Abstract
Diet plays a crucial role in cognitive function. Few studies have examined the relationship between dietary patterns and cognitive functions of older adults in the Korean population. This study aimed to identify the effect of dietary patterns on the risk of mild cognitive impairment. A total of 239 participants, including 88 men and 151 women, aged 65 years and older were selected from health centers in the district of Seoul, Gyeonggi province, and Incheon, in Korea. Dietary patterns were determined using Reduced Rank Regression (RRR) methods with responses regarding vitamin B6, vitamin C, and iron intakes, based on both a one-day 24-h recall and a food frequency questionnaire. Cognitive function was assessed using the Korean-Mini Mental State Examination (K-MMSE). Multivariable logistic regression models were used to estimate the association between dietary pattern score and the risk of mild cognitive impairment. A total of 20 (8%) out of the 239 participants had mild cognitive impairment. Three dietary patterns were identified: seafood and vegetables, high meat, and bread, ham, and alcohol. Among the three dietary patterns, the older adult population who adhered to the seafood and vegetables pattern, characterized by high intake of seafood, vegetables, fruits, bread, snacks, soy products, beans, chicken, pork, ham, egg, and milk had a decreased risk of mild cognitive impairment compared to those who did not (adjusted odds ratios 0.06, 95% confidence interval 0.01–0.72) after controlling for gender, supplementation, education, history of dementia, physical activity, body mass index (BMI), and duration of sleep. The other two dietary patterns were not significantly associated with the risk of mild cognitive impairment. In conclusion, high consumption of fruits, vegetables, seafood, and protein foods was significantly associated with reduced mild cognitive impairment in older Korean adults. These results can contribute to the establishment of dietary guidelines targeting older Korean adults to reduce mild cognitive impairments. Future prospective cohort studies are warranted to examine the effect of the seafood and vegetable dietary pattern on reducing mild cognitive impairment to prove the cause–effect relationship between dietary patterns and cognitive function.
Keywords: cognitive function, dietary patterns, reduced rank regression (RRR), Korean-Mini Mental State Examination (K-MMSE), older Korean adults
1. Introduction
The number of older adults has rapidly risen worldwide, and cognitive decline is one of the major issues in older adults, associated with adverse health effects such as an increased risk for mortality [1], depressive symptoms [2], and metabolic syndrome [3]. Korea is rapidly transitioning from an ageing society to elderly society. The older adult Korean population, aged 65 or older, with mild cognitive impairment was estimated at 28.5% in 2011 [4].
Dietary factors play a key role in the prevention of cognitive decline. Dietary pattern approaches have been introduced to examine the synergistic effects of foods and nutrients, rather than examining a single nutrient in isolation. Some studies have investigated dietary patterns associated with cognitive functions in older Asian adults, including Korean [5,6] and Japanese [7] populations. Using cluster analysis, two major dietary patterns were identified in an older Korean adult population. Among the two dietary patterns, a dietary pattern including consumption of multi-grain rice, fish, dairy products, fruits, and fruit juices was associated with a lower risk of cognitive impairment [6]. To extract dietary patterns in these studies, empirical data-driven approaches, including principal component analysis (PCA) and cluster analysis, were used to input food or nutrient groups as predictors. The reduced rank regression (RRR) approach was used to input both food groups and responses, such as biomarkers and nutrients, as predictors that are known to be associated with disease. RRR is an innovative method that can elucidate the diet-disease pathway compared to classic PCA [8]. RRR has been used in relation to the risk of numerous diseases such as gestational diabetes mellitus [9], type 2 diabetes mellitus [10], depressive symptoms [11], and esophageal cancer [12]. In a study in Japan, the relationship between dietary patterns identified by RRR and the risk of dementia was examined [7]. Because Korea has distinct and traditional dietary patterns and cultures [13] compared to other Asia or western countries, identifying specific dietary patterns of the older Korean adult population is required.
To the best of our knowledge, few studies have identified the dietary patterns of the older adult Korean population using RRR techniques in relation to the degree of cognitive function. The aim of the study was to identify distinct dietary patterns in relation to cognitive impairment using RRR. We hypothesized that dietary patterns derived by RRR are differentially associated with the degree of cognitive function in the adult Korean population.
2. Materials and Methods
2.1. Study Design and Participants
A sample of 316 older adults aged ≥65 years who reside either in the district of Seoul, Gyeonggi province, or Incheon in Korea, were recruited through a one-to-one interview with questionnaires from March 2012 to July 2012. Of the 316 participants, 239 participants, including 88 men and 151 women, completed both a 24-h recall and a food frequency questionnaire (FFQ). The survey was conducted in the University Hospital clinics, the Elderly Welfare Centers, and the Health Welfare Center in the district of Seoul, Gyeonggi province, and Incheon in Korea. The study was reviewed and approved by the Institutional Review Board at Hannam University (2012-01K).
2.2. Identification of Dietary Patterns
Dietary intake data of older adults were collected by using both a one-day 24-h dietary recall and a 63-item FFQ designed to assess food and nutrient intakes among Koreans. The FFQ included 63 food and beverage items with 9 intake frequency and 4 serving size questions. The modified version of the FFQ from the Korea National Health and Nutrition Examination Survey (KNHANES) was previously validated in reference to 3-day diet records with correlation coefficients ranging from 0.3 to 0.7 [14]. Participants were asked to choose from 9 possible frequency responses, ranging from “never” to “3 times a day” for each food or beverage item. The intake was generated by multiplying the frequency of each food or beverage item by portion size of that food or beverage on a daily basis. Intakes of total energy, proteins, fats, carbohydrates, vitamins, and minerals were calculated from the one-day 24-h dietary recall data. In addition to completing FFQ, only one day 24-h recall was conducted to minimize the burden on respondents as they are older adults. A total of 63 food items were aggregated into 26 food groups. RRR was performed to extract dietary patterns of the older adult population with responses including intakes of vitamin C, vitamin B6, and iron. Responses chosen for RRR were nutrients that have been associated with cognitive impairment in an older adult population in the previous literature (vitamin B6, vitamin B12, vitamin C, vitamin E, calcium, zinc, folate, iron, and saturated fat). Analysis using different numbers of responses indicated that the greatest explanation of the total variation in food groups and in responses was obtained using vitamin B6, vitamin C, and iron (14.2% in food groups and 17.8% in responses). Results of this analysis is presented in Supplemental Table S1.
2.3. Assessment of Cognitive Function
Cognitive function was examined by the Korean-adjusted version of the Mini-Mental State Examination (K-MMSE), which is the most widely used screening tool for quantitative assessment of the cognitive status of the older adult population, developed by Kwon and Park [15], adapted from Folstein et al. [16]. The K-MMSE includes 19 items covering the following fields: orientation (10 points), language function (6 points), attention and calculation (5 points), memory (3 points), memory recall (3 points), understanding and judgment (2 point), and visuospatial construction (1 point). The scores range from 0 to 30. A higher K-MMSE score indicates better cognitive function. The study population with normal cognitive function were those who had a K-MMSE score equal or greater than −1.5 standard deviations (SD) of the mean score [17]. The study population with mild cognitive impairment were those who had a K-MMSE less than −1.5 SD of the overall mean MMSE score [17].
2.4. Covariates
The multivariate model was adjusted for sex, supplementation, education, history of dementia, physical activity, body mass index (BMI), and sleep duration. Sex was categorized as men or women. Supplementation was divided into yes or no. Education level was categorized as uneducated, ≤high school, and graduate college or higher. History of dementia was divided into yes or no. Physical activity was categorized as yes or no. BMI (kg/m2) and sleep duration (hours/day) were controlled in a continuous variable.
2.5. Statistical Analyses
Descriptive statistics were used to examine the frequencies and means (SD) of socioeconomic and lifestyle variables in relation to two categories of cognitive function status: mild cognitive function or normal cognitive function. Distributions of socioeconomic status, lifestyle variables, and nutrients were examined in relation to the tertiles of each dietary pattern. The chi-square test for categorical variables and the t-test for continuous variables were used to examine the differences in sociodemographic, lifestyle variables, and nutrients across the tertiles of the dietary pattern scores. Analysis of variance (ANOVA) was conducted to test if any significant differences existed across the tertiles of each dietary pattern score. Multivariable logistic regression was used to calculate adjusted odds ratios with 95% confidence intervals (CI) to examine the relationship between each dietary pattern and the risk of mild cognitive impairment after controlling covariates including sex, daily supplement use, education, history of dementia, physical activity, BMI, and daily sleep duration. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA).
3. Results
Table 1 shows the differences in descriptive statistics of socioeconomic and lifestyle variables between older adults with mild cognitive impairment compared to those with normal cognitive functions. Sex, daily supplement use, education, self-reported health, tooth conditions, physical activity, age, BMI, and sleep duration significantly differ with cognitive function status (p value < 0.05).
Table 1.
Sociodemographic characteristics of study participants between the degrees of cognitive function.
| Sociodemographic Characteristics | K-MMSE | p Value 1 | |||
|---|---|---|---|---|---|
| Mild Cognitive Impairment (n = 20) | Normal (n = 219) | ||||
| n | % | n | % | ||
| Sex | |||||
| Men | 2 | 10.0 | 86 | 39.3 | 0.009 |
| Women | 18 | 90.0 | 133 | 60.7 | |
| Daily Supplements Use | |||||
| Yes | 0 | 0.0 | 108 | 49.3 | <0.0001 |
| No | 20 | 100.0 | 111 | 50.7 | |
| Family Type | |||||
| With spouse | 6 | 30.0 | 102 | 46.6 | 0.215 |
| With children | 7 | 35.0 | 75 | 34.3 | |
| Spouse and children | 1 | 5.0 | 15 | 6.9 | |
| Alone | 5 | 25.0 | 20 | 9.1 | |
| Other | 1 | 5.0 | 7 | 3.2 | |
| Education | |||||
| Uneducated | 15 | 75.0 | 36 | 16.4 | <0.0001 |
| ≤High school Graduate | 5 | 25.0 | 177 | 80.8 | |
| College or Higher | 0 | 0.0 | 6 | 2.7 | |
| Self-Reported Health Status | |||||
| Very good | 0 | 0.0 | 12 | 5.5 | 0.028 |
| Good | 6 | 30.0 | 42 | 19.2 | |
| Fair | 6 | 30.0 | 118 | 53.9 | |
| Poor | 4 | 20.0 | 36 | 16.4 | |
| Very poor | 4 | 20.0 | 11 | 5.0 | |
| Self-Reported Teeth Condition | |||||
| Very good | 2 | 10.0 | 10 | 83.3 | <0.0001 |
| Good | 0 | 0.0 | 45 | 20.6 | |
| Fair | 2 | 10.0 | 78 | 35.6 | |
| Poor | 6 | 30.0 | 66 | 30.1 | |
| Very poor | 10 | 50.0 | 20 | 9.1 | |
| History of Dementia | |||||
| Yes | 2 | 10.0 | 10 | 4.6 | 0.287 |
| No | 18 | 90.0 | 209 | 95.4 | |
| Smoking Status | |||||
| Yes | 18 | 90.0 | 186 | 84.9 | 0.539 |
| No | 2 | 10.0 | 33 | 15.1 | |
| Physical Activity | |||||
| None | 12 | 60.0 | 56 | 25.6 | <0.0001 |
| 1–2x/week | 0 | 0.0 | 86 | 39.3 | |
| 3–4x/week | 0 | 0.0 | 35 | 16.0 | |
| Everyday | 8 | 40.0 | 42 | 19.2 | |
| Variables | Mean | SD | Mean | SD | p Value 1 |
| Age (years) | 80.5 | 5.2 | 73.4 | 5.9 | <0.0001 |
| BMI (kg/m2) | 18.3 | 4.2 | 22.1 | 3.5 | <0.0001 |
| Sleep Duration (hours/day) | 5.2 | 2.7 | 6.9 | 1.6 | 0.0129 |
1 Chi-square test was used for categorical variables, and t-test was used for continuous variables. K-MMSE: Korean-Mini Mental State Examination; BMI: body mass index; SD: standard deviations.
Three dietary patterns were identified using RRR, as presented in Table 2, with factor loadings on each food or beverage group. The first dietary pattern called the “seafood and vegetables pattern” was characterized by a high intake of seafood, vegetables, bread, snacks, soy products, beans, chicken, pork, ham, egg, fruits, and milk. The second pattern, labeled “high meat pattern” was characterized by high consumption of whole grains, beef, and pork, and low consumption of soy products, sweet potatoes, eggs, and milk. The third pattern, named “bread, ham, and alcohol pattern” was characterized by high consumption of bread, ham, and alcohol, and low consumption of beans, sweet potatoes, pork, eggs, and seaweed.
Table 2.
Factor loadings and explained variation in food groups, and response of dietary patterns from reduced rank regression (RRR) in an older Korean adult population.
| Food Groups | Seafood and Vegetables Pattern | High Meat Pattern | Bread, Ham, and Alcohol Pattern |
|---|---|---|---|
| White rice | - | - | - |
| Whole grains | - | 0.26 | - |
| Noodles | - | - | - |
| Bread | 0.21 | - | 0.34 |
| Rice cakes | - | - | - |
| Snacks | 0.25 | - | - |
| Soy products | 0.29 | −0.39 | - |
| Beans | 0.29 | - | −0.26 |
| Potatoes | - | - | - |
| Sweet potatoes | - | −0.28 | −0.29 |
| Beef | - | 0.32 | - |
| Chicken | 0.24 | - | - |
| Pork | 0.23 | 0.43 | −0.24 |
| Ham | 0.22 | - | 0.31 |
| Egg | 0.27 | −0.27 | −0.44 |
| Fish | - | - | - |
| Seafood (Fish cake, squid, clam, salted fish) | 0.33 | - | - |
| Vegetables | 0.37 | - | - |
| Seaweed | - | - | −0.26 |
| Fruits | 0.24 | 0.25 | - |
| Milk | 0.21 | −0.36 | - |
| Yogurt | - | - | - |
| Ice cream | - | - | - |
| Beverages | - | - | - |
| Alcohol | - | - | 0.30 |
| Fast foods | - | - | - |
| Explained variation in: | |||
| Food groups (%) | 5.30 | 3.67 | 3.06 |
| Responses (%) | 15.35 | 3.20 | 2.00 |
The following factors had loadings ≥|0.20| are shown in the table.
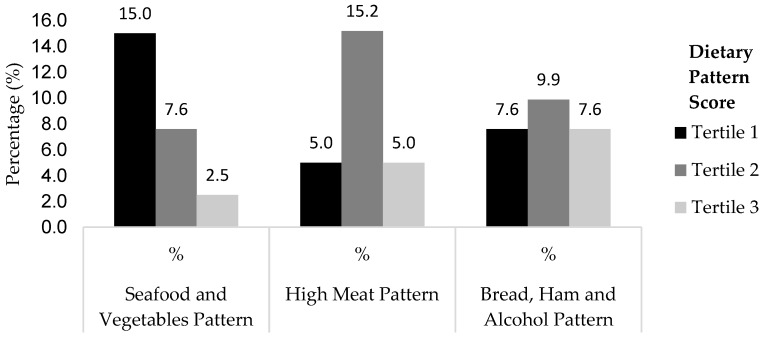
The prevalence of mild cognitive impairment across the tertiles of the dietary pattern score is presented in Figure 1. The prevalence of older adults with mild cognitive impairment decreased with an increase in the seafood and vegetables pattern score, from the lowest tertile (15%) to the highest tertile (2.5%).
Figure 1.
Prevalence of mild cognitive impairment across the tertiles of dietary pattern score.
Socioeconomic status and lifestyle variables across the tertiles of each dietary pattern score is presented in Table 3. The distributions of daily supplement use, history of dementia, physical activity, age, and BMI significantly differed amongst the tertiles of the seafood and vegetables pattern (p value < 0.05). Self-reported health status, history of dementia, and K-MMSE significantly differed among the tertiles of the high meat pattern (p value < 0.05). Distributions of sex, family type, education, self-reported health status, smoking status, age, and K-MMSE significantly differed among the tertiles of the bread, am, and alcohol pattern (p value < 0.05).
Table 3.
Sociodemographics and lifestyles across the tertiles of each dietary pattern score.
| Sociodemographic and Lifestyle Characteristics | Seafood and Vegetables Pattern | High Meat Pattern | Bread, Ham and Alcohol Pattern | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 (n = 80) | Tertile 2 (n = 79) | Tertile 3 (n = 80) | p Value | Tertile 1 (n = 80) | Tertile 2 (n = 79) | Tertile 3 (n = 80) | p Value | Tertile 1 (n = 79) | Tertile 2 (n = 81) | Tertile 3 (n = 79) | p Value | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||||
| Sex | |||||||||||||||||||||
| Men | 36 | 45.0 | 23 | 29.1 | 29 | 36.3 | 0.115 | 26 | 32.5 | 26 | 32.9 | 36 | 45.0 | 0.177 | 24 | 30.4 | 22 | 27.2 | 42 | 53.2 | 0.001 |
| Women | 44 | 55.0 | 56 | 70.9 | 51 | 63.8 | 54 | 67.5 | 53 | 67.1 | 44 | 55.0 | 55 | 69.6 | 59 | 72.8 | 37 | 46.8 | |||
| Supplements | |||||||||||||||||||||
| Yes | 28 | 35.0 | 36 | 45.6 | 44 | 55.0 | 0.039 | 41 | 51.3 | 29 | 36.7 | 38 | 47.5 | 0.161 | 41 | 51.9 | 28 | 51.9 | 39 | 49.4 | 0.058 |
| No | 52 | 65.0 | 43 | 54.4 | 36 | 45.0 | 39 | 48.8 | 50 | 63.3 | 42 | 52.5 | 38 | 48.1 | 53 | 65.4 | 40 | 50.6 | |||
| Family Type | |||||||||||||||||||||
| With spouse | 35 | 43.8 | 34 | 43.0 | 39 | 48.8 | 0.158 | 35 | 43.8 | 36 | 45.6 | 37 | 46.3 | 0.523 | 26 | 32.9 | 44 | 54.3 | 38 | 48.1 | 0.006 |
| With children | 26 | 32.5 | 28 | 35.4 | 28 | 35.0 | 26 | 32.5 | 24 | 30.4 | 32 | 40.0 | 35 | 44.3 | 24 | 29.6 | 23 | 29.1 | |||
| Spouse and children | 4 | 5.0 | 3 | 3.8 | 9 | 11.3 | 6 | 7.5 | 8 | 10.1 | 2 | 2.5 | 3 | 3.8 | 2 | 2.5 | 11 | 13.9 | |||
| Alone | 12 | 15.0 | 11 | 13.9 | 2 | 2.5 | 10 | 12.5 | 7 | 8.9 | 8 | 10.0 | 12 | 15.2 | 7 | 8.6 | 6 | 7.6 | |||
| Other | 3 | 3.8 | 3 | 3.8 | 2 | 2.5 | 3 | 3.8 | 4 | 5.1 | 1 | 1.3 | 3 | 3.8 | 4 | 4.9 | 1 | 1.3 | |||
| Education | |||||||||||||||||||||
| Uneducated | 23 | 28.8 | 15 | 19.0 | 13 | 16.3 | 0.154 | 22 | 27.5 | 16 | 20.3 | 13 | 16.3 | 0.216 | 11 | 13.9 | 26 | 32.1 | 14 | 17.7 | 0.014 |
| ≤High school Graduate | 57 | 71.3 | 61 | 77.2 | 64 | 80.0 | 57 | 71.3 | 62 | 78.5 | 63 | 78.8 | 68 | 86.1 | 52 | 64.2 | 62 | 78.5 | |||
| College or higher | 0 | 0.0 | 3 | 3.8 | 3 | 3.8 | 1 | 1.3 | 1 | 1.3 | 4 | 5.0 | 0 | 0.0 | 3 | 3.7 | 3 | 3.8 | |||
| Self-Reported Health | |||||||||||||||||||||
| Very good | 4 | 5.0 | 4 | 5.1 | 4 | 5.0 | 0.792 | 1 | 1.3 | 2 | 2.5 | 9 | 11.3 | 0.005 | 9 | 11.4 | 0 | 0.0 | 3 | 3.8 | 0.019 |
| Good | 17 | 21.3 | 16 | 20.3 | 15 | 18.8 | 19 | 23.8 | 10 | 12.7 | 19 | 23.8 | 15 | 19.0 | 16 | 19.8 | 17 | 21.5 | |||
| Fair | 41 | 51.3 | 45 | 57.0 | 38 | 47.5 | 39 | 48.8 | 46 | 58.2 | 39 | 48.8 | 38 | 48.1 | 40 | 49.4 | 46 | 58.2 | |||
| Poor | 13 | 16.3 | 12 | 15.2 | 15 | 18.8 | 14 | 17.5 | 19 | 24.1 | 7 | 8.8 | 15 | 19.0 | 17 | 21.0 | 8 | 10.1 | |||
| Very poor | 5 | 6.3 | 2 | 2.5 | 8 | 10.0 | 7 | 8.8 | 2 | 2.5 | 6 | 7.5 | 2 | 2.5 | 8 | 9.9 | 5 | 6.3 | |||
| Teeth Condition | |||||||||||||||||||||
| Very good | 5 | 6.3 | 0 | 0.0 | 7 | 8.8 | 0.144 | 4 | 5.0 | 5 | 6.3 | 3 | 3.8 | 0.132 | 7 | 8.9 | 2 | 2.5 | 3 | 3.8 | 0.197 |
| Good | 14 | 17.5 | 13 | 16.5 | 18 | 22.5 | 14 | 17.5 | 10 | 12.7 | 21 | 26.3 | 14 | 17.7 | 14 | 17.3 | 17 | 21.5 | |||
| Fair | 23 | 28.8 | 29 | 36.7 | 28 | 35.0 | 27 | 33.8 | 23 | 29.1 | 30 | 37.5 | 27 | 34.2 | 21 | 25.9 | 32 | 40.5 | |||
| Poor | 24 | 30.0 | 27 | 34.2 | 21 | 26.3 | 23 | 28.8 | 33 | 41.8 | 16 | 20.0 | 23 | 29.1 | 30 | 37.0 | 19 | 24.1 | |||
| Very poor | 14 | 17.5 | 10 | 12.7 | 6 | 7.5 | 12 | 15.0 | 8 | 10.1 | 10 | 12.5 | 8 | 10.1 | 14 | 17.3 | 8 | 10.1 | |||
| History of Dementia | |||||||||||||||||||||
| Yes | 2 | 2.5 | 8 | 10.1 | 2 | 2.5 | 0.040 | 9 | 11.3 | 3 | 3.8 | 0 | 0.0 | 0.004 | 2 | 2.5 | 7 | 8.6 | 3 | 3.8 | 0.174 |
| No | 78 | 97.5 | 71 | 89.9 | 78 | 97.5 | 71 | 88.8 | 76 | 96.2 | 80 | 100.0 | 77 | 97.5 | 74 | 91.4 | 76 | 96.2 | |||
| Smoking | |||||||||||||||||||||
| Yes | 65 | 81.3 | 70 | 88.6 | 69 | 86.3 | 0.407 | 71 | 88.8 | 69 | 87.3 | 64 | 80.0 | 0.244 | 69 | 87.3 | 74 | 91.4 | 61 | 77.2 | 0.034 |
| No | 15 | 18.8 | 9 | 11.4 | 11 | 13.8 | 9 | 11.3 | 10 | 12.7 | 16 | 20.0 | 10 | 12.7 | 7 | 8.6 | 18 | 22.8 | |||
| Physical Activity | |||||||||||||||||||||
| None | 33 | 41.3 | 11 | 13.9 | 24 | 30.0 | 0.0007 | 15 | 18.8 | 30 | 38.0 | 23 | 28.8 | 0.099 | 19 | 24.1 | 25 | 30.9 | 24 | 30.4 | 0.288 |
| 1–2x/week | 23 | 28.8 | 42 | 53.2 | 21 | 26.3 | 35 | 43.8 | 28 | 35.4 | 23 | 28.8 | 36 | 45.6 | 21 | 25.9 | 29 | 36.7 | |||
| 3–4x/week | 9 | 11.3 | 10 | 12.7 | 16 | 20.0 | 12 | 15.0 | 9 | 11.4 | 14 | 17.5 | 9 | 11.4 | 15 | 18.5 | 11 | 13.9 | |||
| Every day | 15 | 18.8 | 16 | 20.3 | 19 | 23.8 | 18 | 22.5 | 12 | 15.2 | 20 | 25.0 | 15 | 19.0 | 20 | 24.7 | 15 | 19.0 | |||
| Variables | Mean | SD | Mean | SD | Mean | SD | p Value | Mean | SD | Mean | SD | Mean | SD | p Value | Mean | SD | Mean | SD | Mean | SD | p Value |
| Age (years) | 76.2 | 6.5 | 72.8 | 6.0 | 73.1 | 5.3 | 0.0004 | 75.0 | 6.7 | 73.9 | 4.9 | 73.2 | 6.5 | 0.155 | 72.9 | 5.7 | 75.8 | 6.6 | 73.4 | 5.8 | 0.005 |
| BMI (kg/m2) | 20.8 | 3.9 | 21.7 | 3.7 | 23.0 | 3.1 | 0.0004 | 21.4 | 3.3 | 22.1 | 4.3 | 22.0 | 3.4 | 0.411 | 22.1 | 3.2 | 21.3 | 4.1 | 22.1 | 3.7 | 0.254 |
| Sleep Duration (hours/day) | 7.1 | 1.9 | 6.5 | 1.6 | 6.6 | 1.8 | 0.067 | 6.6 | 1.9 | 7.0 | 1.8 | 6.6 | 1.6 | 0.233 | 6.8 | 1.7 | 6.7 | 1.9 | 6.7 | 1.8 | 0.886 |
| MMSE | 25.7 | 4.8 | 26.0 | 5.2 | 26.4 | 4.3 | 0.613 | 25.5 | 4.7 | 25.1 | 5.2 | 27.6 | 4.1 | 0.002 | 26.3 | 4.4 | 25.0 | 5.3 | 26.9 | 4.4 | 0.032 |
MMSE: mini mental state examination.
Energy and nutrient intakes by the tertiles of each dietary pattern score are presented in Table 4. Protein as a percentage of energy, fiber, moisture, vitamin A, vitamin C, vitamin B2, niacin, vitamin B6, folate, calcium, phosphorous, sodium, potassium, iron, and zinc significantly increased as the seafood and vegetables pattern score increased from tertile one to tertile three (p value < 0.05). Vitamin C intake significantly increased from the lowest to the highest tertiles of the high meat pattern score (p value < 0.05). Intakes of fat as a percentage of energy, protein as a percentage of energy, saturated fatty acids, monounsaturated fatty acids, niacin, and vitamin B6 significantly increased, but folate and iron significantly decreased across the tertiles of the bread, ham, and alcohol pattern.
Table 4.
Energy and nutrient intake across the tertiles of each dietary pattern.
| Energy and Nutrient Intake Variables | Seafood and Vegetables Pattern | High Meat Pattern | Bread, Ham and Alcohol Pattern | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | p Value | Tertile 1 | Tertile 2 | Tertile 3 | p Value | Tertile 1 | Tertile 2 | Tertile 3 | p Value | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Energy (kcal/day) | 1519 | 273 | 1783 | 1668 | 1722 | 352 | 0.214 | 1613 | 286 | 1822 | 1672 | 1589 | 331 | 0.268 | 1608 | 283 | 1767 | 1651 | 1645 | 365 | 0.575 |
| Carbohydrate (% of energy) | 70.6 | 7.8 | 69.2 | 10.6 | 67.6 | 7.8 | 0.096 | 69.0 | 8.3 | 67.6 | 10.5 | 70.8 | 7.4 | 0.075 | 69.1 | 7.6 | 70.7 | 10.6 | 67.6 | 8.0 | 0.093 |
| Fat (% of energy) | 14.7 | 6.7 | 15.4 | 6.8 | 16.5 | 6.1 | 0.218 | 15.7 | 6.7 | 16.2 | 6.8 | 14.8 | 6.2 | 0.439 | 16.0 | 6.3 | 14.0 | 6.4 | 16.7 | 6.8 | 0.025 |
| Protein (% of energy) | 14.7 | 2.7 | 15.1 | 3.1 | 16.4 | 3.1 | 0.001 | 15.7 | 3.0 | 15.3 | 3.2 | 15.2 | 3.1 | 0.600 | 15.5 | 2.7 | 14.8 | 3.2 | 16.0 | 3.2 | 0.035 |
| SFA (g/1000 kcal) | 5.5 | 6.9 | 4.4 | 4.8 | 6.1 | 6.6 | 0.241 | 6.1 | 7.3 | 4.6 | 5.4 | 5.3 | 5.7 | 0.280 | 5.3 | 5.5 | 4.1 | 5.4 | 6.6 | 7.3 | 0.042 |
| MUFA (g/1000 kcal) | 7.2 | 9.3 | 6.0 | 6.7 | 7.9 | 8.8 | 0.334 | 8.0 | 10.1 | 5.9 | 7.1 | 7.2 | 7.5 | 0.268 | 7.2 | 7.5 | 5.4 | 7.2 | 8.6 | 9.9 | 0.050 |
| PUFA (g/1000 kcal) | 4.6 | 3.5 | 4.5 | 3.2 | 5.3 | 3.4 | 0.272 | 5.0 | 3.8 | 4.5 | 3.0 | 5.0 | 3.3 | 0.522 | 5.1 | 3.2 | 4.1 | 3.1 | 5.3 | 3.8 | 0.068 |
| Fiber (g/1000 kcal) | 13.7 | 2.9 | 14.7 | 3.5 | 15.1 | 3.7 | 0.029 | 14.3 | 3.7 | 14.4 | 3.6 | 14.9 | 3.0 | 0.458 | 14.9 | 3.2 | 14.2 | 3.4 | 14.3 | 3.6 | 0.370 |
| Moisture (g/1000 kcal) | 381.0 | 108.0 | 413.6 | 119.8 | 441.9 | 102.1 | 0.003 | 396.6 | 98.6 | 419.6 | 127.8 | 420.4 | 109.1 | 0.315 | 411.7 | 116.0 | 405.3 | 110.9 | 419.7 | 111.6 | 0.722 |
| Vitamin A (μg/1000 kcal) | 432.3 | 289.5 | 540.5 | 302.9 | 558.8 | 374.7 | 0.030 | 502.0 | 340.6 | 508.4 | 312.6 | 520.8 | 334.1 | 0.935 | 548.1 | 309.4 | 474.1 | 296.2 | 509.9 | 374.1 | 0.363 |
| Vitamin E (mg/1000 kcal) | 7.1 | 3.3 | 7.8 | 3.6 | 8.0 | 3.0 | 0.164 | 7.7 | 3.2 | 7.7 | 3.3 | 7.5 | 3.5 | 0.947 | 8.0 | 3.2 | 7.2 | 3.5 | 7.6 | 3.3 | 0.362 |
| Vitamin C (mg/1000 kcal) | 55.7 | 24.5 | 62.9 | 29.7 | 75.5 | 32.5 | 0.0001 | 58.4 | 24.4 | 63.8 | 31.1 | 71.9 | 33.0 | 0.016 | 68.1 | 32.4 | 63.5 | 29.6 | 62.5 | 28.2 | 0.458 |
| Thiamin (mg/1000 kcal) | 0.7 | 0.1 | 0.7 | 0.1 | 0.7 | 0.1 | 0.388 | 0.7 | 0.1 | 0.7 | 0.2 | 0.7 | 0.1 | 0.587 | 0.7 | 0.1 | 0.7 | 0.1 | 0.7 | 0.2 | 0.059 |
| Vitamin B2 (mg/1000 kcal) | 0.6 | 0.2 | 0.6 | 0.2 | 0.7 | 0.2 | 0.002 | 0.6 | 0.2 | 0.6 | 0.2 | 0.6 | 0.2 | 0.895 | 0.7 | 0.2 | 0.6 | 0.2 | 0.6 | 0.2 | 0.133 |
| Niacin (mg/1000 kcal) | 8.0 | 2.3 | 8.2 | 2.4 | 9.0 | 2.3 | 0.015 | 8.4 | 2.5 | 8.4 | 2.4 | 8.3 | 2.1 | 0.922 | 8.1 | 2.2 | 8.0 | 2.2 | 9.0 | 2.6 | 0.023 |
| Vitamin B6 (mg/1000 kcal) | 0.9 | 0.3 | 0.9 | 0.2 | 1.0 | 0.2 | 0.018 | 0.9 | 0.2 | 0.9 | 0.3 | 1.0 | 0.2 | 0.519 | 0.9 | 0.2 | 0.9 | 0.2 | 1.0 | 0.3 | 0.004 |
| Folate (μg/1000 kcal) | 337.8 | 91.4 | 359.8 | 118.3 | 387.6 | 131.7 | 0.025 | 370.0 | 137.3 | 338.9 | 95.9 | 376.1 | 109.8 | 0.097 | 389.4 | 136.5 | 350.8 | 110.2 | 345.4 | 95.2 | 0.034 |
| Vitamin B12 (mg/1000 kcal) | 4.8 | 5.9 | 6.6 | 7.4 | 6.3 | 4.9 | 0.127 | 6.7 | 6.7 | 5.2 | 4.5 | 5.8 | 7.0 | 0.320 | 6.3 | 6.5 | 5.8 | 7.0 | 5.6 | 4.8 | 0.756 |
| Calcium (mg/1000 kcal) | 294.3 | 108.6 | 314.6 | 106.6 | 347.9 | 116.3 | 0.009 | 329.6 | 110.6 | 315.9 | 109.3 | 311.3 | 117.5 | 0.566 | 333.7 | 113.9 | 309.7 | 107.1 | 313.7 | 115.9 | 0.353 |
| Phosphorous (mg/1000 kcal) | 610.9 | 138.1 | 626.5 | 143.0 | 663.5 | 125.7 | 0.044 | 633.3 | 131.3 | 630.7 | 143.6 | 636.9 | 137.7 | 0.960 | 628.0 | 126.6 | 623.4 | 144.1 | 649.8 | 139.8 | 0.432 |
| Potassium (mg/1000 kcal) | 1712.2 | 433.4 | 1757.4 | 440.3 | 1915.0 | 514.3 | 0.016 | 1829.8 | 524.9 | 1735.4 | 456.4 | 1819.0 | 424.5 | 0.385 | 1877.8 | 479.9 | 1718.8 | 425.9 | 1790.3 | 495.9 | 0.101 |
| Iron (mg/1000 kcal) | 8.2 | 1.7 | 8.9 | 2.1 | 9.7 | 2.8 | <0.001 | 9.4 | 2.9 | 8.6 | 2.1 | 8.8 | 1.8 | 0.118 | 9.5 | 2.7 | 8.7 | 2.1 | 8.6 | 2.1 | 0.027 |
| Zinc (mg/1000 kcal) | 6.0 | 1.3 | 6.3 | 1.4 | 6.6 | 1.2 | 0.016 | 6.4 | 1.4 | 6.1 | 1.4 | 6.4 | 1.1 | 0.136 | 6.4 | 1.2 | 6.3 | 1.5 | 6.2 | 1.1 | 0.523 |
| Copper (mg/1000 kcal) | 0.8 | 0.2 | 0.8 | 0.2 | 0.8 | 0.2 | 0.530 | 0.8 | 0.2 | 0.8 | 0.3 | 0.8 | 0.2 | 0.698 | 0.8 | 0.2 | 0.8 | 0.2 | 0.8 | 0.2 | 0.597 |
| Cholesterol (mg/1000 kcal) | 145.1 | 103.8 | 135.0 | 90.1 | 128.9 | 73.9 | 0.515 | 143.5 | 102.1 | 134.6 | 84.8 | 130.9 | 82.3 | 0.666 | 144.4 | 97.5 | 129.2 | 87.8 | 135.7 | 84.6 | 0.565 |
Adjusted odds ratios (AOR) and the 95% CI for mild cognitive impairment across the tertiles of each dietary pattern score are presented in Table 5. Older adults in the highest tertile of the seafood and vegetable pattern were less likely to have mild cognitive impairment compared to those older adults in the lowest tertile (AOR 0.06, 95% CI 0.01–0.72) after controlling for covariates (p-for-trend < 0.027). Each unit increase in the seafood and vegetable pattern score significantly lowered the odds for mild cognitive impairment (AOR 0.25, 95% CI 0.08–0.86). The number of older adults affected by mild cognitive impairment reduced from 12 in the lowest tertile of the seafood and vegetable pattern to two in the highest tertile of the seafood and vegetable pattern. The other two dietary patterns, high meat, and bread, ham, and alcohol, were not significantly associated with the risk of mild cognitive impairment.
Table 5.
Adjusted odds ratios (AOR) for mild cognitive impairment across tertiles of each dietary pattern.
| Dietary Patterns | Mild Cognitive Impairment (n)/Total (n) | AOR 1 | 95% CI |
|---|---|---|---|
| Seafood and Vegetables Pattern | |||
| Tertile 1 | 12/80 | 1.00 | |
| Tertile 2 | 6/79 | 0.45 | 0.04–5.63 |
| Tertile 3 | 2/80 | 0.06 | 0.01–0.72 |
| Continuous | 0.25 | 0.08–0.86 | |
| p-for-trend | 0.027 | ||
| High Meat Pattern | |||
| Tertile 1 | 4/80 | 1.00 | |
| Tertile 2 | 12/79 | 13.69 | 0.98–190.94 |
| Tertile 3 | 4/80 | 1.25 | 0.06–25.90 |
| Continuous | 1.11 | 0.34–3.59 | |
| p-for-trend | 0.866 | ||
| Bread, Ham and Alcohol Pattern | |||
| Tertile 1 | 6/79 | 1.00 | |
| Tertile 2 | 8/81 | 0.10 | 0.01–1.06 |
| Tertile 3 | 6/79 | 0.56 | 0.08–3.83 |
| Continuous | 0.78 | 0.29–2.07 | |
| p-for-trend | 0.611 |
1 Adjusted for sex (men/women), supplemental use (yes/no), education (uneducated; ≤high school; graduate college or higher), history of dementia (yes/no), physical activity, BMI (kg/m2), and sleep duration (hours/day). AOR: adjusted odds ratios; 95% CI: 95% confidence intervals.
4. Discussion
We identified three dietary patterns in the older Korean adult population aged 65 or older: seafood and vegetables, high meat, and bread, ham, and alcohol. Among the three patterns, the older adult population who adhered to the seafood and vegetables pattern had a decreased risk for mild cognitive impairment compared to those who did not. In the meantime, the other two dietary patterns were not associated with mild cognitive impairment. The very low value in explained variation for “High Meat” Pattern and “Bread, Ham, and Alcohol” Pattern could partially explain no significant association with cognitive impairment. Previous studies have identified specific dietary patterns related to the degree of cognitive function or Alzheimer’s disease using different dietary approaches, including factor analysis, cluster analysis, RRR, and index analysis. In a French older population [18], participants with the highest adherence to the healthy dietary pattern, characterized by high consumption of fruit, whole grains, fresh dairy products, and vegetables, demonstrated better cognitive functioning compared to those with the least adherence. In a prospective cohort study of U.S. older adults, Gu et al. [19] found that the older adults with higher intakes of salad dressing, nuts, fish, tomatoes, poultry, cruciferous vegetables, fruits, and dark and green leafy vegetables, and a lower intake of high-fat dairy products, red meat, organ meat, and butter, had a reduced risk of Alzheimer’s disease. In a cross-sectional study for older Chinese people [20], older adults with the vegetable-fruits pattern, characterized by high intakes of vegetables and fruits, soy products, and legumes, had a reduced risk of cognitive impairment. Overall, these previous studies indicated that the overlapping food groups that reduced the risk of cognitive impairment were fruits and vegetable containing high antioxidants that are associated with reduced risk of cognitive impairment [21].
In our study, the seafood and vegetable pattern indicating high consumption of fruits and vegetables lowering the risk of mild cognitive impairment is supported by a U.S. prospective cohort study [22] and a populations-based cohort of Australian adults [23]. In the Nurses’ Health Study [22], the effect of daily in fruit and vegetable intake on cognitive function and decline was examined in women aged 70 and older, and a higher vegetable intake was found to be associated with less cognitive decline. In a population of older adults aged 60 and older, the fruit and vegetable pattern, derived by PCA, was associated with a lower likelihood of cognitive impairment. High concentrations of antioxidants in fruits and vegetables demonstrated protective effects of cognitive decline in previous findings.
Older adults who adhered to the seafood and vegetables pattern had significantly higher intakes of protein as a percentage of energy, fiber, moisture, vitamin A, vitamin C, vitamin B2, niacin, vitamin B6, folate, calcium, phosphorous, potassium, iron, and zinc. Our findings align with previous findings. In a study of Italian older adults (mean age: 75.8 years), patients with mild cognitive impairment presented significantly lower plasma levels of vitamin C, vitamin E, and A compared to their counterparts [24]. Vitamin C is a powerful water soluble antioxidant that prevents the oxidative process by donating its electrons [25,26]. Another antioxidant, Vitamin E, has been found to have a beneficial role in reduced cognitive decline [27] by capturing reactive oxygen-free radicals and reducing oxidative stress [28]. Notably, a significantly higher intake of folate and vitamin B6 was found in those who followed the seafood and vegetable pattern compared to those who did not. Folate and vitamin B6 have been shown to have beneficial effects for cognitive decline. Folate deficiency is closely linked to elevated homocysteine, which has been associated with recall memory decline in aging men [29] and increased risk for cognitive impairment in an older adults in The Netherlands [30]. Folate is found in vegetables and may support the beneficial effect of the seafood and vegetables pattern on cognitive decline. Seafood consumption [31], tuna and dark-meat fish (one vs. less than one serving/week) has also been associated with lower decline in verbal memory during a four-year follow-up (OR 0.84, 95% CI 0.60–0.96) of women. In the present study, squid and clams contain high amounts of omega-3 fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which have been to found to reduce amyloid-beta deposition, neuron loss, and eventually improve cognitive functioning as found by a meta-analysis [32].
Our study has several limitations. Firstly, due to the cross-sectional study design, the cause-effect relationship cannot be drawn in this study. Secondly, changes in cognitive functioning could not be captured in this study. However, dietary intake was assessed using both a food frequency questionnaire and 24-h recalls, which captured both short- and long-term dietary intake. Thirdly, although we controlled for a large number of covariates such as sex, supplemental use, education, history of dementia, physical activity, BMI, and sleep duration, we may have not accounted for residual confounding. Lastly, a single, one-day 24-h recall may not be representative of an individual’s usual diet.
5. Conclusions
In conclusion, the seafood and vegetables dietary pattern was significantly associated with reduced mild cognitive impairment in older Korean adults. These results may contribute to the establishment of dietary guidelines targeting older Korean adults to reduce mild cognitive impairment. The RRR is useful over other models since it reveals diet-disease pathway, and they are in line with global findings that may lead to the development of global recommendations. Future prospective cohort studies are warranted to examine the effects of the seafood and vegetable dietary pattern on reducing mild cognitive impairment to prove the cause–effect relationship between dietary patterns and cognitive function.
Acknowledgments
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2016S1A3A2924243).
Supplementary Materials
The following are available online at www.mdpi.com/1660-4601/15/1/100/s1, Table S1: Selection of responses variables in the reduced rank regression (RRR) analysis.
Author Contributions
Dayeon Shin conceptualized the study question, conducted the data analysis and interpretation, and wrote the first draft of the manuscript. Kyung Won Lee provided inputs for data analysis and critically revised the manuscript. Mi-Hye Kim, Hung Ju Kim, and Yun Sook An interpreted the data and contributed to manuscript development and revision. Hae-Kyung Chung guided the manuscript development and critically revised the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Dewey M.E., Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: A systematic review of the literature. Int. J. Geriatr. Psychiatry. 2001;16:751–761. doi: 10.1002/gps.397. [DOI] [PubMed] [Google Scholar]
- 2.Barnes D.E., Alexopoulos G.S., Lopez O.L., Williamson J.D., Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: Findings from the Cardiovascular Health Study. Arch. Gen. Psychiatry. 2006;63:273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K., Kanaya A., Lindquist K., Simonsick E.M., Harris T., Shorr R.I., Tylavsky F.A., Newman A.B. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y.K. Proceedings of the Health-Welfare Policy Forum. Korea Institute for Health and Social Affairs (KiHASA); Sejong Special Self-Governing City, Korea: 2012. Functional Activities and Assistance Needs of the Elderly; pp. 52–63. [Google Scholar]
- 5.Kim J., Yu A., Choi B.Y., Nam J.H., Kim M.K., Oh D.H., Kim K., Yang Y.J. Dietary patterns and cognitive function in Korean older adults. Eur. J. Nutr. 2015;54:309–318. doi: 10.1007/s00394-014-0713-0. [DOI] [PubMed] [Google Scholar]
- 6.Kim J., Yu A., Choi B.Y., Nam J.H., Kim M.K., Oh D.H., Yang Y.J. Dietary Patterns Derived by Cluster Analysis are Associated with Cognitive Function among Korean Older Adults. Nutrients. 2015;7:4154–4169. doi: 10.3390/nu7064154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozawa M., Ninomiya T., Ohara T., Doi Y., Uchida K., Shirota T., Yonemoto K., Kitazono T., Kiyohara Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013;97:1076–1082. doi: 10.3945/ajcn.112.045575. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann K., Schulze M.B., Schienkiewitz A., Nothlings U., Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004;159:935–944. doi: 10.1093/aje/kwh134. [DOI] [PubMed] [Google Scholar]
- 9.Shin D., Lee K.W., Song W.O. Dietary patterns during pregnancy are associated with risk of gestational diabetes mellitus. Nutrients. 2015;7:9369–9382. doi: 10.3390/nu7115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.InterAct Consortium Adherence to predefined dietary patterns and incident type 2 diabetes in European populations: EPIC-InterAct Study. Diabetologia. 2014;57:321–333. doi: 10.1007/s00125-013-3092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeulen E., Stronks K., Visser M., Brouwer I.A., Schene A.H., Mocking R.J., Colpo M., Bandinelli S., Ferrucci L., Nicolaou M. The association between dietary patterns derived by reduced rank regression and depressive symptoms over time: The Invecchiare in Chianti (InCHIANTI) study. Br. J. Nutr. 2016;115:2145–2153. doi: 10.1017/S0007114516001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y., Yngve A., Lagergren J., Lu Y. A dietary pattern rich in lignans, quercetin and resveratrol decreases the risk of oesophageal cancer. Br. J. Nutr. 2014;112:2002–2009. doi: 10.1017/S0007114514003055. [DOI] [PubMed] [Google Scholar]
- 13.Chung H.-K., Yang H.J., Shin D., Chung K.R. Aesthetics of Korean foods: The symbol of Korean culture. J. Ethn. Foods. 2016;3:178–188. doi: 10.1016/j.jef.2016.09.001. [DOI] [Google Scholar]
- 14.Hong S., Choi Y., Lee H.J., Kim S.H., Oe Y., Lee S.Y., Nam M., Kim Y.S. Development and validation of a semi-quantitative food frequency questionnaire to assess diets of korean type 2 diabetic patients. Korean Diabetes J. 2010;34:32–39. doi: 10.4093/kdj.2010.34.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon Y.C., Park J.-H. Korean version of Mini-Mental State Examination (MMSE-K). Part I: Development of the test for the elderly. J. Korean Neuropsychiatr. Assoc. 1989;28:125–135. [Google Scholar]
- 16.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Seoul National University Bundang Hospital . Standardization of Dementia Diagnosis Tool. Ministry for Health, Welfare and Family Affairs; Seoul, Korea: 2009. [Google Scholar]
- 18.Kesse-Guyot E., Andreeva V.A., Jeandel C., Ferry M., Hercberg S., Galan P. A healthy dietary pattern at midlife is associated with subsequent cognitive performance. J. Nutr. 2012;142:909–915. doi: 10.3945/jn.111.156257. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y., Nieves J.W., Stern Y., Luchsinger J.A., Scarmeas N. Food combination and Alzheimer disease risk: A protective diet. Arch. Neurol. 2010;67:699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan R., Chan D., Woo J. A cross sectional study to examine the association between dietary patterns and cognitive impairment in older Chinese people in Hong Kong. J. Nutr. Health Aging. 2013;17:757–765. doi: 10.1007/s12603-013-0348-5. [DOI] [PubMed] [Google Scholar]
- 21.Jiang X., Huang J., Song D., Deng R., Wei J., Zhang Z. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Cognitive Impairment and Dementia: Meta-Analysis. Front. Aging Neurosci. 2017;9:18. doi: 10.3389/fnagi.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang J.H., Cook N.R., Manson J.E., Buring J.E., Albert C.M., Grodstein F. Vitamin E, Vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: The Women’s Antioxidant and Cardiovascular Study. Circulation. 2009;119:2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashby-Mitchell K., Peeters A., Anstey K.J. Role of dietary pattern analysis in determining cognitive status in elderly Australian adults. Nutrients. 2015;7:1052–1067. doi: 10.3390/nu7021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinaldi P., Polidori M.C., Metastasio A., Mariani E., Mattioli P., Cherubini A., Catani M., Cecchetti R., Senin U., Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging. 2003;24:915–919. doi: 10.1016/S0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 25.Zandi P.P., Anthony J.C., Khachaturian A.S., Stone S.V., Gustafson D., Tschanz J.T., Norton M.C., Welsh-Bohmer K.A., Breitner J.C. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch. Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 26.Padayatty S.J., Katz A., Wang Y., Eck P., Kwon O., Lee J.-H., Chen S., Corpe C., Dutta A., Dutta S.K. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 27.Morris M.C., Evans D.A., Bienias J.L., Tangney C.C., Wilson R.S. Vitamin E and cognitive decline in older persons. Arch. Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- 28.Hong J.H., Kim M.J., Park M.R., Kwag O.G., Lee I.S., Byun B.H., Lee S.C., Lee K.B., Rhee S.J. Effects of vitamin E on oxidative stress and membrane fluidity in brain of streptozotocin-induced diabetic rats. Clin. Chim. Acta. 2004;340:107–115. doi: 10.1016/j.cccn.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Tucker K.L., Qiao N., Scott T., Rosenberg I., Spiro A. High homocysteine and low B vitamins predict cognitive decline in aging men: The Veterans Affairs Normative Aging Study. Am. J. Clin. Nutr. 2005;82:627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 30.Mooijaart S.P., Gussekloo J., Frölich M., Jolles J., Stott D.J., Westendorp R.G., de Craen A.J. Homocysteine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: The Leiden 85-Plus study. Am. J. Clin. Nutr. 2005;82:866–871. doi: 10.1093/ajcn/82.4.866. [DOI] [PubMed] [Google Scholar]
- 31.Kim D.H., Grodstein F., Rosner B., Kang J.H., Cook N.R., Manson J.E., Buring J.E., Willett W.C., Okereke O.I. Seafood types and age-related cognitive decline in the Women’s Health Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013;68:1255–1262. doi: 10.1093/gerona/glt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooijmans C.R., Pasker-de Jong P.C., de Vries R.B., Ritskes-Hoitinga M. The effects of long-term omega-3 fatty acid supplementation on cognition and Alzheimer’s pathology in animal models of Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2012;28:191–209. doi: 10.3233/JAD-2011-111217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.