Abstract
Aim:
Drug–drug interactions (DDIs) are one of the major but preventable cause of adverse drug reaction. Study of prevalence and prediction of DDIs will make the physician easier to provide better patient care and mitigate patient's harm. Hence, the study was planned to evaluate the potential DDIs among medication prescribed to hypertensive patients in our hospital.
Materials and Methods:
A prospective, cross-sectional study was conducted among the hypertensive patients in medicine (outpatient/inpatient) department over the period of three months in a tertiary care hospital. Adult hypertensive patients of either sex with comorbidities were included in the study. The prescriptions were collected and analyzed for DDI using Medscape interaction checker. Data were analyzed using SPSS (version 16.0) software and expressed in percentage. Pearson's correlation and regression analysis were done.
Results:
Among 125 patients, 48% were exposed to at least one DDI. Totally 123 DDI were identified and majority of them were significant (85.36%). No serious interactions were identified. Pharmacodynamic and pharmacokinetic drug interactions were found to be 37.39% and 28.76%, respectively. Logistic regression analysis showed advanced male gender and polypharmacy was associated with increased risk of DDI. About 51 interacting pairs of DDI were identified and most frequently occurring pair was amlodipine with atenolol. Aspirin was found to have commonly involved in DDI with enalapril, atenolol, frusemide, spironolactone, carvedilol, and metoprolol.
Conclusion:
The study highlighted that patients with hypertension are particularly vulnerable to DDI. The comorbidities, advanced age, and polypharmacy are the important factors associated with the occurrence of DDI.
Keywords: Antihypertensive drugs, drug–drug interactions, hypertension, Medscape drug interaction checker
INTRODUCTION
Drug–drug interactions (DDIs) are the major concern among the patients receiving multidrug therapy. The World Health Organization emphasizes that adverse drug reactions and its impact can be significantly minimized by implementing careful attention to the population at risk of DDIs.[1] A drug interaction is defined as the qualitative or quantitative modification of the effect of a drug by the simultaneous or successive administration of a different one. This may result in the alteration of therapeutic effect and safety of either or both drugs. Drug interactions can be due to pharmacokinetics (alteration in the delivery of drug to its site of action) or pharmacodynamics (modification of response of drug target) interactions.[2] Since the possibilities of interaction among medications increase day by day due to concurrent use of multiple drugs and new pharmacological agents, the knowledge about the drug interaction may render the framework for prevention.[3] One review reported that DDIs cause approximately 0.054% of emergency room visits, 0.57% of hospital admissions, and 0.12% of rehospitalizations.[4] DDI contributes to 3%–4% of the adverse drug reactions and fourth leading cause of mortality.[5] Hypertension is regarded as a leading cause of cardiovascular death worldwide, and in India, cardiovascular disease is said to contribute to the largest burden of noncommunicable diseases. The prevalence of hypertension among the Indian population is 29.8%.[6] Majority of the hypertensive patients requires medication to maintain the target blood pressure, and among those, about 70% of them requires the use of two or more antihypertensive drugs.[7] Hypertensive patients are particularly vulnerable to DDIs due to age, comorbid conditions, polypharmacy, and long hospital stay.[8] Besides this, drug therapy for other comorbid conditions which may coexist or emerge as a complication of long-term hypertension, such as diabetes mellitus, congestive cardiac failure, coronary artery disease (CAD), and chronic kidney disease (CKD) also contribute for increasing risk of DDI.[9] One study conducted in Gujarat had shown that there was 83.42% potential DDIs (pDDIs) out of 350 prescriptions analyzed among all the patients visiting medicine outpatient department.[10] In this regard, recognition of a pDDI and appropriate improvement in the quality of prescription could decrease the physical and economic burden on patients and their relatives. Literature search revealed only two similar published reports done in India. one study from Rajasthan showed that hypertension-related comorbidities were associated with pDDI,[11] while another study in Telangana had evaluated that comorbidities as well as polypharmacy were related to significant DDI among hypertensive patients.[8] In this regard, due to lack of adequate antihypertensives-related DDI data in Indian population as well as in our hospital, the present study was designed with the aim to assess pattern and rate of pDDI, to identify the associated risk factors, high-risk drugs, and common interacting pairs of drugs involved among hypertensive patients in a tertiary care teaching hospital.
MATERIALS AND METHODS
This prospective, cross-sectional study was conducted among hypertensive patients in a tertiary care teaching hospital situated near Puducherry. The prescriptions were collected from hypertensive patients over the period of 3 months (December 2015–February 2016) from the medicine (outpatient/inpatient) department and analyzed for pattern and rate of pDDI, common interacting pairs, high-risk drugs, and associated risk factors. The study was commenced after getting approval from the Institutional Ethical Committee (Code No: 60/2015) and written informed consent from all the patients. Sample size was calculated using Epi Info 3.4.3 software (Centers for Disease Control and Prevention, Atlanta, USA) considering 91.1%[10] as the prevalence rate of DDI among hypertensive patients with 95% confidence interval and the estimated total sample size was 125. Adult hypertensive patient's prescriptions of both genders with and without comorbidities such as diabetes mellitus, CAD, dyslipidemia, and CKDs were included in the study. Pregnant women and hypertensive patients with acute medical conditions such as acute left ventricular failure and myocardial infarction were excluded from the study. Demographic data, comorbid conditions, diagnosis, and drugs prescribed were noted [Pro forma 1]. Each prescription was screened for pDDI using Medscape database interaction checker.[12] This software has the appropriate specificity and sensitivity to detect possible drug interactions. In Medscape drug interaction checker, drug interactions were categorized based on severity as minor (current medications can be continued), significant (close monitoring is required), and serious (suggested for alternative medication). DDIs were further classified based on the mechanism as pharmacodynamic interactions, pharmacokinetic interactions, and unspecified using Medscape drug interaction checker.[11]
Statistical analysis
Data were collected and entered in Microsoft Excel 2010 spreadsheet, and analysis was done using SPSS for Windows version 16.0 software (SPSS, Inc., Chicago, IL, USA).[13] Frequencies and percentages were used to represent gender, age group, comorbidities, number of interactions, and number of drugs used by the patients. Multivariate logistic regression analysis was done to determine the association of occurrence of pDDIs with specified risk factors including gender, age, comorbidities, and number of drugs prescribed. Pearson's correlation coefficient was used to analyze the correlation between DDI with gender and polypharmacy. P = 0.05 or less was deemed statistically significant.
RESULTS
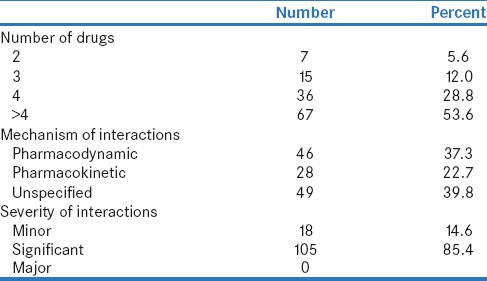
Among the 125 hypertensive patients, the same (125 prescriptions), with a total number of 650 drugs prescribed were analyzed for pDDIs using Medscape interaction checker. Among the study population, females were predominant accounting for 61.6% and 38.4% were males. More than half of the patients (56%) belonged to the age group of 40–60 years. The most frequent comorbid condition was diabetes mellitus (19.2%), CAD (5.6%), and CKD (1.6%), and the average number of comorbid conditions per patient is 0.45 ± 0.70. The average numbers of drugs per prescription were 5.02 ± 2.04 and 82.4% of patients received ≥4 drugs. Demographic details are shown in Table 1. Sixty prescriptions (48%) out of 125 had at least one pDDI, and majority of 85.4% was significant. No serious drug interactions were identified. Exactly 37.3% of DDI was due to pharmacodynamic interactions, 22.7% was pharmacokinetic, and 39.8% was unspecified. The patterns of DDI are shown in Table 2.
Table 1.
Demographic details of study the population
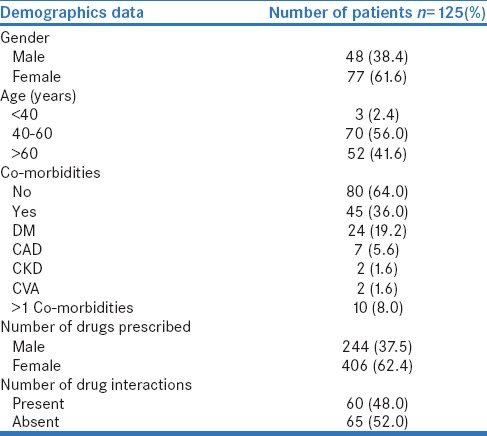
Table 2.
Pattern of potential drug-drug interactions
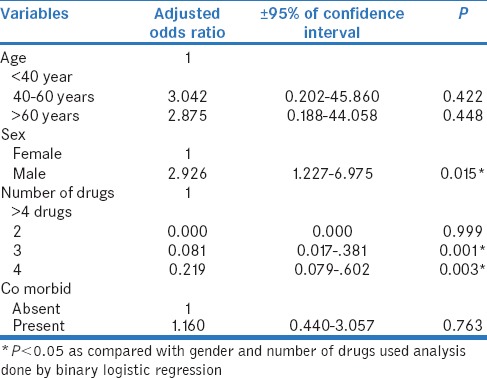
The distribution of antihypertensive drugs with other class of drugs and among the pairs used is shown in Table 3 and Table 4. Among 125 prescriptions, 123 pDDIs and 51 (40.8%) interacting drug pairs were identified. The distribution of antihypertensive drug interactions with other groups of drugs is shown in Table 5. Atenol/amlodipine combination was most commonly used in the study as this combination potentiates antihypertensive effect of each other. Among the antihypertensive drugs and other cardiovascular drugs, the most common drug interaction observed was between enalapril and aspirin. Antihypertensive drugs with high probability of causing DDI are shown in Figure 1. The multivariate logistic regression analysis showed the significant risk of DDI associated with the male gender and polypharmacy [Table 6].
Table 3.
Distribution of antihypertensive drug interaction with other class of drugs
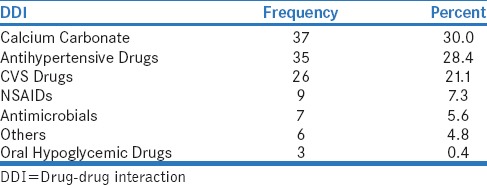
Table 4.
Distribution of potential drug interacting pairs among anti hypertensive drugs group
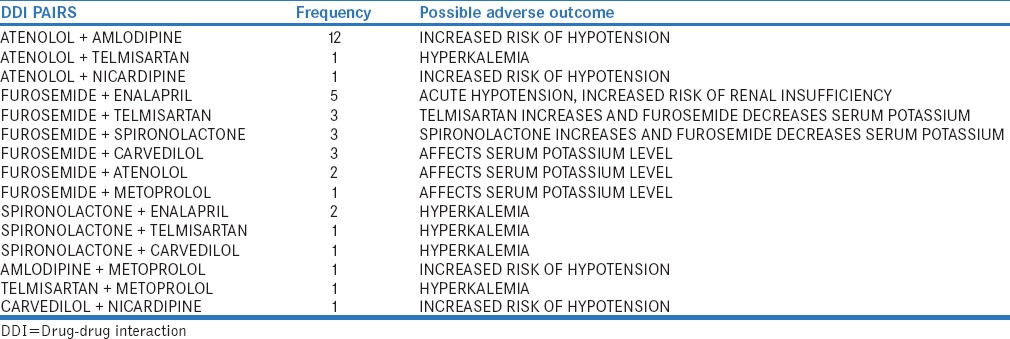
Table 5.
Distribution of potential drug interacting pairs among anti hypertensive drugs with other class of drugs
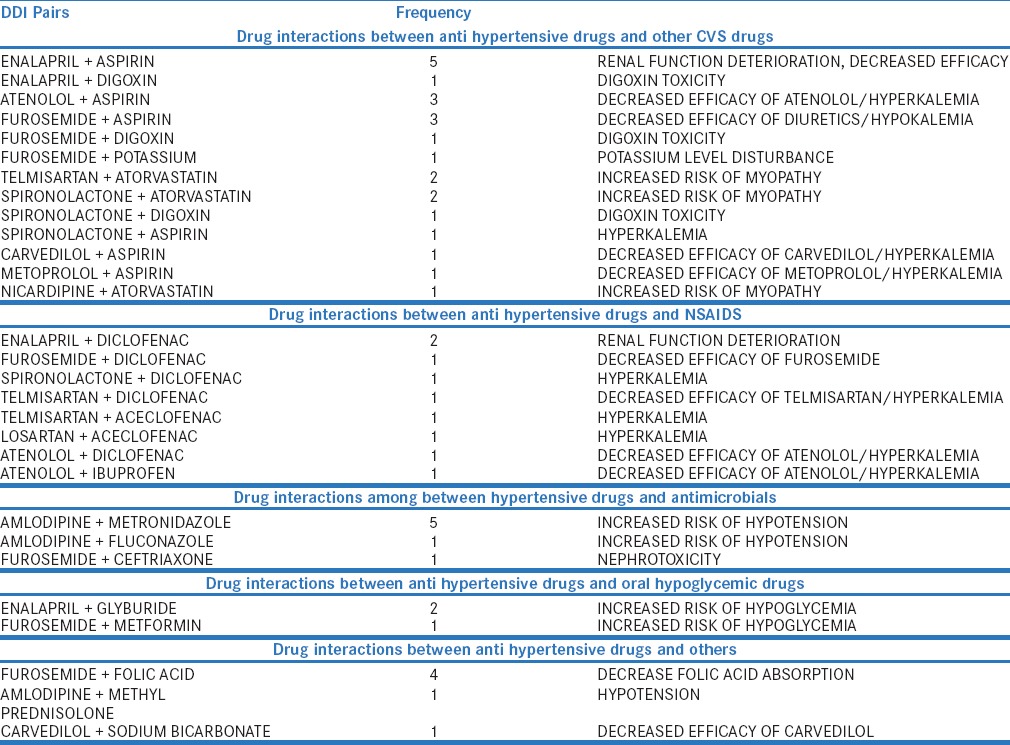
Figure 1.
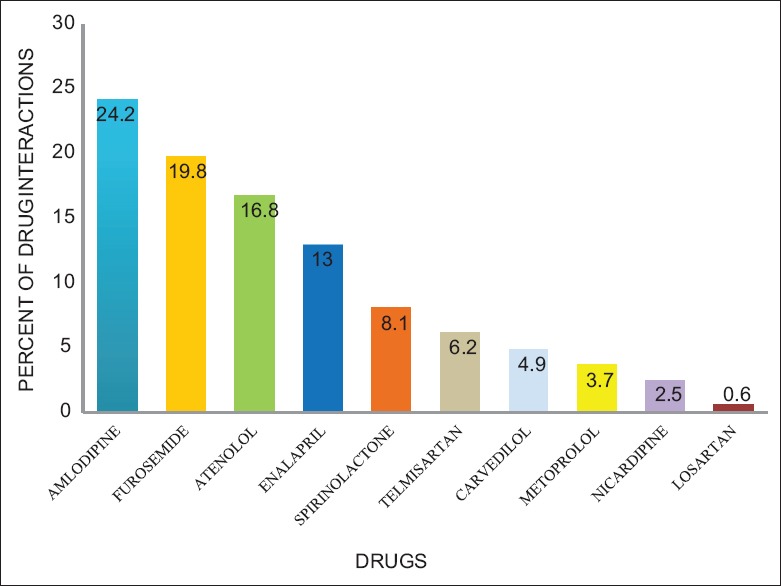
Percent of antihypertensive drugs causing drug interactions
Table 6.
Binary logistic regression analysis for factors associated with drug interactions
DISCUSSION
DDIs occur when the effect of one drug is altered by the coadministration of another drug. The alterations may result either from changes in the drug's effect independent of concentration (pharmacodynamic interaction) or from changes in the drug's concentration (pharmacokinetic interaction). In the present study, the DDIs among hypertensive patients were analyzed with the help of Medscape interaction checker.[10,11]
Among the 125 prescriptions analyzed, the majority (56%) was in the age group of 40–60 years which is similar to the study of Sivva et al.[8] Studies had shown that interactions are common in the elderly age group >60 years.[13,14] The possible reason could be due to decline in the renal and hepatic functions, additional comorbidities, and the drugs prescribed for every aliment. Based on our study, DDIs were common in male patients than compared to females which could be due to smoking, use of tobacco, alcohol, and raised triglyceride and cholesterol levels.[8]
Many studies suggested that polypharmacy contributes to considerable risk of drug interactions in hypertensive patients. Our study also showed that patients prescribed more than four drugs (53.6%) were more prone for pDDIs than the others. The average number of drugs prescribed per patient is 5.02 ± 2.04, and 48% of the patients had at least one drug interactions. A study done by Patel et al. showed a higher percentage of drug interactions (83.42%) in patients with age above 40 years, comorbidities, and polypharmacy than compared to our study (48%).[10]
Among the 123 interactions (minor [18], significant [105]), about 85.4% was significant interactions compared to 73.37% in Patel et al. study and 67.3% in the study done by Chelkeba et al.[10,13] In addition, in the present study, minor interaction was only 14.6%, compared to 22.9% with Patel et al. study and 3.1% with Chelkeba et al. study[10,13] Surprisingly, no serious interaction was found in our study but in other studies it was about 3.6% to 29.6%.[8,10,13] In addition, the present study also showed a significant association between the number of DDIs with male patients (r = 0.196, P = 0.028). The number of pDDIs experienced by the patients was directly proportionate to the number of medications received by them (r = 0.437, P < 0.001). In our study, most of the drug interactions are pharmacodynamic (37.3%) in nature followed by pharmacokinetic interactions (22.7%) similar to a study done by Patel et al.[10] Antihypertensive drugs, calcium supplements, other cardiovascular drugs, nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, and oral hypoglycemic drugs were frequently involved in interactions which is in accordance with the other studies.[8,13] About 30% of DDI is due to interactions between antihypertensive drugs and calcium supplements followed by 28.4% within the antihypertensive drugs.
The common interacting drug pairs among the antihypertensive drugs were atenolol/amlodipine furosemide/enalapril, furosemide/telmisartan, and furosemide/atenolol similar to Chelkeba et al. study.[13] Combination of atenolol with amlodipine has opposite effect on plasma renin activity, and dual mechanism can be beneficial in controlling hypertension. The efficacy of amlodipine could be improved by adding beta blocker (atenolol).[2] Joint National Committee VII recommended antihypertensive drug combinations such as angiotensin receptor blocker with diuretics, beta-blocker with diuretics, and angiotensin-converting enzyme (ACE) inhibitor with diuretics were used in our study. Furosemide/enalapril and furosemide/telmisartan can cause hypotension, which could be related to vasodilatation and relative intravascular volume depletion in addition to their opposing action on serum potassium levels.[15] Spironolactone/enalapril, spironolactone/telmisartan, and spironolactone/carvedilol also had caused pDDIs in our study, and these combinations also increase serum potassium levels.
Among the antihypertensives interacting with other cardiovascular drugs, aspirin was most frequently involved in DDI. Aspirin/enalapril, aspirin/atenolol, aspirin/furosemide, aspirin/spironolactone, aspirin/carvedilol, and aspirin/metoprolol were the common interactions identified. Aspirin blocks the prostaglandins production and could decrease the effectiveness of antihypertensives. A study done by Magro et al. showed that digoxin had caused more than 50% of pDDIs with furosemide and hydrochlorothiazide.[16] In our study, the combination of digoxin/spironolactone, digoxin/enalapril, and digoxin/furosemide was also the potential drug pairs causing drug interactions. Spironolactone could increase digoxin concentration by reducing its clearance.[13]
Among the interaction between the antihypertensive drugs and NSAIDs, frequent DDI was between enalapril and diclofenac. Diclofenac, a cyclooxygenase inhibitor, decreases the efficacy of enalapril by inhibiting the prostaglandin mediated vasodilating effect of ACE inhibitors and also causes an increased risk of renal function deterioration by compromising renal hemodynamics.[17]
Among interaction between antihypertensives and other class of drugs, amlodipine/fluconazole, amlodipine/metronidazole, and enalapril/glyburide were observed in this study. Fluconazole, a CYP3A enzyme inhibitor, causes inhibition of amlodipine metabolism leading to increased risk of hypotension.[18] ACE inhibitors administered along with the sulfonylureas may increase the insulin sensitivity through vasodilatation and potentiate the risk of hypoglycemia.[19]
These above results may provide baseline data that can be applied in finding the prevalence of pDDIs in patients with hypertension and identify factors associated with these interactions which can help in designing and implementing appropriate interventions, educational programs, and carrying out other related studies. The limitation of the present study was the software used in this study will provide only the potential estimate of DDI and was conducted only in one institution. Meticulous monitoring and continuous follow-up over longer duration are required to identify the pDDIs. Controlled studies were required in the future to evaluate whether precise clinical management of pDDIs can abate medication-related morbidity and mortality.
CONCLUSION
The study highlighted that patients having hypertension are particularly vulnerable to DDI. pDDIs though common in this study is comprised mainly of minor and moderate types. The comorbidities, advanced age, and polypharmacy are the important factors determining the DDI. The knowledge about the drug interaction may render the framework for prevention. Computer-based screening could add potential knowledge and help the treating physicians to determinate clinically significant interactions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. WHO Drug Information. Vol. 19. Geneva: World Health Organization; 2005. pp. 3–4. [Google Scholar]
- 2.Osterhoudt KC, Penning JC. Drug toxicity and poisoning. In: Brunton LL, Chabner AB, Knollmann CB, editors. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011. pp. 77–8.pp. 763 [Google Scholar]
- 3.Percha B, Altman RB. Informatics confronts drug-drug interactions. Trends Pharmacol Sci. 2013;34:178–84. doi: 10.1016/j.tips.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalisations and emergency department visits due to drug-drug interactions: A literature review. Pharmacoepidemiol Drug Saf. 2007;16:641–51. doi: 10.1002/pds.1351. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. Preventable Adverse Drug Reactions: A Focus on Drug Interactions. [Last accessed on 2016 Oct 30]. Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm110632.htm .
- 6.Anchala R, Kannuri NK, Pant H, Khan H, Franco OH, Di Angelantonio E, et al. Hypertension in India: A systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32:1170–7. doi: 10.1097/HJH.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang Cheung T, Man Yung Cheung B. Identifying patients with resistant hypertension and options for clinical management. Future Cardiol. 2012;8:837–46. doi: 10.2217/fca.12.66. [DOI] [PubMed] [Google Scholar]
- 8.Sivva D, Mateti UV, Neerati VM, Thiruthopu NS, Martha S. Assessment of drug-drug interactions in hypertensive patients at a superspeciality hospital. Avicenna J Med. 2015;5:29–35. doi: 10.4103/2231-0770.154194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, Jr, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: A scientific statement from the American Heart Association council for high blood pressure research and the councils on clinical cardiology and epidemiology and prevention. Circulation. 2007;115:2761–88. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 10.Patel PS, Rana DA, Suthar JV, Malhotra SD, Patel VJ. A study of potential adverse drug-drug interactions among prescribed drugs in medicine outpatient department of a tertiary care teaching hospital. J Basic Clin Pharm. 2014;5:44–8. doi: 10.4103/0976-0105.134983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kothari N, Ganguly B. Potential drug – Drug interactions among medications prescribed to hypertensive patients. J Clin Diagn Res. 2014;8:HC01–4. doi: 10.7860/JCDR/2014/10032.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drug Interaction Checker. New York: Medscape; 1994. [Last accessed on 2016 Oct 30]. Available from: http://www.reference.medscape.com/drug-interactionchecker . [Google Scholar]
- 13.Chelkeba L, Alemseged F, Bedada W. Assessment of potential drug-drug interactions among outpatients receiving cardiovascular medications at Jimma University specialized hospital, South West Ethiopia. Int J Basic Clin Pharmacol. 2013;2:144. [Google Scholar]
- 14.Nag KA, Umesh M, Churi S. Assessment of drug-drug interactions in hospitalised patients in India. Asian J Pharm Clin Res. 2011;4:62–6. [Google Scholar]
- 15.Marquito AB, Fernandes NM, Colugnati FA, de Paula RB. Identifying potential drug interactions in chronic kidney disease patients. J Bras Nefrol. 2014;36:26–34. doi: 10.5935/0101-2800.20140006. [DOI] [PubMed] [Google Scholar]
- 16.Magro L, Conforti A, Del Zotti F, Leone R, Iorio ML, Meneghelli I, et al. Identification of severe potential drug-drug interactions using an Italian general-practitioner database. Eur J Clin Pharmacol. 2008;64:303–9. doi: 10.1007/s00228-007-0394-1. [DOI] [PubMed] [Google Scholar]
- 17.Juhlin T, Björkman S, Höglund P. Cyclooxygenase inhibition causes marked impairment of renal function in elderly subjects treated with diuretics and ACE-inhibitors. Eur J Heart Fail. 2005;7:1049–56. doi: 10.1016/j.ejheart.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Kroner BA. Common drug pathways and interactions. Diabetes Spectr. 2002;15:249–55. [Google Scholar]
- 19.May M, Schindler C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther Adv Endocrinol Metab. 2016;7:69–83. doi: 10.1177/2042018816638050. [DOI] [PMC free article] [PubMed] [Google Scholar]


