Abstract
Recent epidemiological evidence suggests that exposure to particulates may be a factor in the etiology of metabolic syndrome (MetS). In this novel study, we investigated the relationship between particulate levels and prevalence of MetS component abnormalities (hypertension, hyperglycemia, obesity) in a recruited cohort (N = 2025) in Jeddah, Saudi Arabia. We observed significant associations between a 10 μg/m3 increase in PM2.5 and increased risks for MetS (Risk Ratio (RR): 1.12; 95% Confidence Interval (CI): 1.06–1.19), hyperglycemia (RR: 1.08; 95% CI: 1.03–1.14), and hypertension (RR: 1.09; 95% CI: 1.04–1.14). PM2.5 from soil/road dust was found to be associated with hyperglycemia (RR: 1.12; 95% CI: 1.06–1.19) and hypertension (RR: 1.11; 95% CI: 1.05–1.18), while PM2.5 from traffic was associated with hyperglycemia (RR: 1.33; 95% CI: 1.05–1.71). We did not observe any health associations with source-specific mass exposures. Our findings suggest that exposure to specific elemental components of PM2.5, especially Ni, may contribute to the development of cardiometabolic disorders.
Keywords: air pollution, particulate matter, metabolic syndrome, hypertension, diabetes, hyperglycemia
1. Introduction
Jeddah, a major metropolitan city of nearly 3.4 million residents, serves as a commercial, economic and cultural hub of Saudi Arabia and the Middle East [1]. Rapid population growth and expansion of the city have resulted in deteriorating air quality, raising concerns about the potential health effects.
Ambient air pollution is linked with adverse health effects, with numerous studies establishing causal associations between exposure to particulate matter (PM) and elevated risk for cardiovascular morbidity and mortality [2]. Preliminary epidemiological and mechanistic evidence have suggested that ambient air pollution exposure is also involved in the development of cardiometabolic disorders [3]. Metabolic syndrome (MetS) is a cluster of risk factors, with the Adult Treatment Panel III formally defining MetS as having at least three of the five out of hypertension, abdominal obesity, elevated fasting glucose, high serum triglycerides and low circulating high-density lipoprotein [4]. MetS is a precursor of developing cardiovascular disease and type II diabetes mellitus, and the presence of component abnormalities of MetS may impart individuals with greater susceptibility to PM-related health effects [5]. The prevalence of MetS has rapidly risen in Saudi Arabia over the past two decades, with studies estimating prevalence in the country as high as 41% [6].
Past epidemiologic investigation of the association between air pollution and cardiometabolic disorders have shown mixed, but generally positive associations. For example, short [7], long [8], long and short [9], intermittent [10]. Exposure to PM with an aerodynamic diameter equal to or less than 2.5 µm (PM2.5) was found to contribute to increased incidence and/or prevalence of diabetes mellitus (DM) among U.S. adults. Short-term exposure to PM2.5 has been found to be associated with immediate elevation of blood pressure [11,12]. Long-term exposure has been associated with the development of hypertension [13]. Associations between air pollution and formally defined MetS have not been examined extensively to date, although recently a significant association between PM10 and prevalence of MetS, defined via multiple definitions, was reported in a cohort of Swiss adults [14].
In animal studies, exposure to PM2.5 increased blood glucose, adipose inflammation, and insulin resistance [15]. It impaired energy metabolism and increased inflammation in insulin responsive organs, resulting in imbalances in circulating leptin and adiponectin levels [16]. By triggering autonomic nervous system imbalance, PM2.5 exposure also promoted vasoconstriction and reduced insulin sensitivity [17]. PM2.5 impaired renal handling of excess sodium, inhibiting normal nocturnal reduction in blood [18].
As PM represents a complex mixture that is directly released from both geogenic and anthropogenic sources, or formed in the atmosphere from pollutant gases into secondary aerosols through chemical reactions, the chemical and physical characteristics, as well as the associated health effects, of the PM air pollution mixture are variable depending on its source(s), and this is thought to also influence PM toxicity [19]. Only a few studies have partitioned the health effects to source-related PM components [20,21,22], and none that we are aware of have been conducted to date in the Middle East.
In this innovative study, based upon our prior quantification of elemental components and source apportionment of PM with an aerodynamic diameter equal to or less than 10 µm in size (PM10) and PM2.5 in Jeddah [23], we investigated the epidemiological relationships of the previously estimated PM exposures from various sources in Jeddah with the prevalence of MetS in a local cohort, as well as with three component cardiometabolic abnormalities (hyperglycemia, hypertension, and obesity), within this recruited cohort.
2. Materials and Methods
2.1. Air Quality Measurements in Jeddah
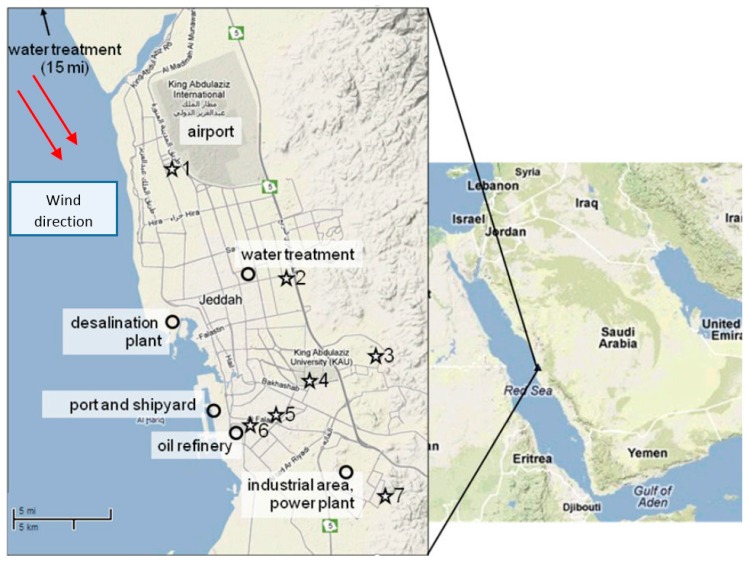
The primary mobile source of pollution in Jeddah is traffic, with more than 1.4 million vehicles fueled mainly by unleaded gasoline and diesel oil, while major stationary sources in Jeddah include an oil refinery, a major seaport, a desalinization plant, a power generation plant, and several manufacturing industries (Figure 1). As elaborated in more details in Khodeir et al. [23], air samplers were installed at seven locations across Jeddah. Samples were collected every 24 h from midnight to midnight every other day, using PM10 and PM2.5 Harvard impactors [24] and calibrated vacuum pumps to draw air at a rate of 10.0 L/min onto Teflon filters (GelmanTeflo, 37 mm, 0.2 μm pore-size). The seven sampling sites were located throughout the city (Figure 1), and were selected based on varying traffic and/or population density: four in urban areas (University Campus, Al-Nuzlah, Pitrumin, and Al-Rughama), and three in residential areas (Al-Muhammadiyah, Al-Rehab and Al-Alfiyyah).
Figure 1.
Locations of sampling sites (stars) and notable stationary industrial sources (circles) in Jeddah; 1 = Al-Muhammadiyah; 2 = Al-Rehab; 3 = Al-Rughama; 4 = University Campus; 5 = Al-Nuzlah Al Yamaneyyah; 6 = Pitrumin; 7 = Al-Alfiyyah.
2.2. Source Identification
We applied the widely employed Absolute Principal Components Analysis (APCA) method developed by Thurston and Spengler [25] in order to identify major PM source categories in each size fraction. This provided positive indices of daily source impacts, upon which daily PM mass concentrations were regressed to achieve a source apportionment for application to this research. Estimates of mass contributed by the identified source categories for each of the locations were calculated using factor weights, and the regression models developed in a previous source apportionment study conducted in Jeddah [23]. For PM2.5, four source factors were identified based on the factor eigenvalues and factor source interpretability as follows: soil/road dust (loadings on Al, Ca, Cr, Fe, K, Mg, Mn, Si, Sr, Ti), residual oil (Ni, S, V), solid waste incineration (Cu, Zn), and traffic (Pb and S); for PM10, three factors were identified: soil/road dust, traffic, and solid waste incineration.
2.3. Study Population Recruitment, Exposure Assessment, and Health Measurements
Participants (N = 2686) were recruited from interviewing individuals at two mega-malls, one located in North Jeddah, and the other located in South Jeddah, from June 2011 to May 2012. Participants were enrolled by trained personnel based on their willingness to participate in the study, excluding only those having lived in the area of study for less than 15 years. Confidentiality was maintained in the completed questionnaires, and the identity of the participants remained anonymous. The questionnaire included information on age, type of residence, occupation, education, smoking habits, sleeping hours, physical activity, hours since the last meal, past history of diabetes, hypertension, and other chronic diseases, and weekly frequency of food intakes by type. Physical activity was defined as ‘yes’ if participants were involved in walking, swimming, running, and biking. After the interview, body weight and height were measured without shoes using electronic measuring scale. Body mass index (BMI) was calculated as weight in kilograms divided by height in m2 and obesity was defined as BMI > 30 for both men and women. Blood pressure was monitored in three measurements taken in 5-min intervals and averaged. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg; those reporting use of anti-hypertensive drugs were considered as hypertensive regardless of their recorded blood pressure. Random blood sugar levels were measured and hyperglycemia was defined if the values were equal to or exceeded 126 mg/dL; those reporting use of diabetes control medications were considered as having diabetes regardless of recorded blood sugar levels. Presence of MetS was defined as having all three of the cardiometabolic abnormalities measured in the study: obesity, hypertension, and hyperglycemia [26]. Individual exposure to air pollution was linked to the subjects’ district of residence, using averaged values from the samplers that were installed at seven sites throughout Jeddah districts as described previously (Figure 1). An informed verbal consent was obtained from each participant, and the study proposal was approved by the King Abdulaziz University Research Ethics Committee (number 700-12).
2.4. Statistical Analyses
The associations between PM concentration levels and prevalence of MetS, as well as with individual components of MetS, were investigated using a generalized linear mixed effects model, adjusted for each individual’s age, sex, type of residence (house, apartment, villa), marriage status (currently married/no), occupation (unemployed, labor work, desk work), education level (<secondary, secondary, university and post graduate), physical activity (no/yes), diet (weekly consumption of fresh fruits and vegetables), current smoking status (currently smoking/no), and district location (as a random effect). Detailed cohort characteristics, by location of residence, are displayed in Table 1. Subjects with incomplete individual covariates data were excluded, the total number of subjects available for analysis was N = 2025. All statistical analyses were conducted in SAS (R) Studio 3.1 (SAS Institute Inc., Cary, NC, USA).
Table 1.
Study cohort characteristics by location.
| Total | Al-Nuzlah | Al-Alfiyya | Al-Rehab | Pitrumin | Al-Rughama | University | Al-Muhammadiyah | ||
|---|---|---|---|---|---|---|---|---|---|
| N = 2025 | N = 266 | N = 157 | N = 409 | N = 104 | N = 103 | N = 697 | N = 298 | ||
| Age | Mean (SD) | 31.46 (10.69) | 29.43 (11.36) | 35.26 (11.73) | 32.28 (10.41) | 30.66 (10.80) | 28.38 (8.73) | 31.37 (10.98) | 31.75 (9.06) |
| Sex | Male N (%) | 996 (49.2) | 185 (70.1) | 130 (86.1) | 175 (42.8) | 42 (40.4) | 61 (59.2) | 246 (35.3) | 157 (52.9) |
| Female N (%) | 1029 (50.8) | 79 (29.9) | 21 (13.9) | 234 (57.2) | 62 (59.6) | 42 (40.8) | 451 (64.7) | 140 (47.1) | |
| Smoking | Non-smoker N (%) | 1493 (73.7) | 219 (82.9) | 103 (68.2) | 296 (72.4) | 80 (76.9) | 59 (57.3) | 526 (75.5) | 210 (70.7) |
| Smoker N (%) | 532 (26.3) | 45 (17.0) | 48 (31.8) | 113 (27.6) | 24 (23.1) | 44 (42.7) | 171 (24.5) | 87 (29.3) | |
| Fruit and Vegetable Intake | ≤4 times/week N (%) | 959 (47.4) | 164 (62.1) | 88 (58.3) | 143 (35.0) | 46 (44.2) | 49 (476.) | 342 (49.1) | 127 (42.8) |
| 5–6 times/week N (%) | 787 (38.9) | 82 (31.1) | 55 (36.4) | 170 (41.6) | 49 (47.1) | 39 (37.9) | 276 (39.6) | 116 (39.1) | |
| ≥7 times/week N (%) | 279 (13.8) | 18 (6.8) | 9 (5.3) | 96 (23.5) | 9 (8.6) | 15 (14.6) | 79 (11.3) | 54 (18.2) | |
| Residence | House N (%) | 681 (40.0) | 152 (57.6) | 25 (16.6) | 92 (22.5) | 74 (71.2) | 58 (56.3) | 221 (31.7) | 59 (19.9) |
| Apartment N (%) | 1261 (68.9) | 108 (40.9) | 87 (57.6) | 301 (73.6) | 30 (28.8) | 44 (42.7) | 467 (67.0) | 224 (75.4) | |
| Villa N (%) | 83 (3.1) | 4 (1.5) | 39 (25.8) | 16 (3.9) | 0 (0.0) | 1 (0.1) | 9 (1.3) | 14 (4.7) | |
| Marietal Status | No N (%) | 971 (48.0) | 164 (62.1) | 44 (29.1) | 163 (39.9) | 57 (54.8) | 63 (61.2) | 351 (50.3) | 129 (43.4) |
| Yes N (%) | 1054 (52.0) | 100 (37.9) | 107 (70.9) | 246 (60.1) | 47 (45.2) | 40 (38.8) | 346 (49.6) | 168 (56.6) | |
| Type of Work | No Work N (%) | 523 (25.8) | 73 (27.7) | 30 (19.9) | 146 (35.7) | 32 (30.8) | 23 (22.3) | 129 (18.5) | 90 (30.3) |
| Labor Work N (%) | 1339 (66.1) | 160 (60.6) | 76 (50.3) | 223 (54.5) | 69 (66.3) | 80 (77.7) | 562 (80.6) | 169 (56.9) | |
| Desk Work N (%) | 163 (8.1) | 31 (11.7) | 45 (29.8) | 40 (9.8) | 3 (2.9) | 0 (0.0) | 6 (0.9) | 38 (12.8) | |
| Education Level | Illiterate N (%) | 67 (3.3) | 17 (6.4) | 2 (1.3) | 15 (3.7) | 3 (2.9) | 2 (1.9) | 28 (4.0) | 0 (0.0) |
| Can Read & Write N (%) | 59 (2.9) | 8 (3.0) | 2 (1.3) | 11 (2.7) | 3 (2.9) | 0 (0.0) | 26 (3.7) | 9 (3.0) | |
| Primary School N (%) | 30 (1.5) | 9 (3.5) | 2 (1.3) | 3 (0.7) | 6 (5.8) | 0 (0.0) | 8 (1.1) | 2 (0.7) | |
| Prepatory School N (%) | 116 (5.7) | 33 (12.5) | 1 (0.7) | 23 (5.6) | 10 (9.6) | 5 (4.9) | 31 (4.5) | 13 (4.4) | |
| Secondary School N (%) | 758 (37.5) | 109 (41.3) | 52 (34.4) | 172 (42.1) | 43 (41.3) | 38 (36.9) | 230 (32.9) | 114 (38.4) | |
| University Degree N (%) | 969 (47.9) | 85 (32.2) | 90 (59.6) | 174 (42.5) | 38 (36.5) | 57 (55.3) | 369 (52.9) | 156 (52.5) | |
| Post Graduate N (%) | 26 (1.3) | 3 (1.1) | 2 (1.3) | 11 (2.7) | 1 (1.0) | 1 (1.0) | 5 (0.7) | 3 (1.0) | |
| Physical Activity | No N (%) | 1315 (64.9) | 143 (64.2) | 37 (24.5) | 323 (78.9) | 56 (53.8) | 54 (52.5) | 496 (71.2) | 206 (69.4) |
| Yes N (%) | 710 (35.1) | 121 (45.8) | 114 (75.5) | 86 (21.0) | 48 (46.2) | 49 (47.5) | 201 (28.8) | 91 (30.6) | |
| Metabolic Syndrome | No N (%) | 1656 (81.8) | 232 (87.9) | 133 (88.1) | 290 (70.9) | 86 (82.7) | 91 (88.3) | 607 (87.1) | 217 (73.1) |
| Yes N (%) | 369 (18.2) | 32 (12.1) | 18 (11.9) | 119 (29.1) | 18 (17.3) | 12 (11.7) | 90 (12.9) | 80 (26.9) | |
| Hyperglycemia | No N (%) | 941 (46.5) | 186 (70.5) | 122 (80.8) | 215 (52.6) | 77 (74.0) | 74 (71.8) | 491 (70.4) | 158 (53.2) |
| Yes N (%) | 1084 (53.5) | 78 (29.5) | 29 (19.2) | 194 (47.4) | 27 (26.0) | 29 (28.2) | 206 (29.6) | 139 (46.8) | |
| Hypertension | No N (%) | 1323 (65.3) | 132 (50.0) | 86 (56.9) | 147 (35.9) | 45 (43.3) | 45 (43.7) | 357 (51.2) | 129 (43.4) |
| Yes N (%) | 702 (34.7) | 132 (50.0) | 65 (43.1) | 262 (64.1) | 59 (56.7) | 58 (56.3) | 340 (48.8) | 168 (56.6) | |
| Obesity | No N (%) | 659 (32.5) | 112 (42.4) | 64 (42.4) | 129 (31.5) | 29 (27.9) | 41 (39.8) | 226 (32.4) | 58 (19.5) |
| Yes N (%) | 1366 (67.5) | 152 (57.6) | 87 (57.6) | 280 (68.5) | 75 (72.1) | 62 (60.2) | 471 (67.6) | 239 (80.5) |
3. Results and Discussion
Summary characteristics of the seven monitoring sites are presented in Table 2. As seen in Table 2, the overall average concentrations of PM2.5 (38.6 μg/m3, SD = 21.4 μg/m3) and PM10 (85.1 μg/m3, SD = 30.30 μg/m3) measured in the present study period across the sampling sites greatly exceeded the recommended annual levels by the World Health Organization for PM2.5 (10 μg/m3) and PM10 (20 μg/m3) [27]. Highest concentrations for PM2.5 (73.2 μg/m3) and PM10 (141.3 μg/m3) were observed in the Al-Rughama, while lowest concentrations for PM2.5 (15.8 μg/m3) and PM10 (47.0 μg/m3) were observed in Al-Muhammadiyah. Al-Rughama is a suburban area characterized by intensive traffic, construction activities, small workshops and open burning of solid waste, while al-Muhammadiyah is a quiet typical residential area with light traffic.
Table 2.
PM and elemental constituents concentration levels (Mean ± S.D., μg/m3) by location.
| Total | Al-Nuzlah | Al-Alfiyya | Al-Rehab | |||||
| PM2.5 | PM10 | PM2.5 | PM10 | PM2.5 | PM10 | PM2.5 | PM10 | |
| PM | 29.07 (18.68) | 85.12 (30.30) | 29.10 (14.11) | 73.51 (10.13) | 24.51 (11.77) | 99.42 (43.94) | 18.04 (3.96) | 68.95 (20.48) |
| Al | 0.89 (1.35) | 3.42 (2.05) | 0.28 (0.066) | 2.06 (0.46) | 0.58 (0.36) | 4.79 (2.50) | 0.28 (0.12) | 2.41 (0.86) |
| Ca | 0.63 (0.77) | 4.18 (1.33) | 0.31 (0.037) | 3.55 (0.49) | 0.36 (0.18) | 4.48 (2.35) | 0.28 (0.18) | 3.48 (1.32) |
| Cr | 0.0028 (0.0031) | 0.0093 (0.0058) | 0.0018 (0.00077) | 0.0066 (0.0011) | 0.0021 (0.00087) | 0.013 (0.0073) | 0.0014 (0.00099) | 0.0059 (0.0023) |
| Cu | 0.0061 (0.0041) | 0.017 (0.0076) | 0.0024 (0.0023) | 0.012 (0.0048) | 0.013 (0.0097) | 0.022 (0.015) | 0.0018 (0.0014) | 0.013 (0.0038) |
| Fe | 0.72 (1.18) | 3.32 (2.24) | 0.17 (0.027) | 1.84 (0.38) | 0.37 (0.21) | 4.25 (2.43) | 0.23 (0.15) | 2.29 (0.86) |
| K | 0.21 (0.17) | 0.72 (0.27) | 0.15 (0.033) | 0.52 (0.083) | 0.013 (0.063) | 0.86 (0.51) | 0.11 (0.030) | 0.56 (0.19) |
| Mg | 0.33 (0.43) | 1.44 (0.52) | 0.20 (0.079) | 1.17 (0.16) | 0.23 (0.15) | 1.93 (0.87) | 0.11 (0.057) | 1.21 (0.54) |
| Mn | 0.022 (0.033) | 0.10 (0.063) | 0.0054 (0.0018) | 0.053 (0.012) | 0.012 (0.0057) | 0.12 (0.062) | 0.009 (0.0048) | 0.069 (0.028) |
| Ni | 0.0071 (0.0028) | 0.011 (0.0039) | 0.0071 (0.0023) | 0.099 (0.0027) | 0.0064 (0.0033) | 0.012 (0.0067) | 0.0042 (0.00091) | 0.0073 (0.0025) |
| Pb | 0.13 (0.14) | 0.16 (0.17) | 0.037 (0.049) | 0.039 (0.044) | 0.49 (0.44) | 0.47 (0.47) | 0.086 (0.14) | 0.11 (0.15) |
| S | 3.48 (0.75) | 3.39 (0.51) | 4.04 (0.45) | 3.76 (0.47) | 3.87 (2.34) | 3.47 (2.17) | 2.51 (0.35) | 2.69 (0.15) |
| Si | 2.47 (3.99) | 11.19 (6.70) | 0.72 (0.23) | 7.08 (1.36) | 1.44 (0.89) | 15.29 (8.10) | 0.68 (0.43) | 7.69 (2.80) |
| Sr | 0.0049 (0.0061) | 0.026 (0.0085) | 0.0021 (0.00066) | 0.022 (0.0034) | 0.003 (0.0019) | 0.029 (0.015) | 0.0018 (0.0020) | 0.023 (0.015) |
| Ti | 0.070 (0.13) | 0.32 (0.25) | 0.011 (0.0027) | 0.15 (0.028) | 0.032 (0.021) | 0.42 (0.26) | 0.017 (0.014) | 0.21 (0.08) |
| V | 0.025 (0.010) | 0.032 (0.012) | 0.029 (0.0098) | 0.33 (0.0096) | 0.023 (0.012) | 0.031 (0.015) | 0.015 (0.0038) | 0.021 (0.06) |
| Zn | 0.038 (0.023) | 0.073 (0.044) | 0.017 (0.0069) | 0.039 (0.012) | 0.056 (0.064) | 0.13 (0.17) | 0.019 (0.0088) | 0.049 (0.011) |
| Pitrumin | Al-Rughama | University | Al-Muhammadiyah | |||||
| PM2.5 | PM10 | PM2.5 | PM10 | PM2.5 | PM10 | PM2.5 | PM10 | |
| PM | 31.14 (5.85) | 107.13 (28.75) | 73.16 (65.08) | 141.27 (124.20) | 29.91 (11.67) | 85.67 (33.09) | 15.78 (3.05) | 47.01 (6.52) |
| Al | 0.41 (0.22) | 3.51 (1.07) | 3.93 (4.07) | 7.17 (6.07) | 0.63 (1.02) | 3.07 (1.77) | 0.15 (0.43) | 0.91 (0.41) |
| Ca | 0.53 (0.17) | 6.11 (1.42) | 2.36 (2.76) | 4.95 (2.94) | 0.39 (0.30) | 4.77 (2.18) | 0.19 (0.15) | 1.96 (0.61) |
| Cr | 0.0015 (0.0010) | 0.0089 (0.0031) | 0.0097 (0.010) | 0.021 (0.020) | 0.0019 (0.0021) | 0.0083 (0.0053) | 0.00092 (0.00058) | 0.0022 (0.00081) |
| Cu | 0.0054 (0.0053) | 0.029 (0.0096) | 0.0098 (0.0057) | 0.016 (0.011) | 0.0077 (0.011) | 0.019 (0.016) | 0.0029 (0.0037) | 0.0048 (0.0022) |
| Fe | 0.36 (0.15) | 3.59 (1.16) | 3.37 (3.94) | 7.71 (7.73) | 0.39 (0.64) | 2.81 (1.59) | 0.12 (0.093) | 0.75 (0.22) |
| K | 0.21 (0.091) | 0.91 (0.29) | 0.59 (0.62) | 1.14 (0.93) | 0.16 (0.13) | 0.69 (0.31) | 0.12 (0.024) | 0.35 (0.075) |
| Mg | 0.19 (0.098) | 1.55 (0.47) | 1.29 (1.21) | 2.21 (1.51) | 0.25 (0.33) | 1.41 (0.60) | 0.058 (0.025) | 1.55 (0.16) |
| Mn | 0.014 (0.0073) | 0.11 (0.035) | 0.097 (0.11) | 0.23 (0.21) | 0.015 (0.021) | 0.095 (0.052) | 0.0051 (0.0028) | 0.11 (0.0079) |
| Ni | 0.012 (0.0048) | 0.018 (0.0044) | 0.0097 (0.0058) | 0.13 (0.0088) | 0.0058 (0.0022) | 0.011 (0.0044) | 0.0043 (0.00096) | 0.018 (0.0021) |
| Pb | 0.031 (0.020) | 0.041 (0.022) | 0.17 (0.27) | 0.021 (0.34) | 0.21 (0.42) | 0.27 (0.52) | 0.0066 (0.0067) | 0.0093 (0.0063) |
| S | 4.51 (0.97) | 4.06 (0.67) | 3.65 (1.32) | 2.82 (1.25) | 3.11 (1.19) | 3.71 (0.99) | 2.63 (0.47) | 3.19 (0.73) |
| Si | 1.16 (0.62) | 12.18 (3.96) | 11.49 (12.57) | 23.35 (20.07) | 1.47 (2.46) | 10.07 (5.42) | 0.34 (0.21) | 2.64 (1.04) |
| Sr | 0.0044 (0.0017) | 0.036 (0.021) | 0.019 (0.022) | 0.036 (0.025) | 0.0029 (0.0027) | 0.027 (0.014) | 0.0021 (0.00097) | 0.012 (0.003) |
| Ti | 0.025 (0.014) | 0.31 (0.095) | 0.36 (0.43) | 0.83 (0.90) | 0.034 (0.069) | 0.25 (0.17) | 0.012 (0.0094) | 0.07 (0.03) |
| V | 0.045 (0.0099) | 0.055 (0.0072) | 0.028 (0.015) | 0.031 (0.014) | 0.019 (0.0069) | 0.029 (0.0099) | 0.016 (0.0037) | 0.021 (0.0078) |
| Zn | 0.073 (0.13) | 0.13 (0.17) | 0.043 (0.027) | 0.061 (0.036) | 0.044 (0.0.76) | 0.079 (0.13) | 0.0099 (0.0044) | 0.022 (0.005) |
Sulfur (3.39 μg/m3) was the dominant element found in PM2.5, representing 26.8% of total elemental mass, whereas silicon (10.4 μg/m3) was the dominant element found in PM10, representing 34.53% of total elemental mass. The elemental components profiles of each location generally reflected the environmental and physical characteristics of their respective surroundings; samples from locations closest to the non-developed lands east of the city—Al-Alfiyyah, Al-Rehab, and Al-Rughama—had elevated concentrations of crustal elements, such as Al, Ca, Mg, and Si. High concentrations of Ni, S, and V, markers of residual oil combustion were measured in Pitrumin, Al-Nuzlah and Al-Rughama, which are the sampling locations closest to the port and oil refinery. Al-Rehab and Al-Alfiyyah, closest to water treatment and industrial power plant areas, had high concentrations of Cu and Zn, elements commonly associated with open and/or industrial waste burning. Samples from Al-Rughama, Al-Alfiyahh, and University, which are located nearby the major highway, had elevated concentrations of Pb, a marker for traffic even after discontinued leaded gasoline use, due to accumulation in soil [28].
The degree of metal contamination in atmospheric PM2.5 and PM10 of the study areas can be assessed by comparing their measured concentrations with regulatory standards. Such an approach, however, cannot be adopted in the present study because standards are not available for the measured metals. An alternative approach would be to compare the heavy metal concentrations in the study area with the safe limits proposed by international agencies. World Health Organization (WHO) [27] standards for atmospheric Pb, Mn, Cr and Ni are 500, 150, 1100 and 0.38 ng/m3, while those reported by the Agency for Toxic Substances and Disease Registry (ATSDR) [29] are 1500, 500, 100 and 0.24 ng/m3 for the same metals in the same order. In the present study, only Ni levels in both PM2.5 and PM10 were many times higher than the proposed WHO and ATSDR standards, and Mn in PM10 at Al-Rughama was higher than WHO standards. The average concentrations of Pb, Cr and Mn were even lower than the WHO and ATSDR standards. Associations between ambient air nickel and cardiovascular disease have become a research issue in the new millennium [21,30,31,32]. At the molecular level and for Saudi Arabia specifically, Brocato et al. [33] reported that in vivo exposures to particulate matter collected from Jeddah or nickel chloride display similar dysregulation of metabolic syndrome genes.
Results from the statistical modeling are presented in Table 3. After adjusting for confounders, we found that PM10 was not associated with MetS (RR: 1.03; 95% CI: 0.95–1.13), hyperglycemia (RR: 0.98; 95% CI: 0.92–1.05), hypertension (RR: 1.06; 95% CI: 1.00–1.13), and BMI (RR: 0.95; 95% CI: 0.89–1.02). This is not in accordance with the results of Brocato et al. [34] who reported that acutely exposing mice to PM10 collected from Jeddah induced genes involved in inflammation, cholesterol and lipid metabolism, and atherosclerosis. On the other hand, we found that a 10 μg/m3 increase in PM2.5 was significantly associated with MetS prevalence (RR: 1.12; 95% CI: 1.06–1.19), hyperglycemia (RR: 1.08; 95% CI: 1.03–1.13) and hypertension (RR: 1.09; 95% CI: 1.01–1.18), but not with BMI (RR: 0.95; 95% CI: 0.91–1.00). This proves that, for chronic exposure, PM10 is less risky concerning cardio metabolic effects.
Table 3.
Risk Ratios (RR) and 95% Confidence Interval (CI) per 10 μg/m3 increment of particulate matter (PM) and factor mass estimates.
| Metabolic Syndrome | Hyperglycemia | Hypertension | BMI | |
|---|---|---|---|---|
| PM2.5 | 1.11 (1.05–1.18) | 1.08 (1.03–1.14) | 1.09 (1.04–1.14) | 0.95 (0.91–1.00) |
| Factor 1: Soil & Road Dust | 1.16 (1.08–1.25) | 1.12 (1.06–1.19) | 1.11 (1.05–1.18) | 0.95 (0.90–1.01) |
| Factor 2: Residual Oil | 0.94 (0.88–1.00) | 0.88 (0.77–1.01) | 0.98 (0.96–1.01) | 1.00 (0.98–1.03) |
| Factor 3: Incineration | 0.99 (0.92–1.07) | 0.98 (0.91–1.05) | 1.00 (0.96–1.04) | 0.85 (0.76–0.95) |
| Factor 4: Traffic | 1.28 (0.94–1.76) | 1.33 (1.05–1.71) | 1.12 (0.89–1.12) | 0.74 (0.55–1.00) |
| PM10 | 1.03 (0.95–1.13) | 0.98 (0.92–1.05) | 1.06 (1.00–1.13) | 0.95 (0.89–1.02) |
| Factor 1: Soil & Road Dust | 1.03 (0.95–1.10) | 0.99 (0.95–1.05) | 1.05 (1.00–1.10) | 0.99 (0.89–1.10) |
| Factor 2: Incineration | 1.02 (0.82–1.26) | 0.90 (0.76–1.06) | 1.08 (0.93–1.26) | 0.84 (0.61–1.17) |
| Factor 3: Traffic | 1.01 (0.56–1.83) | 1.02 (0.67–1.57) | 0.77 (0.53–1.12) | 1.13 (0.90–1.40) |
When the source of PM was studied again, none of the identified source mass estimates showed significance with neither MetS nor the specific endpoints for PM10. For PM2.5, soil/road dust mass was significantly associated with MetS (RR: 1.16; 95% CI: 1.08–1.25), hyperglycemia (RR: 1.12; 95% CI: 1.06–1.19), and hypertension (RR: 1.11; 95% CI: 1.05–1.18). This suggests that crustal elements as Al and Si originating from the surrounding deserts and partially generated as road dust may potentially be responsible for the cardiometabolic effects. Other investigations found that Al and Si induced markers of oxidative stress and inflammation in animals and humans [35,36,37], a major mechanism for the cardiovascular effects induced by particulate matter exposure [38,39]. Moreover, the deleterious role of Si in the development of chronic renal disease [40] might suggest its implication in hypertension. Also, a significant association (RR: 1.33; 95% CI: 1.05–1.71) between PM2.5 traffic source components (Pb, S) and hyperglycemia was observed. Although Pb exposure has been falling, it is likely that even very low levels of exposure are hazardous to health [41]. Pb exposure exerts its effect on cardiovascular diseases by increasing the risk of hypertension, and through its proinflammatory properties affecting all stages of atherogenesis [42]. The actual contribution of Pb exposure to diabetes risk in the general population is difficult to assess. However, numerous studies proved a strong association between low levels of lead exposure and markers of oxidative stress. Oxidative stress is known to promote the development of diabetes through multiple mechanisms. Inhibition of several key components of insulin signaling pathway resulting in insulin resistance, is one of them [43]. Our source-specific findings could thus potentially explain the inconsistent results from the aforementioned studies examining the relationships between DM/hypertension incidence and an index of overall air pollution (e.g., PM2.5 mass), in which some studies observed stronger associations with NO2, a proxy for traffic-related air pollution, than PM2.5 [44,45]. While a direct effect of NO2 cannot be ruled out, results from our study suggest that elemental components generated from traffic-related PM2.5 could potentially be responsible for the observed health effects, confirming that PM might be the major component of air pollution producing the most deleterious effects on human health [46].
The major strength of the present study is that, up to our knowledge, is the first epidemiological study investigating the relationship between source-specific elemental components of PM and metabolic syndrome. However, there are several limitations of this study. First, the retrospective and cross-sectional nature of the study design precludes establishing a definitive causal link between PM2.5 exposure and the outcomes considered here. Second, several elements, carbonaceous species (elemental and organic carbon), and gases that were either not measured, or not considered in our source apportionment, could potentially be causal predictors of health outcomes of interest. For example, due to missing data in the original APCA, some elements (As, Cd, Cl, Co, Ga, Se, Rb) were excluded in the subsequent analysis, thereby potentially influencing factor mass estimates and loadings, and in turn, the associations between source-specific mass estimates and outcomes. A lack of monitoring data for gases and/or carbonaceous species also raise the possibility that the source categories identified in our study are serving as surrogates for other unmeasured correlated constituents actually responsible for the observed adverse health effects.
4. Conclusions
This is the first study to have partitioned cardiometabolic health effects of PM pollution to specific sources of PM in a Middle Eastern city, suggesting that the toxicity of PM did vary by composition. PM2.5 exposure increased the prevalence of MetS, hyperglycemia and hypertension. PM2.5 trace elements from soil/road dust, such as Al and Si, were significantly associated with hyperglycemia and hypertension. Also, a significant association between PM2.5 traffic source components (Pb, S) and hyperglycemia was observed. Ni content in PM might be responsible for the observed effects. This may help decision makers to take strict measures towards limiting exposure to specified components like Ni for example. A larger and more robust study with greater spatial variability for pollutants and detailed adjustments for ecological confounding could enable a more definitive evaluation of the PM-MetS relationship.
Acknowledgments
This study was funded by King Abdulaziz University (KAU), Jeddah, under grant 4/00/00/252 and by NIEHS grant under P30ES000260. The sponsors did not have any role in the study design.
Author Contributions
Magdy Shamy, Mansour Alghamdi, Mamdouh I. Khoder, Jason Brocato, George D. Thurston, Abdularahman K. Alkhalaf, Abdullah M. Mohorjy and Max Costa designed the study and supervised execution of the work. Alser A. Alkhatim, Lung Chi Chen, Chris C. Lim executed the work and analyzed the data. Magdy Shamy, Mamdouh I. Khoder, Mansour Alghamdi and Chris C. Lim wrote the manuscript. Magdy Shamy and Mamdouh I. Khoder contributed to the manuscript preparation and proofreading. All authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Alghamdi M., Alam M., Yin J., Stark C., Jang E., Harrison R., Shamy M., Khoder K., Shabbaj I. Receptor modeling study of polycyclic aromatic hydrocarbons in Jeddah, Saudi Arabia. Sci. Total Environ. 2015;506–507:401–408. doi: 10.1016/j.scitotenv.2014.10.056. [DOI] [PubMed] [Google Scholar]
- 2.Brook R., Rajagopalan S., Pope C., Brook J., Bhatnagar A., Diez-Roux A., Holguin F., Hong Y., Luepker R., Mittleman M., et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 3.Park S., Auchincloss A., O’neill M., Prineas R., Correa J., Keeler J., Roux A. Particulate air pollution, metabolic syndrome, and heart rate variability: The Multi-Ethnic Study of Atherosclerosis (MESA) Environ. Health Perspect. 2010;118:1406–1411. doi: 10.1289/ehp.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cholesterol Education Program (NCEP) Executive Summary of the Third Report of the National Cholesterol Education Program. Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Pope C., Turner M., Burnett R., Jerrett M., Gapstur S., Diver W., Krewski D., Brook R. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ. Res. 2015;116:108–115. doi: 10.1161/CIRCRESAHA.116.305060. [DOI] [PubMed] [Google Scholar]
- 6.Alzahrani A., Karawagh A., Alshahrani F., Naser T., Ahmed A., Alsharef E. Prevalence and predictors of metabolic syndrome among healthy Saudi Adults. Br. J. Diabetes Vasc. Dis. 2012;12:78–80. doi: 10.1177/1474651412441953. [DOI] [Google Scholar]
- 7.Pearson J., Bachireddy C., Shyamprasad S., Goldfine A., Brownstein J. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care. 2010;33:2196–2201. doi: 10.2337/dc10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H., Burnett R., Kwong J., Villeneuve P., Goldberg M., Brook R., Van Donkelaat A., Jerrett M., Martin R., Brook J., et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ. Health Perspect. 2013;121:804–810. doi: 10.1289/ehp.1205958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S., Wang W. Ambient air pollution and type 2 diabetes mellitus: A systematic review of epidemiologic research. Curr. Environ. Health Rep. 2014;1:275–286. doi: 10.1007/s40572-014-0017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yitshak Sade M., Kloog I., Liberty I., Schwartz J., Novack V. The association between air pollution exposure and glucose and lipids levels. J. Clin. Endocrinol. Metab. 2016;101:2460–2467. doi: 10.1210/jc.2016-1378. [DOI] [PubMed] [Google Scholar]
- 11.Auchincloss A., Diez Roux A., Dvonch J., Brown P., Barr R.G., Daviglus M., Goff D., Kaufman G., O’neill M. Associations between recent exposure to ambient fine particulate matter and blood pressure in the multi-ethnic study of atherosclerosis (MESA) Environ. Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S., Deng F., Huang J., Wang H., Shima M., Wang X., Qin Y., Zheng C., Wei H., Hao Y., et al. Blood pressure changes and chemical constituents of particulate air pollution: Results from the Healthy Volunteer Natural Relocation (HVNR) Study. Environ. Health Perspect. 2013;121:66–72. doi: 10.1289/ehp.1104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H., Burnett R., Kwong J., Villeneuve P., Goldberg M., Brook R., Van Donkelaar A., Jerrett M., Martin R., Kopp A., et al. Spatial association between ambient fine particulate matter and incident hypertension. Circulation. 2014;129:562–569. doi: 10.1161/CIRCULATIONAHA.113.003532. [DOI] [PubMed] [Google Scholar]
- 14.Eze I., Schaffner E., Foraster M., Imboden M., Von Eckardstein A., Gerbase M., Rothe T., Rochat T., Kunzli N., Schindler C., et al. Long-term exposure to ambient air pollution and metabolic syndrome in adults. PLoS ONE. 2015;10:e0130337. doi: 10.1371/journal.pone.0130337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Q., Yue P., Deiuliis J., Lumeng C., Kampfrath T., Mikolaj M., Caj Y., Ostrowsky M., Parthasarathy S., Brook R., et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C., Bai Y., Xu X., Sun L., Wang A., Wang T., Maurya S., Periasamy M., Morishita M., Harkema J., et al. Exaggerated effects of particulate matter air pollution in genetic type II diabetes mellitus. Part. Fibre Toxicol. 2014;11:27. doi: 10.1186/1743-8977-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindmark S., Wiklund U., Bjerle P., Eriksson J. Does the autonomic nervous system play a role in the development of insulin resistance? A study on heart rate variability in first-degree relatives of type 2 diabetes patients and control subjects. Diabet. Med. 2003;20:399–405. doi: 10.1046/j.1464-5491.2003.00920.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsai D., Riediker M., Wuerzner G., Maillard M., Marques-Vidal P., Paccaud F., Vollenweider P., Burnier M., Bochud M. Short-term increase in particulate matter blunts nocturnal blood pressure dipping and daytime urinary sodium excretion. Hypertension. 2012;60:1061–1069. doi: 10.1161/HYPERTENSIONAHA.112.195370. [DOI] [PubMed] [Google Scholar]
- 19.National Research Council (NRC) Research Priorities for Airborne Particulate Matter: Continuing Research Progress. National Academies Press; Washington, DC, USA: 2004. [Google Scholar]
- 20.Ostro B., Roth L., Malig B., Marty M. The effects of fine particle components on respiratory hospital admissions in children. Environ. Health Perspect. 2009;117:475–480. doi: 10.1289/ehp.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell M., Ebisu K., Leaderer B., Gent J., Lee H., Koutrakis P., Wang Y., Dominici F., Peng R. Associations between PM2.5 constituents and sources with hospital admissions: Analysis of four counties in Connecticut and Massachusetts (USA) for persons >65 years of age. Environ. Health Perspect. 2014;122:138–144. doi: 10.1289/ehp.1306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurston G., Burnett R., Turner M., Shi Y., Krewski D., Lall R., Ito K., Jerrett M., Gapstur S., Diver W., et al. Ischemic heart disease mortality and long-term exposure to source-related components of U.S. fine particle air pollution. Environ. Health Perspect. 2016;124:785–794. doi: 10.1289/ehp.1509777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khodeir M., Shamy M., Alghamdi M., Zhong M., Sun H., Costa M., Chen L., Maciejczyk P. Source apportionment and elemental composition of PM2.5 and PM10 in Jeddah City, Saudi Arabia. Atmos. Pollut. Res. 2012;3:331–340. doi: 10.5094/APR.2012.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner W., Olson B., Allen G. Calibration of sharp cut impactors for indoor and outdoor particle sampling. J. Air Waste Manag. Assoc. 2000;50:484–487. doi: 10.1080/10473289.2000.10464043. [DOI] [PubMed] [Google Scholar]
- 25.Thurston G., Spengler J. A quantitative assessment of source contributions to inhalable particulate matter in metropolitan Boston, Massachusetts. Atmos. Environ. 1985;19:9–21. doi: 10.1016/0004-6981(85)90132-5. [DOI] [Google Scholar]
- 26.Bahijri S., Al Raddadi R., Jambi H., Alaamer M., Ferns G. The prevalence of metabolic syndrome in an apparently healthy, normotensive and non-diabetic population in Saudi Arabia by two definitions: Implications for local practice. Open J. Endocr. Metab. Dis. 2013;3:18–24. doi: 10.4236/ojemd.2013.31003. [DOI] [Google Scholar]
- 27.WHO. World Health Organization Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide. Global Update. Summary of Risk Assessment 2006. [(accessed on 13 July 2016)]; Available online: http://apps.who.int/iris/bitstream/10665/69477/1/WHO_SDE_PHE_OEH_06.02_eng.pdf.
- 28.Mielke H., Laidlaw M., Gonzales C. Lead (Pb) legacy from vehicle traffic in eight California urbanized areas: Continuing influence of lead dust on children’s health. Sci. Total Environ. 2010;408:3965–3975. doi: 10.1016/j.scitotenv.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 29.ATSDR Agency for Toxic Substances and Disease Registry. Regulations and Guidelines Applicable. [(accessed on 13 July 2016)]; Available online: http://www.atsdr.cdc.gov/toxprofiles/tp11-c8.pdf.
- 30.Peng R., Dominici F., Pastor-Barriuso R., Zeger S., Samet S. Seasonal analyses of air pollution and mortality in 100 US cities. Am. J. Epidemiol. 2005;161:585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- 31.Dominici F., Peng R., Zeger S., White R., Samet J. Particulate air pollution and mortality in the United States: Did the risks change from 1987 to 2000? Am. J. Epidemiol. 2007;166:880–888. doi: 10.1093/aje/kwm222. [DOI] [PubMed] [Google Scholar]
- 32.Pun V., Yu I., Qiu H., Ho K., Sun Z., Louie P., Wong T., Tian L. Short-term associations of cause-specific emergency hospitalizations and particulate matter chemical components in Hong Kong. Am. J. Epidemiol. 2014;179:1086–1095. doi: 10.1093/aje/kwu026. [DOI] [PubMed] [Google Scholar]
- 33.Brocato J., Hernandez M., Laulicht F., Sun H., Shamy M., Alghamdi M., Khoder M., Kluz T., Chen L., Costa M. In vivo exposures to particulate matter collected from Saudi Arabia or nickel chloride display similar dysregulation of metabolic syndrome genes. J. Toxicol. Environ. Health A. 2015;78:1421–1436. doi: 10.1080/15287394.2015.1095689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brocato J., Sun H., Shamy M., Kluz T., Alghamdi M., Khoder M., Chen L., Costa M. Particulate matter from Saudi Arabia induces genes involved in inflammation, metabolic syndrome and atherosclerosis. J. Toxicol. Environ. Health A. 2014;77:751–766. doi: 10.1080/15287394.2014.892446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z., Rong Y., Cui X., Shen Y., Zhou Y., Xiao L., Chen W. Oxidative stress and mitochondrion-related cell apoptosis in human bronchial epithelial 16HBE cells induced by silica dust. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2015;33:801–805. [PubMed] [Google Scholar]
- 36.Schmidt P., Escobar A., Torres J., Martinez C., Rizzetti D., Kunz S., Vassallo D., Alonso M., Peçanha F., Wiggers G. Aluminum exposure for one hour decreases vascular reactivity in conductance and resistance arteries in rats. Toxicol. Appl. Pharmacol. 2016;15:109–118. doi: 10.1016/j.taap.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Srikanth K., Trindade T., Duarte A., Pereira E. Cytotoxicity and oxidative stress responses of silica-coated iron oxide nanoparticles in CHSE-214 cells. Environ. Sci. Pollut. Res. Int. 2017;24:2055–2064. doi: 10.1007/s11356-016-7870-z. [DOI] [PubMed] [Google Scholar]
- 38.Peters A. Ambient particulate matter and the risk for cardiovascular disease. Prog. Cardiovasc. Dis. 2011;53:327–333. doi: 10.1016/j.pcad.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Brook R., Sun Z., Brook J., Zhao X., Yan J., Mukherjee B., Rao X., Duan F., Sun L., Liang R., et al. Extreme air pollution conditions adversely affect blood pressure and insulin resistance: The air pollution and cardiometabolic disease study. Hypertension. 2016;67:77–85. doi: 10.1161/HYPERTENSIONAHA.115.06237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mascarenhas S., Mutnuri S., Ganguly A. Deleterious role of trace elements—Silica and lead in the development of chronic kidney disease. Chemosphere. 2017;177:239–249. doi: 10.1016/j.chemosphere.2017.02.155. [DOI] [PubMed] [Google Scholar]
- 41.Grandjean P. Even low-dose lead exposure is hazardous. Lancet. 2010;376:855–856. doi: 10.1016/S0140-6736(10)60745-3. [DOI] [PubMed] [Google Scholar]
- 42.Poręba R., Gać P., Poręba M., Andrzejak R. Environmental and occupational exposure to lead as a potential risk factor for cardiovascular disease. Environ. Toxicol. Pharmacol. 2011;31:267–277. doi: 10.1016/j.etap.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Tyrell J., Hafida S., Stemmer P., Adhami A., Leff T. Lead (Pb) exposures promotes diabetes in obese rodents. J. Trace Elem. Med. Biol. 2017;39:221–226. doi: 10.1016/j.jtemb.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Puett R., Hart J., Schwartz J., Hu F., Liese A., Laden F. Are particulate matter exposures associated with risk of type 2 diabetes? Environ. Health Perspect. 2011;119:384–389. doi: 10.1289/ehp.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coogan P., White L., Jerrett M., Brook R., Su J., Seto E., Burnett R., Palmer J., Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song C., He J., Wu L., Jin T., Chen X., Li R., Ren P., Zhang L., Mao H. Health burden attributable to ambient PM2.5 in China. Environ. Pollut. 2017;223:575–586. doi: 10.1016/j.envpol.2017.01.060. [DOI] [PubMed] [Google Scholar]