Abstract
Aim:
The aim of this study was to determine remineralizing potential of grape seed extract (GSE) compared to casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) and calcium glycerophosphate (CaGP) through pH-cycling model and subsequent evaluation using polarized light microscope (PLM).
Subjects and Methods:
Twenty sound human teeth fragments of ten teeth were obtained from the cervical portion of the roots and were stored in demineralizing solution for 96 h at 37°C to induce artificial root carious lesion. The sections then were divided into four treatment groups including: 6.5% GSE, CPP-ACP, 0.5% CaGP, and control group (no treatment). The demineralized samples were then pH cycled through treatment solutions, acidic buffer, and neutral buffer for 8 days at six cycles per day. The samples were subsequently evaluated using PLM.
Statistical Analysis Used:
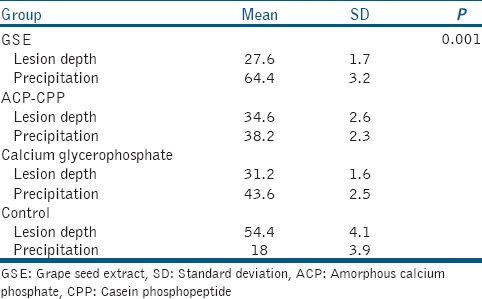
Data were analyzed using ANOVA and Scheffe post hoc comparison test (P < 0.001).
Results:
PLM data revealed a significantly thicker mineral precipitation band on the surface layer of the GSE-treated lesions compared to the other groups (P < 0.001).
Conclusion:
GSE positively affects the demineralization and/or remineralization processes of artificial root caries lesions.
Keywords: Grape seed extract, polarized light microscopy, proanthocyanidin, remineralization
INTRODUCTION
Root caries is more prevalent among elderly individuals due to gingival recession and subsequent exposure of susceptible root surface.[1] The process of root caries involves dissolution of dentin mineral by the acid produced from the oral bacterial biofilm and subsequent degradation of demineralized dentin matrix thereby allowing the bacteria to infiltrate the intertubular dentin.[2] It has been revealed in the literature that reduction in the progression of erosion in dentin is attributed to the presence of organic matrix.[3] The novel phenomenon regarding preventive aspects for root caries is to promote remineralization of demineralized dentin.[1]
The literature search has revealed that a plethora of materials, namely, fluorides, calcium glycerophosphate (CaGP), casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), and xylitol have been used to promote remineralization. More recently, a variety of naturally occurring vegetables and food supplements has shown to promote health and is now suggested that antimicrobial compounds of plant origin can be considered alternative to the commonly used chemicals for controlling dental plaque and dental diseases.[4] One among these is grape seed extract (GSE).[4,5,6]
GSE contains 98% of proanthocyanidin (PA). PA widely available in fruits, vegetables, nuts, seeds, and flowers is a natural plant metabolite, antioxidant, and free radical scavenger. The large molecule structure of PA, a bioflavonoid consists of flavin. PA from fruits and vegetables has been found to prevent acid production by Streptococcus mutans as well as increased collagen synthesis by preventing conversion of soluble collagen to insoluble.[7]
The present study was done to compare and evaluate remineralizing potential of GSE with CPP-ACP and CaGP through pH-cycling model and subsequent evaluation using polarized light microscope (PLM).
SUBJECTS AND METHODS
Materials
The GSE used in the present study consisted of 98% PA according to the manufacturer (Navchetana Kendra, Delhi, India). A 6.5% solution in phosphate buffer (0.025M KH2 PO4 at pH 7.4) was prepared and used in the study. The other materials that were used are GC Tooth Mousse (Recaldent, Europe) and CaGP (Chemsworth, Surat, India).
Specimen preparation
Ten sound human permanent mandibular first molar teeth were selected. They were cleaned, and the organic contaminants were removed with an ultrasonic scaler (P6 Unicorn Medident, India). Twenty root fragments from below the cementoenamel junction measuring about 5 mm × 5 mm were obtained and polished with a polishing discs (Sof-le × 3M ESPE). Root fragments were sealed with an acid-resistant nail varnish except for 3 mm × 4 mm window [Figure 1].
Figure 1.
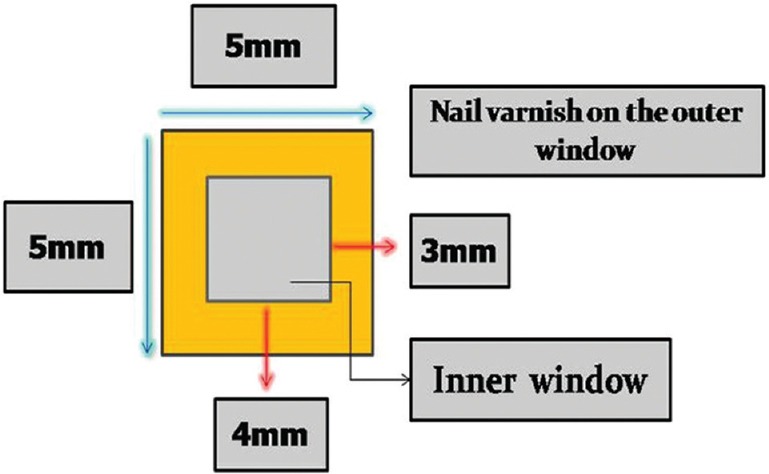
Digrammatic representation of the specimen used in the study
Lesion formation
Root fragments were placed in a demineralizing solution (2.2 mM CaCl2,2H2O [RANKEM, India], 2.2 mM KH2 PO2 [MOLYCHEM, Mumbai, India], and 50 mM sodium acetate [Research Lab Fine Chem Industries]) at pH 4.6 for 96 h at 37°C to create artificial carious lesions. Following lesion development, the fragments were rinsed thoroughly with deionized water (dH2O).
Remineralization regimen
The demineralized root fragments were randomly divided into four groups (n = 5) based on treatment as follows:
Group A: 6.5% (w/v) GSE solution in phosphate buffer 0.025M KH2PO4 (MOLYCHEM, Mumbai, India), 0.025M K2HPO4 (Thomas-Baker Lab Chemicals, India) at pH 7.4
Group B: GC Tooth Mousse (RECALDENT, Pvt Ltd.)
Group C: 0.5% CaGP (Chemsworth, Surat, India)
Group D: dH2O, no treatment.
All the samples were pH cycled through treatment solutions (10 min), acidic buffer (50 mM acetate; 2.25 mM CaCl2·2H2O; 1.35 mM KH2 PO4; 130 mM KCl; pH 5.0; 30 min) and neutral buffer (20 mM HEPES; 2.25 mM CaCl2·2H2O; 1.35 mM KH2 PO4; 130 mM KCl; pH 7.0; 10 min) for 8 days. For in vitro pH-cycling experiments, 250 ml polystyrene jars were used. All the solutions were freshly made before use. Six cycles per day were performed. Root fragments were kept in a neutral buffer overnight [Figure 2].
Figure 2.
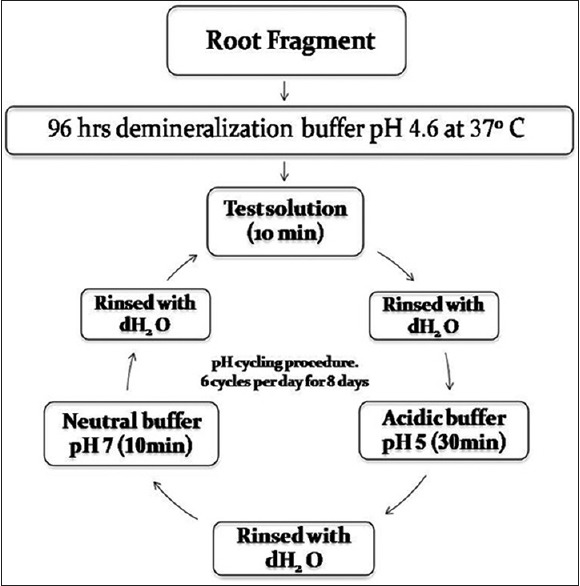
In-vitro artificial lesion formation and pH- cycling procedure
Posttreatment analysis
Following pH cycling, root fragments were rinsed with dH2O for 2 min.
PLM (LABOMED) and image analysis:
100 um thick longitudinal sections through the lesion were obtained using hard tissue microtome (LEICA SP1600)
Lesion depths and the depth of the remineralized band were quantified using PLM and an image analysis system (Adobe Photoshop CS.2, version 9, USA).
Statistical analysis
For each sample group, the mean and standard deviation were calculated for all measured parameters. The data collected from PLM were analyzed using one-way ANOVA and Scheffe post hoc comparison test [Figure 3 and Table 1].
Figure 3.
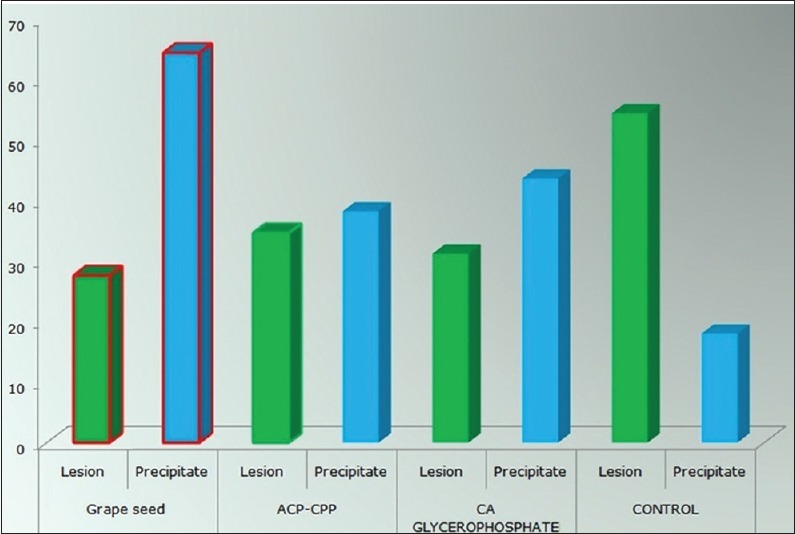
Effect of GSE,CPP-ACP,0.5% Calcium Glycerophosphate on artificial root lesion depth as determined by PLM
Table 1.
Statistically significant difference detected between the grape seed extract group and other groups (P<0.001)
RESULTS
Representative PLM micrographs of root fragment sections from each group were viewed.
The profile obtained from PLM showed:
Upon pH cycling, a mineral precipitation band was evident on the superficial layers in the control and treated lesions upon pH cycling [Figure 4, P]
An increase in advanced demineralization band was also noted in every sample due to acid challenge during pH-cycling procedure [Figure 4, AD]
The lesion depths data obtained from PLM of GSE group treated were significantly smaller than other groups [Figures 3 and 4a]
A significantly wider (P < 0.001) mineral precipitation band was observed in the GSE-treated group when compared to other and control groups [Figures 3 and 4a].
Figure 4.
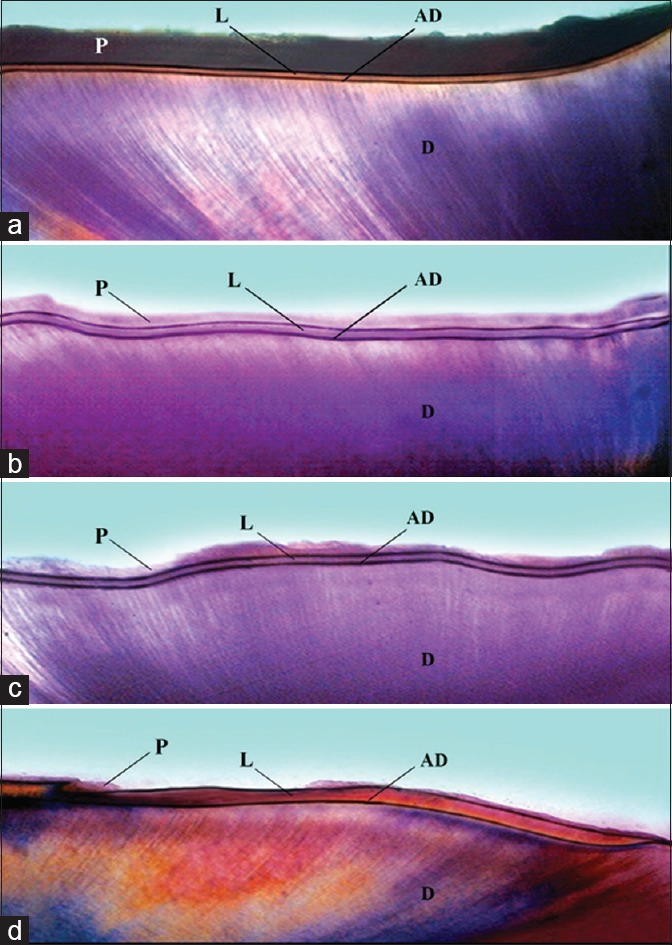
Photomicrographs of sections of artificial root lesion (a) GSE (b) CPP-ACP (c) 0.5% Calcium Glycerophosphate (d) Control. (L: Lesion, (d) Sound Dentin, P: Mineral precipitation band, AD: Advanced demineralized band)
DISCUSSION
Remineralization is a process, whereby calcium and phosphate ions are supplied from an external source to the tooth, thereby converting ion deposition into crystal voids in demineralized enamel, thus producing net mineral gain.[5] Remineralization of carious lesions may be possible with a variety of remineralizing agents such as fluoride, CPP-ACP, self-assembling peptide, CaGP, bioactive glass, tricalcium phosphate, and xylitol.[5,6]
The caries-preventive effect has been proven earlier. Anticariogenic potential of CaGP can be attributed to various reasons that include elevation of plaque calcium and phosphate concentrations, buffering plaque pH, reduction of plaque mass, and direct interaction with dental hard tissues as they act on hydroxyapatite thereby reducing the extent of demineralization.[8] There is also extensive clinical as well as laboratory evidence for the effects of CPP-ACP as remineralizing agent. It has been proved that CPP-ACP binds readily to pellicle, plaque, soft tissue, and even hydroxyapatite crystals when applied within the oral cavity.[9]
GSE used in the present study consists of 98% of PA. PA is naturally occurring plant metabolite widely available in fruits, vegetables, nuts, seeds, flowers, and bark. It is commonly used as natural antioxidants and free radical scavengers.[10] GSE is a rich source of PA, which has been reported to strengthen collagen-based tissues by increasing collagen cross-links. PA from cranberries inhibited the surface-adsorbed glucosyltransferases and acid production by S. mutans.[1]
PA-treated collagen matrices are nontoxic and inhibit enzymatic activity of glucosyltransferase, F-ATPase, and amylase. Glucosyltransferase which is produced by S. mutans polymerizes the glucosyl moiety from sucrose and starch carbohydrates into glucans. This constitutes the sucrose-dependent pathway for S. mutans to establish on the tooth surface and is of prime importance in plaque formation and development of caries. The adherent glucans also contribute to the formation of dental plaque, in which accumulation of acid leads to localized decalcification of enamel surface by facilitating bacterial adherence of the tooth surfaces, interbacterial adhesion, and accumulation of biofilms. Hence, inhibition of glucosyltransferases by PA, in turn, inhibits caries.[8,11]
Xie et al. suggested that GSE could contribute not only the deposition of mineral on the superficial layer of the lesion but also may interact with the organic portion of root dentin through PA-collagen interact stabilizing the exposed collagen matrix.[1] Bedran-Russo et al. reported that GSE treatment significantly increased the ultimate tensile strength at demineralized dentin, indicating the potential of GSE to induce cross-links in the dentin collagen.[12,13]
In the present study, remineralization potential of GSE was compared to CPP-ACP and 0.5% CaGP using PLM. The results of this in vitro study revealed the highest mineral precipitation band with GSE as against CPP-ACP and CaGP.
CONCLUSION
Considering the results of this in vitro study, it can be assumed that the GSE prevents the demineralization and also help in remineralization of artificial root carious lesions making it a promising naturally occurring remineralizing agent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Xie Q, Bedran-Russo AK, Wu CD. in vitro remineralization effects of grape seed extract on artificial root caries. J Dent. 2008;36:900–6. doi: 10.1016/j.jdent.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ten Cate AR. St. 5th ed. St Louis: Mosby; 1998. Oral Histology: Development, Structure, and Function; p. 150. [Google Scholar]
- 3.Vanuspong W, Eisenburger M, Addy M. Cervical tooth wear and sensitivity: Erosion, softening and rehardening of dentine; effects of pH, time and ultrasonication. J Clin Periodontol. 2002;29:351–7. doi: 10.1034/j.1600-051x.2002.290411.x. [DOI] [PubMed] [Google Scholar]
- 4.Silva AP, Gonçalves RS, Borges AF, Bedran-Russo AK, Shinohara MS. Effectiveness of plant-derived proanthocyanidins on demineralization on enamel and dentin under artificial cariogenic challenge. J Appl Oral Sci. 2015;23:302–9. doi: 10.1590/1678-775720140304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naveena Preethi P, Nagarathana C, Sakunthala BK. Remineralizing agent – Then and now – An update. Dentistry. 2014;4:9. [Google Scholar]
- 6.Tyagi S, Garg P, Sinha D, Singh U. An update on remineralizing agents. J Interdiscip Dent. 2013;3:151–8. [Google Scholar]
- 7.Parashar K, Zaidka S, Somani R, Jayanti S. Anti-cariogenic effects of polyphenol plant products – A review. Int J Res Ayurveda Pharm. 2011;2:736–42. [Google Scholar]
- 8.Benjamin S, Sharma R, Thomas SS, Nainan MT. Grape seed extract as a potential remineralizing agent: A comparative in vitro study. J Contemp Dent Pract. 2012;13:425–30. doi: 10.5005/jp-journals-10024-1162. [DOI] [PubMed] [Google Scholar]
- 9.Moezizadeh M, Moayedi S. Anticariogenic effect of amorphous calcium phosphate stabilized by casein phosphopeptid: A review. Res J Biol Sci. 2009;4:132–6. [Google Scholar]
- 10.Sarni-Manchado P, Cheynier V, Moutounet M. Interactions of grape seed tannins with salivary proteins. J Agric Food Chem. 1999;47:42–7. doi: 10.1021/jf9805146. [DOI] [PubMed] [Google Scholar]
- 11.Ferrazzano GF, Amato I, Ingenito A, Zarrelli A, Pinto G, Pollio A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules. 2011;16:1486–507. doi: 10.3390/molecules16021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res B Appl Biomater. 2008;86:330–4. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 13.Wu CD. Grape products and oral health. J Nutr. 2009;139:1818S–23S. doi: 10.3945/jn.109.107854. [DOI] [PMC free article] [PubMed] [Google Scholar]


