Abstract
Background:
Even after rapid progress in contemporary dental practice, we encounter the failures due to endodontic, periodontal, or combined lesions. Complex anatomy of tooth and resistant microbes demands the development of new treatment strategies.
Aim:
The aim of this study is to biosynthesize silver nanoparticles (AgNPs) using fungi and determine the antibacterial efficacy against Porphyromonas gingivalis, Bacillus pumilus, and Enterococcus faecalis.
Materials and Methods:
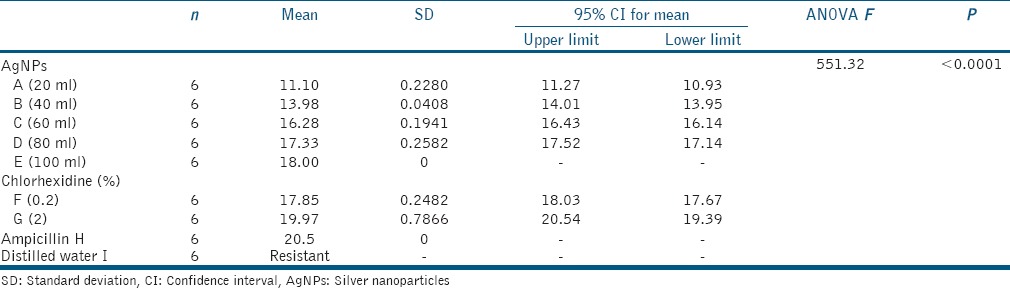
Fungi isolated from healthy leaves of Withania somnifera were used to biosynthesize AgNPs. The biosynthesized AgNPs were characterized by different methods, and antibacterial efficacy was evaluated by agar well diffusion method measuring the zone of inhibition. Test microorganisms were divided as Group 1: B. pumilus 27142 (American Type Culture Collection [ATCC]), Group 2: E. faecalis 29212 (ATCC), and Group 3: P. gingivalis 33277 (ATCC). Agents used for antibacterial efficacy were grouped as: AgNPs: A (20 μl), B (40 μl), C (60 μl), D (80 μl), E (100 μl), F (0.2% chlorhexidine [CHX]), G (2% CHX), H (Ampicillin), and I (sterile distilled water).
Results:
Characterization studies showed the color change from colorless to reddish brown color; ultraviolet spectrum showed peak at 420 nm, transmission electron microscope revealed the particles spherical in shape and 10–20 nm size. Fourier transform infrared spectroscopy analysis revealed the presence of functional groups. Data collected for antibacterial efficacy were analyzed using one-way ANOVA and post hoc Tukey's multiple shows no significant difference among three groups (P < 0.0001). AgNPs were as effective as CHX and positive control ampicillin. No zones were seen for I (distilled water).
Conclusion:
Biosynthesized AgNPs showed efficient antibacterial efficacy. Therefore, it creates a new horizon in the management of endodontic, periodontal, and combined lesions.
Keywords: Antibacterial efficacy; biosynthesized silver nanoparticles; characterization; endo-perio lesions; Enterococcus faecalis and Bacillus pumilus; fungi, Porphyromonas gingivalis
INTRODUCTION
The pulp and periodontium have embryonic, anatomic, and functional interrelationship. There are various pathways for the exchange of infectious elements and irritants from the pulp to periodontium or vice versa, leading to the development of endo-perio lesions.[1] The main etiological factors are the microorganisms. The microorganisms of endodontic and periodontal diseases are similar to some aspect. According to Zehnder,[2] all bacteria's found within root canals were present in the periodontal pocket such as Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens, Aggregatibacter actinomycetemcomitans, Cochracea, Actinomyces naeslundii, and Streptococcus sanguinis; however, periodontal pocket presents a greater variety of microorganisms which is similar to the findings of the study by Kerekes and Olsen[3] P. gingivalis is considered as an important periodontal pathogen, which is also a member of red complex and causes endodontic and periradicular infections.[4] Bacillus pumilus is seen in both root canal system and also in periodontal pockets resulting in severe marginal periodontitis.[5,6] Enterococcus faecalis is the most frequently detected species in root-filled teeth with persistent apical or lateral periodontitis.[7] According Sunde et al.[8] microbiota in the periapical tissues beyond the root apex of periapical lesions refractory to endodontic therapy consists of the microorganisms such as Staphylococcus, E. faecalis, Enterobacter, Pseudomonas, P. gingivalis, Stenotrophomonas, Sphingomonas, Bacillus, and Candida species. Hence, these lesions left untreated may communicate through the different pathways and affect both tissues leading to combined endo-perio lesions.[9]
The main objective of the treatment is to completely eliminate the bacterial infection; however, complete eradication of bacteria is not possible because of the complex anatomy of the tooth system and increase in the number of resistant strains.[9] Antibiotic resistance is often seen with long-term antibiotic therapy. Recent studies have focused on developing newer antimicrobial agents which are highly effective against microbes while being nontoxic, noninvasive, and without causing drug resistance.[10,11]
Nanotechnology has evolved as a favorable tool in medical field and dentistry using nanoparticles (NPs) for the treatment of several diseases.[12] NPs are clusters of atoms in the size range of 1–100 nm. Among the Nobel metals, silver (Ag) is considered in the field of biological system, living organisms, and medicine.[13] Silver NPs (AgNPs) can be effectively used alternative to available antimicrobial agents because of their broad spectrum of activity and biocompatibility.[14]
Biosynthesis of AgNPs is in demand due to the growing need to develop safe, cost-effective, and environment friendly technologies without causing hazards to human health and environment. The synthesis of AgNPs using fungi is most ongoing research as fungi possess several advantages; most of the fungi are easy to handle, require simple nutrients to grow, possess high wall-binding capacity, possess intracellular metal uptake capabilities, secrete higher amounts of bioactive substances, therefore, suitable for large-scale production hence, considered as naturally occurring nanofactories.[15] Different steps are included in AgNPs biosynthesis using fungi such as isolation, screening and identification of the fungi, biosynthesis and characterization of the produced AgNPs.[16]
Antimicrobial studies of biosynthesized AgNPs have been reported against human pathogens.[15,17] However, very few studies have been reported against the dental pathogens, and in particular, the pathogens involved in endodontic, periodontal, or the combined endo-perio lesions.[18,19] This article aims at producing the AgNPs using the fungi and determine the antibacterial efficacy of biosynthesized AgNPs against the endo-perio pathogens P. gingivalis, B. pumilus, and E. faecalis.
MATERIALS AND METHODS
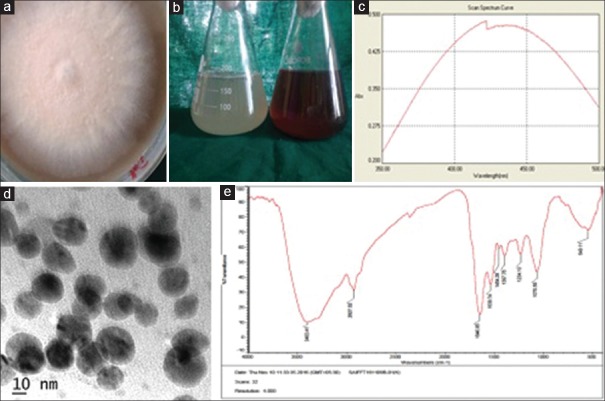
Healthy fresh leaves of Withania somnifera (Ashwagandha) were used to isolate the fungi. The leaves were gently washed with sterile distilled water several times and cut into small pieces and sterilized by immersing into 70% ethanol for 30 s, 0.01% mercuric chloride for 5 min, and sodium hypochlorite for another 3 min. Finally, rinsed with sterile distilled water and blot dried. The cut leaf segments were placed on a Potato dextrose agar supplemented with streptomycin sulfate (250 mg/ml) Petri dish and incubated at 28°C for 5–6 days. Isolated fungi were identified based on morphological and microscopic characteristics [Figure 1a].
Figure 1.
Biosynthesis and characterization of silver nanoparticles. (a) Isolated colonies of Fusarium semitectum. (b) Visible observation indicating color change. (c) Ultraviolet – Vis spectrum with peak at 420 nm. (d) Transemission electron microscope micrograph. (e) Fourier transform Infrared spectroscopy spectrum showing bands of different biomolecules
For synthesizing AgNPs, fungi cultures were inoculated in Erlenmeyer flask containing 100 ml malt glucose yeast peptone broth and incubated at 29°C for 72 h. After incubation, the fungal biomass was harvested, filtered using Whatman filter paper No. 1 and washed with sterile distilled water several times. About 25 g fungal biomass (wet weight) was then placed in flasks containing 100 ml of sterile distilled water and incubated for 48 h which was then filtered and the filtrate was used discarding the biomass. Aqueous solution of silver nitrate (AgNO3) of 1 mm concentration was then added to the fungal filtrate and kept in dark at 29°C for 24 h for formation of AgNPs.[17]
AgNPs was initially monitored by visual observation for color change of the test solution (with AgNO3), and control solution (without AgNO3) were monitored for 24 h [Figure 1b]. The AgNPs were further monitored by recording absorbance peaks at wavelengths between 200 and 600 nm using a double-beam ultraviolet (UV) – Vis spectrophotometer (T90+ UV–Vis, USA) [Figure 1c].
Transmission electron microscope (TEM) (JEOL/JEM 2100, USA) was used to determine the size and shape of AgNPs. The samples were prepared by transferring aliquot of aqueous suspension of AgNPs onto a carbon-coated copper grid and air-dried under vacuum. TEM micrographs were taken at 100 kV [Figure 1d].
Fourier transform infrared spectroscopy (FTIR) (Thermo Nicolet Avatar 370, USA) was used to determine the presence of biomolecules. About 2 mg powder of biosynthesized AgNPs mixed with 200 mg potassium bromide (KBr) (FT-IR grade), pressed into a pellet and placed into the sample holder. Spectra were scanned in the range 4000–400/cm at a resolution of 4/cm and the images were analyzed [Figure 1e].
Antibacterial efficacy of AgNPs was evaluated by agar diffusion method. Test microorganisms were divided into three groups: Group 1: B. pumilus 27142 (American Type Culture Collection [ATCC]), Group 2: E. faecalis 29212 (ATCC), and Group 3: P. gingivalis 33277 (ATCC). Agents used for antibacterial efficacy were grouped as follows: AgNPs of different concentrations: A (20 μl), B (40 μl), C (60 μl), D (80 μl), E (100 μl), and F (0.2% chlorhexidine [CHX]), G (2% CHX), H (Ampicillin), I (sterile distilled water).
B. pumilus and E. faecalis strains were subcultured from the stock cultures on trypticase soy agar (TSA) plates incubated at 35°C for 24 h. Inoculum was prepared by transferring the microbial colonies from the plates with a sterilized inoculation loop to the trypticase soya broth (TSA) and incubated for 6–7 h. P. gingivalis strains were subcultured on sterilized TSA supplemented with 5% sheep blood, hemin (5 μg/ml), vitamin K1 (0.5 μg/ml), and yeast extract (10 mg/ml). The plates were incubated at 37°C for 72 h under anaerobic atmosphere (10% CO2, 5% H2, and 85% N2). Inoculum was prepared by transferring the colonies from the plates to the supplemented TSA broth. The cultures of each strain were confirmed by Gram-staining and density of all test microorganisms was standardized equal to 0.5 McFarland constant which is equivalent to 1.5 × 108 colony-forming units/mL using a spectrophotometer. The entire surface of the TSA plates for B. pumilus and E. faecalis and supplemented TSA plate for P. gingivalis were swabbed, rotating plates approximately 60° between streaking to ensure even distribution. The wells of 5 mm diameter were prepared using a sterile punching device in the agar plates. Test solutions of different concentrations of AgNPs: A (20 μl), B (40 μl), C (60 μl), D (80 μl), E (100 μl), F (0.2% CHX), G (2% CHX), H (Ampicillin), and I (sterile distilled water) were added into the respective wells in the agar plates. The plates were incubated for 18–24 h at 37°C aerobically for B. pumilus and E. faecalis and anaerobically for P. gingivalis. The plates were read only if the lawn of growth was confluent. Thediameter of the inhibition zone was measured using theVernier calipers [Figure 2]. The experiment was carried out six times for each agent.
Figure 2.
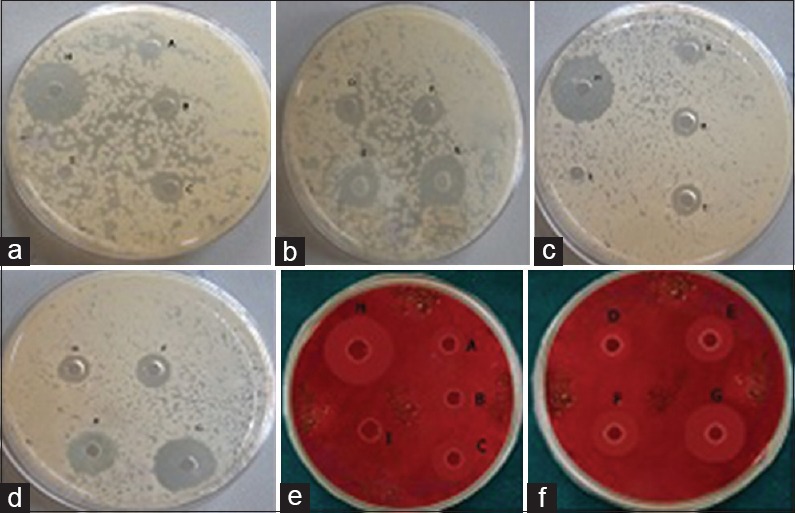
Zones of inhibition of test agents A (20 μl), B (40 μl), C (60 μl), D (80 μl), and E (100μl). Chlorhexidine – F (0.2%), G (2%), H (Ampicillin), I (Distilled water) – No zone against test microbes. (a and b): Zones of Inhibition of Bacillus pumilus. (c and d) Zones of Inhibition of Enterococcus faecalis. (e and f): Zones of inhibition of Porphyromonas gingivalis
RESULTS
The fungal isolate was identified as Fusarium semitectum [Figure 1a]. Visual observation of color change from colorless to reddish brown color was observed after completion of the reaction for the test solution (with AgNO3). The control (without AgNO3) showed no color change of the solution [Figure 1b]. Maximum absorbance peak at 420 nm after 24 h of incubation was observed by UV-Vis spectrum [Figure 1c]. TEM micrographs revealed the particles are spherical in shape, 10–20 nm in size and uniformly distributed without agglomeration [Figure 1d]. FTIR spectrum shows the presence of bands at 1646.85, 1539.74, 2927.52, 1234.10, 1454.26, 3403/cm [Figure 1e]. The bands 1646.85 and 1539.74/cm correspond to amide I and II linkages of proteins while 2927.52/cm represents the alkane linkages of proteins due to carbonyl stretch and N–H stretch vibrations. The bands at 1454.26, 1234.10 represent the CH2 and C-N stretching vibrations of aromatic and aliphatic amines and N-H stretching vibrations of the primary amines were observed at 3403/cm. These observations indicate the presence of fungal-derived biomolecules.
For evaluating the antibacterial efficacy, the collected data were statistically analyzed using one-way ANOVA and post hoc Tukey's multiple comparison test and the results are summarized in [Tables 1ߝ4 and Graphs 1ߝ3].
Table 1.
Mean, standard deviation, and one-way analysis of variance of zone of inhibition for Group I (Bacillus pumilus 27142 (ATCC))
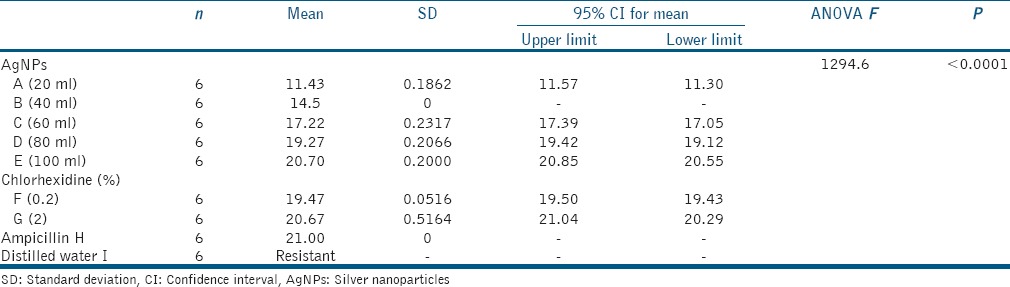
Table 4.
P value by post hoc Tukey's multiple comparison test between groups

Graph 1.

Means of zone of inhibition for Group I (Bacillus pumilus)
Graph 3.

Means of zone of inhibition for Group III (Porphyromonas gingivalis)
Table 2.
Mean, standard deviation, and one-way analysis of variance of zone of inhibition for Group II (Enterococcus faecalis 29212 (ATCC))
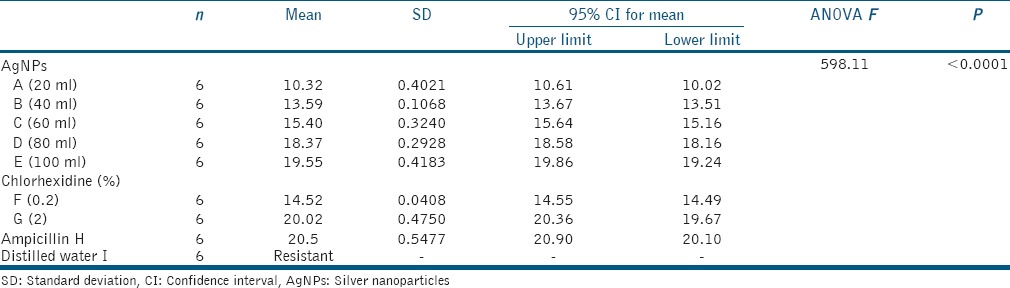
Table 3.
Mean, standard deviation, and one-way analysis of variance of zone of inhibition for Group II (Porphyromonas gingivalis 33277 (ATCC))
Graph 2.
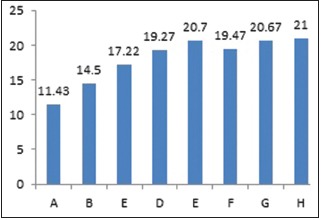
Means of zone of inhibition for Group II (Enterococcus faecalis)
DISCUSSION
Endo-perio lesions are very complex lesions with the involvement of both pulp and periodontium with several paths of communication.[1] Management of these lesions is most difficult because of the complex anatomy of the tooth system and also, increase in the number of resistant strains persisting in tissues which are difficult to eradicate with existing antimicrobial agents.[9,11] Therefore, it is extremely important to develop newer antimicrobial agents to overcome these limitations. Nanoparticles such as AgNPs have emerged as novel agents because of their unique properties. AgNPs have greater efficiency in mediating their antimicrobial activity and biocompatibility.[13,20] AgNPs acts synergistically in distinct targets, and there is no interference with antimicrobial resistance mechanisms.[2] AgNPs can penetrate the tissues owing to their extremely small size and high surface area, hence, has potential to use for resistant microbes, and therefore, offers an attractive alternative to conventional antimicrobial agents.[21]
The results of the present study show the color change from colorless to reddish-brown after addition of AgNO3 to the cell-free fungal filtrate which is the first indication of the formation of AgNPs.[15,17] The biological reaction takes place between the fungal filtrate and the silver ion solution. The silver ions were reduced in the presence of enzyme nitrate reductase secreted by the fungi leading to the formation of AgNPs stabilized by the capping peptide.[22]
Fundamental of the nanotechnology lies in the fact that properties of materials change dramatically when their size is reduced to the nanometer range (1–100 nm). Characterization techniques of AgNPs allow the better understanding of morphology, size, and dimensions of the nanoparticles.[16] The results of different characterization techniques of our study revealed the AgNPs are spherical in shape, ranged 10–20 nm in size, and also, the presence of functional groups in FTIR spectrum indicates the fungal-derived proteins act as capping agents and stabilization of AgNPs.
Most antimicrobial investigations of biosynthesized AgNPs were studied by the agar well diffusion method.[15,17] The available literature regarding the application of biosynthesized AgNPs in the management of endodontic, periodontal, and combined lesions is scarce. P. gingivalis, B. pumilus, and E. faecalis were found associated with endodontic, periodontal, and combined lesions hence used as test microbes in the present study.
CHX is the most effective antimicrobial agent used for endodontic and periodontal disinfection. Its effectiveness is attributed to its antibacterial activity and substantivity within the oral cavity. It is recommended to use 0.2%–0.12% CHX for plaque control.[23] Two percent CHX is recommended as a final root canal irrigant due to substantivity and residual effect on the root dentin and relative absence of cytotoxicity.[24] Therefore, both 0.2% and 2% CHX are used in the present study.
Jaffat et al. reported the antimicrobial activity of biosynthesized AgNPs against multidrug-resistant bacteria including E faecalis and 12–16 mm zones of inhibition were found.[18] Bahadoor et al. reported the antibacterial activity of nanosilver Iranian mineral trioxide aggregate (MTA) (NS-IMTA) produced using fungi Aspergillus terreus derived AgNPs with mean zone of inhibition of 9 and 13 mm against P. gingivalis.[19] The results of the present study showed the AgNPs were effective even at the lower concentrations. Highest zones of inhibition were seen at 100 μl of AgNPs for all the microorganisms. AgNPs were as effective as CHX and positive control Ampicillin. For group 2 (E. faecalis), the zone of inhibition with 0.2% CHX is 14.52 mm, whereas with 2% CHX 20.02 mm indicating 2% CHX is more effective against E. faecalis. Studies recommend 2% CHX to be used as root canal irrigant.[24,25] For group 3 (P. gingivalis) AgNPs were as effective as CHX.
The antibacterial mechanisms of AgNPs against bacteria are not yet fully elucidated. AgNPs interact with three components of the cells: (a) peptidoglycan cell wall; (b) cytoplasmic membrane modifying the chemical and physical properties resulting in an imbalance of permeability, osmolality, electron transport; and (c) ribosomal DNA, molecular sites of proteins, especially in enzymes involved in the electron transport chain.[26] Other factors such as the concentration, size, and shape of particles might also affect. AgNPs possess high antimicrobial properties due to increased surface area, biocompatibility, and the shape and size of the particles causing numerous highly reactive corners.[27,28] AgNPs synthesized biologically were found to possess high bactericidal activity against Gram-positive and Gram-negative bacteria, including highly multi-resistant strains.[17]
The results of the present study indicate biosynthesized AgNPs can be used in endodontics as root canal irrigants, intracanal medicaments, root canal sealers, etc.[16] Nanoparticles have more advantages compared to other drug delivery systems in periodontal therapy such as increased stability, controlled release rate, high dispersibility in an aqueous medium, penetrate deeper regions, and reduce the frequency of administration and provide uniform distribution of the active agents.[12] Therefore, biosynthesized AgNPs can be used alternative to topical antiseptics and antimicrobial agents and for local drug delivery during periodontal therapy increasing the success rate.
CONCLUSION
Biosynthesized AgNPs show effective antibacterial efficacy against the microbes P. gingivalis, B. pumilus, and E. faecalis. Therefore, it shows an insight for the use of these nanoparticles in the treatment of endodontic, periodontal, and the combined lesions. However, further in vivo and in vitro studies should be conducted for the effective use of these nanoparticles.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank University Science Instrumentation Centre (USIC) Gulbarga University, Kalaburgi, and SAIF, STIC, Cochin University of Science and Technology, Cochin for technical support.
REFERENCES
- 1.Mjör IA, Nordahl I. The density and branching of dentinal tubules in human teeth. Arch Oral Biol. 1996;41:401–12. doi: 10.1016/0003-9969(96)00008-8. [DOI] [PubMed] [Google Scholar]
- 2.Zehnder M. Endodontic infection caused by localized aggressive periodontitis: A case report and bacteriologic evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:440–5. doi: 10.1067/moe.2001.117270. [DOI] [PubMed] [Google Scholar]
- 3.Kerekes K, Olsen I. Similarities in the microfloras of root canals and deep periodontal pockets. Endod Dent Traumatol. 1990;6:1–5. doi: 10.1111/j.1600-9657.1990.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 4.Ozbek SM, Ozbek A. Real-time polymerase chain reaction of “red complex” (Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola) in periradicular abscesses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:670–4. doi: 10.1016/j.tripleo.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Tokunaga C, Crozeta BM, Bonato MC, Coelho BS, Filho FB, Tomazinho FS. Microbiological aspects of endoperiodontal lesion. RSBO. 2013;10:176–81. [Google Scholar]
- 6.Johnson BT, Shaw LN, Nelson DC, Mayo JA. Extracellular proteolytic activities expressed by Bacillus pumilus isolated from endodontic and periodontal lesions. J Med Microbiol. 2008;57:643–51. doi: 10.1099/jmm.0.47754-0. [DOI] [PubMed] [Google Scholar]
- 7.Halkai R, Hegde MN, Halkai K. Evaluation of the presence of Enterococcus faecalis in root cementum: A confocal laser scanning microscope analysis. J Conserv Dent. 2014;17:119–23. doi: 10.4103/0972-0707.128039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunde PT, Olsen I, Debelian GJ, Tronstad L. Microbiota of periapical lesions refractory to endodontic therapy. J Endod. 2002;28:304–10. doi: 10.1097/00004770-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348–81. doi: 10.1177/154411130401500604. [DOI] [PubMed] [Google Scholar]
- 10.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 11.del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007;82:204–9. doi: 10.1038/sj.clpt.6100247. [DOI] [PubMed] [Google Scholar]
- 12.Sapra P, Patel BD, Patel DV, Borkhataria CH. Review: Recent advances in periodontal formulations. Int J Pharm Chem Anal. 2014;1:65–74. [Google Scholar]
- 13.Parashar V, Parashar R, Sharma B, Pandy AC. Parthenium leaf extract mediated synthesis of silver nanoparticles, a novel approach toward weed utilization digest. J Nanomater Biostruct. 2009;4:45–90. [Google Scholar]
- 14.Neal AL. What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology. 2008;17:362–71. doi: 10.1007/s10646-008-0217-x. [DOI] [PubMed] [Google Scholar]
- 15.Singh D, Rathod V, Ninganagouda S, Herimath J, Kulkarni P. Biosynthesis of silver nano particle by endophytic fungi Pencillium sp. isolated from Curcuma longa (Turmeric) and its antibacterial activity against pathogenic gram negative bacteria. J Pharm Res. 2013;7:448–53. [Google Scholar]
- 16.Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai R. Biosynthesised silver nanoparticles from fungi as antimicrobial agents for endo-perio lesions – A review. Annu Res Rev Biol. 2016;10:1–7. [Google Scholar]
- 17.Ninganagouda S, Rathod V, Jyoti H, Singh D, Prema K, Haq MU, et al. Extracellular biosynthesis of silver nanoparticles using Aspergillus flavus and their antimicrobial activity against gram negative MDR strains. Int J Pharma Bio Sci. 2013;4:222–9. [Google Scholar]
- 18.Jaffat HS, Alduja NH, Abdul Hassan AJ. Antimicrobial activity of silver nano particles biosynthesized by lactobacillus mixtures. RJPBCS. 2017;8:1911–24. [Google Scholar]
- 19.Bahadoor A, Esmaeili D, Khaledi A, Ghorbanzadeh R. An in vitro assessment of the antibacterial properties of nanosilver Iranian MTA against Porphyromonas gingivalis. J Chem Pharm Res. 2013;5:65–71. [Google Scholar]
- 20.Ansari MA, Khan HM, Khan AA, Malik A, Sultan A, Shahid M, et al. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MRSA on isolates from skin infections. Biol Med. 2011;3:141–6. [Google Scholar]
- 21.Sree L, Balasubramanian Deepa. Nanotechnology in dentistry – A review. Int J Dent Sci Res. 2013;1:40–4. [Google Scholar]
- 22.Arasu T, Prabhu D, Soniya M. Stable silver nanoparticle synthesizing methods and its applications. J Bio Sci Res. 2010;1:259–70. [Google Scholar]
- 23.Keijser JA, Verkade H, Timmerman MF, Van der Weijden FA. Comparison of 2 commercially available chlorhexidine mouth rinses. J Periodontol. 2003;74:214–8. doi: 10.1902/jop.2003.74.2.214. [DOI] [PubMed] [Google Scholar]
- 24.Leonardo MR, Filho MT, Silva LA, Filho PN, Bonifacio KC, Ito IY. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25:167–71. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 25.Luddin N, Ahmed HM. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J Conserv Dent. 2013;16:9–16. doi: 10.4103/0972-0707.105291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28:580–8. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Kuku E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]