipizide (8), voglibose (7), and sitagliptin (2).
Table 2.
Antidiabetic drugs used to treat type 2 diabetes mellitus in the study population (n=200)
Quality of life
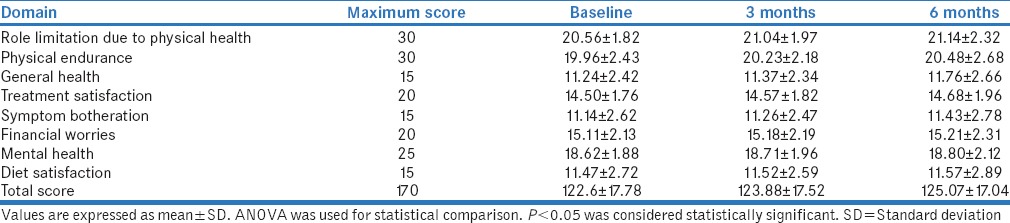
It was observed that the QOL parameters improved at 3 and 6 months as compared to baseline, but the difference was statistically nonsignificant. Further analysis of QOL in relation to drug treatment was carried out [Table 3].
Table 3.
Mean Quality of Life Instrument for Indian Diabetes scores of type 2 diabetes mellitus patients (n=200)
Impact of drug treatment on quality of life parameters
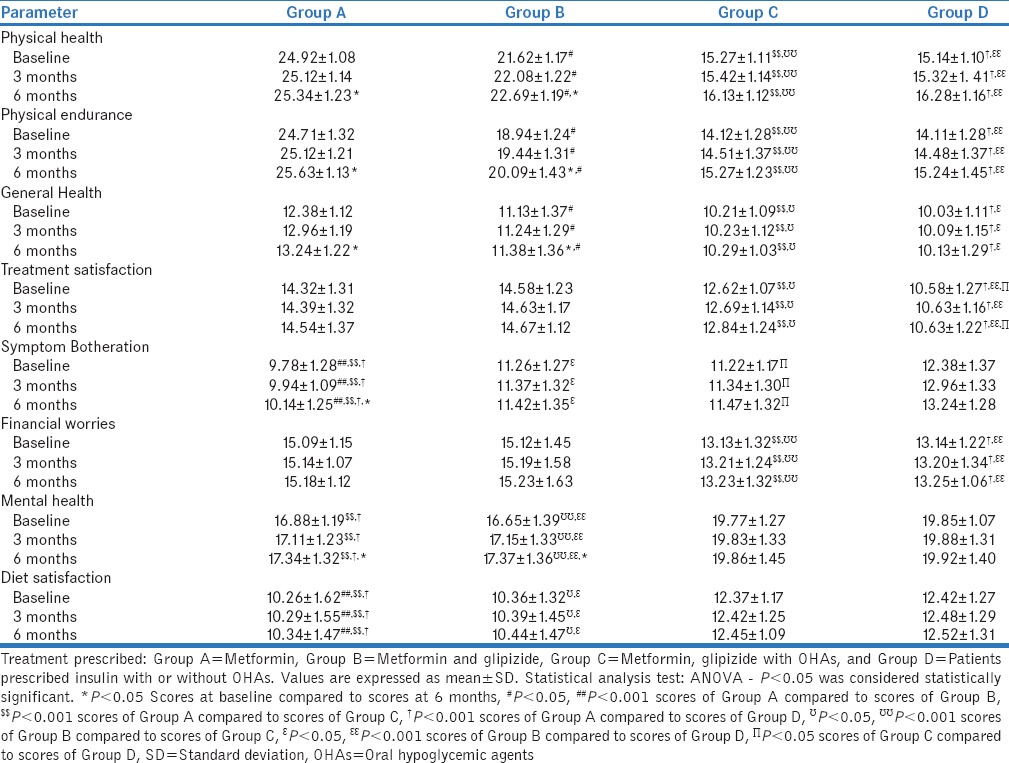
Patients were divided into four groups based on drug treatment they received, namely: Group A: metformin alone (n = 40), Group B: metformin and glipizide (n = 47), Group C: metformin, glipizide with other OHAs (n = 78), and Group D: insulin with or without OHAs (n = 35). An intergroup comparison was carried out at baseline, 3 months, and 6 months. Their impact on various QOL parameters is shown in Table 4.
Table 4.
Impact of drug treatment on quality of life parameters in patients of type 2 diabetes mellitus (n=200)
Role limitation due to physical health
The mean scores of Group A (metformin) and B (metformin and glipizide) were significantly higher than C (metformin, glipizide with other OHAs) and D (insulin with or without OHAs) (P < 0.001) at baseline, 3 months, and 6 months. Moreover, the score of Group A was found to be significantly higher than Group B at respective periods (P < 0.005).
Physical endurance
The mean scores of Groups A and B at baseline, 3 months, and 6 months were significantly higher than those of Group C (P < 0.001) and Group D (P < 0.001) at similar periods. The scores of Group A were found to be significantly higher than scores of Group B (P < 0.05) at respective periods. No significant difference was observed between scores of Group C and Group D at respective periods (P > 0.05). Intragroup comparison of mean scores at baseline, 3 months, and 6 months showed that the scores improved at 6 months as compared to baseline (P < 0.05), while no significant difference was observed in scores at 3 months than at baseline (P > 0.05) in Groups A and B.
General health
The mean scores of Group A at baseline, 3 months and 6 months were significantly higher than those of Groups B (P < 0.05), C (P < 0.001), and D (P < 0.001) at similar periods. The scores of Group B were significantly higher than mean scores of Group C (P < 0.05) and Group D (P < 0.05) at respective visits. No significant difference was observed between scores of Group C and Group D at respective visits (P > 0.05). Intragroup comparison of mean scores at baseline, 3 months, and 6 months showed that the scores improved at 6 months as compared to baseline (P < 0.05), while no significant difference was observed in scores at 3 months as compared to baseline scores (P > 0.05) in Groups A and B.
Treatment satisfaction
The mean scores of Groups A and B at baseline, 3 months, and 6 months were significantly higher than those of Groups C (P < 0.001) and D (P < 0.001) at similar periods, respectively. No significant difference was observed between scores of Groups A and B and those of Groups C and D at respective visits (P > 0.05). Intragroup comparison of mean scores showed no significant difference in all the groups (P > 0.05).
Symptom botheration
The mean scores of Group D at baseline, 3 months, and 6 months were significantly higher than those of Groups A (P < 0.001), B (P < 0.05), and C (P < 0.05) at similar periods. The mean scores of Group B and Group C were also significantly higher than mean scores of Group A (P < 0.001) at respective visits. No significant difference was observed between scores of Group B and Group C. Intragroup comparison of mean scores at baseline, 3 months, and 6 months showed that the scores improved at 6 months as compared to baseline (P < 0.05), while no significant difference was observed in scores at 3 months as compared to baseline (P > 0.05) in Group A. Intragroup comparisons were found to be statistically nonsignificant in Groups B, C, and D (P > 0.05) during the study period.
Financial worries
The mean scores of Group A and Group B at baseline, 3 months, and 6 months were significantly higher than those of Groups C (P < 0.001) and D (P < 0.001) at similar periods. No significant difference was observed between scores of Group A and B and between scores of Groups C and D at respective periods (P > 0.05). Intragroup comparison of mean scores showed no significant difference in all groups (P > 0.05).
Mental health
Intergroup comparison of “mental health” showed that mean scores of Groups D and C at baseline, 3 months, and 6 months were significantly higher than those of Groups A (P < 0.001) and B (P < 0.001) at similar periods, respectively. No significant difference was observed between scores of Group C and Group D and between scores of Group A and Group B (P > 0.05). Intragroup comparison of mean scores at baseline, 3 months, and 6 months showed that the scores improved at 6 months as compared to baseline (P < 0.05), while no significant difference was observed in scores at 3 months as compared to baseline (P > 0.05) in Groups A and B. Intragroup comparisons were found to be statistically nonsignificant in Groups C and D (P > 0.05).
Diet satisfaction
The mean scores of Groups D and C at baseline, 3 months, and 6 months were significantly higher than those of Groups A (P < 0.001) and B (P < 0.05) at similar periods. The mean scores of Group B were also significantly higher than scores of Group A at respective visits (P < 0.001). No significant difference was observed between scores of Group C and Group D (P > 0.05). Thus, it was inferred that diet satisfaction was same for patients receiving insulin and combination of OHAs. Intragroup comparison of mean scores showed no significant difference in all the groups (P > 0.05).
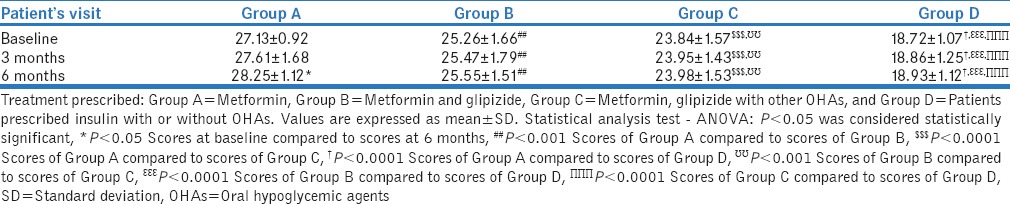
Treatment satisfaction
The scores of Group A were significantly higher than scores of Group B (P < 0.001), Group C (P < 0.0001), and Group D (P < 0.0001) at baseline, 3 months, and 6 months at similar periods. Scores of Group B were found to be significantly higher than scores of Group C (P < 0.001) and Group D (P < 0.0001) at respective visits. Scores of Group C were found to be higher than scores of Group D at respective visits (P < 0.0001). Intragroup comparison of mean scores of DTSQ between baseline and 6 months score was found significant in Group A (P < 0.05), while it was not significant between baseline and 3 months (P > 0.05) [Table 5].
Table 5.
Treatment satisfaction in patients with diabetes mellitus
DISCUSSION
A mean age of patients with DM above 50 years, like in this study, has been observed in studies conducted in developed countries by various researchers[11,12] as well as in India.[13] This is also similar to a study conducted in Africa that reported a mean age of patients with diabetes to be more than 40 years.[11] The prevalence of type 2 DM increases with age; hence, screening and intervention for detection of DM should begin early.
The mean weight of the patients was 64.04 ± 6.17 kg and mean BMI was 23.75 ± 2.11 kg/m2, which was well within the normal range. The reason for this may be that most of the patients reporting to the study site (a tertiary care public hospital) belonged to lower to middle socioeconomic class who are prone to be malnourished and underweight. Similar observations were reported in an Indian study conducted in the same settings.[14] The average duration of diabetes in this study was 10.96 ± 5.99 years which correlates well with other studies,[14,15,16] which reported an average duration of diabetes reporting to hospitals to be more than 10 years. HT was the most common concomitant disease reported in 76 (38%) patients. The association between diabetes and HT is well known. Diabetes and HT coexist in approximately 40%–60% of patients with type 2 diabetes.[17,18] Sixty-five percent patients had a relative or sibling who also suffered from DM. This represents a strong association of inheritance of DM, a finding similar to other studies,[19,20] which reported that family history of diabetes as associated with an increased prevalence of the disease.
Clinical characteristics
The most common presenting complaint in this study was polyuria, followed by polydipsia, polyphagia, vision problems, and neuropathic pain. The symptoms were assessed at baseline and at 3 and 6 months. An amelioration of symptoms was observed at the end of 3 months and 6 months, an observation similar to another Indian study.[14] The improvement in symptoms of DM is attributed to the glycemic control achieved by the antidiabetic treatment. This fact is suggested by our study, which showed good glycemic control as evidenced by an improvement in FBS, PPBS, and HbA1c values over time with treatment.
Quality of life
The QOL was assessed at the baseline and at the 3 and 6 months of treatment using QOLID.
The role limitation due to physical health was greater in patients receiving insulin with or without OHAs compared to those receiving metformin alone or in combination with other OHAs. Similar results were found in a European study.[21] Insulin is usually required in patients with long-standing uncontrolled DM. These patients are also more likely to be suffering from the complications of the disease, which affects their physical health. Physical endurance was better in patients receiving metformin alone or in combination with other OHAs as compared to patients receiving insulin with or without OHAs. The chronic and long-standing nature of the disease and associated complications determine the use of insulin with or without OHAs, and these patients are likely to exhibit lesser physical endurance. The general health was better in patients receiving metformin alone or in combination with other OHAs as compared to patients receiving insulin with or without OHAs. Similar results were found in a European study.[21] While these drugs do not affect the general health, the chronicity and severity of disease are greater in patients who require insulin with or without OHAs, and hence the observation.
Treatment satisfaction of patients receiving metformin alone or in combination with glipizide was better than that of patients receiving other OHAs and/or insulin at respective visits. Similar findings were observed in a European study.[22] Patients receiving metformin alone or in combination with glipizide spend lesser time to manage their illness, have better compliance and flexibility in dosing schedules, as compared to patients receiving other OHAs and/or insulin.[23]
Patients who received insulin with or without OHAs exhibited lesser botheration due to symptoms than patients who received metformin and/or glipizide with other OHAs. It was also less in patients receiving a combination of OHAs as compared to patients receiving metformin. Similar results were reported in the retrospective cohort study on 75 patients suffering from type 2 DM in the USA.[24] It is possible that patients receiving insulin and combination of OHAs were probably better adjusted to the disease and hence were less bothered by the symptoms of diabetes.
Patients receiving metformin alone or in combination with glipizide exhibited lesser financial worries than the patients who received insulin with or without OHAs. Metformin and glipizide were dispensed free of cost from the hospital while patients had to purchase newer OHAs such as voglibose and sitagliptin. Similar results were observed in an Asian study.[25] Financial worries are determined by multiple variables and factors including the annual income, family size, expenditure on medicines, and medical insurance; hence, these findings warrant further evaluation.
Patients who received insulin with or without OHAs reported better mental health than patients who received metformin, glipizide with other OHAs. Furthermore, mental health was better in patients receiving combination of OHAs than patients receiving metformin alone or in combination with glipizide. However, an American study on 58 type 2 DM patients to assess treatment satisfaction found no significant difference in mental health scores of patients receiving insulin as compared to patients receiving OHAs.[26] Diet satisfaction was better in patients receiving insulin with or without OHAs. It was also better in patients receiving combination of OHAs as compared to patients receiving metformin alone or in combination with glipizide. However, the Asian study reported higher diet satisfaction in patients receiving OHAs.[27]
Treatment satisfaction
The DTSQ scores of patient receiving metformin alone were significantly better than patients receiving metformin and glipizide, metformin, glipizide with other OHAs, and those receiving insulin with or without OHAs suggesting that patients who received metformin alone had better treatment satisfaction than who received one or more drugs. Similar results were obtained in a European study.[28] Patients receiving more than one drug are likely to suffer from severe or uncontrolled DM or complications of the disease. Furthermore, lesser number of drugs means lesser side effects and better compliance.[29] It was also observed that treatment satisfaction in patients receiving metformin, glipizide with other OHAs was significantly better than patients receiving insulin with or without OHAs. A multicentric study carried out in Austria, France, India, Belgium, Mexico, and the United States in patients suffering from type 2 DM using DTSQ observed that patients receiving insulin had lesser treatment satisfaction compared to patients receiving OHAs.[30] Patients receiving insulin have reported difficulty and inconvenience in self-administration of the drug with respect to injecting the drug themselves and also maintaining the schedules with respect to the diet to prevent insulin-induced hypoglycemia. These patients are also prone to frequent adverse events such as hypoglycemia and lipodystrophy. Treatment satisfaction improved over time in patients receiving metformin alone.
Adverse drug reactions
A total of 39 ADRs were reported during this study. A greater percentage of patients receiving insulin suffered from ADRs as compared to patients receiving OHAs which is in congruence with the known characteristics of these drugs. However, a study carried out in Texas, USA, on patients of type 2 DM to compare treatment satisfaction and adverse events caused by insulin and OHAs found no significant difference in the ADRs caused by insulin and OHAs.[26]
Hence, better QOL was observed in patients receiving single- or two-drug regimens as compared to patients receiving combination regimen of OHAs and insulin. Complex drug regimens and multidrug therapies must be avoided unless the severity or complications of the disease warrant otherwise. Periodic QOL and treatment satisfaction assessments are recommended.
Our preliminary exploratory study has certain limitations that include a small sample drawn (especially in subgroups receiving treatment other than OHAs) from a single public health facility. Newer OHAs are not frequently prescribed in our public health facility hence were not evaluated in the present study. However, this is one of the few studies in India that evaluates QOL in patients of type 2 DM and links prescribed treatment with QOL and treatment satisfaction.
CONCLUSIONS
Therapeutic outcomes are not measured by laboratory values and glycemic control alone but also by improved QOL. The findings of this study may direct future research on QOL and treatment satisfaction in DM. Further studies in a larger sample of patients receiving OHA other than metformin and glipizide are warranted to make definitive conclusions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Diabetes Action Online. Geneva: World Health Organization; 1999. [Last cited on 2015 Jan 11]. Available from: http://www.who.int/diabetes/collaboratingcentres/en/ [Google Scholar]
- 2.Fauci AS, Kasper DL, Braunwald E, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrrison's Principles of Internal Medicine. 17th ed. New York: McGraw-Hills; 2009. [Google Scholar]
- 3.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 4.Carr AJ, Higginson IJ. Are quality of life measures patient centred? BMJ. 2001;322:1357–60. doi: 10.1136/bmj.322.7298.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghanbari A, Yekta P, AtrkarRoushan Z. Determine of the pattern of effective factors on quality of life in diabetic patients. J Guilan Univ Med Sci. 2001;10:82–9. [Google Scholar]
- 6.Nagpal J, Kumar A, Kakar S, Bhartia A. The development of Quality of Life Instrument for Indian Diabetes patients (QOLID): A validation and reliability study in middle and higher income groups. J Assoc Physicians India. 2010;58:295–304. [PubMed] [Google Scholar]
- 7.Bradley C, editor. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Chur, Switzerland: Harwood Academic Publishers; 1994. The diabetes treatment satisfaction questionnaire: DTSQ. [Google Scholar]
- 8.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. Amethod for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 9.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 10.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- 11.Nyanzi R, Wamala R, Atuhaire LK. Diabetes and quality of life: A Ugandan perspective. J Diabetes Res. 2014;20:76–86. doi: 10.1155/2014/402012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ, 3rd, O'Brien PC. Risk factors for severity of diabetic polyneuropathy: Intensive longitudinal assessment of the Rochester diabetic neuropathy study cohort. Diabetes Care. 1999;22:1479–86. doi: 10.2337/diacare.22.9.1479. [DOI] [PubMed] [Google Scholar]
- 13.Dutta A, Naorem S, Singh TP, Wangjam K. Prevalence of peripheral neuropathy in newly diagnosed type 2 diabetes. Int J Diabetes Dev Ctries. 2005;25:30–3. [Google Scholar]
- 14.Patel N, Mishra V, Patel P, Dikshit RK. A study of the use of carbamazepine, pregabalin and alpha lipoic acid in patients of diabetic neuropathy. J Diabetes Metab Disord. 2014;13:62. doi: 10.1186/2251-6581-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janghorbani M, Rezvanian H, Kachooei A, Ghorbani A, Chitsaz A, Izadi F, et al. Peripheral neuropathy in type 2 diabetes mellitus in Isfahan, Iran: Prevalence and risk factors. Acta Neurol Scand. 2006;114:384–91. doi: 10.1111/j.1600-0404.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 16.Pradeepa R, Rema M, Vignesh J, Deepa M, Deepa R, Mohan V. Prevalence and risk factors for diabetic neuropathy in an urban South Indian population: The Chennai Urban Rural Epidemiology Study (CURES-55) Diabet Med. 2008;25:407–12. doi: 10.1111/j.1464-5491.2008.02397.x. [DOI] [PubMed] [Google Scholar]
- 17.Kirpichnikov D, Sowers JR. Diabetes mellitus and diabetes-associated vascular disease. Trends Endocrinol Metab. 2001;12:225–30. doi: 10.1016/s1043-2760(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 18.Arauz-Pacheco C, Parrott MA, Raskin P. The treatment of hypertension in adult patients with diabetes. Diabetes Care. 2002;25:134–47. doi: 10.2337/diacare.25.1.134. [DOI] [PubMed] [Google Scholar]
- 19.Valdez R, Yoon PW, Liu T, Khoury MJ. Family history and prevalence of diabetes in the U.S. population: The 6-year results from the National Health and Nutrition Examination Survey (1999-2004) Diabetes Care. 2007;30:2517, 22. doi: 10.2337/dc07-0720. [DOI] [PubMed] [Google Scholar]
- 20.Papazafiropoulou A, Sotiropoulos A, Skliros E, Kardara M, Kokolaki A, Apostolou O, et al. Familial history of diabetes and clinical characteristics in Greek subjects with type 2 diabetes. BMC Endocr Disord. 2009;9:12. doi: 10.1186/1472-6823-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fal AM, Jankowska B, Uchmanowicz I, Sen M, Panaszek B, Polanski J. Type 2 diabetes quality of life patients treated with insulin and oral hypoglycemic medication. Acta Diabetol. 2011;48:237–42. doi: 10.1007/s00592-010-0244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart HE, Redekop WK, Bilo HJ, Meyboom-de Jong B, Berg M. Health related quality of life in patients with type I diabetes mellitus: Generic and disease-specific measurement. Indian J Med Res. 2007;125:203–16. [PubMed] [Google Scholar]
- 23.Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. doi: 10.2147/PPA.S24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna A, Bush AL, Swint JM, Peskin MF, Street RL, Jr, Naik AD. Hemoglobin A1c improvements and better diabetes-specific quality of life among participants completing diabetes self-management programs: A nested cohort study. Health Qual Life Outcomes. 2012;10:48. doi: 10.1186/1477-7525-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andayani TM, Ibrahim MI, Asdie HA. The association of diabetes related factor and quality of life in type 2 diabetes mellitus. Int J Pharm Sci. 2010;2:139–45. [Google Scholar]
- 26.Lingvay I, Legendre JL, Kaloyanova PF, Zhang S, Adams-Huet B, Raskin P. Insulin-based versus triple oral therapy for newly diagnosed type 2 diabetes: Which is better? Diabetes Care. 2009;32:1789–95. doi: 10.2337/dc09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Byati AI, Atheem MA. Quality of life and diet satisfaction in type II diabetes. Food Sci Qual Manage. 2014:18–35. [Google Scholar]
- 28.Schmidt WE, Christiansen JS, Hammer M, Zychma MJ, Buse JB. Patient-reported outcomes are superior in patients with type 2 diabetes treated with liraglutide as compared with exenatide, when added to metformin, sulphonylurea or both: Results from a randomized, open-label study. Diabet Med. 2011;28:715–23. doi: 10.1111/j.1464-5491.2011.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamis L, Hyer S, Shehata H. Metformin effects on treatment satisfaction and quality of life in gestational diabetes. Br J Diabetes Vasc Dis. 2013;13:178–82. [Google Scholar]
- 30.Naegeli AN, Hayes RP. Expectations about and experiences with insulin therapy contribute to diabetes treatment satisfaction in insulin-naïve patients with type 2 diabetes. Int J Clin Pract. 2010;64:908–16. doi: 10.1111/j.1742-1241.2010.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


